Functional Bi2O3/Gd2O3 Silica-Coated Structures for Improvement of Early Age and Radiation Shielding Performance of Cement Pastes
Abstract
:1. Introduction
2. Research Significance
3. Materials and Methods
3.1. Materials
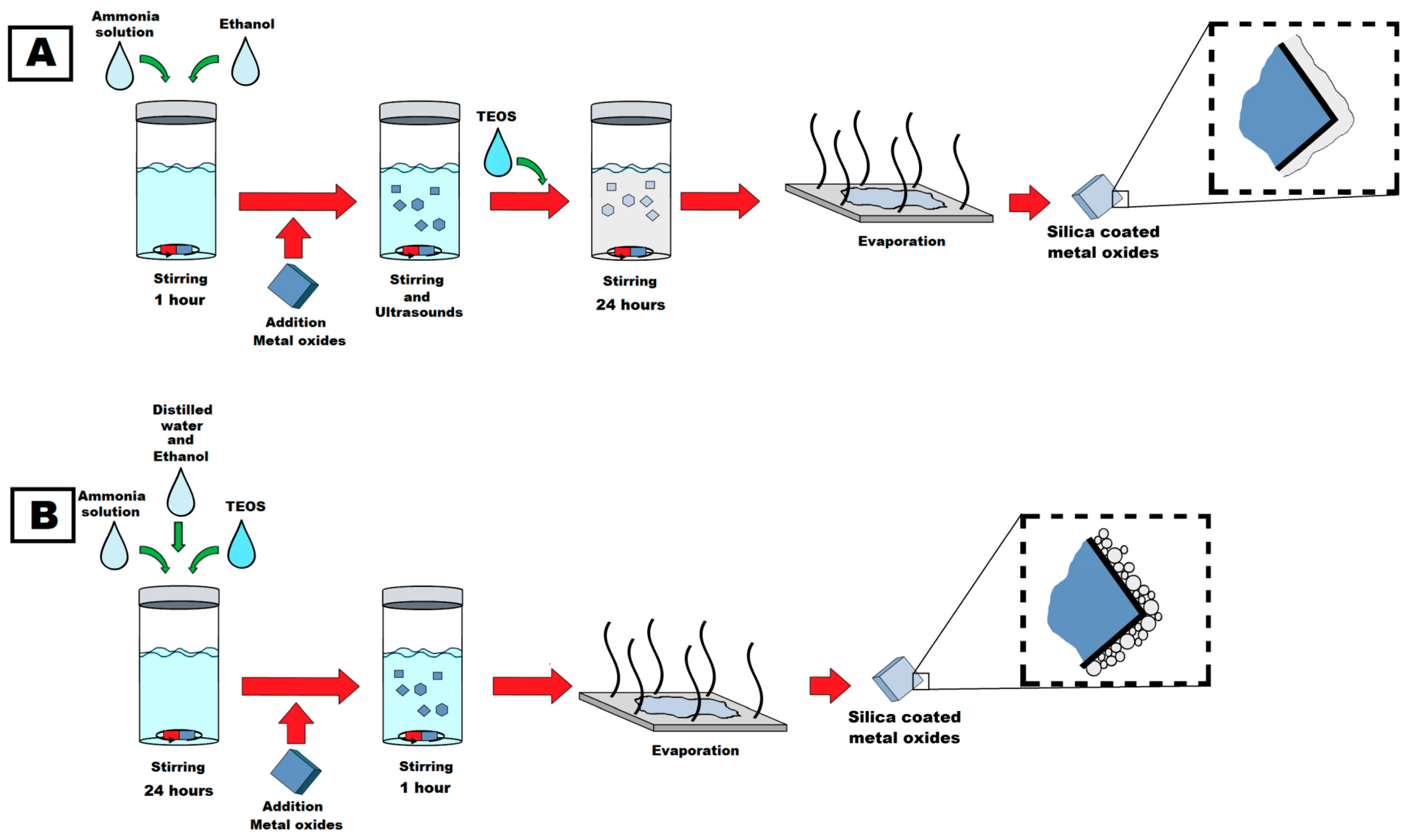
3.2. Synthesis Process of Functional Additives
3.3. Mixture Design and Specimen Preparation
3.4. Methods
3.4.1. Nanomaterials Characterization
