Switchable Optical Properties of Dyes and Nanoparticles in Electrowetting Devices
Abstract
:1. Introduction
2. Materials and Methods
3. Results
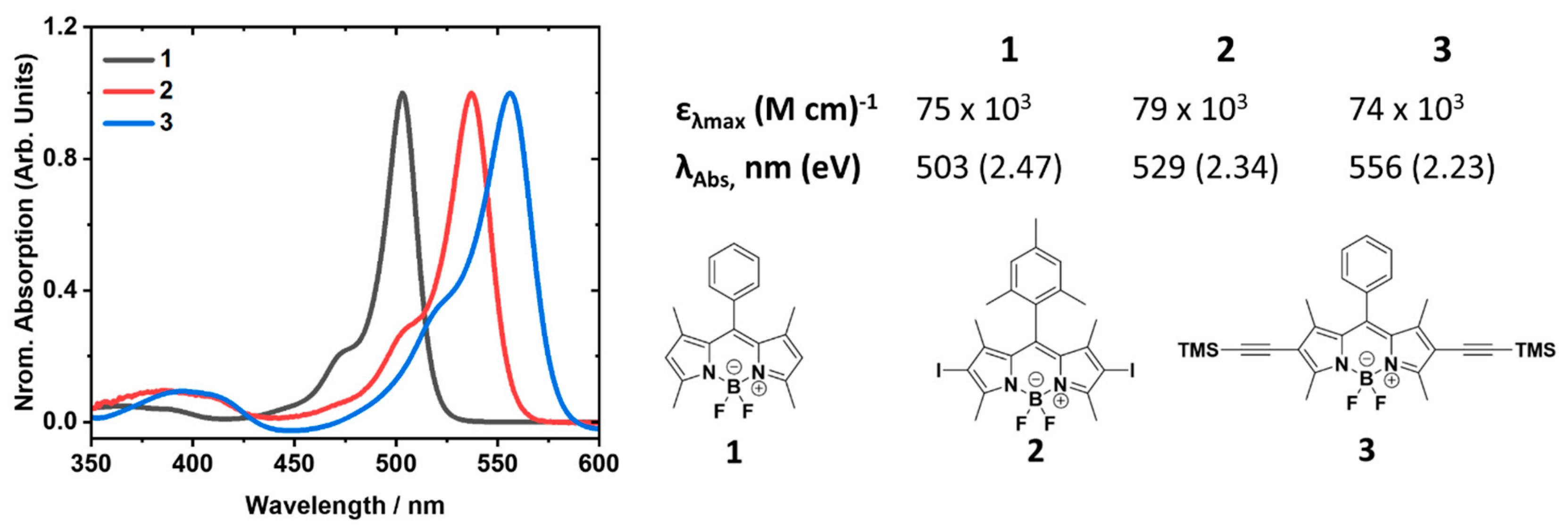
3.1. Dye Absorption Spectra, Structures and Extinction Coefficients
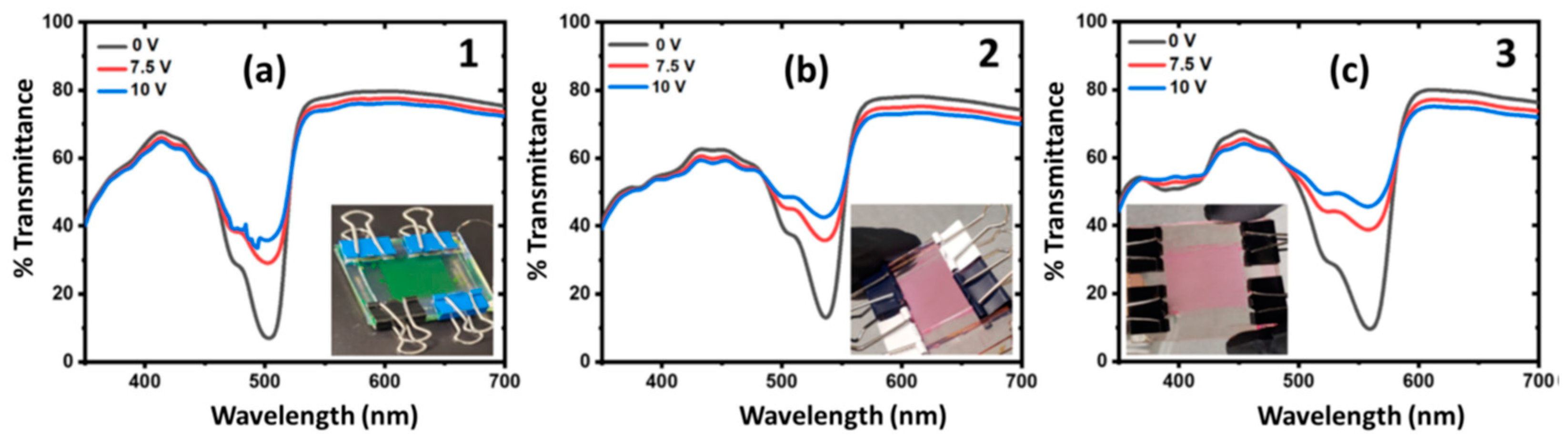
3.2. Actuation of Dyes in 300 × 900 µm (Pixel Dimensions) Devices
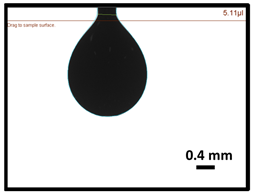
3.3. Surface Tension of Dyes 1, 2 and 3
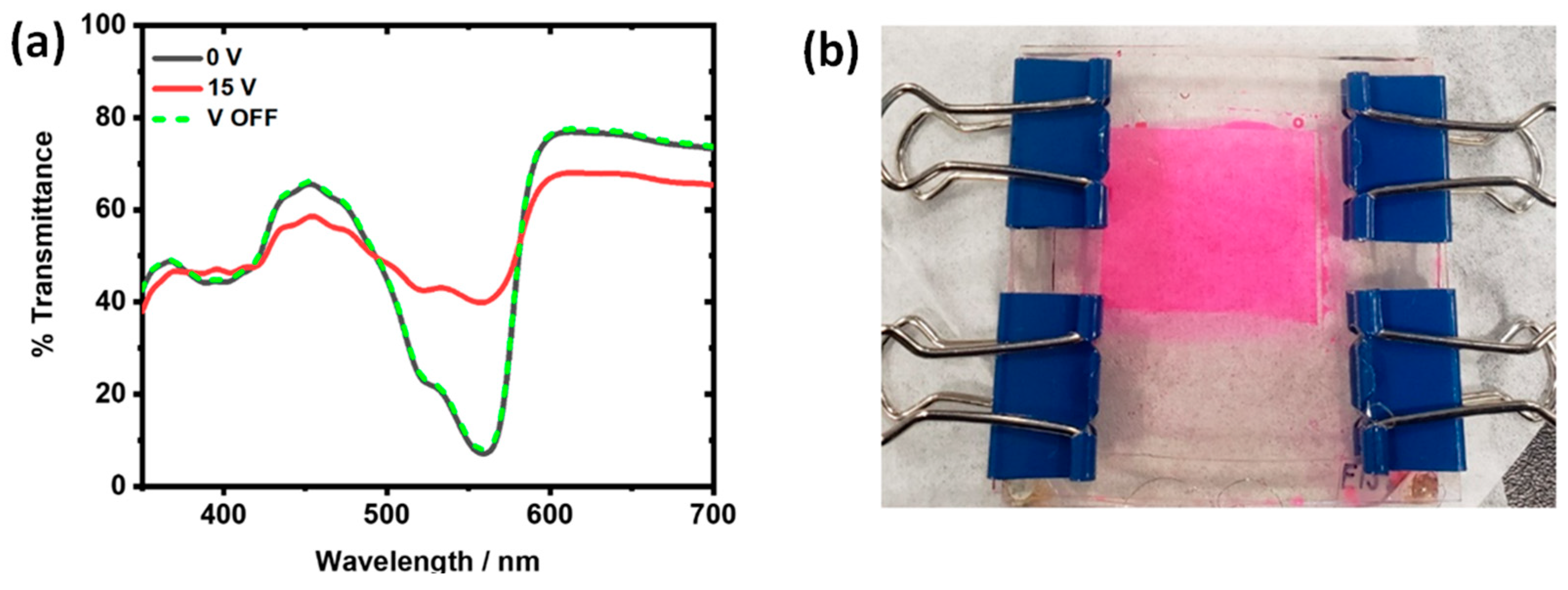
3.4. Actuation of Dye 3 in Devices with 200 × 600 and 150 × 450 µm Pixel Dimensions
3.5. Devices Dosed with Nanoparticles
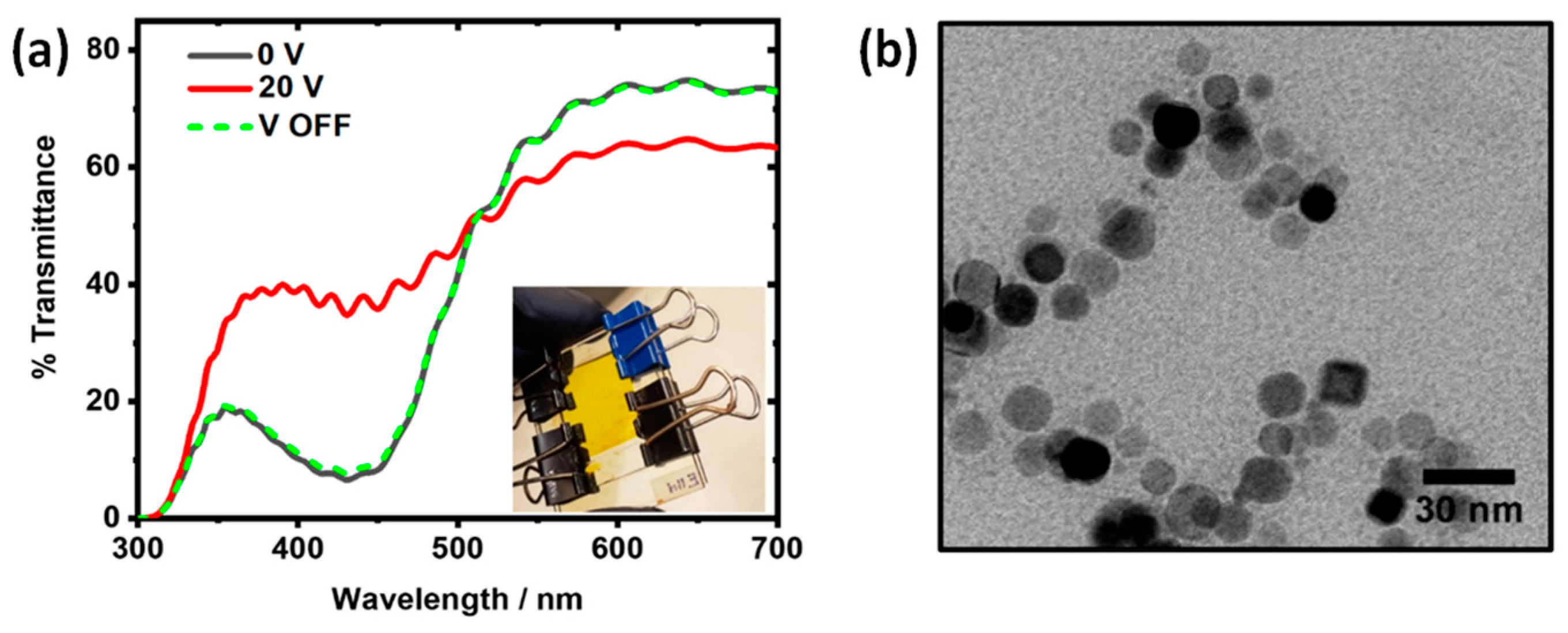
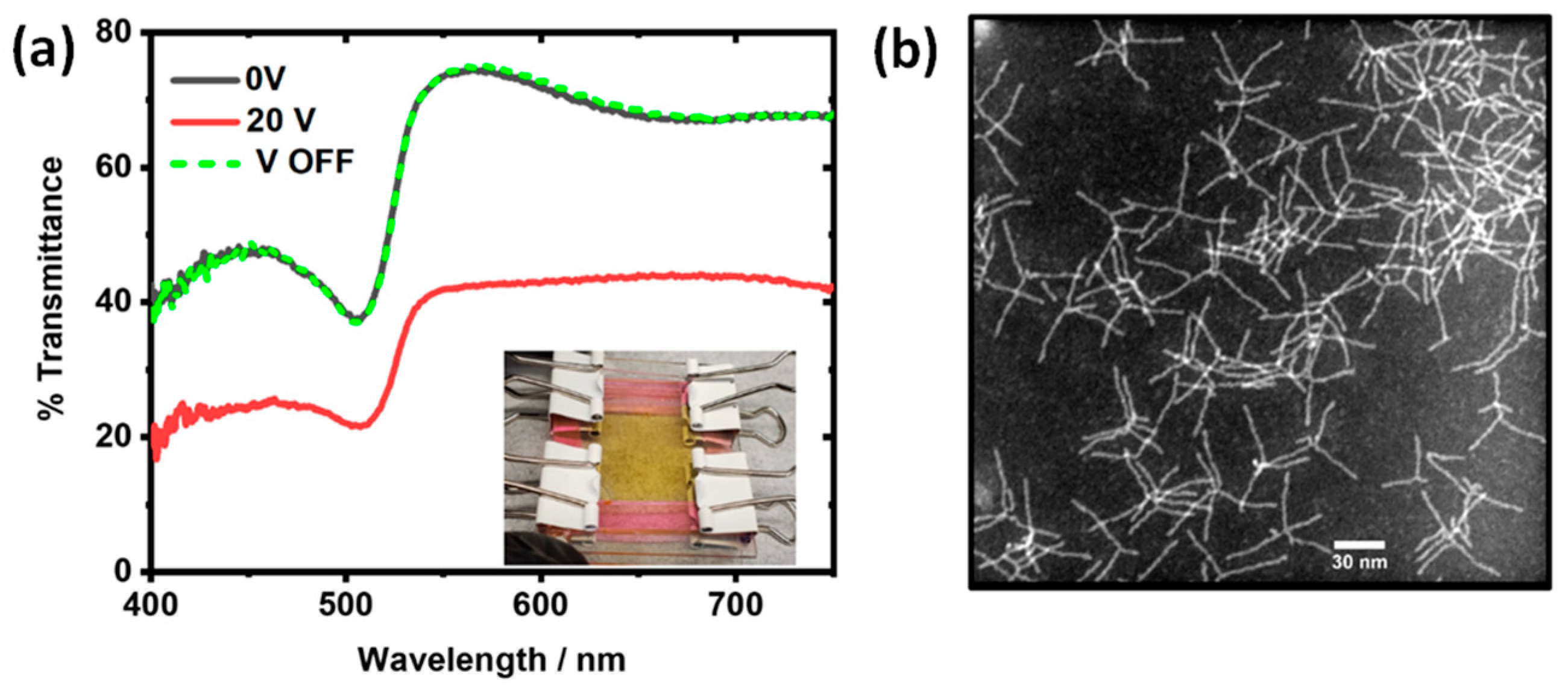
3.5.1. Ag Nanoparticles in EW Device
3.5.2. CdSe Nanotetrapods in EW Device
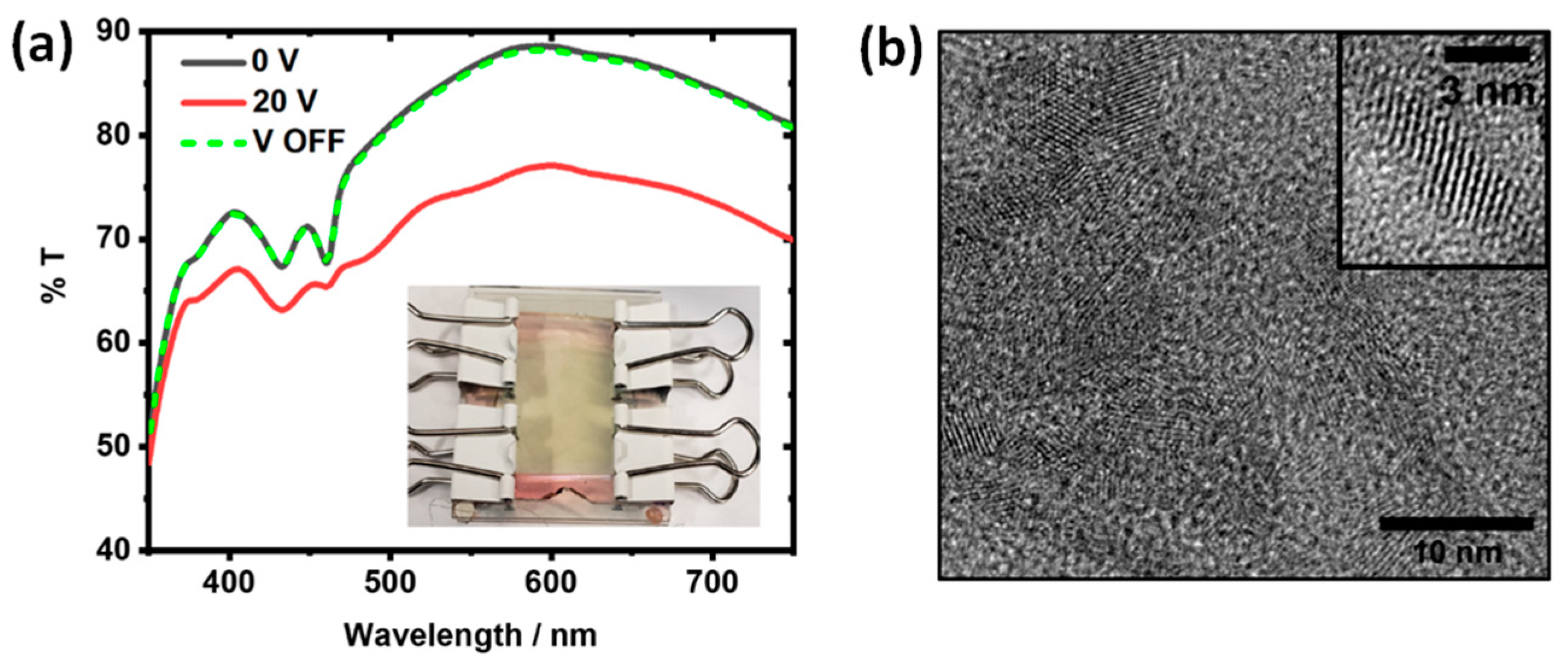
3.5.3. CdSe Nanoplatelets in EW Device
3.6. Electro-Optic Response Times for Representative Dye and Nanoparticle Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pollack, M.G.; Shenderov, A.D.; Fair, R.B. Electrowetting-based actuation of droplets for integrated microfluidics. Lab A Chip 2002, 2, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Roques-Carmes, T.; Palmier, S.; Hayes, R.A.; Schlangen, L.J.M. The effect of the oil/water interfacial tension on electrowetting driven fluid motion. Colloids Surf. A Physicochem. Eng. Asp. 2005, 267, 56–63. [Google Scholar] [CrossRef]
