Transport of Nanoparticles into Plants and Their Detection Methods
Abstract
:1. Introduction
2. Transport of Nanoparticles into Plants
2.1. Types of Nanoparticles Used for Transport
| Factor | Description |
|---|---|
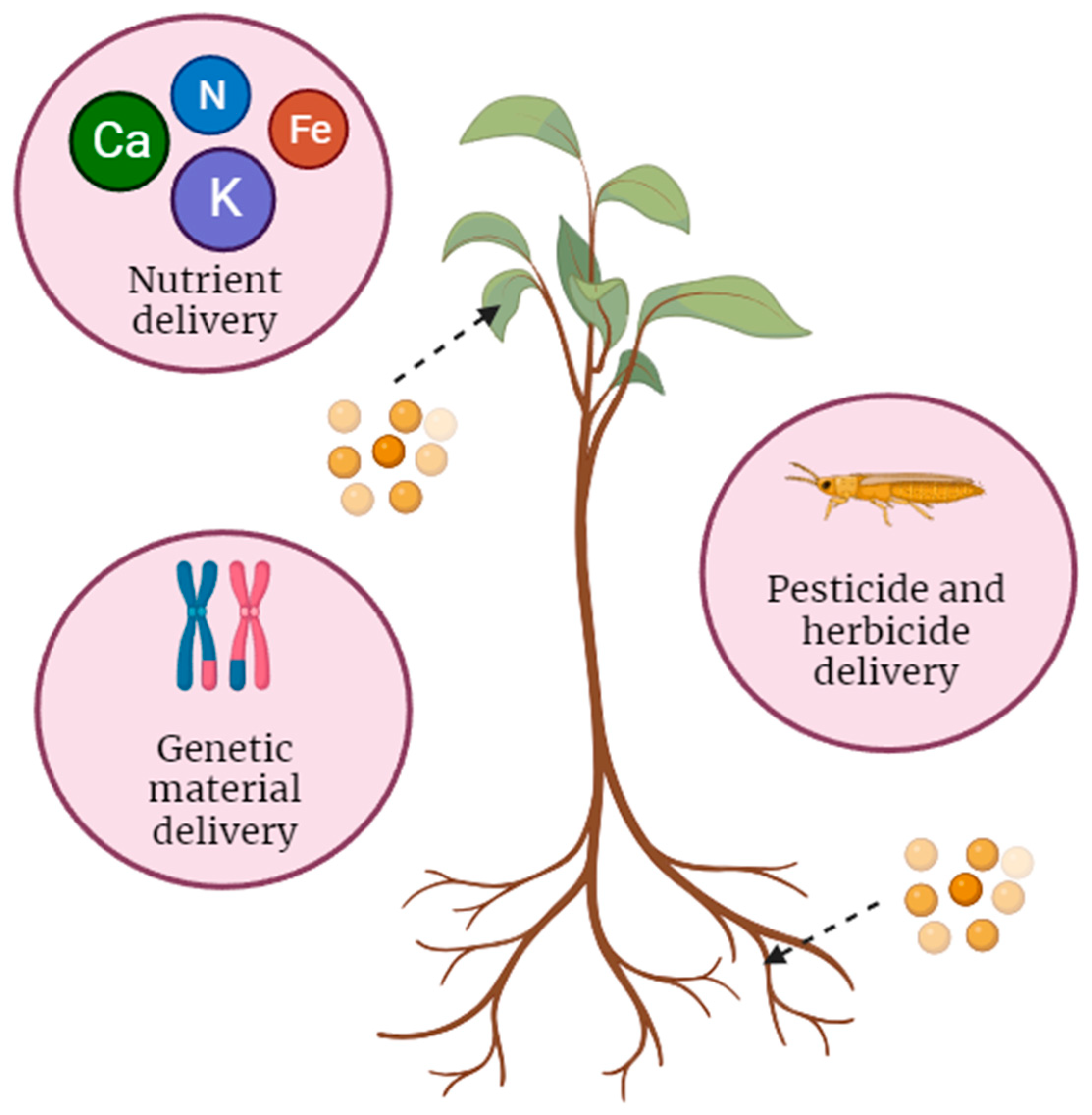
| Desired application | Different applications, such as nutrient delivery, genetic material transfer, or pesticide transport, require specific nanoparticle types with suitable properties [25,28,31]. |
| Payload type | The nature of the payload, whether it is nutrients, genetic material (DNA or RNA), pesticides, or other substances, influences the selection of the appropriate nanoparticle [23,24,29]. |
| Payload size and solubility | The size and solubility of the payload may determine the choice of nanoparticle, as some nanoparticles are better suited for carrying particular types of cargo [40]. |
| Targeted delivery | If precise delivery to specific plant tissues or cells is required, the nanoparticle type should allow for targeted delivery [41]. |
| Biocompatibility | Some applications, such as those involving genetic material delivery or interactions with living organisms, necessitate biocompatible nanoparticles [42]. |
| Environmental considerations | The environmental impact of nanoparticle use, including factors like biodegradability and safety, is crucial in agriculture and ecological applications [43]. In addition to these considerations, environmental conditions such as soil pH, temperature, and relative humidity play pivotal roles in determining the fate and impact of nanoparticles [44]. |
| Size and shape | The size and shape of nanoparticles can influence their ability to enter plant cells or tissues. In some cases, specific shapes or sizes may be more effective [29,45]. |
| Crop or plant type | Different plant species or crops may have varying requirements and responses to nanoparticle-based applications, affecting the selection of nanoparticle types [46]. |
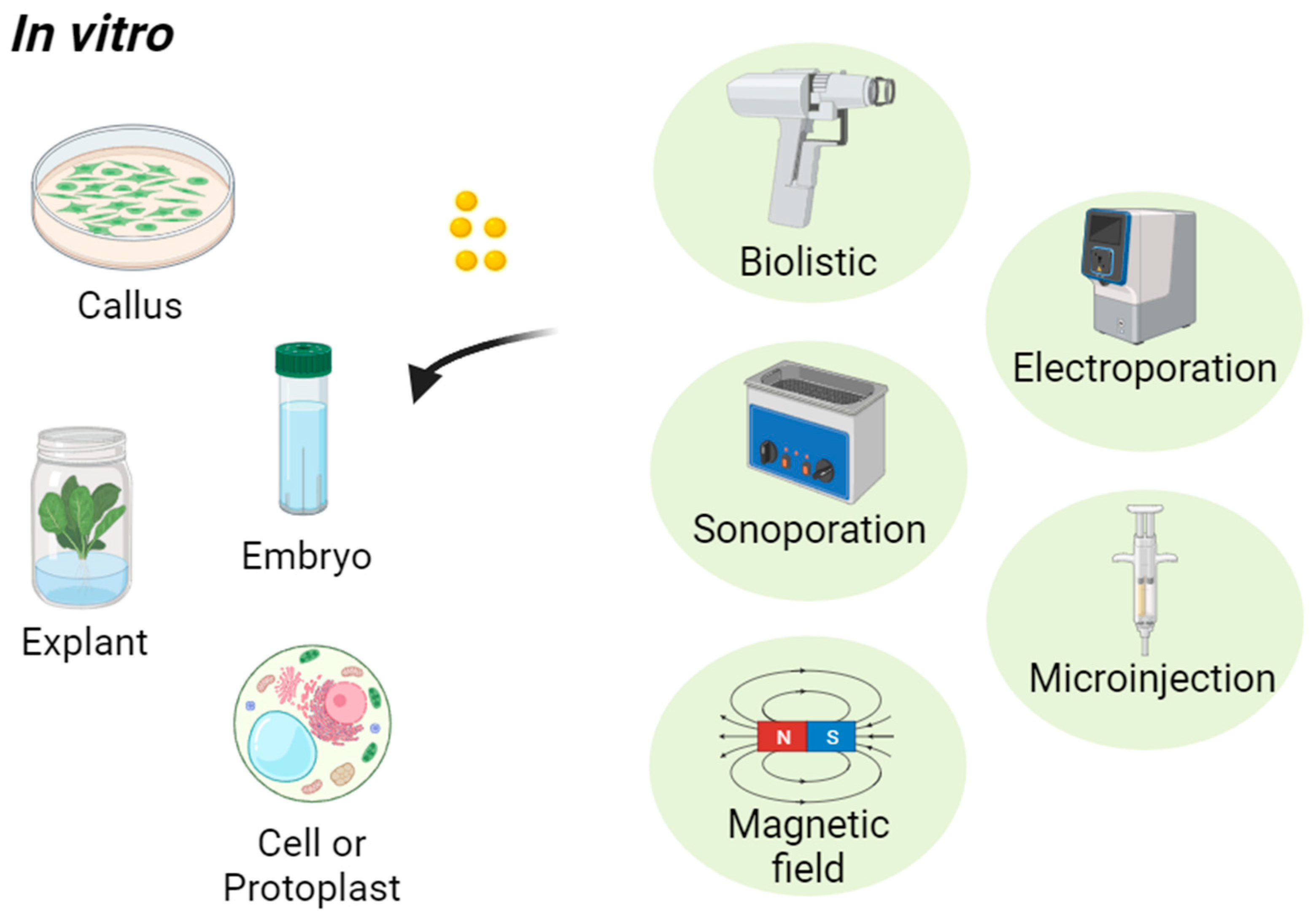
2.2. Modes of Transport of Nanoparticles into Plants
| Method | NPs | Size (nm) | Plants | Target | Ref. |
|---|---|---|---|---|---|
| Biolistic (gene gun) | Ag | 100 | Nicotiana tabacum | Leaf explants | [94] |
| Au | 712 ± 95 | Nicotiana benthamiana | Leaf explants | [106] | |
| Au-MSN | 600 | Nicotiana tabacum, Teosinte | Leaf explants | [111] | |
| Au-MSN | 600 | Zea mays | Embryos | [103] | |
| Fe | 255 ± 170 | Nicotiana benthamiana | Leaf explants | [106] | |
| Sonoporation | hPAMAM-G2 | 123 ± 21 | Medicago sativa | Cells | [112] |
| PEI-MSN | 100 ± 87 | Nicotiana tabacum | Cells | [96] | |
| Magnetic field | γ-Fe2O3 | 21.2 ± 3 | Zea mays | Roots | [113] |
| Carbon-coated iron | 10–50 | Cucurbita pepo | Roots | [114] | |
| Fe2O3 | 10 | Solanum lycopersicum | Roots | [115] | |
| Fe3O4 | 13 | Hordeum vulgare | Roots | [116] | |
| Electroporation | CPNs | 60–80 | Tobacco | Protoplasts | [105] |
| Microinjection | mGNPs | 20–30 | Brassica napus | Cells | [117] |
| SWNTs | 500 | Nicotiana tabacum | Cells | [118] |
2.3. Challenges during Nanoparticle Transport
3. Detection of Nanoparticles in Plants
3.1. Type of Detection Methods
3.2. Challenges in Using Selected Detection Methods
4. Plant Stems as Future Recognition Sites for Transport
| Plants | Xylem Diameter (μm) | Phloem Diameter (μm) | Ref. |
|---|---|---|---|
| Arabidopsis thaliana | 16 | - | [227] |
| Cucurbita maxima | - | 5 | [228] |
| Ipomoea hederifolia | - | 323–358 | [229] |
| Larix sibirica | - | 24–29 | [230] |
| Phaseolus vulgaris | - | 1.5–20 | [228] |
| Populus trichocarpa | 29–104 | 16–43 | [231] |
| Portulaca grandiflora | 121.5 | - | [232] |
| Portulaca oleracea | 98 | - | [232] |
| Portulaca quadrifida | 101.9 | - | [232] |
| Quercus chapmanni | 333 | - | [233] |
| Quercus falcata | 492 | - | [233] |
| Quercus hemisphaerica | 474 | - | [233] |
| Quercus incana | 470 | - | [233] |
| Quercus laevis | 469 | - | [233] |
| Quercus margaretta | 345 | - | [233] |
| Quercus myrtifolia | 455 | - | [233] |
| Quercus nigra | 517 | - | [233] |
| Quercus pubescens | 202.4 | 35.8 | [234] |
| Quercus sessiliflora | 1837.6 | 286.8 | [235] |
| Quercus austrina | 340 | - | [233] |
| Quercus geminata | 273 | - | [233] |
| Quercus michauxii | 332 | - | [233] |
| Quercus shumardii | 569 | - | [233] |
| Quercus virginiana | 286 | - | [233] |
| Ricinus communis | 300 | 100–150 | [236] |
| Rosmarinus officinalis | 467.3 | 7.2 | [237] |
| Solanum lycopersicum | - | 1.5–20 | [228] |
| Talinum fruticosum | 133.2 | - | [232] |
| Tectona grandis | 110.2–212.5 | - | [238] |
| Vitis vinifera | 33.6–35.8 | - | [239] |
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczak, A.; Osiak, M.; Cárdenas-Pérez, S.; Lubińska-Mielińska, S.; Piernik, A. Osmotic stress or ionic composition: Which affects the early growth of crop species more? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Choi, H.S.; Cho, H.T. Root hairs enhance Arabidopsis seedling survival upon soil disruption. Sci. Rep. 2019, 9, 11181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro-and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.; Espen, L.; Prinsi, B. Nitrogen uptake in plants: The plasma membrane root transport systems from a physiological and proteomic perspective. Plants 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G.; et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Kurotani, K.I.; Notaguchi, M. Cell-to-cell connection in plant grafting—Molecular insights into symplasmic reconstruction. Plant Cell Physiol. 2021, 62, 1362–1371. [Google Scholar] [CrossRef]
- Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The apoplast: A key player in plant survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef]
- van Bel, A.J. The plant axis as the command centre for (re) distribution of sucrose and amino acids. J. Plant Physiol. 2021, 265, 153488. [Google Scholar] [CrossRef]
- Wang, M.; Dinh, Q.T.; Qi, M.; Wang, M.; Yang, W.; Zhou, F.; Liang, D. Radicular and foliar uptake, and xylem-and phloem-mediated transport of selenium in maize (Zea mays L.): A comparison of five Se exogenous species. Plant Soil 2020, 446, 111–123. [Google Scholar] [CrossRef]
- Brewer, K.; Clulow, A.; Sibanda, M.; Gokool, S.; Odindi, J.; Mutanga, O.; Naiken, V.; Chimonyo, V.G.P.; Mabhaudhi, T. Estimation of maize foliar temperature and stomatal conductance as indicators of water stress based on optical and thermal imagery acquired using an unmanned aerial vehicle (UAV) platform. Drones 2022, 6, 169. [Google Scholar] [CrossRef]