3.4.2. Rheological Properties
3.4.3. Isothermal Calorimetry
3.4.4. Thermogravimetric Analysis (TGA)
3.4.5. Mechanical Performance
3.4.6. Mercury Intrusion Porosimetry
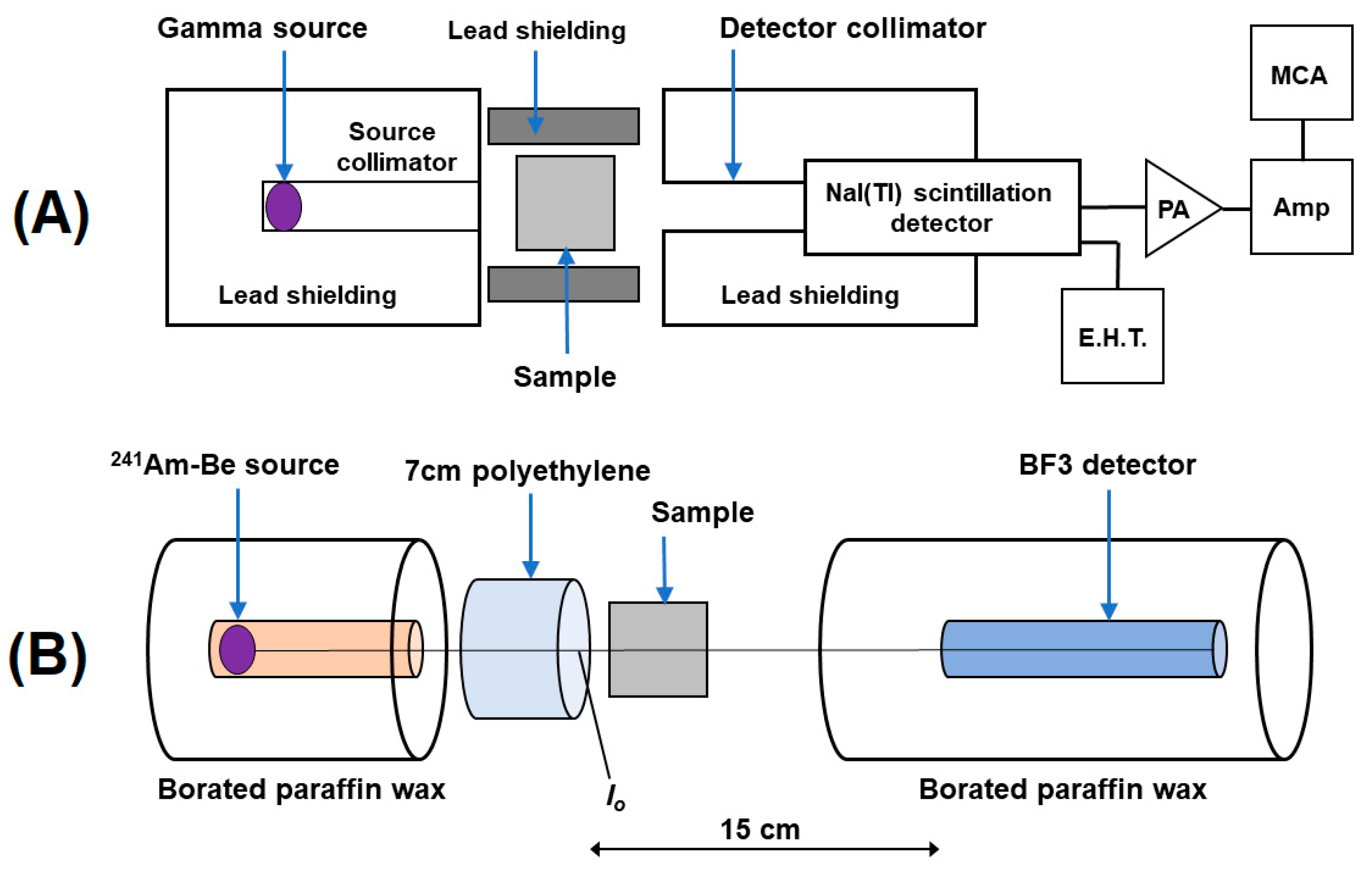
3.5. Radiation Shielding Performance Test
4. Results
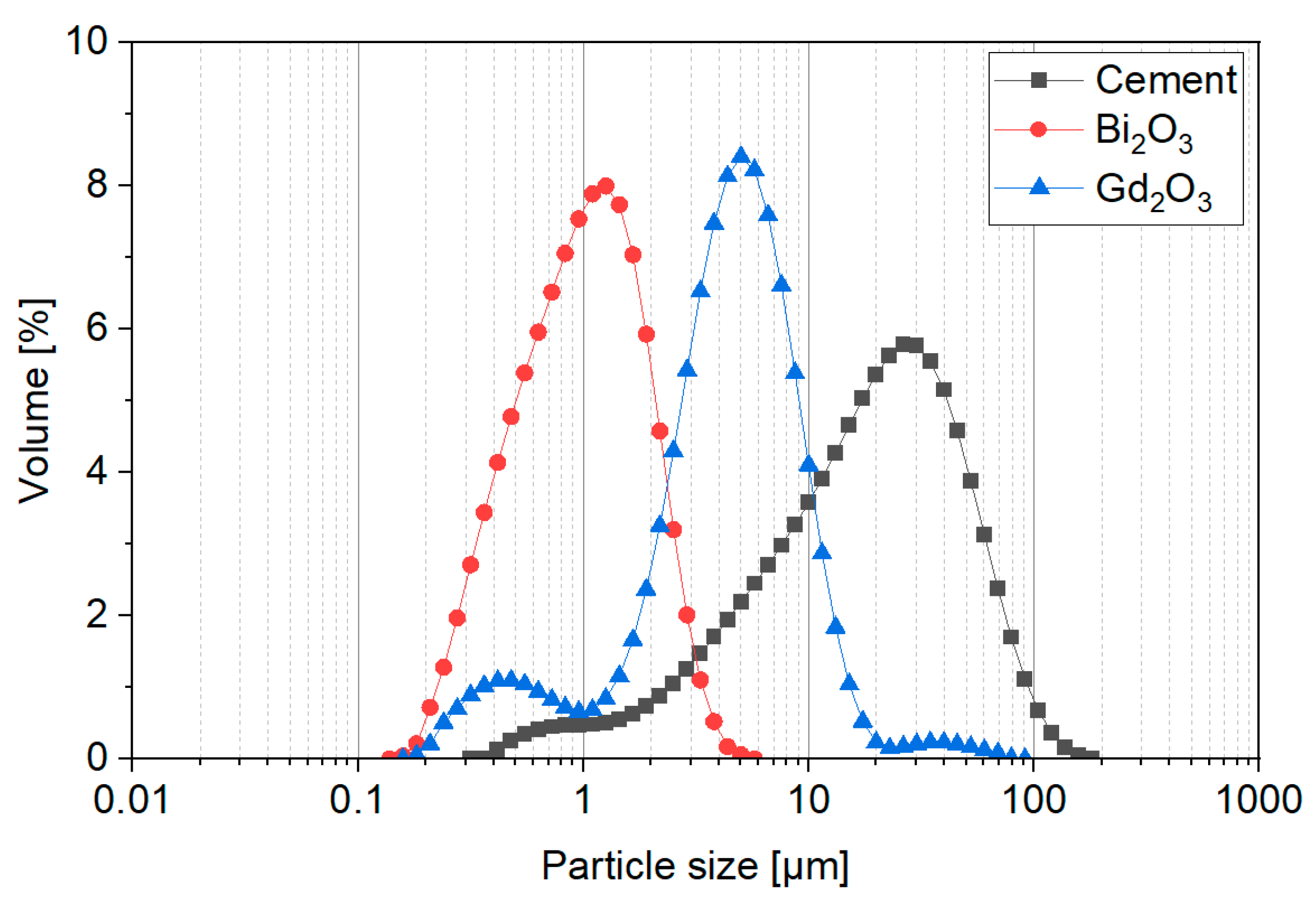
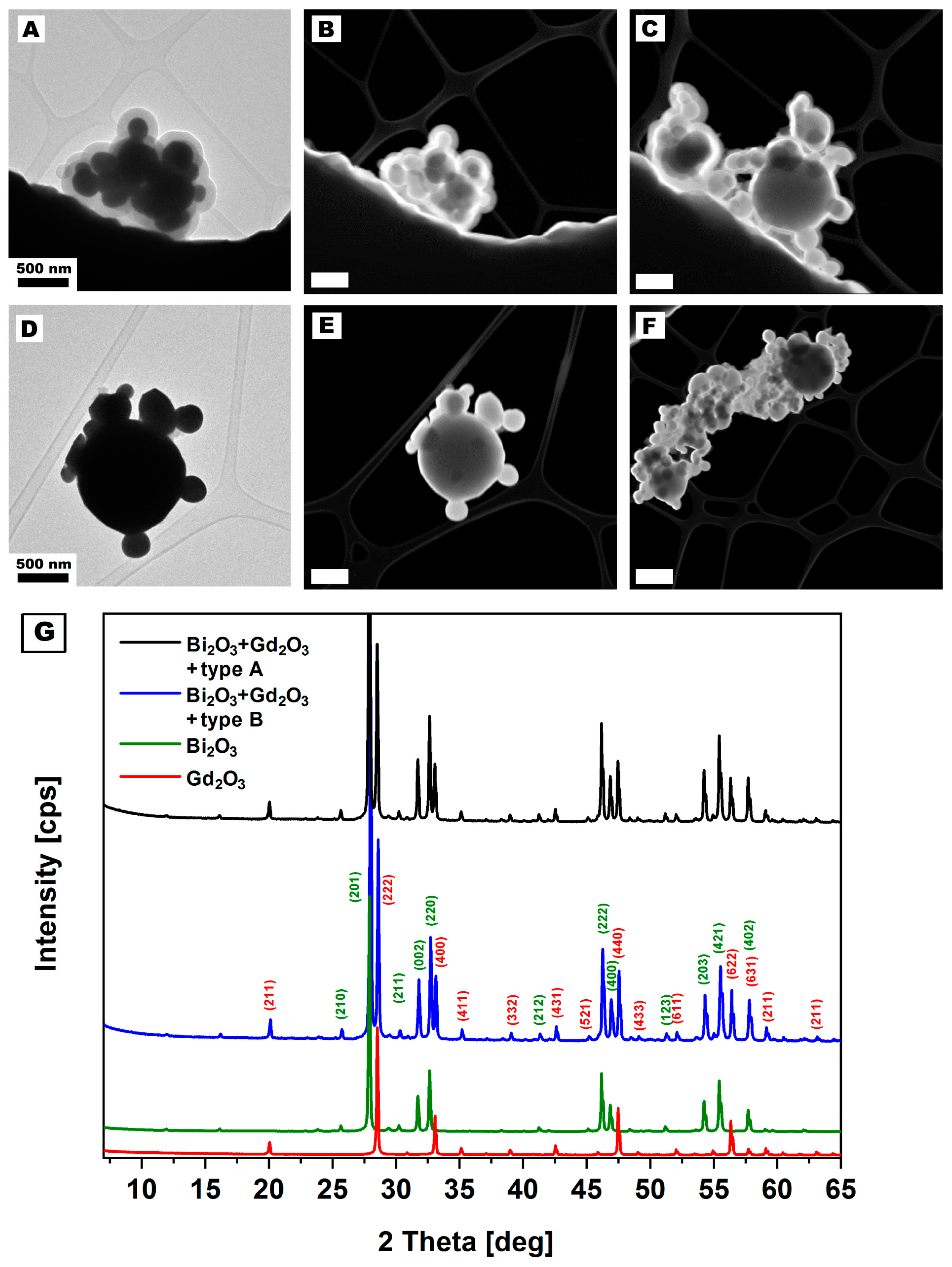
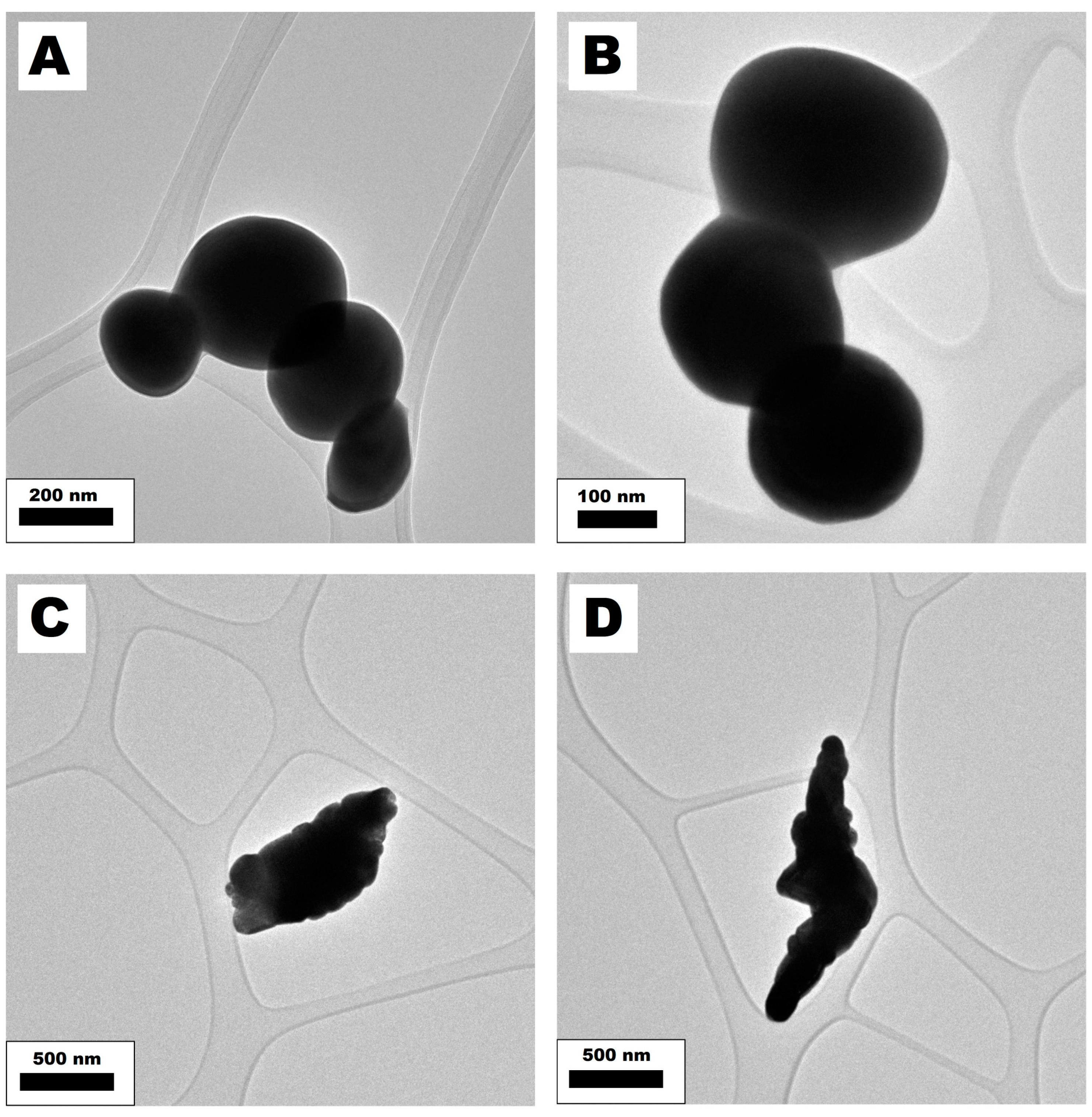
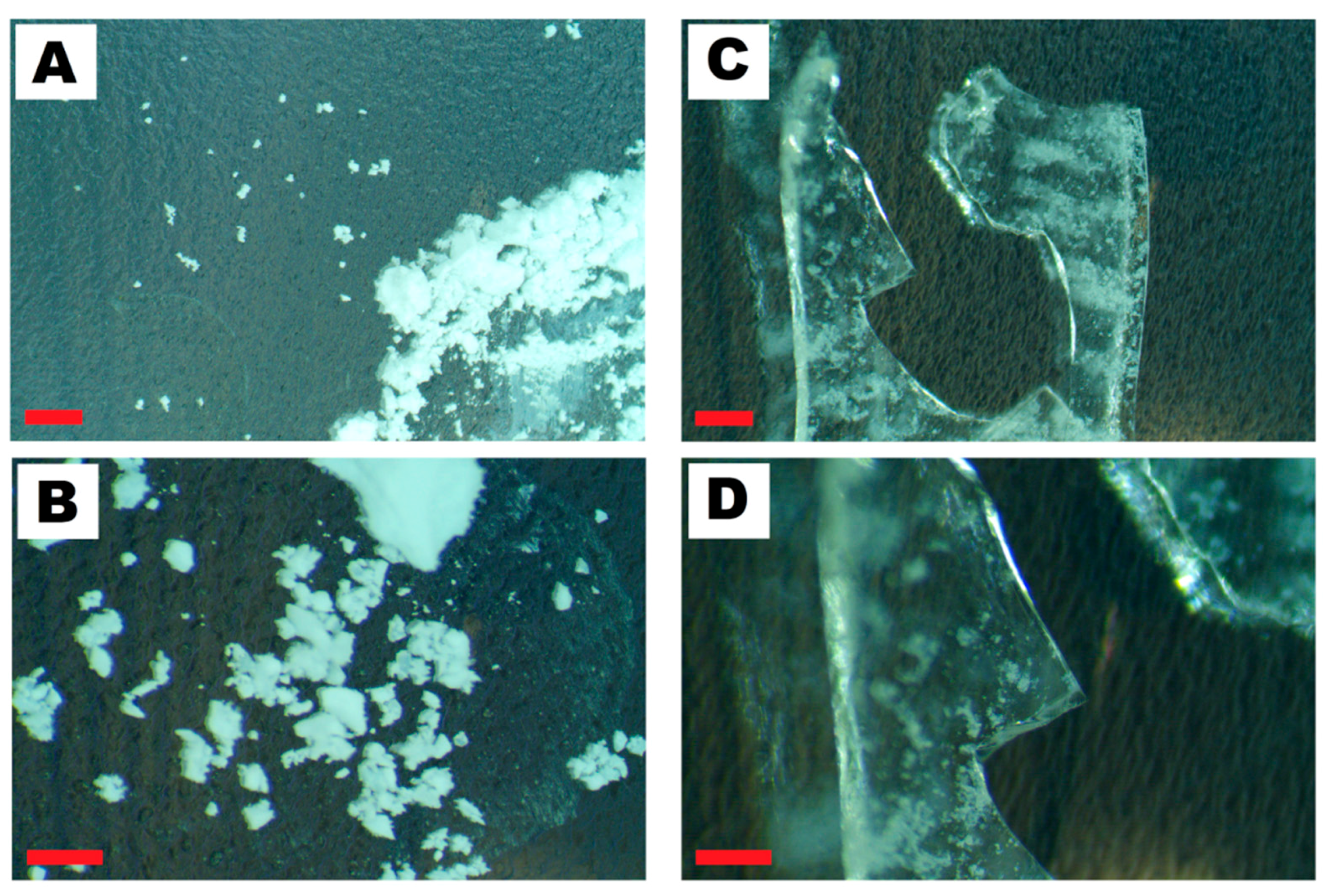