- Kavousanakis, M.E.; Chamakos, N.T.; Ellinas, K.; Tserepi, A.; Gogolides, E.; Papathanasiou, A.G. How to Achieve Reversible Electrowetting on Superhydrophobic Surfaces. Langmuir 2018, 34, 4173–4179. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.K.; Borca-Tasciuc, T.; Purkayastha, A.; Ramanath, G. Electrowetting on dielectric-actuation of microdroplets of aqueous bismuth telluride nanoparticle suspensions. Nanotechnology 2007, 18, 475711. [Google Scholar] [CrossRef]
- Roques-Carmes, T.; Aldeek, F.; Balan, L.; Corbel, S.; Schneider, R. Aqueous dispersions of core/shell CdSe/CdS quantum dots as nanofluids for electrowetting. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 269–277. [Google Scholar] [CrossRef]
- Khan, A.; Habib, M.R.; Kumar, R.R.; Islam, S.M.; Arivazhagan, V.; Salman, M.; Yang, D.; Yu, X. Wetting behaviors and applications of metal-catalyzed CVD grown graphene. J. Mater. Chem. A 2018, 6, 22437–22464. [Google Scholar] [CrossRef]
- Arscott, S. Electrowetting and semiconductors. RSC Adv. 2014, 4, 29223–29238. [Google Scholar] [CrossRef]
- Sukhatme, S.A.; Agarwal, A. Digital Microfluidics: Techniques, Their Applications and Advantages. J. Bioeng. Biomed. Sci. 2013, 8, 2. [Google Scholar]
- Moon, H.; Cho, S.K.; Garrell, R.L.; Kim, C.-J.C. Low voltage electrowetting-on-dielectric. J. Appl. Phys. 2002, 92, 4080–4087. [Google Scholar] [CrossRef]