- Wheeler, T.D.; Stroock, A.D. The transpiration of water at negative pressures in a synthetic tree. Nature 2008, 455, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Dontsova, T.A.; Nahirniak, S.V.; Astrelin, I.M. Metaloxide nanomaterials and nanocomposites of ecological purpose. J. Nanomater. 2019, 2019, 5942194. [Google Scholar] [CrossRef]
- Handy, R.D.; Von der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle size effects in biomedical applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Xin, X.; Judy, J.D.; Sumerlin, B.B.; He, Z. Nano-enabled agriculture: From nanoparticles to smart nanodelivery systems. Environ. Chem. 2020, 17, 413–425. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology strategies for plant genetic engineering. Adv. Mater. 2022, 34, 2106945. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Grandio, E.G.; Newkirk, G.M.; Demirer, G.S.; Butrus, S.; Giraldo, J.P.; Landry, M.P. Nanoparticle-mediated genetic engineering of plants. Mol. Plant 2019, 12, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; Espirito Santo Pereira, A.D.; Sharif, R.; Jogaiah, S.; Paidi, M.K.; Wang, L.; Ali, Q.; et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: Current trends and future directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Monreal, C.M.; DeRosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Ercan, I.; Alshammari, T.; Tombuloglu, G.; Slimani, Y.; Almessiere, M.; Baykal, A. Incorporation of micro-nutrients (nickel, copper, zinc, and iron) into plant body through nanoparticles. J. Soil Sci. Plant Nutr. 2020, 20, 1872–1881. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Jat, S.K.; Bhattacharya, J.; Sharma, M.K. Nanomaterial based gene delivery: A promising method for plant genome engineering. J. Mater. Chem. B 2020, 8, 4165–4175. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. Nanomaterials 2016, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Lenggoro, I.W. Comparative analysis of germination performance from several species of seeds under influence of silica nanoparticles. IOP Conf. Ser. Earth Environ. Sci. 2023, 1271, 012085. [Google Scholar] [CrossRef]
- Sembada, A.A.; Maki, S.; Faizal, A.; Fukuhara, T.; Suzuki, T.; Lenggoro, I.W. The role of silica nanoparticles in promoting the germination of tomato (Solanum lycopersicum) seeds. Nanomaterials 2023, 13, 2110. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Yu, F.; Chen, C.; Faraz, A.; Hayat, S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: A review. Curr. Protein Pept. Sci. 2021, 22, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on natural, incidental, bioinspired, and engineered nanomaterials: History, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Santana, I.; Wu, H.; Hu, P.; Giraldo, J.P. Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 2020, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Chanana, M. Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, K.; Tan, K.X.; Loo, S.C.J. Developing nano-delivery systems for agriculture and food applications with nature-derived polymers. Iscience 2020, 23, 101055. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Kim, K.H.; Deep, A.; Das, P.; Bhattacharya, S.S.; Kumar, S.; Adelodun, A.A. Engineered nano particles: Nature, behavior, and effect on the environment. J. Environ. Manag. 2017, 196, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Flores, A.D.R.; Zhai, R.; et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Antisari, L.V.; Carbone, S.; Gatti, A.; Vianello, G.; Nannipieri, P. Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ. Sci. Pollut. Res. 2014, 22, 1841–1853. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337. [Google Scholar] [CrossRef]
- Lee, W.M.; Kwak, J.I.; An, Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Koelmel, J.; Leland, T.; Wang, H.; Amarasiriwardena, D.; Xing, B. Investigation of gold nanoparticles uptake and their tissue level distribution in rice plants by laser ablation-inductively coupled-mass spectrometry. Environ. Pollut. 2013, 174, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sabo-Attwood, T.; Unrine, J.M.; Stone, J.W.; Murphy, C.J.; Ghoshroy, S.; Blom, D.; Bertsch, P.M.; Newman, L.A. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 2012, 6, 353–360. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef]
- Lizzi, D.; Mattiello, A.; Adamiano, A.; Fellet, G.; Gava, E.; Marchiol, L. Influence of cerium oxide nanoparticles on two terrestrial wild plant species. Plants 2021, 10, 335. [Google Scholar] [CrossRef]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Rico, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef]
- Rico, C.M.; Morales, M.I.; McCreary, R.; Castillo-Michel, H.; Barrios, A.C.; Hong, J.; Tafoya, A.; Lee, W.Y.; Varela-Ramirez, A.; Peralta-Videa, J.R.; et al. Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ. Sci. Technol. 2013, 47, 14110–14118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, X.; Zhang, W.; Pei, H.; Chen, Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 2012, 4, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhang, H.; Ma, Y.; Zhang, P.; Ding, Y.; Zhao, Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, V.; Guo, Y.; Rajput, V.D.; Chalenko, E.; Yadronova, O.; Zaruba, T.; Varduny, T.; Kirichenko, E. Changes of dorsoventral asymmetry and anoxygenic photosynthesis in response of Chelidonium majus leaves to the SiO2 nanoparticle treatment. Photosynthetica 2023, 61, 275–284. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Wang, S.; Kurepa, J.; Smalle, J.A. Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ. 2011, 34, 811–820. [Google Scholar] [CrossRef]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of carbon nanoparticles to improve soil fertility, crop growth and nutrient uptake by corn (Zea mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Dasgupta-Schubert, N.; Villaseñor Cendejas, L.M.; Villegas, J.; Carreto Montoya, L.; Borjas García, S.E. Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl. Nanosci. 2014, 4, 577–591. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B.; Matsuoka, M.; Akasaka, T.; Watari, F. Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage. Appl. Surf. Sci. 2012, 262, 120–124. [Google Scholar] [CrossRef]