4.1. Nanomaterials Characterization
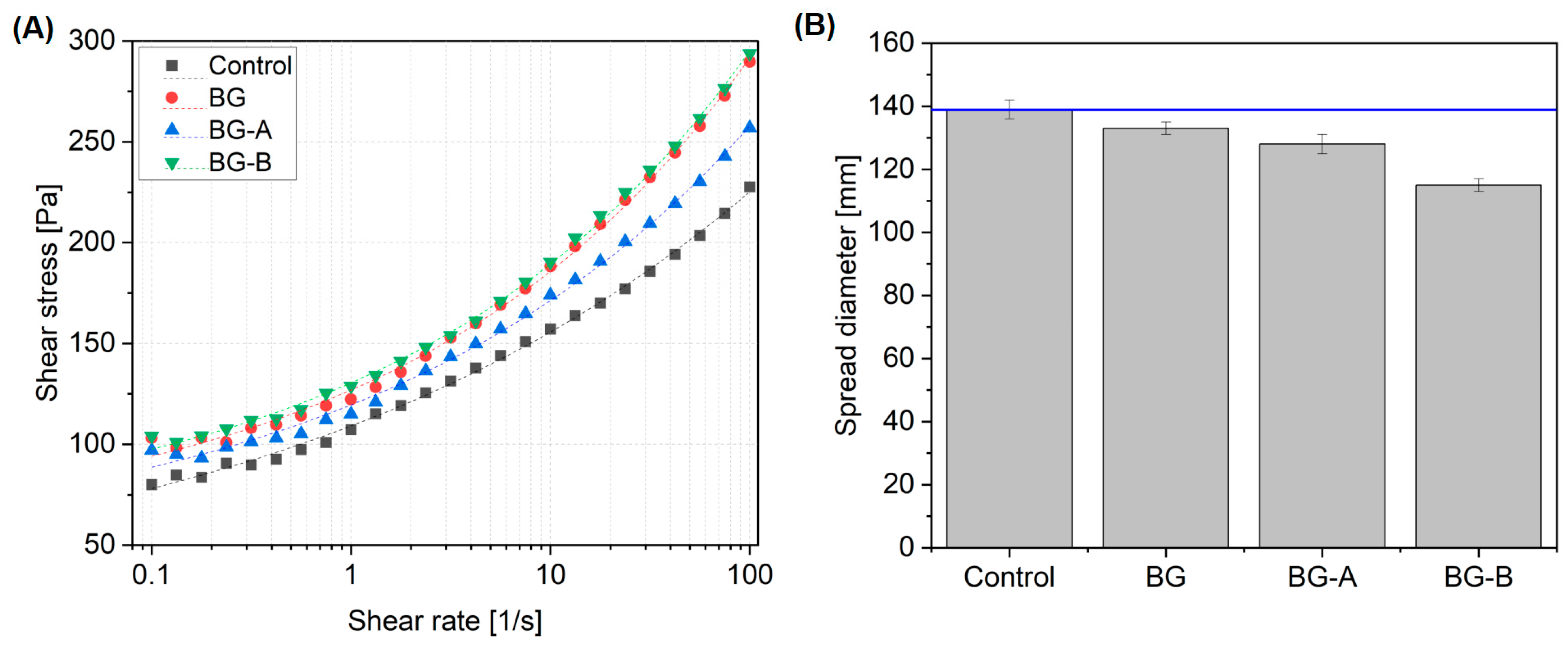
4.2. Fresh Properties
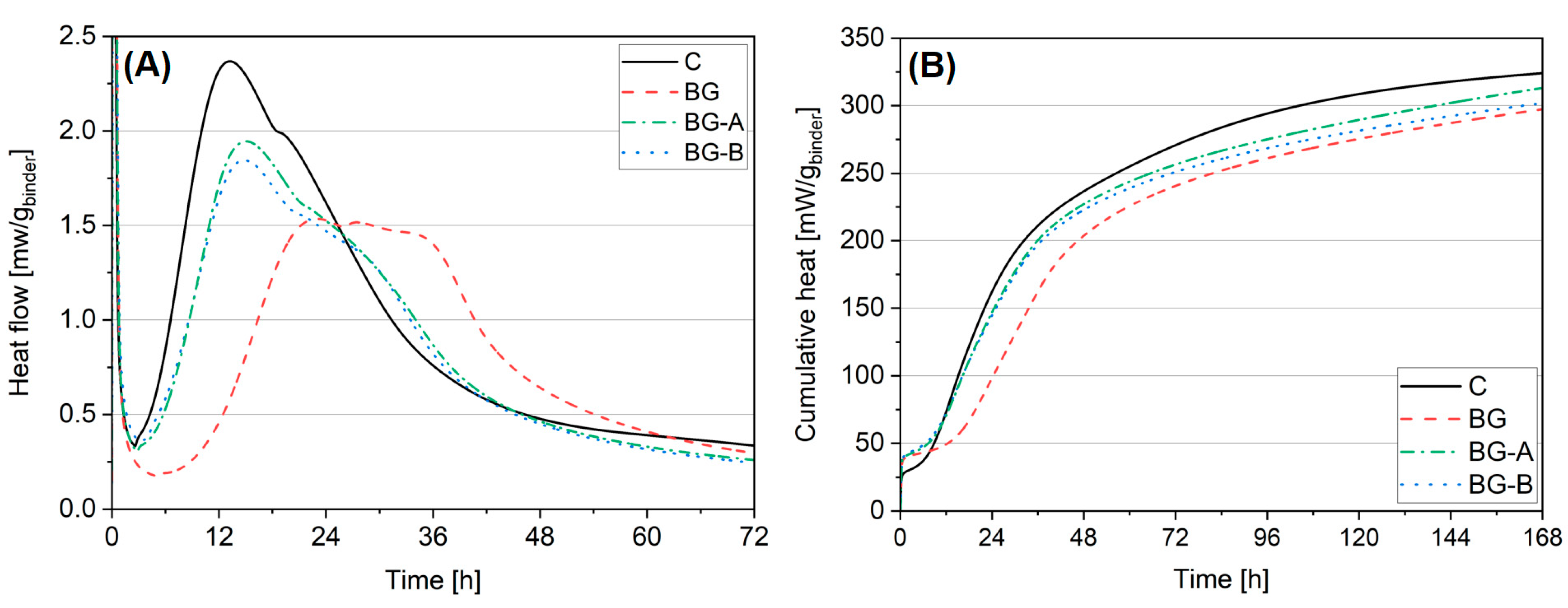
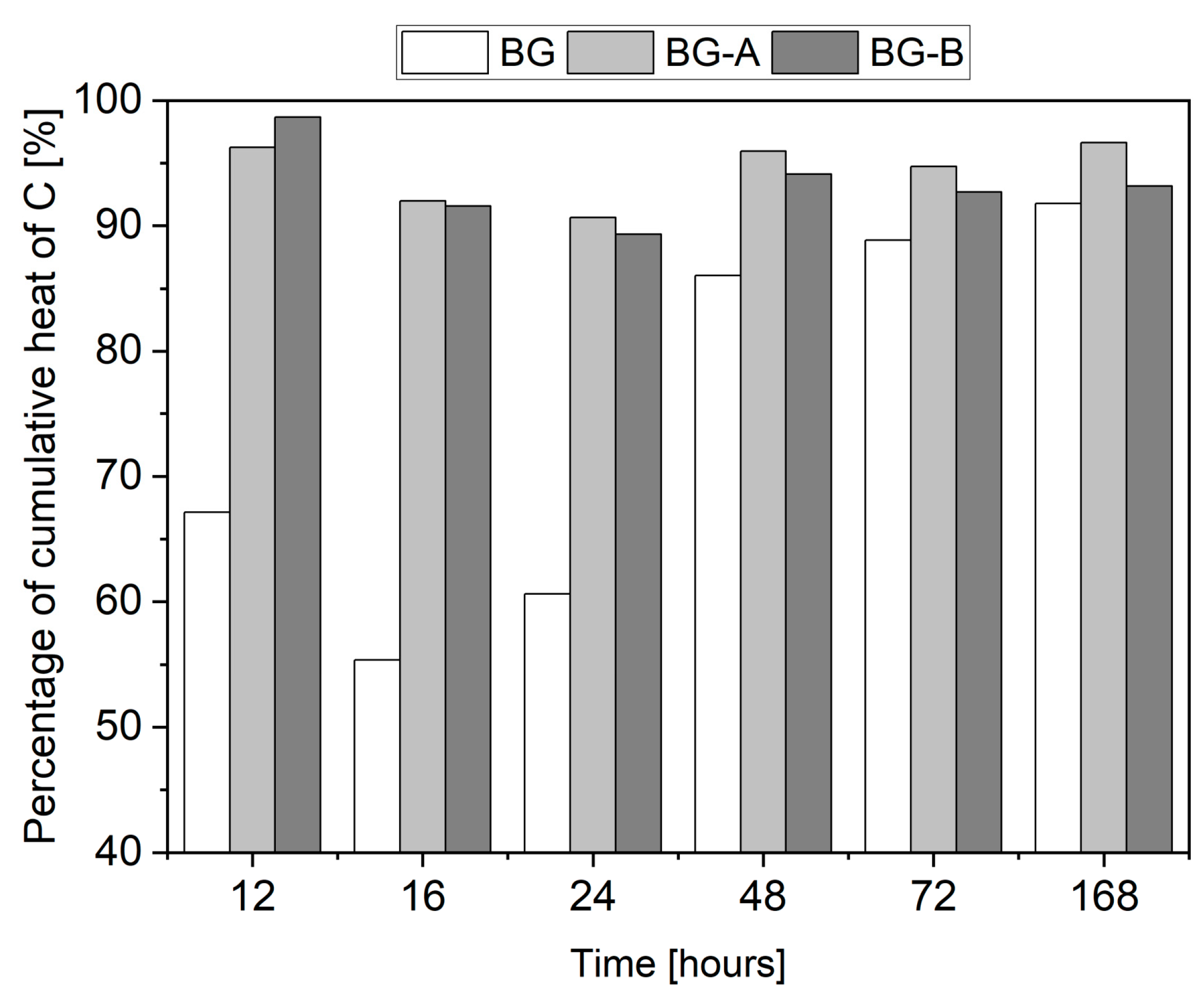
4.3. Isothermal Calorimetry
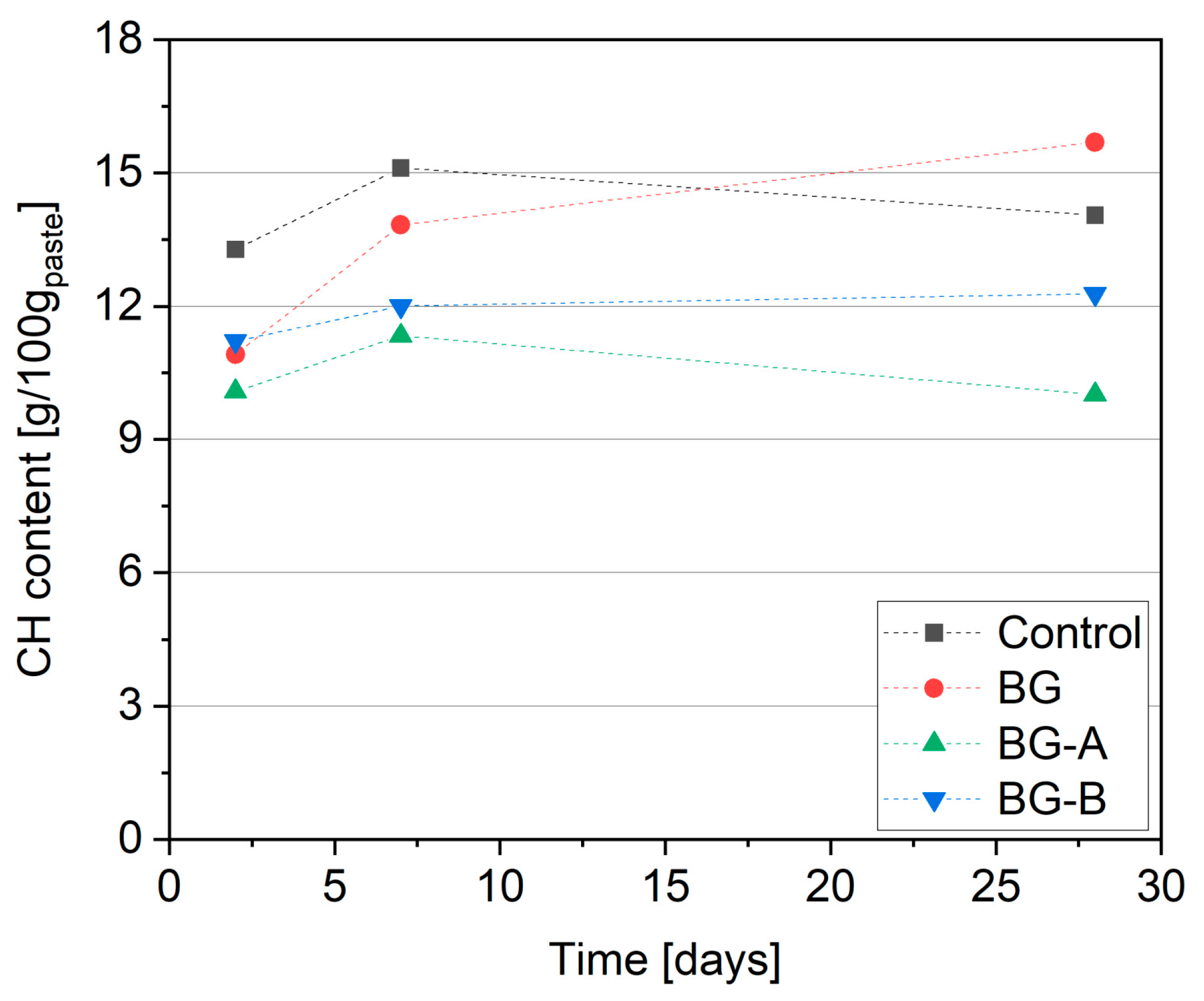
4.4. Thermogravimetric Analysis (CH Content)
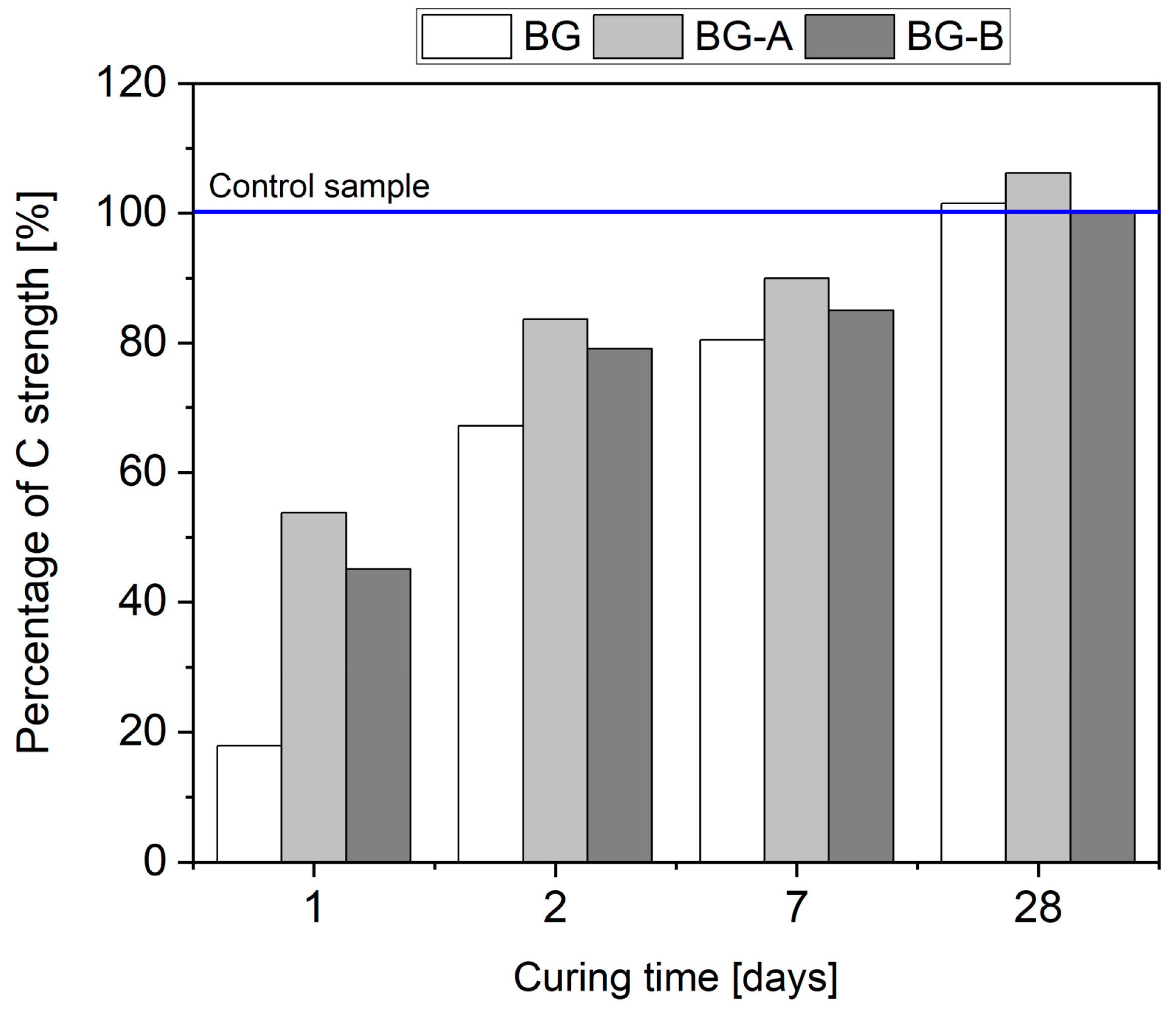
4.5. Mechanical Performance
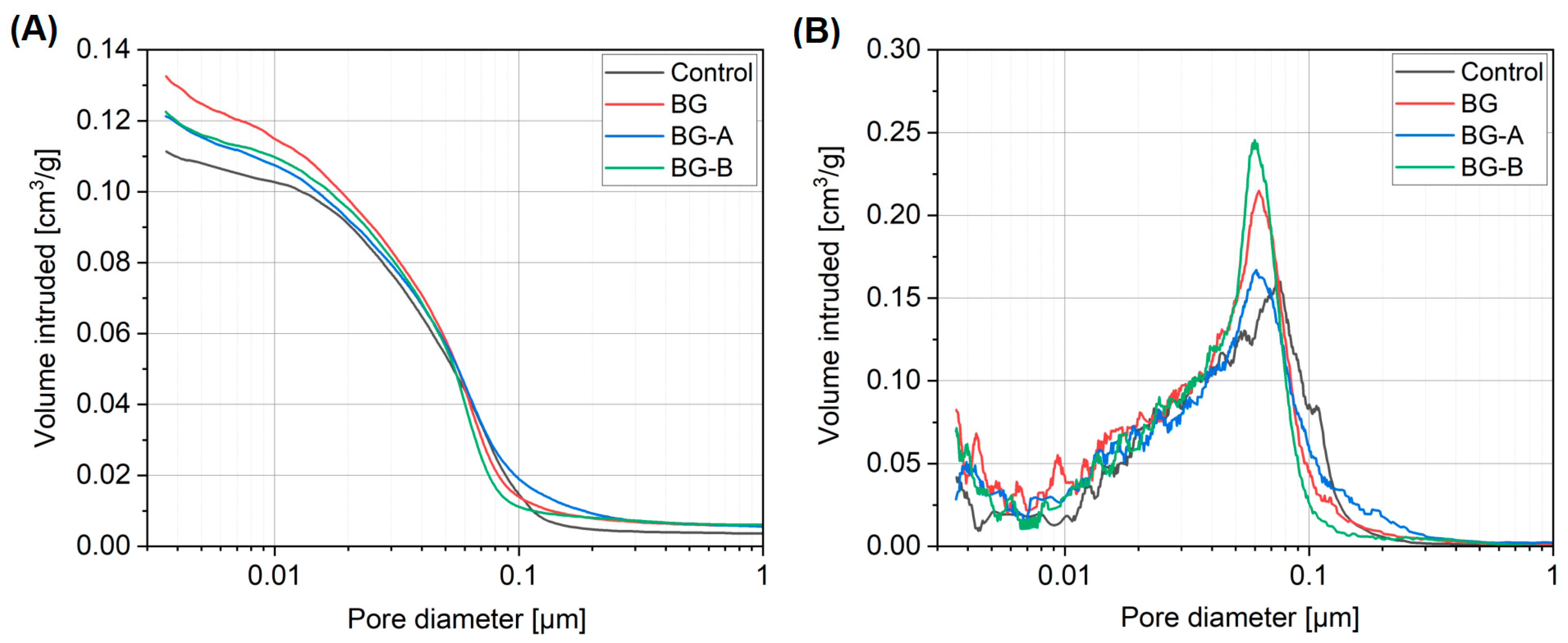
4.6. Mercury Intrusion Porosimetry
4.7. Radiation Shielding Results
4.7.1. Gamma-Ray Attenuation Performance
4.7.2. Slow Neutron Attenuation Performance
5. Conclusions
- Two types of silica-coated Bi2O3/Gd2O3 structures were synthesized and varied in terms of coating structures, thickness, porosity and surface area, allowing the modification of selected properties such as hydration process, rheology, early strength development and radiation shielding. Proposed structures were found to be beneficial both to early age strength development and radiation shielding properties of cement pastes. Thanks to the introduction of high-Z materials Bi2O3/Gd2O3 along with the material of neutron capture cross-section (Gd2O3), simultaneous improvement of both gamma-ray and neutron attenuation can be achieved.