- Günther, S.; Endrödy, C.; Si, S.; Weinberger, S.; Claes, R.; Justo, Y.; Heer, H.D.; Neft, A.; Hoffmann, M. EWOD system designed for optical switching. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 1329–1332. [Google Scholar]
- Tohgha, U.N.; Alvino, E.L.; Jarnagin, C.C.; Iacono, S.T.; Godman, N.P. Electrowetting Behavior and Digital Microfluidic Applications of Fluorescent, Polymer-Encapsulated Quantum Dot Nanofluids. ACS Appl. Mater. Interfaces 2019, 11, 28487–28498. [Google Scholar] [CrossRef]
- Tohgha, U.N.; Watson, A.M.; Godman, N.P. Tuning the electrowetting behavior of quantum dot nanofluids. J. Colloid Interface Sci. 2021, 584, 395–402. [Google Scholar] [CrossRef]
- Ribet, F.; De Luca, E.; Ottonello-Briano, F.; Swillo, M.; Roxhed, N.; Stemme, G. Zero-insertion-loss optical shutter based on electrowetting-on-dielectric actuation of opaque ionic liquid microdroplets. Appl. Phys. Lett. 2019, 115, 073502. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Jin, B.; Ren, H. Infrared Optical Switch Using a Movable Liquid Droplet. Micromachines 2015, 6, 186–195. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Wang, Q.-H. Bidirectional optical switch based on electrowetting. J. Appl. Phys. 2013, 113, 193106. [Google Scholar] [CrossRef]
