Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles’ Physical and Chemical Parameters
2.2. Bacterial Strains and Culture Conditions
2.3. Antimicrobial Activity of AgNPs
2.4. Antibiofilm Activity of AgNPs
2.5. The Effect of Silver Nanoparticles on Biofilms Structure
2.6. Statistical Analysis
3. Results
3.1. Silver Nanoparticles’ Physical and Chemical Parameters
3.2. Bacterial Strains
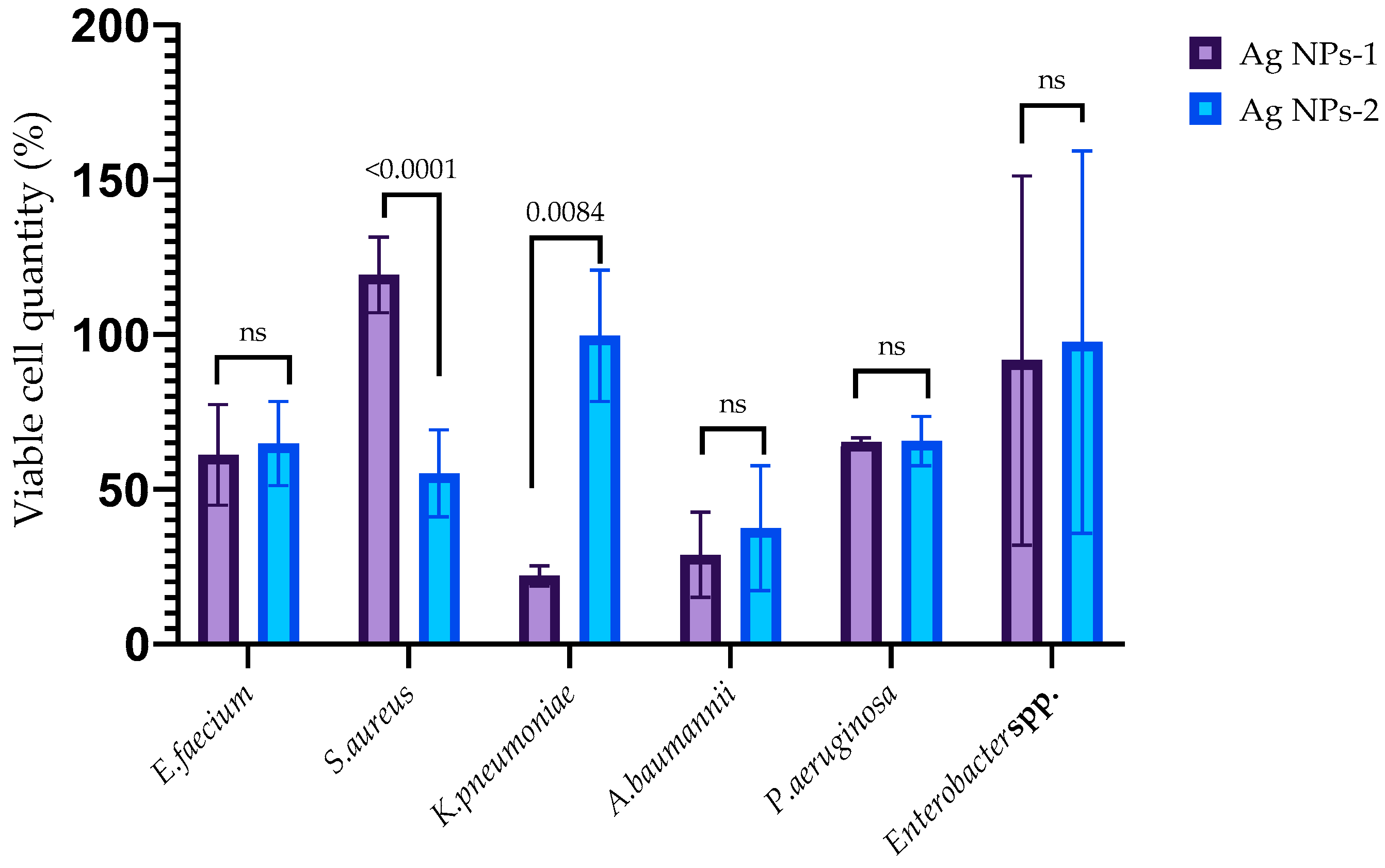
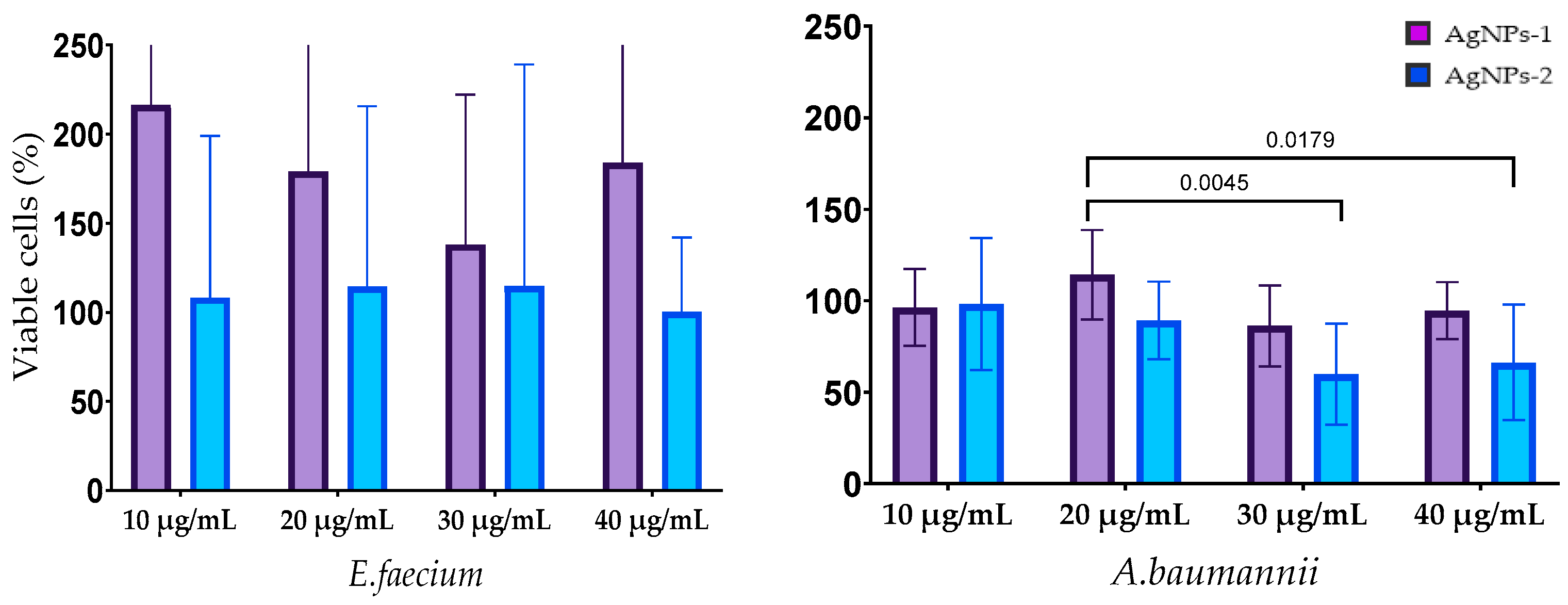
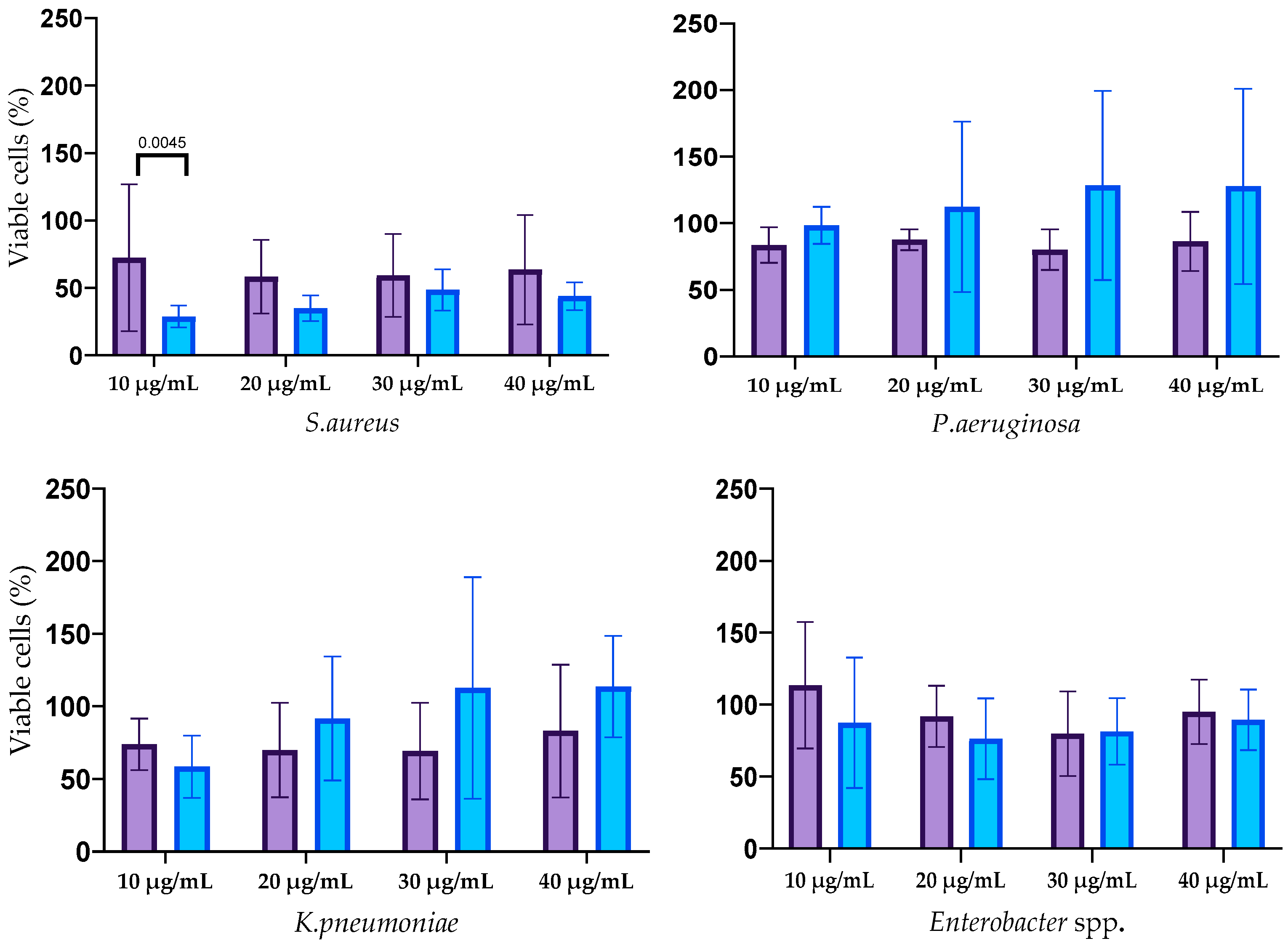
3.3. Antimicrobial Activity of AgNPs
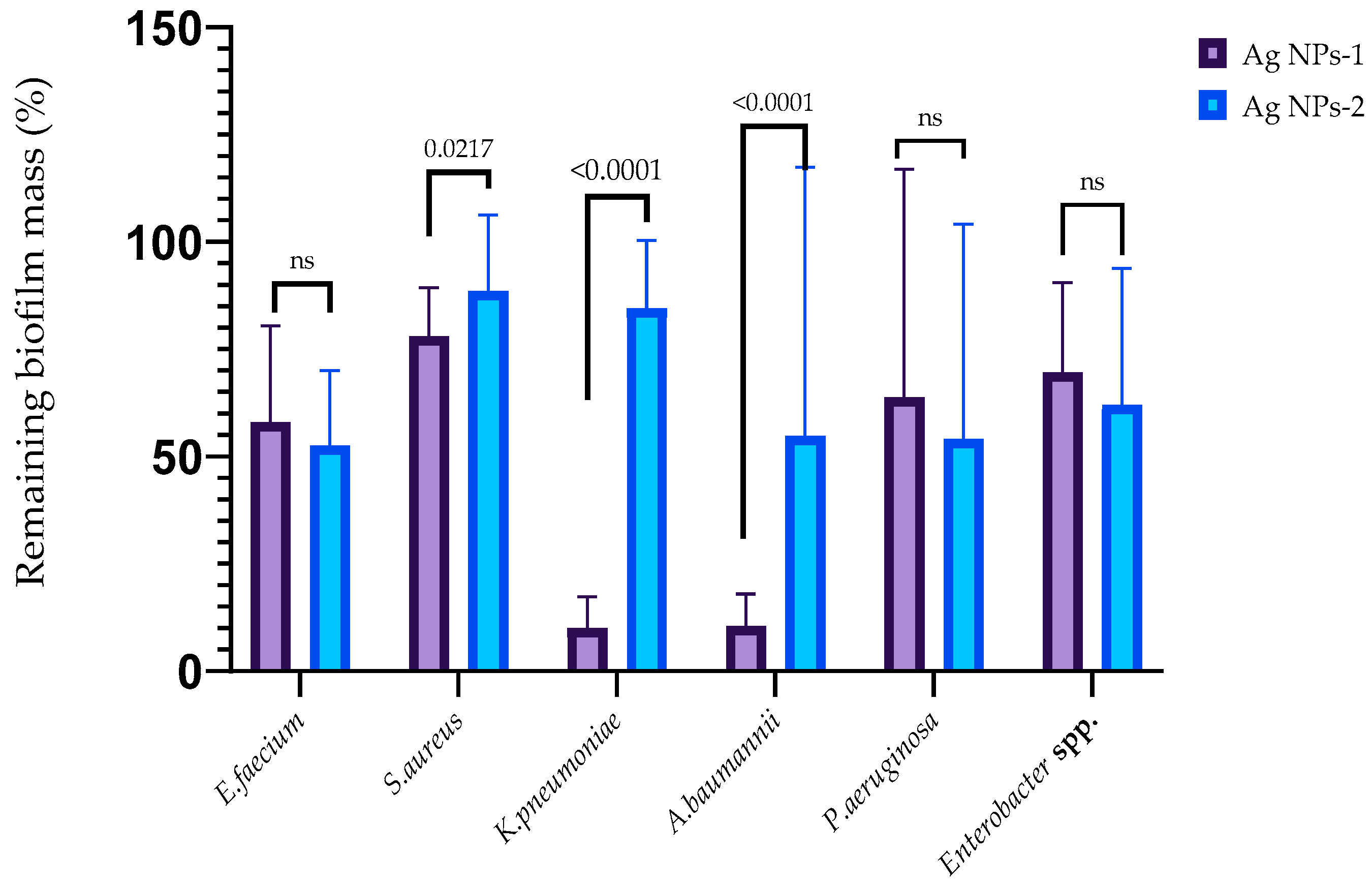
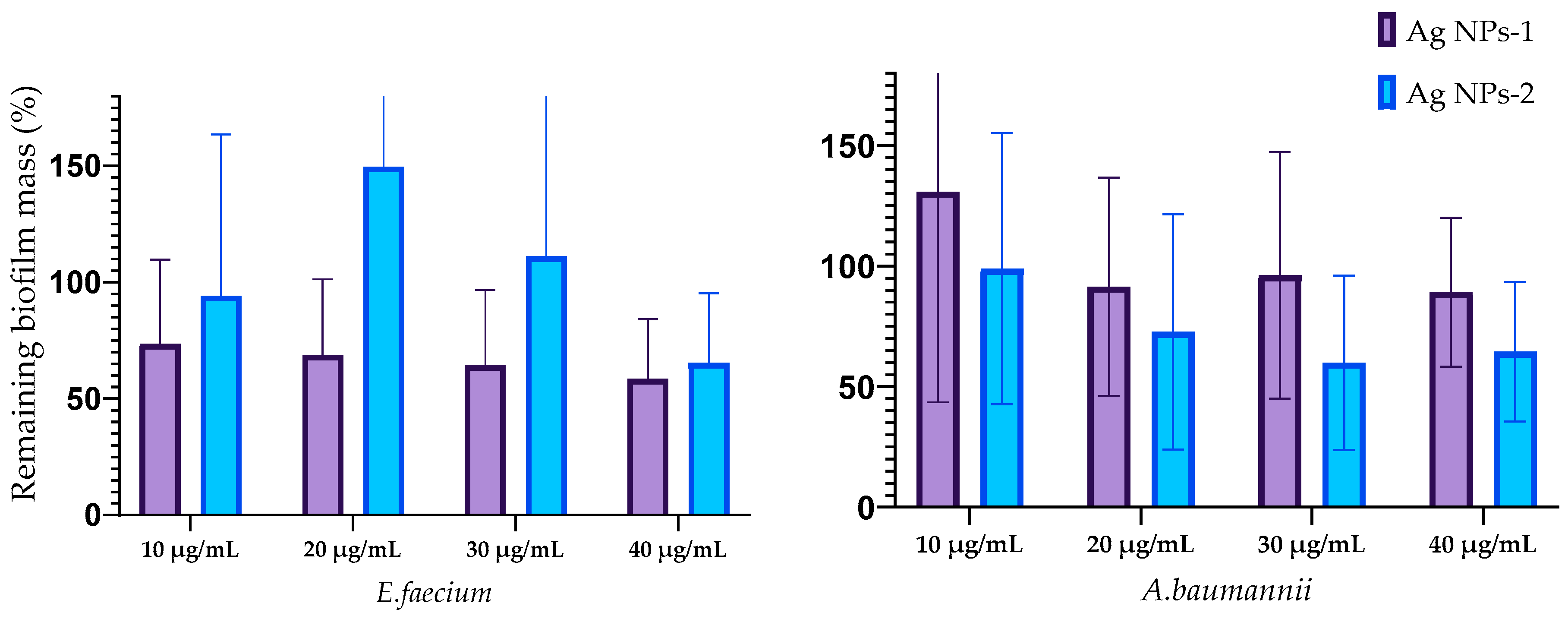
3.4. Antibiofilm Activity of AgNPs
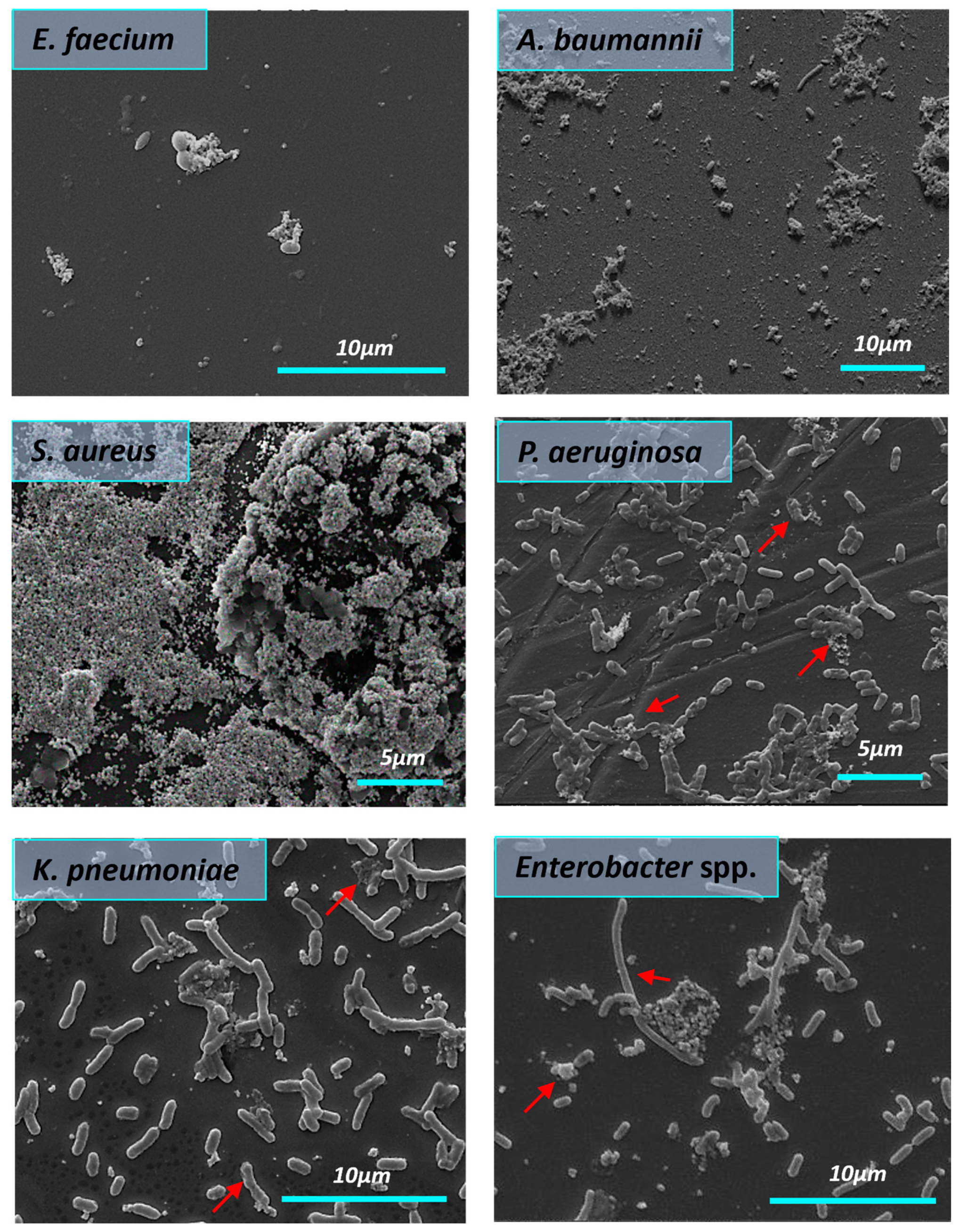
3.5. Effect of Silver Nanoparticles on the Biofilms Structure
4. Discussion
4.1. Nanoparticle Size and Shape
4.2. Antibacterial Mechanisms
4.3. Biofilm Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- E Marturano, J.; Lowery, T.J. ESKAPE Pathogens in Bloodstream Infections Are Associated With Higher Cost and Mortality but Can Be Predicted Using Diagnoses Upon Admission. Open Forum Infect. Dis. 2019, 6, ofz503. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Banerji, R.; Kanojiya, P.; Saroj, S.D. Foodborne ESKAPE Biofilms and Antimicrobial Resistance: Lessons Learned from Clinical Isolates. Pathog. Glob. Health 2021, 115, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.J.; Dubern, J.-F.; Chan, W.C.; Chhabra, S.R.; Williams, P. Pseudomonas aeruginosa PQS quorum-sensing system inhibitor with anti-staphylococcal activity sensitizes polymicrobial biofilms to tobramycin. Cell Chem. Biol. 2022, 29, 1187–1199.e6. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Mittal, A.; Malik, D.K. Antimicrobial potential and in vitro cytotoxicity study of polyvinyl pyrollidone-stabilised silver nanoparticles synthesised from Lysinibacillus boronitolerans. IET Nanobiotechnol. 2021, 15, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Klink, M.J.; Laloo, N.; Taka, A.L.; Pakade, V.E.; Monapathi, M.E.; Modise, J.S. Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens. Molecules 2022, 27, 3532. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi 2022, 28, 911. [Google Scholar] [CrossRef] [PubMed]
- Žalnėravičius, R.; Pakštas, V.; Grincienė, G.; Klimas, V.; Paškevičius, A.; Timmo, K.; Kauk-Kuusik, M.; Franckevičius, M.; Niaura, G.; Talaikis, M.; et al. Antimicrobial particles based on Cu2ZnSnS4 monograins. Colloids Surf. B Biointerfaces 2023, 225, 113275. [Google Scholar] [CrossRef] [PubMed]