- Higher reactivity of synthesized structures, and thus higher acceleration of hydration process and strength development, was obtained in the case of type A silica coating of Bi2O3/Gd2O3. Due to the pozzolanic activity of silica coating, lower CH contents were found in specimens containing silica-coated structures when compared to specimens containing uncoated particles. Moreover, higher microstructure refinement in specimens BG-A and BG-B was found compared to specimen BG. Although method B shows slightly lower reactivity in the cement hydration process, it offers advantages related to the additional alteration of rheological properties (increased yield shear stress) which can be useful in selected applications such as 3D printing. At the same time, coating type B has the advantage of lower synthesis time and cost reduction (reduction of ethanol content required for synthesis by 80%).
- Rheology tests are an essential and effective tool for selecting nanoparticle candidates and determining the most suitable silica coating methods for specific applications. The incorporation of Bi2O3 and Gd2O3 particles in pastes increases yield shear stress and consistency coefficient. By employing the silica coating method A on BG particles, the yield shear stress of the resulting pastes decreased by around 19%. On the contrary, the application of method B resulted in a slight increase of 6% compared to the plain BG mixture. These findings highlight the significant impact of silica coating on the properties of BG particles, which can ultimately enhance the performance of resulting composites in the hardened state.
- Both pristine and silica-coated structures can be effectively used as a cement filler at a rate of 10 wt% without deterioration of 28 days compressive strength. However, due to the extremely retarding effect of the hydration process attributed to pristine Bi2O3, early age performance of cement pastes containing uncoated structures is deficient. Therefore, the introduction of silica coatings can overcome the retardation of hydration, leading to higher hydration heat and strength gain, especially in the first two days of hydration. Thus, specimens BG-A and BG-B exhibited 300% and 251% (after one day) and 25% and 18% (after two days) higher compressive strength than BG specimens.
- The incorporation of pristine Bi2O3 and Gd2O3 to cement pastes enhanced radiation shielding as expected. The introduction of silica-coated structures resulted in further improvement of the shielding performance of specimens. However, the silica-coated structure synthesized by method B is superior for radiation shielding. The specimens BG-A and BG-B exhibited 7.5% and 13.5% higher LAC, respectively, than the BG specimen at a photon energy of 80 keV. Additionally, the performance of slow neutrons was successfully improved by silica-coated structures. For instance, specimens BG-A and BG-B showed 7.9% and 34.8% higher Σs, respectively, compared to the BG specimen.
- As an outcome, silica-coated structures can be successfully used in cement-based composites with demanding early age performances, e.g. repairing mortars, prefabrication technology or additive manufacturing (3D printing) where early setting times and high early strengths are crucial factors for these technologies.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdullah, M.A.H.; Rashid, R.S.M.; Amran, M.; Hejazii, F.; Azreen, N.M.; Fediuk, R.; Voo, Y.L.; Vatin, N.I.; Idris, M.I. Recent Trends in Advanced Radiation Shielding Concrete for Construction of Facilities: Materials and Properties. Polymers 2022, 14, 2830. [Google Scholar] [CrossRef] [PubMed]
- Badarloo, B.; Lehner, P.; Bakhtiari Doost, R. Mechanical Properties and Gamma Radiation Transmission Rate of Heavyweight Concrete Containing Barite Aggregates. Materials 2022, 15, 2173. [Google Scholar] [CrossRef] [PubMed]
- Zeyad, A.M.; Hakeem, I.Y.; Amin, M.; Tayeh, B.A.; Agwa, I.S. Effect of aggregate and fibre types on ultra-high-performance concrete designed for radiation shielding. J. Build. Eng. 2022, 58, 104960. [Google Scholar] [CrossRef]
- Ban, C.C.; Khalaf, M.A.; Ramli, M.; Ahmed, N.M.; Ahmad, M.S.; Ahmed Ali, A.M.; Dawood, E.T.; Ameri, F. Modern heavyweight concrete shielding: Principles, industrial applications and future challenges; review. J. Build. Eng. 2021, 39, 102290. [Google Scholar] [CrossRef]
- Piotrowski, T.; Tefelski, D.; Polański, A.; Skubalski, J. Monte Carlo simulations for optimization of neutron shielding concrete. Open Eng. 2012, 2, 296–303. [Google Scholar] [CrossRef]
- Gokul, P.; Ashok Kumar, J.; Preetha, R.; Chattopadhyaya, S.; Mini, K.M. Additives in concrete to enhance neutron attenuation characteristics—A critical review. Results Eng. 2023, 19, 101281. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, X.; Li, M.; Yang, R.; Jiang, T.; Lv, J. Investigation of gamma ray shielding efficiency and mechanical performances of concrete shields containing bismuth oxide as an environmentally friendly additive. Radiat. Phys. Chem. 2016, 127, 188–193. [Google Scholar] [CrossRef]
- Sikora, P.; El-Khayatt, A.M.; Saudi, H.A.; Chung, S.-Y.; Stephan, D.; Abd Elrahman, M. Evaluation of the effects of bismuth oxide (Bi2O3) micro and nanoparticles on the mechanical, microstructural and γ-ray/neutron shielding properties of Portland cement pastes. Constr. Build. Mater. 2021, 284, 122758. [Google Scholar] [CrossRef]
- Gouda, M.M.; El-Khatib, A.M.; Abbas, M.I.; Al-Balawi, S.M.; Alabsy, M.T. Gamma Attenuation Features of White Cement Mortars Reinforced by Micro/Nano Bi2O3 Particles. Materials 2023, 16, 1580. [Google Scholar] [CrossRef]
- El-Nahal, M.A.; Elsafi, M.; Sayyed, M.I.; Khandaker, M.U.; Osman, H.; Elesawy, B.H.; Saleh, I.H.; Abbas, M.I. Understanding the Effect of Introducing Micro- and Nanoparticle Bismuth Oxide (Bi2O3) on the Gamma Ray Shielding Performance of Novel Concrete. Materials 2021, 14, 6487. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Mohebbi, R.; Dezhampanah, S.; Mehdipour, S.; Mohammadi, R.; Nejat, T. Gamma ray shielding properties of heavy-weight concrete containing Nano-TiO2. Radiat. Phys. Chem. 2019, 162, 157–167. [Google Scholar] [CrossRef]
- Nikbin, I.; Mehdipour, S.; Dezhampanah, S.; Mohammadi, R.; Mohebbi, R.; Moghadam, H.; Sadrmomtazi, A. Effect of high temperature on mechanical and gamma ray shielding properties of concrete containing nano-TiO2. Radiat. Phys. Chem. 2020, 174, 108967. [Google Scholar] [CrossRef]
- Ramadan, M.; Kohail, M.; Abadel, A.A.; Alharbi, Y.R.; Tuladhar, R.; Mohsen, A. De-aluminated metakaolin-cement composite modified with commercial titania as a new green building material for gamma-ray shielding applications. Case Stud. Constr. Mater. 2022, 17, e01344. [Google Scholar] [CrossRef]
- Dezhampanah, S.; Nikbin, I.M.; Mehdipour, S.; Mohebbi, R.; Moghadam, H. Fiber- reinforced concrete containing nano—TiO2 as a new gamma-ray radiation shielding materials. J. Build. Eng. 2021, 44, 102542. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Almousa, N.; Elsafi, M. Preparation of Mortar with Fe2O3 Nanoparticles for Radiation Shielding Application. Coatings 2022, 12, 1329. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; El-Hosiny, F.I.; El-Gamal, S.; Amin, M.S.; Ramadan, M. Gamma radiation shielding, fire resistance and physicochemical characteristics of Portland cement pastes modified with synthesized Fe2O3 and ZnO nanoparticles. Constr. Build. Mater. 2018, 173, 687–706. [Google Scholar] [CrossRef]
- Al-Tersawy, S.H.; El-Sadany, R.A.; Sallam, H. Experimental gamma-ray attenuation and theoretical optimization of barite concrete mixtures with nanomaterials against neutrons and gamma rays. Constr. Build. Mater. 2021, 289, 123190. [Google Scholar] [CrossRef]
- Al-Ghamdi, H.; Elsafi, M.; Sayyed, M.I.; Almuqrin, A.H.; Tamayo, P. Performance of newly developed concretes incorporating WO3 and barite as radiation shielding material. J. Mater. Res. Technol. 2022, 19, 4103–4114. [Google Scholar] [CrossRef]