- Tohgha, U.N.; Watson, A.M.; Godman, N.P. Low voltage electrowetting of non-aqueous fluorescent quantum dot nanofluids. J. Mol. Liq. 2022, 359, 119086. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H. Dielectrically actuated attenuator at 1.55 μm. J. Phys. Commun. 2018, 2, 085026. [Google Scholar] [CrossRef]
- Deng, Y.; Ye, D.; Guo, Y.; Zhou, G.; Jiang, H. Multi-Chromophore Dyes for Improving Light Stability of Electro-Fluidic Displays. Front. Phys. 2021, 9, 737205. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.; Jang, D.; Chung, S.K. Switchable liquid shutter operated by electrowetting for security of mobile electronics. Rev. Sci. Instrum. 2021, 92, 055009. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.; Chung, S.K. Switchable liquid shutter for security and design of mobile electronic devices. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; pp. 14–16. [Google Scholar]
- Heikenfeld, J.; Zhou, K.; Kreit, E.; Raj, B.; Yang, S.; Sun, B.; Milarcik, A.; Clapp, L.; Schwartz, R. Electrofluidic displays using Young–Laplace transposition of brilliant pigment dispersions. Nat. Photonics 2009, 3, 292. [Google Scholar] [CrossRef]
- Zhou, K.; Heikenfeld, J.; Dean, K.A.; Howard, E.M.; Johnson, M.R. A full description of a simple and scalable fabrication process for electrowetting displays. J. Micromech. Microeng. 2009, 19, 065029. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, K.; Lao, Y.; Heikenfeld, J.; Cheng, W. Scalable fabrication of electrowetting displays with self-assembled oil dosing. Appl. Phys. Lett. 2007, 91, 011106. [Google Scholar] [CrossRef]