- Mondal, A.; Basu, R.; Das, S.; Nandy, P. Beneficial role of carbon nanotubes on mustard plant growth: An agricultural prospect. J. Nanopart. Res. 2011, 13, 4519–4528. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Liu, X.; Gu, X.; Chen, K.; Lu, D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012, 14, 841. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef] [PubMed]
- Modlitbová, P.; Novotný, K.; Pořízka, P.; Klus, J.; Lubal, P.; Zlámalová-Gargošová, H.; Kaiser, J. Comparative investigation of toxicity and bioaccumulation of Cd-based quantum dots and Cd salt in freshwater plant Lemna minor L. Ecotoxicol. Environ. Saf. 2018, 147, 334–341. [Google Scholar] [CrossRef]
- Modlitbová, P.; Pořízka, P.; Novotný, K.; Drbohlavová, J.; Chamradová, I.; Farka, Z.; Zlámalová-Gargošová, H.; Romih, T.; Kaiser, J. Short-term assessment of cadmium toxicity and uptake from different types of Cd-based Quantum Dots in the model plant Allium cepa L. Ecotoxicol. Environ. Saf. 2018, 153, 23–31. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Zhu, H.; Braam, J.; Schnoor, J.L.; Alvarez, P.J. Uptake, translocation, and transformation of quantum dots with cationic versus anionic coatings by Populus deltoides× nigra cuttings. Environ. Sci. Technol. 2014, 48, 6754–6762. [Google Scholar] [CrossRef]
- Lian, F.; Wang, C.; Wang, C.; Gu, S.; Cao, X. Variety-dependent responses of rice plants with differential cadmium accumulating capacity to cadmium telluride quantum dots (CdTe QDs): Cadmium uptake, antioxidative enzyme activity, and gene expression. Sci. Total Environ. 2019, 697, 134083. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Murugan, I.; Selvaraj, M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr. Polym. 2019, 212, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; Nair, S.J.; Jisha, M.S. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019, 124, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Wang, Q.; Ebbs, S.D.; Chen, Y.; Ma, X. Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 2013, 5, 753–759. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.; Schwab, A.P.; Ma, X. Mutual effects and in planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Rani, N.; Kumari, K.; Sangwan, P.; Barala, P.; Yadav, J.; Vijeta; Rahul; Hooda, V. Nano-iron and nano-zinc induced growth and metabolic changes in Vigna radiata. Sustainability 2022, 14, 8251. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Kralova, K.; Jampilek, J. Responses of medicinal and aromatic plants to engineered nanoparticles. Appl. Sci. 2021, 11, 1813. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.H. Advantage of nanotechnology-based genome editing system and its application in crop improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A. Nanobiolistics: New generation transfection system for animals and plants. J. Mol. Cell. Biol. Forecast 2020, 3, 1020. [Google Scholar]
- Rajkumari, N.; Alex, S.; Soni, K.B.; Anith, K.N.; Viji, M.M.; Kiran, A.G. Silver nanoparticles for biolistic transformation in Nicotiana tabacum L. 3 Biotech 2021, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Gupta, P.; Chakraborty, D. Physical methods of gene transfer: Kinetics of gene delivery into cells: A Review. Agric. Rev. 2015, 36, 61–66. [Google Scholar] [CrossRef]
- Zolghadrnasab, M.; Mousavi, A.; Farmany, A.; Arpanaei, A. Ultrasound-mediated gene delivery into suspended plant cells using polyethyleneimine-coated mesoporous silica nanoparticles. Ultrason. Sonochem. 2021, 73, 105507. [Google Scholar] [CrossRef] [PubMed]
- González-Melendi, P.; Fernández-Pacheco, R.; Coronado, M.J.; Corredor, E.; Testillano, P.S.; Risueño, M.C.; Marquina, C.; Ibarra, M.R.; Rubiales, D.; Pérez-de-Luque, A. Nanoparticles as smart treatment-delivery systems in plants: Assessment of different techniques of microscopy for their visualization in plant tissues. Ann. Bot. 2008, 101, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.; Rodriguez Mariblanca, I.; Pini, V.; Dias, M.; Cebrian, V.; Thon, A.; Saad, A.; Salvador-Matar, A.; Ahumada, Ó.; Silván, M.M.; Saunders, A.E.; et al. Novel characterization techniques for multifunctional plasmonic–magnetic nanoparticles in biomedical applications. Nanomaterials 2023, 13, 2929. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic materials and systems: Domain structure visualization and other characterization techniques for the application in the materials science and biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A review of the current state of magnetic force microscopy to unravel the magnetic properties of nanomaterials applied in biological systems and future directions for quantum technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Adhikari, S.; Orrit, M. Progress and perspectives in single-molecule optical spectroscopy. J. Chem. Phys. 2022, 156, 160903. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, K.; Frey, N.; Zienicke, B.; Siebenbrock, J.; Schunck, T.; Fischer, R.; Bräse, S.; Birtalan, E.; Nann, T.; Nick, P. Use of nanoparticles to study and manipulate plant cells. Adv. Eng. Mater. 2010, 12, B406–B412. [Google Scholar] [CrossRef]
- Martin-Ortigosa, S.; Peterson, D.J.; Valenstein, J.S.; Lin, V.S.Y.; Trewyn, B.G.; Lyznik, L.A.; Wang, K. Mesoporous silica nanoparticle-mediated intracellular Cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014, 164, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Moradi, M.; Garousi, G.A. Elicitation of stevia glycosides using salicylic acid and silver nanoparticles under callus culture. Sugar Tech 2019, 21, 569–577. [Google Scholar] [CrossRef]
- Silva, A.T.; Nguyen, A.; Ye, C.; Verchot, J.; Moon, J.H. Conjugated polymer nanoparticles for effective siRNA delivery to tobacco BY-2 protoplasts. BMC Plant Biol. 2010, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Makhotenko, A.V.; Snigir, E.A.; Kalinina, N.O.; Makarov, V.V.; Taliansky, M.E. Data on a delivery of biomolecules into Nicothiana benthamiana leaves using different nanoparticles. Data Br. 2018, 16, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Q.; Huang, T.; Ling, D.; Gao, J. New insights into biocompatible iron oxide nanoparticles: A potential booster of gene delivery to stem cells. Small 2020, 16, 2001588. [Google Scholar] [CrossRef]
- Ahmad, A.; Hashmi, S.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef]