- Žalnėravičius, R.; Klimas, V.; Paškevičius, A.; Grincienė, G.; Karpicz, R.; Jagminas, A.; Ramanavičius, A. Highly efficient antimicrobial agents based on sulphur-enriched, hydrophilic MoS2 nano/microparticles and heterostructured Pd/MoS2/Ti coatings. J. Colloid Interface Sci. 2021, 591, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Jiménez, M. Silver Nanoparticle-Based Therapy: Can It Be Useful to Combat Multi-Drug Resistant Bacteria? Antibiotics 2022, 11, 1205. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Comparative study of Antifungal Activity of Silver and Gold Nanoparticles Synthesized by Facile Chemical Approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.N.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Alshareef, A.; Laird, K. Shape-dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl. Surf. Sci. 2017, 424, 310–315. [Google Scholar] [CrossRef]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Oladzadabbasabadi, N.; Alsaedi, A.; Braim, F.S.; Jameel, M.S.; Ramizy, A.; Alrosan, M.; Almajwal, A.M. Comparative Analysis of Stable Gold Nanoparticles Synthesized Using Sonochemical and Reduction Methods for Antibacterial Activity. Molecules 2023, 28, 3931. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Alghoraibi, I.; Soukkarieh, C.; Ismail, M.T.; Alahmad, A. Influence of Polyvinylpyrrolidone Concentration on Properties and Anti-Bacterial Activity of Green Synthesized Silver Nanoparticles. Micromachines 2022, 13, 777. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Khan, M.H.; Unnikrishnan, S.; Ramalingam, K. Bactericidal potential of silver-tolerant bacteria derived silver nanoparticles against multi drug resistant ESKAPE pathogens. Biocatal. Agric. Biotechnol. 2019, 18, 100939. [Google Scholar] [CrossRef]
- Musthafa, M.; Gobianand, K.; Manohar, M. Anti-ESKAPE activity of green synthesized silver nanoparticles from Picrorhiza Kurroa royle ex benth. Int. J. Pharm. Sci. Res. 2020, 11, 5004–5009. [Google Scholar] [CrossRef]
- Myronov, P.; Bugaiov, V.; Holubnycha, V.; Sikora, V.; Deineka, V.; Lyndin, M.; Opanasyuk, A.; Romaniuk, A.; Pogorielov, M. Low-frequency ultrasound increase efectiveness of silver nanoparticles in a purulent wound model. Biomed. Eng. Lett. 2020, 10, 621–631. [Google Scholar] [CrossRef]