- Lao, Y.; Sun, B.; Zhou, K.; Heikenfeld, J. Ultra-High Transmission Electrowetting Displays Enabled by Integrated Reflectors. J. Disp. Technol. 2008, 4, 120–122. [Google Scholar] [CrossRef]
- Wu, H.; Shui, L.; Li, F.; Hayes, R.; Henzen, A.; Mugele, F.; Zhou, G. Large-Area High-Contrast Hydrophobic/Hydrophilic Patterned Surface for Robust Electrowetting Devices. ACS Appl. Nano Mater. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Wu, H.; Hayes, R.A.; Li, F.; Henzen, A.; Shui, L.; Zhou, G. Influence of fluoropolymer surface wettability on electrowetting display performance. Displays 2018, 53, 47–53. [Google Scholar] [CrossRef]
- He, T.; Jin, M.; Eijkel, J.C.T.; Zhou, G.; Shui, L. Two-phase microfluidics in electrowetting displays and its effect on optical performance. Biomicrofluidics 2016, 10, 011908. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Zhao, L.; Huang, P.; Han, B.; Lv, J.; Qiu, X.; Wei, S.-H.; Tang, Z. Distinct Excitonic Circular Dichroism between Wurtzite and Zincblende CdSe Nanoplatelets. Nano Lett. 2018, 18, 6665–6671. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, F.; Xu, D.; Shen, S.; Wang, Q. Metal ion redox potential plays an important role in high-yield synthesis of monodisperse silver nanoparticles. Chem. Commun. 2012, 48, 4728–4730. [Google Scholar] [CrossRef]