- Kjeldskov, J.; Skov, M.B. Studying usability in sitro: Simulating real world phenomena in controlled environments. Int. J. Hum. Comput. Interact. 2007, 22, 7–36. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Martin-Ortigosa, S.; Valenstein, J.S.; Lin, V.S.Y.; Trewyn, B.G.; Wang, K. Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv. Funct. Mater. 2012, 22, 3576–3582. [Google Scholar] [CrossRef]
- Amani, A.; Zare, N.; Asadi, A.; Zakaria, R.A. Ultrasound-enhanced gene delivery to alfalfa cells by hPAMAM dendrimer nanoparticles. Turk. J. Biol. 2018, 42, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, J.; Ma, C.; Wang, Y.; Wu, C.; Huang, J.; Xing, B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 2016, 159, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I. Gene transfer to plants by electroporation: Methods and applications. Mol. Biol. Rep. 2020, 47, 3195–3210. [Google Scholar] [CrossRef] [PubMed]
- Shankramma, K.; Yallappa, S.; Shivanna, M.B.; Manjanna, J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 2016, 6, 983–990. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Baykal, A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 2019, 226, 110–122. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, X.; Shi, Y.; Song, S.; Xing, J.; Marowitch, J.; Chen, J.; Chen, J. Magnetic gold nanoparticles as a vehicle for fluorescein isothiocyanate and DNA delivery into plant cells. Botany 2013, 91, 457–466. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Anjum, N.A.; Rodrigo, M.A.M.; Moulick, A.; Heger, Z.; Kopel, P.; Zítka, O.; Kizek, R. Transport phenomena of nanoparticles in plants and animals/humans. Environ. Res. 2016, 151, 233–243. [Google Scholar] [CrossRef]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.J.; Goh, N.S.; Demirer, G.S.; Matos, J.L.; Landry, M.P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018, 36, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.L.; Gómez-Lim, M.; Fernández, F.; Loske, A.M. Physical methods for genetic plant transformation. Phys. Life Rev. 2012, 9, 308–345. [Google Scholar] [CrossRef] [PubMed]
- O’brien, J.A.; Lummis, S.C. Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat. Protoc. 2006, 1, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Jiang, R.; Chen, J.; Chen, W. Nanoparticle-mediated gene transformation strategies for plant genetic engineering. Plant J. 2020, 104, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Kendall, K.; Toloueinia, P.; Mehrabadi, Y.; Gupta, G.; Newton, J. Aggregation and adhesion of gold nanoparticles in phosphate buffered saline. J. Nanopart. Res. 2012, 14, 758. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Rich, J.; Tian, Z.; Huang, T.J. Sonoporation: Past, present, and future. Adv. Mater. Technol. 2022, 7, 2100885. [Google Scholar] [CrossRef]
- Snipstad, S.; Hanstad, S.; Bjørkøy, A.; Mørch, Ý.; de Lange Davies, C. Sonoporation using nanoparticle-loaded microbubbles increases cellular uptake of nanoparticles compared to co-incubation of nanoparticles and microbubbles. Pharmaceutics 2021, 13, 640. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [PubMed]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar]
- Zhang, Y.; Yu, L.C. Microinjection as a tool of mechanical delivery. Curr. Opin. Biotechnol. 2008, 19, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Selvaganapathy, P.R.; Wilson, J. Microinjection in a microfluidic format using flexible and compliant channels and electroosmotic dosage control. Lab Chip 2009, 9, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Narita, F.; Wang, Y.; Kurita, H.; Suzuki, M. Multi-scale analysis and testing of tensile behavior in polymers with randomly oriented and agglomerated cellulose nanofibers. Nanomaterials 2020, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Magazzù, A.; Marcuello, C. Investigation of soft matter nanomechanics by atomic force microscopy and optical tweezers: A comprehensive review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, L.; Reggente, M.; Passeri, D.; Natali, M.; Rossi, M. Identification of nanoparticles and nanosystems in biological matrices with scanning probe microscopy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1521. [Google Scholar] [CrossRef]
- Okpara, E.C.; Nde, S.C.; Fayemi, O.E.; Ebenso, E.E. Electrochemical characterization and detection of lead in water using SPCE modified with BiONPs/PANI. Nanomaterials 2021, 11, 1294. [Google Scholar] [CrossRef]
- Zhang, K.; Jariwala, B.; Li, J.; Briggs, N.C.; Wang, B.; Ruzmetov, D.; Burke, R.A.; Lerach, J.O.; Ivanov, T.G.; Haque, M.; et al. Large scale 2D/3D hybrids based on gallium nitride and transition metal dichalcogenides. Nanoscale 2018, 10, 336–341. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.C.; Wells, B.; Roberts, K. Direct visualization of cross-links in the primary plant cell wall. J. Cell Sci. 1990, 96, 323–334. [Google Scholar] [CrossRef]
- Fujino, T.; Itoh, T. Changes in pectin structure during epidermal cell elongation in pea (Pisum sativum) and its implications for cell wall architecture. Plant Cell Physiol. 1998, 39, 1315–1323. [Google Scholar] [CrossRef]
- Rose, J.K.; Bennett, A.B. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999, 4, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Goldbach, H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces–further evidence for a stomatal pathway. Physiol. Plant. 2008, 132, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Malejko, J.; Godlewska-Żyłkiewicz, B.; Vanek, T.; Landa, P.; Nath, J.; Dror, I.; Berkowitz, B. Uptake, translocation, weathering and speciation of gold nanoparticles in potato, radish, carrot and lettuce crops. J. Hazard. Mater. 2021, 418, 126219. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical review: Role of inorganic nanoparticle properties on their foliar uptake and in planta translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Deans, R.M.; Brodribb, T.J.; Busch, F.A.; Farquhar, G.D. Optimization can provide the fundamental link between leaf photosynthesis, gas exchange and water relations. Nat. Plants 2020, 6, 1116–1125. [Google Scholar] [CrossRef]