- Krolicka, A.; Banasiuk, R.; Frackowiak, J.E.; Krychowiak, M.; Matuszewska, M.; Kawiak, A.; Ziabka, M.; Lendzion-Bielun, Z.; Narajczyk, M. Synthesis of antimicrobial silver nanoparticles through a photomediated reaction in an aqueous environment. Int. J. Nanomedicin. 2016, 11, 315–324. [Google Scholar] [CrossRef]
- Limbago, B. M100-S11, Performance standards for antimicrobial susceptibility testing. Clin. Microbiol. Newsl. 2001, 23, 49. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and Caveats in Combating ESKAPE Pathogens against Nosocomial Infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.D.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Rukayadi, Y. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Elgorban, A.M.; El-Samawaty, A.E.R.M.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M.; Bakri, M.; Khan, M. Antifungal Silver Nanoparticles: Synthesis, Characterization and Biological Evaluation. Biotechnol. Biotechnol. Equip. 2016, 30, 56–62. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Trivinõ, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of Silver Nanoparticle Synthesis by Chemical Reduction and Evaluation of Its Antimicrobial and Toxic Activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef]
- Fernandes, M.; González-Ballesteros, N.; da Costa, A.; Machado, R.; Gomes, A.C.; Rodríguez-Argüelles, M.C. Antimicrobial and Anti-Biofilm Activity of Silver Nanoparticles Biosynthesized with Cystoseira Algae Extracts. J. Biol. Inorg. Chem. 2023, 28, 439–450. [Google Scholar] [CrossRef]
- Sayed, F.A.Z.; Eissa, N.G.; Shen, Y.; Hunstad, D.A.; Wooley, K.L.; Elsabahy, M. Morphologic Design of Nanostructures for Enhanced Antimicrobial Activity. J. Nanobiotechnol. 2022, 20, 536. [Google Scholar] [CrossRef]
- Yuan, Q.; Xiao, R.; Afolabi, M.; Bomma, M.; Xiao, Z. Evaluation of Antibacterial Activity of Selenium Nanoparticles against Food-Borne Pathogens. Microorganisms 2023, 11, 1519. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, S.; Lozano-Iturbe, V.; García, B.; Andrés, L.J.; Menéndez, M.F.; Rodríguez, D.; Vazquez, F.; Vazquez, F.; Martín, C.; Quirós, L.M. Antibacterial Effect of Silver Nanorings. BMC Microbiol. 2020, 20, 172. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-Dependent Antimicrobial Activities of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, A.P.; Gonçalves, S. Studies on interaction of green silver nanoparticles with whole bacteria by surface characterization techniques. BBA-Biomembr. 2019, 1861, 1086–1092. [Google Scholar] [CrossRef]