- Gavrish, V.; Cherkashina, N.; Chayka, T. Investigations of the influence of tungsten carbide and tungsten oxide nanopowders on the radiation protection properties of cement matrix-based composite materials. J. Phys. Conf. Ser. 2020, 1652, 12008. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Shad, M.; Jafarzadeh, G.A.; Dezhampanah, S. An experimental investigation on combined effects of nano-WO3 and nano-Bi2O3 on the radiation shielding properties of magnetite concretes. Prog. Nucl. Energy 2019, 117, 103103. [Google Scholar] [CrossRef]
- Tekin, H.O.; Singh, V.P.; Manici, T. Effects of micro-sized and nano-sized WO3 on mass attenauation coefficients of concrete by using MCNPX code. Appl. Radiat. Isot. 2017, 121, 122–125. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; ALMisned, G.; Issa, S.A.M.; Ivanov, V.; Tekin, H.O. Towards a better understanding of filler size on radiation shielding enhancement: Impact of micro- and nano-WO3/PbO particle reinforcement on ILC concrete. J. Aust. Ceram. Soc. 2023, 59, 127–135. [Google Scholar] [CrossRef]
- Sarıyer, D.; Küçer, R.; Küçer, N. Neutron Shielding Properties of Concrete and Ferro-Boron. Acta Phys. Pol. A 2015, 128, B-201–B-203. [Google Scholar] [CrossRef]
- Piotrowski, T. Neutron shielding evaluation of concretes and mortars: A review. Constr. Build. Mater. 2021, 277, 122238. [Google Scholar] [CrossRef]
- Abdulrahman, S.T.; Ahmad, Z.; Thomas, S.; Rahman, A.A. Introduction to neutron-shielding materials. In Micro and Nanostructured Composite Materials for Neutron Shielding Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–23. ISBN 9780128194591. [Google Scholar]
- Moe, H.J.; Vallario, E.J. Operational Health Physics Training; ANL-88-26, DE89 004616; Argonne National Laboratory: Lemont, IL, USA, 1988. [Google Scholar]
- Mughabghab, S.F. Thermal Neutron Capture Cross-Sections: Resonance Integrals and g-Factors; IAEA Nuclear Data Section; International Atomic Energy Agency: Vienna, Austria, 2003. [Google Scholar]
- Sayyed, M.I.; Al-Ghamdi, H.; Almuqrin, A.H.; Yasmin, S.; Elsafi, M. A Study on the Gamma Radiation Protection Effectiveness of Nano/Micro-MgO-Reinforced Novel Silicon Rubber for Medical Applications. Polymers 2022, 14, 2867. [Google Scholar] [CrossRef] [PubMed]
- Mesbahi, A.; Verdipoor, K.; Zolfagharpour, F.; Alemi, A. Investigation of fast neutron shielding properties of new polyurethane-based composites loaded with B4C, BeO, WO3, ZnO, and Gd2O3 micro-and nanoparticles. Pol. J. Med. Phys. Eng. 2019, 25, 211–219. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Kothan, S.; Kedkaew, C.; Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 2020, 172, 108791. [Google Scholar] [CrossRef]
- Çağlar, M.; Karabul, Y.; Kılıç, M.; Özdemir, Z.G.; İçelli, O. Na2Si3O7/Ag micro and nano-structured glassy composites: The experimental and MCNP simulation surveys of their radiation shielding performances. Prog. Nucl. Energy 2021, 139, 103855. [Google Scholar] [CrossRef]
- Elsafi, M.; Almuqrin, A.H.; Almutairi, H.M.; Al-Saleh, W.M.; Sayyed, M.I. Grafting red clay with Bi2O3 nanoparticles into epoxy resin for gamma-ray shielding applications. Sci. Rep. 2023, 13, 5472. [Google Scholar] [CrossRef]
- Gouda, M.M.; Abbas, M.I.; Eid, M.H.; Ziedan, M.S.; Ibrahim, M.A.; Tawfik, M.M.; El-Khatib, A.M. Impact of micro/nano cadmium oxide on shielding properties of cement-ball clay matrix. Sci. Rep. 2023, 13, 18224. [Google Scholar] [CrossRef]
- Almatari, M.; Koraim, Y.; Saleh, I.H.; Sayyed, M.I.; Uddin Khandaker, M.; Elsafi, M. Investigation of the photon shielding capability of kaolin clay added with micro and nanoparticles of Bi2O3. Radiat. Phys. Chem. 2022, 200, 110191. [Google Scholar] [CrossRef]
- Bawazeer, O.; Makkawi, K.; Aga, Z.B.; Albakri, H.; Assiri, N.; Althagafy, K.; Ajlouni, A.-W. A review on using nanocomposites as shielding materials against ionizing radiation. J. Umm Al-Qura Univ. Appll. Sci. 2023, 9, 325–340. [Google Scholar] [CrossRef]
- Hu, G.; Shi, G.; Hu, H.; Yang, Q.; Yu, B.; Sun, W. Development of gradient composite shielding material for shielding neutrons and gamma rays. Nucl. Eng. Technol. 2020, 52, 2387–2393. [Google Scholar] [CrossRef]
- Huo, Z.; Zhao, S.; Zhong, G.; Zhang, H.; Hu, L. Surface modified-gadolinium/boron/polyethylene composite with high shielding performance for neutron and gamma-ray. Nucl. Mater. Energy 2021, 29, 101095. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.I.; Prabhu, N.S.; Kamath, S.D. Influence of Bi2O3 on Mechanical Properties and Radiation-Shielding Performance of Lithium Zinc Bismuth Silicate Glass System Using Phys-X Software. Materials 2022, 15, 1327. [Google Scholar] [CrossRef]
- Al-Saleh, W.M.; Almutairi, H.M.; Sayyed, M.I.; Elsafi, M. Multilayer radiation shielding system with advanced composites containing heavy metal oxide nanoparticles: A free-lead solution. Sci. Rep. 2023, 13, 18429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jia, C.; Li, J.; Wang, W. Shielding composites for neutron and gamma-radiation with Gd2O3@W core-shell structured particles. Mater. Lett. 2020, 276, 128082. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Pan, G.; Xu, S.; Yao, L. Preparation of Core–Shell Structure W/Gd2O3 and Study on the Properties of Radiation Protection Materials. Coatings 2022, 12, 851. [Google Scholar] [CrossRef]
- Alhindawy, I.G.; Sayyed, M.I.; Almuqrin, A.H.; Mahmoud, K.A. Optimizing gamma radiation shielding with cobalt-titania hybrid nanomaterials. Sci. Rep. 2023, 13, 8936. [Google Scholar] [CrossRef]
- Soni, B.K.; Makwana, R.; Mukherjee, S.; Barala, S.S.; Parashari, S.; Chauhan, R.; Jodha, A.S.; Katovsky, K. Novel concrete compositions for γ-rays and neutron shielding using WC and B4C. Results Mater. 2021, 10, 100177. [Google Scholar] [CrossRef]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent Progress in Nanomaterials for Modern Concrete Infrastructure: Advantages and Challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pérez, S.P.; Gonzales-Pérez, Y.M.; Pardo-Muñoz, T.E. The use of Nanomaterials in the construction sector: A literary review. DYNA 2022, 89, 101–109. [Google Scholar] [CrossRef]
- Franco-Luján, V.A.; Montejo-Alvaro, F.; Ramírez-Arellanes, S.; Cruz-Martínez, H.; Medina, D.I. Nanomaterial-Reinforced Portland-Cement-Based Materials: A Review. Nanomaterials 2023, 13, 1383. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, K.; Federowicz, K.; Techman, M.; Chougan, M.; Kędzierski, T.; Sanytsky, M.; Mijowska, E.; Sikora, P. Enhancing the Fresh and Early Age Performances of Portland Cement Pastes via Sol-Gel Silica Coating of Metal Oxides (Bi2O3 and Gd2O3). Coatings 2023, 13, 1698. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. The hydration chemistry of ProRoot MTA. Dent. Mater. J. 2015, 34, 458–465. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C-S-H Gel Structure of White Portland Cement. J. Funct. Biomater. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Grazziotin-Soares, R.; Nekoofar, M.H.; Davies, T.E.; Bafail, A.; Alhaddar, E.; Hübler, R.; Busato, A.L.S.; Dummer, P.M.H. Effect of bismuth oxide on white mineral trioxide aggregate: Chemical characterization and physical properties. Int. Endod. J. 2014, 47, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, T.; Glinicka, J.; Glinicki, M.A.; Prochoń, P. Influence of gadolinium oxide and ulexite on cement hydration and technical properties of mortars for neutron radiation shielding purposes. Constr. Build. Mater. 2019, 195, 583–589. [Google Scholar] [CrossRef]
- Bolhassani, M.; Sayyahmanesh, M. A study on mechanical properties of cement paste using magnetite-silica nano-composites. Adv. Cem. Res. 2015, 27, 571–580. [Google Scholar] [CrossRef]
- Han, B.; Li, Z.; Zhang, L.; Zeng, S.; Yu, X.; Han, B.; Ou, J. Reactive powder concrete reinforced with nano SiO2-coated TiO2. Constr. Build. Mater. 2017, 148, 104–112. [Google Scholar] [CrossRef]