- García-Gómez, C.; Pérez, R.A.; Albero, B.; Obrador, A.; Almendros, P.; Fernández, M.D. Interaction of ZnO nanoparticles with metribuzin in a soil–plant system: Ecotoxicological effects and changes in the distribution pattern of Zn and metribuzin. Agronomy 2023, 13, 2004. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.L. Seed variation in wild radish: Effect of seed size on components of seedling and adult fitness. Ecology 1984, 65, 1105–1112. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Palta, J.; Prasad, P.V.; Siddique, K.H. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143. [Google Scholar] [CrossRef]
- Lombi, E.; Susini, J. Synchrotron-based techniques for plant and soil science: Opportunities, challenges and future perspectives. Plant Soil 2009, 320, 1–35. [Google Scholar] [CrossRef]
- Deng, Y.; Petersen, E.J.; Challis, K.E.; Rabb, S.A.; Holbrook, R.D.; Ranville, J.F.; Nelson, B.C.; Xing, B. Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environ. Sci. Technol. 2017, 51, 10615–10623. [Google Scholar] [CrossRef]
- Fouad, A.; Hegazy, A.E.; Azab, E.; Khojah, E.; Kapiel, T. Boosting of antioxidants and alkaloids in Catharanthus roseus suspension cultures using silver nanoparticles with expression of CrMPK3 and STR genes. Plants 2021, 10, 2202. [Google Scholar] [CrossRef]
- Pilalis, E.; Chatziioannou, A.; Thomasset, B.; Kolisis, F. An in silico compartmentalized metabolic model of Brassica napus enables the systemic study of regulatory aspects of plant central metabolism. Biotechnol. Bioeng. 2011, 108, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Taguchi, M.; Burrs, S.L.; Hauser, B.A.; Salim, W.W.A.W.; Claussen, J.C.; McLamore, E.S. Emerging technologies for non-invasive quantification of physiological oxygen transport in plants. Planta 2013, 238, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef] [PubMed]
- De la Calle, I.; Menta, M.; Séby, F. Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: A review. Spectrochim. Acta B At. Spectrosc. 2016, 125, 66–96. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Root system architecture, copper uptake and tissue distribution in soybean (Glycine max (L.) Merr.) grown in copper oxide nanoparticle (CuONP)-amended soil and implications for human nutrition. Plants 2020, 9, 1326. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; AlShammari, T.M.; Bargouti, M.; Ozdemir, M.; Tombuloglu, G.; Akhtar, S.; Sabit, H.; Hakeem, K.R.; Almessiere, M.; et al. Uptake, translocation, and physiological effects of hematite (α-Fe2O3) nanoparticles in barley (Hordeum vulgare L.). Environ. Pollut. 2020, 266, 115391. [Google Scholar] [CrossRef]
- Bao, D.; Oh, Z.G.; Chen, Z. Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis. Front. Plant Sci. 2016, 7, 32. [Google Scholar] [CrossRef]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: Transport and accumulation. Sci. Rep. 2019, 9, 10372. [Google Scholar] [CrossRef]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.; Bruce, N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013, 15, 1417. [Google Scholar] [CrossRef]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnology 2014, 12, 50. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, S.; Luo, L.; Zhang, J.; Yang, K.; Christie, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68–77. [Google Scholar] [CrossRef]
- López-Luna, J.; Cruz-Fernández, S.; Mills, D.S.; Martínez-Enríquez, A.I.; Solís-Domínguez, F.A.; del Carmen Ángeles González-Chávez, M.; Carrillo-González, R.; Martinez-Vargas, S.; Mijangos-Ricardez, O.F.; del Carmen Cuevas-Díaz, M. Phytotoxicity and upper localization of Ag@CoFe2O4 nanoparticles in wheat plants. Environ. Sci. Pollut. Res. 2020, 27, 1923–1940. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef]
- Zhang, W.; Dan, Y.; Shi, H.; Ma, X. Elucidating the mechanisms for plant uptake and in-planta speciation of cerium in radish (Raphanus sativus L.) treated with cerium oxide nanoparticles. J. Environ. Chem. Eng. 2017, 5, 572–577. [Google Scholar] [CrossRef]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef]
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech 2017, 7, 263. [Google Scholar] [CrossRef]
- Yang, Q.; Shan, W.; Hu, L.; Zhao, Y.; Hou, Y.; Yin, Y.; Liang, Y.; Wang, F.; Cai, Y.; Liu, J.; et al. Uptake and transformation of silver nanoparticles and ions by rice plants revealed by dual stable isotope tracing. Environ. Sci. Technol. 2018, 53, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Ma, X.; Zhang, W.; Liu, K.; Stephan, C.; Shi, H. Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO2 nanoparticles. Anal. Bioanal. Chem. 2016, 408, 5157–5167. [Google Scholar] [CrossRef]
- Hayder, M.; Wojcieszek, J.; Asztemborska, M.; Zhou, Y.; Ruzik, L. Analysis of cerium oxide and copper oxide nanoparticles bioaccessibility from radish using SP-ICP-MS. J. Sci. Food Agric. 2020, 100, 4950–4958. [Google Scholar] [CrossRef]
- Neves, V.M.; Heidrich, G.M.; Rodrigues, E.S.; Enders, M.S.; Muller, E.I.; Nicoloso, F.T.; Pereira de Carvalho, H.W.; Dressler, V.L. La2O3 nanoparticles: Study of uptake and distribution in Pfaffia glomerata (spreng.) pedersen by LA-ICP-MS and μ-XRF. Environ. Sci. Technol. 2019, 53, 10827–10834. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszek, J.; Jiménez-Lamana, J.; Ruzik, L.; Asztemborska, M.; Jarosz, M.; Szpunar, J. Characterization of TiO2 NPs in radish (Raphanus sativus L.) by single-particle ICP-QQQ-MS. Front. Environ. Sci. 2020, 8, 100. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Santana, I.; Dansie, J.; Giraldo, J.P. In vivo delivery of nanoparticles into plant leaves. Curr. Protoc. Chem. Biol. 2017, 9, 269–284. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle uptake in plants: Gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Howard, D.; Unrine, J.M.; Lowry, G.V. Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum). Environ. Sci. Technol. 2017, 51, 7361–7368. [Google Scholar] [CrossRef]
- Peng, C.; Tong, H.; Shen, C.; Sun, L.; Yuan, P.; He, M.; Shi, J. Bioavailability and translocation of metal oxide nanoparticles in the soil-rice plant system. Sci. Total Environ. 2020, 713, 136662. [Google Scholar] [CrossRef]
- Larue, C.; Veronesi, G.; Flank, A.M.; Surble, S.; Herlin-Boime, N.; Carrière, M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A 2012, 75, 722–734. [Google Scholar] [CrossRef]
- Shan, Q.; Liu, Y.; Zhang, X.; Shao, J.; Hei, D.; Ling, Y.; Jia, W. EDXRF analysis of TiO2 nanoparticles bioaccumulation in aquatic plant, Salvinia natans. Microchem. J. 2020, 155, 104784. [Google Scholar] [CrossRef]