- Das, B.; Kumar, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Samrot, A.V.; Mohamed, A.A.; Faradjeva, E.; Jie, L.S.; Sze, C.H.; Arif, A.; Sean, T.C.; Michael, E.N.; Mun, C.Y.; Qi, N.X.; et al. Mechanisms and Impact of Biofilms and Targeting of Biofilms Using Bioactive Compounds—A Review. Medicina 2021, 57, 839. [Google Scholar] [CrossRef]
- Coriolano, D.d.L.; de Souza, J.B.; Bueno, E.V.; Medeiros, S.M.d.F.R.d.S.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bose, S.; Shaoo, A.; Das, S.K. Nanotechnology based therapeutic approaches: An advanced strategy to target the biofilm of ESKAPE pathogens. Mater. Adv. 2023, 4, 2544. [Google Scholar] [CrossRef]







| AgNPs-1 | AgNPs-2 | ||
|---|---|---|---|
| Average radius (r) ± SD, nm | Average SA/V ± SD | Average length (a) ± SD, nm | Average SA/V ± SD |
| 22.3 ± 3.22 | 0.14 ± 0.021 | 70 ± 35 * | 0.1 ± 0.03 * |
| AgNPs-1 | AgNPs-2 | |||
|---|---|---|---|---|
| µg/mL | % | µg/mL | % | |
| Silver content in the working solution | 129.0 | - | 194.7 | - |
| The content of silver ions in the supernatant on the first day after preparation | 2.3 | 1.7 | 19.7 | 10.1 |