- Lynn Ko, W.Y.; Bagaria, H.G.; Asokan, S.; Lin, K.-J.; Wong, M.S. CdSe tetrapod synthesis using cetyltrimethylammonium bromide and heat transfer fluids. J. Mater. Chem. 2010, 20, 2474–2478. [Google Scholar] [CrossRef]
- Tohgha, U.N.; Crenshaw, E.P.; McConney, M.E.; Lee, K.M.; Godman, N.P. Tuning of optical properties and phase behavior of Nanomaterial-stabilized blue phase liquid crystals. J. Colloid Interface Sci. 2023, 639, 401–407. [Google Scholar] [CrossRef]
- Ly, J.T.; Presley, K.F.; Cooper, T.M.; Baldwin, L.A.; Dalton, M.J.; Grusenmeyer, T.A. Impact of iodine loading and substitution position on intersystem crossing efficiency in a series of ten methylated-meso-phenyl-BODIPY dyes. Phys. Chem. Chem. Phys. 2021, 23, 12033–12044. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Zhong, R.; Gao, Z.; Chen, Z.; Zhang, M.; Wang, F. Photoresponsiveness of Anthracene-Based Supramolecular Polymers Regulated via a σ-Platinated 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene Photosensitizer. Inorg. Chem. 2019, 58, 12407–12414. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Tian, D.; Fu, H.; Wang, S. Recent progress of electrowetting for droplet manipulation: From wetting to superwetting systems. Mater. Chem. Front. 2020, 4, 140–154. [Google Scholar] [CrossRef]