- Muccifora, S.; Castillo-Michel, H.; Barbieri, F.; Bellani, L.; Ruffini Castiglione, M.; Spanò, C.; Pradas del Real, A.E.; Giorgetti, L.; Tassi, E.L. Synchrotron radiation spectroscopy and transmission electron microscopy techniques to evaluate TiO2 NPs incorporation, speciation, and impact on root cells ultrastructure of Pisum sativum L. plants. Nanomaterials 2021, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Wu, X.; Zhang, H.; Shen, X.; Zhang, M.; Chen, W.; Gao, Q.; White, J.C.; Tao, S.; Wang, X. Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). Nanoimpact 2017, 5, 101–108. [Google Scholar] [CrossRef]
- Navarro, D.A.; Bisson, M.A.; Aga, D.S. Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J. Hazard. Mater. 2012, 211, 427–435. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Varela-Ramirez, A.; Castillo-Michel, H.; Li, C.; Zhang, J.; Aguilera, R.J.; Keller, A.A.; Gardea-Torresdey, J.L. Effect of surface coating and organic matter on the uptake of CeO2 NPs by corn plants grown in soil: Insight into the uptake mechanism. J. Hazard. Mater. 2012, 225, 131–138. [Google Scholar] [CrossRef]
- Movafeghi, A.; Khataee, A.; Abedi, M.; Tarrahi, R.; Dadpour, M.; Vafaei, F. Effects of TiO2 nanoparticles on the aquatic plant Spirodela polyrrhiza: Evaluation of growth parameters, pigment contents and antioxidant enzyme activities. J. Environ. Sci. 2008, 64, 130–138. [Google Scholar] [CrossRef]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef]
- Praetorius, A.; Gundlach-Graham, A.; Goldberg, E.; Fabienke, W.; Navratilova, J.; Gondikas, A.; Kaegi, R.; Günther, D.; Hofmann, T.; von der Kammer, F. Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils. Environ. Sci. Nano 2017, 4, 307–314. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Nima, Z.A.; Lahiani, M.H.; Watanabe, F.; Xu, Y.; Khodakovskaya, M.V.; Biris, A.S. Plasmonically active nanorods for delivery of bio-active agents and high-sensitivity SERS detection in planta. RSC Adv. 2014, 4, 64985–64993. [Google Scholar] [CrossRef]
- Nečemer, M.; Kump, P.; Ščančar, J.; Jaćimović, R.; Simčič, J.; Pelicon, P.; Budnar, M.; Jeran, Z.; Pongrac, P.; Regvar, M.; et al. Application of X-ray fluorescence analytical techniques in phytoremediation and plant biology studies. Spectrochim. Acta B At. Spectrosc. 2008, 63, 1240–1247. [Google Scholar] [CrossRef]
- Leppard, G.G. Nanoparticles in the environment as revealed by transmission electron microscopy: Detection, characterisation and activities. Curr. Nanosci. 2008, 4, 278–301. [Google Scholar] [CrossRef]
- Schrand, A.M.; Schlager, J.J.; Dai, L.; Hussain, S.M. Preparation of cells for assessing ultrastructural localization of nanoparticles with transmission electron microscopy. Nat. Protoc. 2010, 5, 744–757. [Google Scholar] [CrossRef]
- Denk, W.; Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004, 2, e329. [Google Scholar] [CrossRef]
- Khwaja, H.A.; Parekh, P.P.; Khan, A.R.; Hershey, D.L.; Naqvi, R.R.; Malik, A.; Khan, K. An in-depth characterization of urban aerosols using electron microscopy and energy dispersive x-ray analysis. Clean (Weinh) 2009, 37, 544–554. [Google Scholar] [CrossRef]
- Jaques, V.A.J.; Zikmundová, E.; Holas, J.; Zikmund, T.; Kaiser, J.; Holcová, K. Conductive cross-section preparation of non-conductive painting micro-samples for SEM analysis. Sci. Rep. 2022, 12, 19650. [Google Scholar] [CrossRef]
- Rodushkin, I.; Engström, E.; Baxter, D.C. Isotopic analyses by ICP-MS in clinical samples. Anal. Bioanal. Chem. 2013, 405, 2785–2797. [Google Scholar] [CrossRef]
- Fabricius, A.L.; Duester, L.; Meermann, B.; Ternes, T.A. ICP-MS-based characterization of inorganic nanoparticles—Sample preparation and off-line fractionation strategies. Anal. Bioanal. Chem. 2014, 406, 467–479. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.; Luo, G.; Wu, J. Three-dimensional reconstruction of light microscopy image sections: Present and future. Front. Med. 2015, 9, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Prikler, S.; Pick, D.; Einax, J.W. Comparing different means of signal treatment for improving the detection power in HPLC-ICP-MS. Anal. Bioanal. Chem. 2012, 403, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Liu, J.; Chen, Y.; Guan, R.; Ouyang, C.; Zhu, Y.; Ji, L.; Chao, H. Cyclometalated iridium (III) complexes as AIE phosphorescent probes for real-time monitoring of mitophagy in living cells. Sci. Rep. 2016, 6, 22039. [Google Scholar] [CrossRef] [PubMed]
- Klockenkämper, R.; Von Bohlen, A.; Moens, L. Analysis of pigments and inks on oil paintings and historical manuscripts using total reflection x-ray fluorescence spectrometry. X-ray Spectrom. 2000, 29, 119–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, X.; Li, J.; Zhong, Z.; Huang, Q.; Fan, C. Synchrotron-based X-ray microscopic studies for bioeffects of nanomaterials. Nanomedicine 2014, 10, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, S.H. Selective fluorescence and fluorescence-free detection of single biomolecules on nanobiochips. J. Biomed. Nanotechnol. 2014, 10, 2620–2640. [Google Scholar] [CrossRef]
- Ozawa, T.; Yoshimura, H.; Kim, S.B. Advances in fluorescence and bioluminescence imaging. Anal. Chem. 2013, 85, 590–609. [Google Scholar] [CrossRef]
- Jarvi, M.T.; Patterson, M.S.; Wilson, B.C. Insights into photodynamic therapy dosimetry: Simultaneous singlet oxygen luminescence and photosensitizer photobleaching measurements. Biophys. J. 2012, 102, 661–671. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Barroso, D.; Komárek, M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut Res. 2016, 23, 1732–1741. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Lal, S.K.; Lakra, N.; Mehta, S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 253, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kong, G.; Li, Y. Long-distance communication through systemic macromolecular signaling mediates stress defense responses in plants. Physiol. Plant. 2021, 173, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; Hove, C.A.T.; Hogeweg, P.; Marée, A.F.M.; Scheres, B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008, 6, e307. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Fukuhara, T.; Suzuki, T.; Lenggoro, I.W. Stem cutting: A novel introduction site for transporting water-insoluble particles into tomato (Solanum lycopersicum) seedlings. Plant Physiol. Biochem. 2024, 206, 108297. [Google Scholar] [CrossRef] [PubMed]