| The content of silver ions in the supernatant on the second day after preparation | 1.1 | 0.85 | 25.7 | 13.1 |
| Strain | Profile of Strains Sensitivity to Antibiotics | |||||||
|---|---|---|---|---|---|---|---|---|
| Amo | Imi | Van | Gat | Cep | Cet | Ami | Azi | |
| E. faecium | R | R | S | S | R | S | S | R |
| S. aureus | R | R | R | S | R | R | R | R |
| K. pneumoniae | R | S | - | R | R | R | S | R |
| A. baumannii | R | R | - | S | R | R | S | R |
| P. aeruginosa | R | S | - | R | R | R | R | S |
| Enterobacter spp. | R | R | - | - | R | S | S | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. https://doi.org/10.3390/nano14020137
Holubnycha V, Husak Y, Korniienko V, Bolshanina S, Tveresovska O, Myronov P, Holubnycha M, Butsyk A, Borén T, Banasiuk R, et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials. 2024; 14(2):137. https://doi.org/10.3390/nano14020137
Chicago/Turabian StyleHolubnycha, Viktoriia, Yevheniia Husak, Viktoriia Korniienko, Svetlana Bolshanina, Olesia Tveresovska, Petro Myronov, Marharyta Holubnycha, Anna Butsyk, Thomas Borén, Rafal Banasiuk, and et al. 2024. "Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria" Nanomaterials 14, no. 2: 137. https://doi.org/10.3390/nano14020137