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123, 3543–3624. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Uddin, A.; Wang, H. ZnO Tetrapods: Synthesis and Applications in Solar Cells. Nanomater. Nanotechnol. 2015, 5, 19. [Google Scholar] [CrossRef]


| Sample | Concentration (mg/mL) | Measured S.T in Air (mN/m) |
|---|---|---|
| 1 | 8 | 21.3 |
| 2 | 8 | 21.7 |
| 3 | 8 | 30.5 |
 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohgha, U.N.; Ly, J.T.; Lee, K.M.; Marsh, Z.M.; Watson, A.M.; Grusenmeyer, T.A.; Godman, N.P.; McConney, M.E. Switchable Optical Properties of Dyes and Nanoparticles in Electrowetting Devices. Nanomaterials 2024, 14, 142. https://doi.org/10.3390/nano14020142
Tohgha UN, Ly JT, Lee KM, Marsh ZM, Watson AM, Grusenmeyer TA, Godman NP, McConney ME. Switchable Optical Properties of Dyes and Nanoparticles in Electrowetting Devices. Nanomaterials. 2024; 14(2):142. https://doi.org/10.3390/nano14020142
Chicago/Turabian StyleTohgha, Urice N., Jack T. Ly, Kyung Min Lee, Zachary M. Marsh, Alexander M. Watson, Tod A. Grusenmeyer, Nicholas P. Godman, and Michael E. McConney. 2024. "Switchable Optical Properties of Dyes and Nanoparticles in Electrowetting Devices" Nanomaterials 14, no. 2: 142. https://doi.org/10.3390/nano14020142