- Tixier, A.; Cochard, H.; Badel, E.; Dusotoit-Coucaud, A.; Jansen, S.; Herbette, S. Arabidopsis thaliana as a model species for xylem hydraulics: Does size matter? J. Exp. Bot. 2013, 64, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Mullendore, D.L.; Windt, C.W.; Van As, H.; Knoblauch, M. Sieve tube geometry in relation to phloem flow. Plant Cell 2010, 22, 579–593. [Google Scholar] [CrossRef]
- Rajput, K.S.; Patil, V.S.; Rao, K.S. Wood anatomy and the development of interxylary phloem of Ipomoea hederifolia Linn. (Convolvulaceae). J. Plant Growth Regul. 2013, 32, 654–662. [Google Scholar] [CrossRef]
- Antonova, G.F.; Stasova, V.V. Seasonal development of phloem in Siberian larch stems. Russ. J. Dev. Biol. 2008, 39, 207–218. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Valdovinos-Ayala, J.; Rodriguez-Zaccaro, F.D.; Hill-Crim, M.A.; Percolla, M.I.; Venturas, M.D. Intra-organismal variation in the structure of plant vascular transport tissues in poplar trees. Trees 2018, 32, 1335–1346. [Google Scholar] [CrossRef]
- Pal, K.; Rahaman, C.H. Studies on foliar epidermal micromorphology, vegetative anatomy and xylem elements of four members of Protulacaceae. Int. J. Curr. Res. 2014, 6, 4968–4975. [Google Scholar]
- Romero, C.; Bolker, B.M.; Edwards, C.E. Stem responses to damage: The evolutionary ecology of Quercus species in contrasting fire regimes. New Phytol. 2009, 182, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Gričar, J.; Lavrič, M.; Ferlan, M.; Vodnik, D.; Eler, K. Intra-annual leaf phenology, radial growth and structure of xylem and phloem in different tree parts of Quercus pubescens. Eur. J. For. Res. 2017, 136, 625–637. [Google Scholar] [CrossRef]
- Gričar, J. Xylem and phloem formation in sessile oak from Slovenia in 2007. Wood Res. 2010, 55, 15–22. [Google Scholar]
- Peuke, A.D.; Rokitta, M.; Zimmermann, U.; Schreiber, L.; Haase, A. Simultaneous measurement of water flow velocity and solute transport in xylem and phloem of adult plants of Ricinus communis over a daily time course by nuclear magnetic resonance spectrometry. Plant Cell Environ. 2001, 24, 491–503. [Google Scholar] [CrossRef]
- Elsayed, A.A.; Ahmed, E.G.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Dié, A.; Kitin, P.; Kouamé, F.N.G.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann. Bot. 2012, 110, 861–873. [Google Scholar] [CrossRef]
- Santarosa, E.; de Souza, P.V.D.; de Araujo Mariath, J.E.; Lourosa, G.V. Physiological interaction between rootstock-scion: Effects on xylem vessels in Cabernet Sauvignon and Merlot grapevines. Am. J. Enol. Vitic. 2016, 67, 65–76. [Google Scholar] [CrossRef]
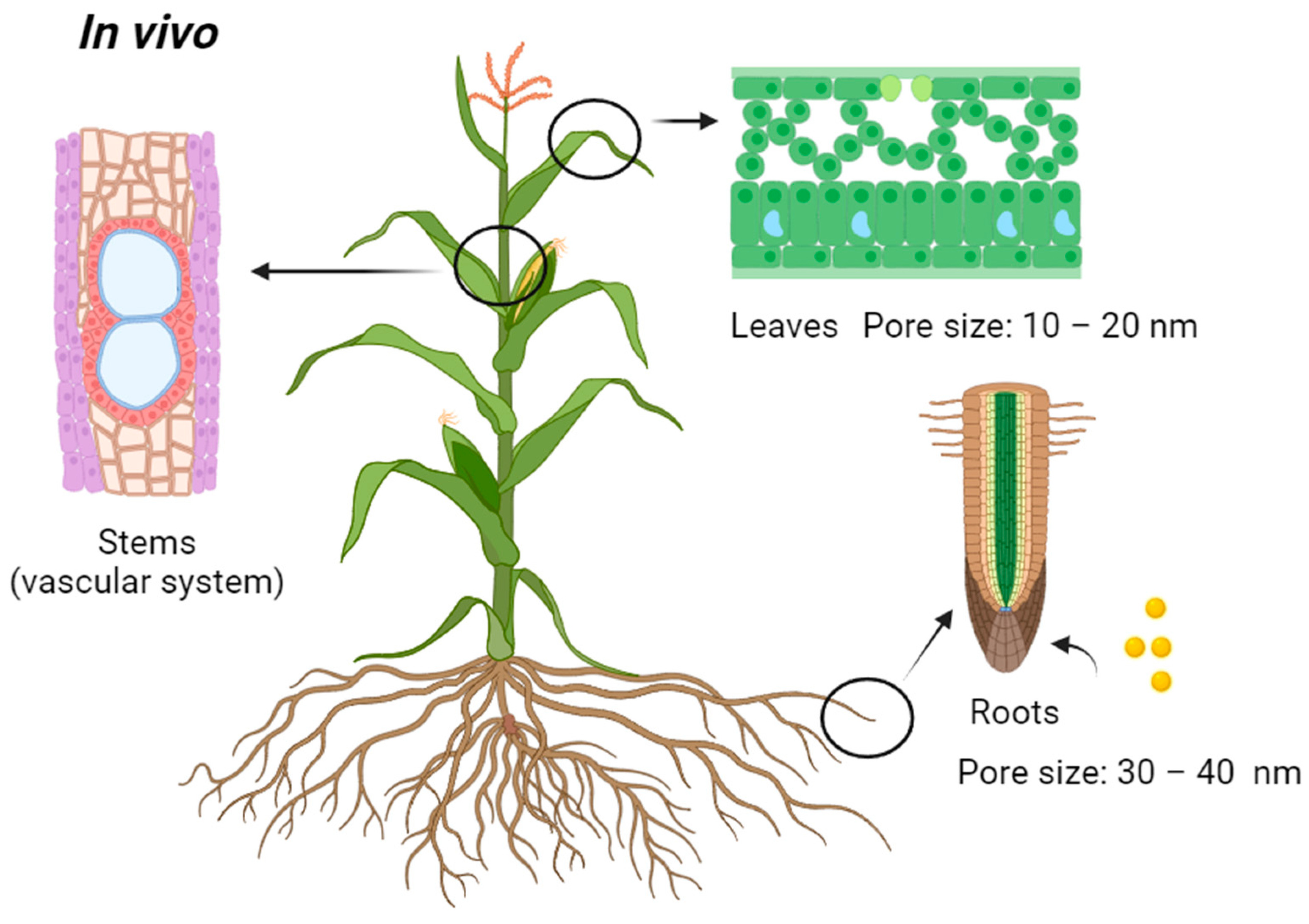
| Type | NPs | Size (nm) | Plants | Introduction Site | Ref. |
|---|---|---|---|---|---|
| Metal or metalloid | Ag | 1–10 | Tomato | Roots | [47] |
| Ag | 10 | Triticum aestivum | Roots | [48] | |
| Ag | 20 ± 3 | Linum usitatissimum, Lolium perenne, Hordeum vulgare | Roots | [49] | |
| Ag | 20 | Arabidopsis thaliana | Roots | [50] | |
| Ag | 10 | Phaseolus radiatus, Sorghum bicolor | Roots | [51] | |
| Ag | 10–15 | Lycopersicum esculentum | Roots | [52] | |
| Ag | 27.3 ± 6 | Populus deltoides, Arabidopsis thaliana | Roots | [53] | |
| Al | 18 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| Au | 1–3 | Oryza sativa | Roots | [55] | |
| Au | 3.5 and 18 | Nicotiana xanthi | Roots | [56] | |
| Au | 6–10 | Oryza sativa, Lolium perenne, Raphanus sativus, Cucurbita mixta | Roots | [57] | |
| Co | 28 | Tomato | Roots | [47] | |
| Ni | 28 | Tomato | Roots | [47] | |
| Si | 14 | Arabidopsis thaliana | Roots | [58] | |
| Zn | 35 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| Metal or metalloid oxide | CeO2 | 25 | Holcus lanatus, Diplotaxis tenuifolia | Roots | [59] |
| CeO2 | 8 | Glycine max | Roots | [60] | |
| CeO2 | 8 ± 1 | Oryza sativa | Roots | [61] | |
| CeO2 | 20 ± 2 | Solanum lycopersicum | Roots | [62] | |
| CeO2 | 6.6 ± 1; 25.2 ± 2 | Cucumis sativus | Roots | [63] | |
| Fe3O4 | 20–30 | Tomato | Roots | [47] | |
| Fe3O4 | 8 | Cucurbita maxima | Roots | [64] | |
| SiO2 | 10–20 | Chelidonium majus | Leaves | [65] | |
| SiO2 | 20 | Cucumis sativus | Leaves | [66] | |
| TiO2 | 20 | Tomato | Roots | [47] | |
| TiO2 | 20 ± 5 | Triticum aestivum | Roots | [67] | |
| TiO2 | 27 ± 4 | Cucumis sativus | Roots | [68] | |
| TiO2 | 27 | Lycopersicum esculentum | Roots | [52] | |
| TiO2 | 2.8 ± 1 | Arabidopsis thaliana | Roots | [69] | |
| ZnO | 10 | Glycine max | Roots | [60] | |
| ZnO | 20 ± 5 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| Carbon-based | C | 20 | Zea mays | Roots | [70] |
| Carbon nanotubes (CNT) | 10–30 | Cicer arietinum | Roots | [71] | |
| Multi-walled carbon nanotubes (MWCNT) | 10–20 | Brassica napus, Raphanus sativus, Lolium perenne, Lactuca sativa, Zea mays, Cucumis sativus | Roots | [54] | |
| MWCNT | 6–9 | Zea mays | Roots | [72] | |
| MWCNT | 4–13 | Lactuca sativa, Oryza sativa, Cucumis sativus, Amaranthus tricolor, Abelmoschus esculentus, Capsicum annuum, Glycine max | Roots | [73] | |
| MWCNT | 30 | Brassica juncea | Roots | [74] | |
| MWCNT | 6–13 | Triticum aestivum | Roots | [75] | |
| Single-walled carbon nanotubes (SWCNT) | 20 | Nicotiana benthamiana | Leaves | [76] | |
| Semiconductor | 3-mercaptopropionic acid (MPA) quantum dots (QDs) | 4–5.4 | Lemna minor | Leaves | [77] |
| Cd-based QDs | 1.9 and 2.4 | Allium cepa | Roots | [78] | |
| CdSe/CdZnS QDs | 19.5 ± 7 | Populus deltoides | Roots | [79] | |
| CdTe QDs | 4 | Oryza sativa | Roots | ||
| Glutathione (GSH) QDs | 4–4.4 | Lemna minor | Leaves | [77] | |
| Polymeric | Chitosan | 19–21 | Oryza sativa | Roots | [80] |
| Thiamine loaded chitosan | 10 | Cicer arietinum | Roots | [81] | |
| Magnetic | Superparamagnetic iron oxide (SPION) | 9 | Glycine max | Roots | [82] |
| Method of Assisted Delivery | Advantages | Disadvantages |
|---|---|---|
| Biolistic (gene gun) |
| |
| Sonoporation |
| |
| Magnetic field |
|
|
| Electroporation |
|
|
| Microinjection |
|
|
| Passive Delivery | Advantages | Disadvantages |
|---|---|---|
| Methodology | ||
| Root uptake |
|
|
| Foliar uptake |
|
|
| Plant developmental stages | ||
| Seed germination |
|
|
| Seedling growth |
| |
| Factors | Description |
|---|---|
| Methodological considerations |
Considerations: May be challenging to observe and quantify; has potential effects on plant health [122].
Considerations: May not fully represent the complexity of the in vivo environment [108].
Considerations: Requires accurate input parameters; simplifications may limit realism [161]. |
| Particle properties | The size and composition of nanoparticles may influence the choice of detection method. Some methods are better suited for specific sizes or materials [162]. |
| Objectives | |
| Non-invasive vs. invasive | Some methods are non-invasive and allow real-time monitoring, while others require destructive sampling [164]. |
| Sensitivity and precision | Considers the required sensitivity to detect low concentrations of nanoparticles [165]. |
| Sample preparation | Evaluates the ease and compatibility of sample preparation with the chosen method. Some methods may require complex sample processing [166]. |
| Methods | Description |
|---|---|
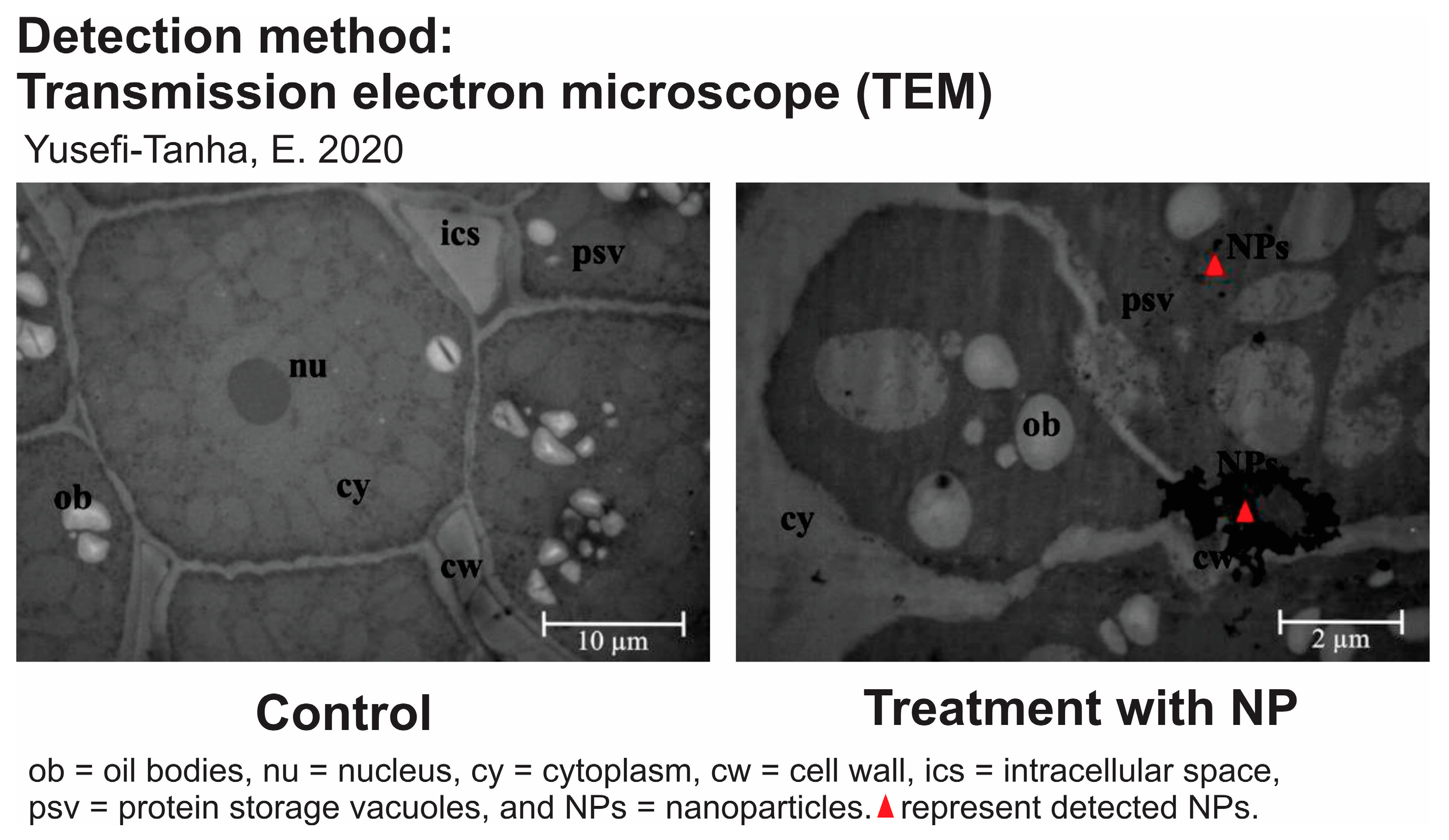
| Transmission electron microscopy (TEM) | TEM allows us to visualize nanoparticles at the nanoscale within plant tissues. By preparing ultrathin sections of plant material and using TEM, the internal distribution and movement of nanoparticles can be observed in different plant structures [167]. |
| Scanning electron microscopy (SEM) | SEM is another microscopy technique that can be used to study the surface morphology of plant tissues and detect the presence of nanoparticles. It provides high-resolution images and can help identify the localization of nanoparticles on the plant’s surface [168]. |
| Inductively coupled plasma mass spectrometry (ICP-MS) | ICP-MS is a highly sensitive analytical technique used to quantify the elemental composition of samples. By digesting plant tissues and then subjecting them to ICP-MS analysis, the presence and concentration of specific nanoparticles can later be determined [169]. |
| Confocal laser scanning microscopy (CLSM) | CLSM is a non-destructive imaging technique that can be used to track the movement of fluorescently labeled nanoparticles within plant tissues over time. This method is particularly useful for studying the dynamics of nanoparticle transport [163]. |
| X-ray fluorescence (XRF) | XRF can be employed to analyze the elemental composition of plant tissues and identify the presence of nanoparticles. It can provide information about the distribution of specific elements, including those contained in nanoparticles [33]. |
| Fluorescence and luminescence techniques (Flo-Lum) | Utilizing fluorescent or luminescent tags on nanoparticles, researchers can track the movement of nanoparticles through plants using fluorescence microscopy or other imaging techniques [170]. |
| Detection Method | Detected NPs | Size (nm) | Plants | Introduction Site | Ref. |
|---|---|---|---|---|---|
| TEM | Ag | 7 and 18 | Medicago sativa | Roots | [171] |
| Ag | 10 | Arabidopsis thaliana | Roots | [169] | |
| Au | 3.5 and 18 | Nicotiana xanthi | Roots | [56] | |
| CeO2 | 6.6 ± 1; 25.2 ± 2 | Cucumis sativus | Roots | [63] | |
| MgO | 27.7 | Watermelon | Leaves | [172] | |
| SiO2 | 30 | Cotton | Roots | [173] | |
| TiO2 | 24.5 | Watermelon | Leaves | [172] | |
| TiO2 | 27 ± 4 | Cucumis sativus | Roots | [68] | |
| ZnO | 20 | Ryegrass | Roots | [174] | |
| ZnO | 30 ± 5 | Zea mays | Roots | [175] | |
| SEM | α-Fe2O3 | 14 | Hordeum vulgare | Roots | [168] |
| Ag@CoFe2O4 | 10 | Triticum aestivum | Roots | [176] | |
| Ag2S | 35 | Cucumis sativus, Triticum aestivum | Roots | [177] | |
| CeO2 | 15.5 | Raphanus sativus | Roots | [178] | |
| Fe3O4 | 12–20 | Lactuca sativa | Roots | [179] | |
| MgO | 15–20 | Arachis hypogaea | Roots | [180] | |
| TiO2 | 19 | Oryza sativa | Roots | [159] | |
| TiO2 | 12–20 | Lactuca sativa | Roots | [179] | |
| ICP-MS | Ag | 10 | Arabidopsis thaliana | Roots | [169] |
| Ag | 7.6 ± 2 | Oryza sativa | Roots | [181] | |
| Au | 2 | Oryza sativa | Roots | [55] | |
| CeO2 | 30 | Solanum lycopersicum, Cucumis sativus, Cucurbita pepo, Glycine max | Roots | [182] | |
| CeO2 | 30 | Raphanus sativus | Roots | [183] | |
| CuO | 25 | Raphanus sativus | Roots | [183] | |
| La2O3 | 20–30 | Pfaffia glomerata | Roots | [184] | |
| TiO2 | 30 | Raphanus sativus | Roots | [185] | |
| CLSM | CeO2 | 1.7–18 | Gossypium hirsutum, Zea mays | Leaves | [186] |
| MSNs | 20 | Lupin, wheat, maize | Roots | [163] | |
| QD | 10 | Arabidopsis thaliana | Leaves | [187] | |
| SiO2 | 1.7–18 | Gossypium hirsutum, Zea mays | Leaves | [186] | |
| ZnO | 30 | Triticum aestivum | Leaves | [188] | |
| XRF | Au | 13.4 ± 1 | Arabidopsis thaliana | Roots | [189] |
| CeO2 | 12 ± 3 | Triticum aestivum | Roots | [190] | |
| CeO2 | 4 | Zea mays, Lactuca sativa, Solanum lycopersicum, Oryza sativa | Roots | [190] | |
| CuO | 30.7 ± 4 | Oryza sativa | Roots | [191] | |
| SiO2 | 10 | Solanum lycopersicum | Roots | [33] | |
| TiO2 | 14 and 25 | Brassica napus, Triticum aestivum | Roots | [192] | |
| TiO2 | 5–10 | Salvinia natans | Roots | [193] | |
| TiO2 | 30 | Pisum sativum | Roots | [194] | |
| TiO2 | 14 | Oryza sativa | Roots | [195] | |
| ZnO | 24.5 ± 4 | Oryza sativa | Roots | [191] | |
| Flo-Lum | Ag | 35 ± 15 | Stevia rebaudiana | Roots | [170] |
| CdSe/ZnS QD | 6.3 ± 1 | Arabidopsis thaliana | Roots | [196] | |
| CeO2 | 8 ± 1 | Zea mays | Roots | [197] | |
| TiO2 | 8 | Spirodela polyrrhiza | Roots | [198] | |
| TiO2 | 2.8 ± 1 | Arabidopsis thaliana | Roots | [199] |
| Detection Method | Advantages | Disadvantages |
|---|---|---|
| TEM | ||
| SEM | ||
| ICP-MS |
|
|
| CLSM | ||
| XRF | ||
| Flo-Lum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sembada, A.A.; Lenggoro, I.W. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials 2024, 14, 131. https://doi.org/10.3390/nano14020131
Sembada AA, Lenggoro IW. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials. 2024; 14(2):131. https://doi.org/10.3390/nano14020131
Chicago/Turabian StyleSembada, Anca Awal, and I. Wuled Lenggoro. 2024. "Transport of Nanoparticles into Plants and Their Detection Methods" Nanomaterials 14, no. 2: 131. https://doi.org/10.3390/nano14020131
APA StyleSembada, A. A., & Lenggoro, I. W. (2024). Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials, 14(2), 131. https://doi.org/10.3390/nano14020131