- Stynoski, P.; Mondal, P.; Wotring, E.; Marsh, C. Characterization of silica-functionalized carbon nanotubes dispersed in water. J. Nanopart Res. 2013, 15, 1396. [Google Scholar] [CrossRef]
- Pi, Z.; Xiao, H.; Liu, R.; Li, H. Combination usage of nano-SiO2-coated steel fiber and silica fume and its improvement effect on SFRCC. Compos. Part. B Eng. 2021, 221, 109022. [Google Scholar] [CrossRef]
- Suo, Y.; Guo, R.; Xia, H.; Yang, Y.; Yan, F.; Ma, Q. Study on modification mechanism of workability and mechanical properties for graphene oxide-reinforced cement composite. Nanomater. Nanotechnol. 2020, 10, 184798042091260. [Google Scholar] [CrossRef]
- Chougan, M.; Lamastra, F.R.; Bolli, E.; Caschera, D.; Kaciulis, S.; Mazzuca, C.; Montesperelli, G.; Ghaffar, S.H.; Al-Kheetan, M.J.; Bianco, A. Extra-Low Dosage Graphene Oxide Cementitious Nanocomposites: A Nano- to Macroscale Approach. Nanomaterials 2021, 11, 3278. [Google Scholar] [CrossRef] [PubMed]
- Chougan, M.; Lamastra, F.R.; Caschera, D.; Kaciulis, S.; Bolli, E.; Mazzuca, C.; Ghaffar, S.H.; Al-Kheetan, M.J.; Montesperelli, G.; Bianco, A. Cementitious nanocomposites engineered with high-oxidized graphene oxide: Spotting the nano to macro correlation. Ceram. Int. 2023, 49, 964–973. [Google Scholar] [CrossRef]
- Schwartzentruber, L.D.; Le Roy, R.; Cordin, J. Rheological behaviour of fresh cement pastes formulated from a Self Compacting Concrete (SCC). Cem. Concr. Res. 2006, 36, 1203–1213. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351228497. [Google Scholar]
- El-Khayatt, A.M. Nxcom—A program for calculating attenuation coefficients of fast neutrons and gamma-rays. Ann. Nucl. Energy 2011, 38, 128–132. [Google Scholar] [CrossRef]
- El-Khayatt, A.M. Radiation shielding of concretes containing different lime/silica ratios. Ann. Nucl. Energy 2010, 37, 991–995. [Google Scholar] [CrossRef]
- Masoud, M.A.; El-Khayatt, A.M.; Shahien, M.G.; Bakhit, B.R.; Suliman, I.I.; Zayed, A.M. Radiation Attenuation Assessment of Serpentinite Rocks from a Geological Perspective. Toxics 2022, 10, 697. [Google Scholar] [CrossRef]
- Cendrowski, K.; Sikora, P.; Horszczaruk, E.; Mijowska, E. Waste-free synthesis of silica nanospheres and silica nanocoatings from recycled ethanol–ammonium solution. Chem. Pap. 2017, 71, 841–848. [Google Scholar] [CrossRef]
- Cendrowski, K.; Sikora, P.; Zielinska, B.; Horszczaruk, E.; Mijowska, E. Chemical and thermal stability of core-shelled magnetite nanoparticles and solid silica. Appl. Surf. Sci. 2017, 407, 391–397. [Google Scholar] [CrossRef]
- Cendrowski, K.; Chen, X.; Zielinska, B.; Kalenczuk, R.J.; Rümmeli, M.H.; Büchner, B.; Klingeler, R.; Borowiak-Palen, E. Synthesis, characterization, and photocatalytic properties of core/shell mesoporous silica nanospheres supporting nanocrystalline titania. J. Nanopart Res. 2011, 13, 5899–5908. [Google Scholar] [CrossRef]
- Yun, K.K.; Kim, J.B.; Song, C.S.; Hossain, M.S.; Han, S. Rheological Behavior of High-Performance Shotcrete Mixtures Containing Colloidal Silica and Silica Fume Using the Bingham Model. Materials 2022, 15, 428. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Ma, H.; Wang, L.; Qian, X.; Qiao, P. Performance enhancement of silica fume blended mortars using bio-functionalized nano-silica. Constr. Build. Mater. 2021, 312, 125467. [Google Scholar] [CrossRef]
- Alferes Filho, R.d.S.; Yu, Q.; Brouwers, J. Rheological Study Comparing Different Superplasticizers Used for 3D Printable Concrete Mixes: Change on Fresh State Behaviour. SSRN 2023. [Google Scholar] [CrossRef]
- Nair, P.A.K.; Vasconcelos, W.L.; Paine, K.; Calabria-Holley, J. A review on applications of sol-gel science in cement. Constr. Build. Mater. 2021, 291, 123065. [Google Scholar] [CrossRef]
- Rai, S.; Tiwari, S. Nano Silica in Cement Hydration. Mater. Today Proc. 2018, 5, 9196–9202. [Google Scholar] [CrossRef]
- Sanytsky, M.; Kropyvnytska, T.; Vakhula, O.; Bobetsky, Y. Nanomodified Ultra High-Performance Fiber Reinforced Cementitious Composites with Enhanced Operational Characteristics. In Proceedings of CEE 2023: Civil and Environmental Engineering and Architecture; Blikharskyy, Z., Koszelnik, P., Lichołai, L., Nazarko, P., Katunský, D., Eds.; Springer: Cham, Switzerland, 2024; pp. 362–371. ISBN 978-3-031-44954-3. [Google Scholar]
- Bai, S.; Guan, X.; Li, H.; Ou, J. Effect of the specific surface area of nano-silica particle on the properties of cement paste. Powder Technol. 2021, 392, 680–689. [Google Scholar] [CrossRef]
- Maagi, M.T.; Jun, G. Effect of the particle size of nanosilica on early age compressive strength in oil-well cement paste. Constr. Build. Mater. 2020, 262, 120393. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Yang, Q.; Du, X.; Li, C.; Cheng, X. Effects of particle size of colloidal nanosilica on hydration of Portland cement at early age. Adv. Mech. Eng. 2019, 11, 168781401982894. [Google Scholar] [CrossRef]
- Khan, K.; Ahmad, W.; Amin, M.N.; Nazar, S. Nano-Silica-Modified Concrete: A Bibliographic Analysis and Comprehensive Review of Material Properties. Nanomaterials 2022, 12, 1989. [Google Scholar] [CrossRef] [PubMed]
- Kryvenko, P.V.; Sanytsky, M.; Kropyvnytska, T. The Effect of Nanosilica on the Early Strength of Alkali-Activated Portland Composite Cements. SSP 2019, 296, 21–26. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide–cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Popławski, J.; Lelusz, M. Influence of Different Types of Nanosilica on Compressive Strength Development of Cement Matrix Composites. Arch. Civ. Eng. 2020, 66, 169–181. [Google Scholar] [CrossRef]
- Chougan, M.; Marotta, E.; Lamastra, F.R.; Vivio, F.; Montesperelli, G.; Ianniruberto, U.; Bianco, A. A systematic study on EN-998-2 premixed mortars modified with graphene-based materials. Constr. Build. Mater. 2019, 227, 116701. [Google Scholar] [CrossRef]
- Du, Y.; Pundienė, I.; Pranckevičienė, J.; Zujevs, A.; Korjakins, A. Study on the Pore Structure of Lightweight Mortar with Nano-Additives. Nanomaterials 2023, 13, 2942. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Ma, B.; Tan, H. Cement-Based Materials Modified by Colloidal Nano-Silica: Impermeability Characteristic and Microstructure. Nanomaterials 2022, 12, 3176. [Google Scholar] [CrossRef]
- Ren, J.; Luo, X.; Bai, R.; Pan, C.; Zhang, J. Pore characteristics of different phase in nano-modified concrete and their influences on the compressive strength. J. Build. Eng. 2022, 46, 103784. [Google Scholar] [CrossRef]

| Mix | Cement | Water | Bi2O3 | Gd2O3 | Bi2O3/Gd2O3-SiO2-Type A | Bi2O3/Gd2O3-SiO2-Type B |
|---|---|---|---|---|---|---|
| Control | 1 | 0.4 | - | - | - | - |
| BG | 0.9 | 0.4 | 0.05 | 0.05 | - | - |
| BG-A | 0.9 | 0.4 | - | - | 0.1 1 | - |
| BG-B | 0.9 | 0.4 | - | - | - | 0.1 1 |
| Parameter | Symbol | Unit | Equation | Explanation |
|---|---|---|---|---|
| Linear attenuation coefficient (LAC) | cm−1 | x: sample thickness. I0 (incident) and I (transmitted) photon intensities. | ||
| Mass attenuation coefficient (MAC) | cm2/g | µm = µ/ρ | ρ: sample density | |
| Macroscopic Slow neutron cross-section | cm2/g | (incident) and (transmitted) neutron fluxes | ||
| Half-value layer | HVL | cm | HVL = ln2/µ ln2/Σs | - |
| Mean free path | MFP | cm | MFP = 1/µ; 1/Σs | - |
| Sample | Specific Gravity [g/cm3] | BET Surface Area [m2/g] | Median Pore Width [Å] | DFT Total Volume in Pores [cm3/g] |
|---|---|---|---|---|
| Bi2O3 | 8.68 | 1.1007 | 9.116 | 0.00042 |
| Gd2O3 | 7.28 | 0.1666 | 7.551 | 0.00007 |
| Bi2O3/Gd2O3/SiO2—A | 5.46 | 8.0129 | 7.652 | 0.00230 |
| Bi2O3/Gd2O3/SiO2—B | 5.35 | 45.0256 | 10.180 | 0.04682 |
| Sample Designation | Yield Shear Stress τ0 (Pa) | Consistency Coefficient K (Pa·sn) | Rheological Index n | R2 |
|---|---|---|---|---|
| C | 15.9 | 93.3 | 0.17 | 0.99796 |
| BG | 53.2 | 73.7 | 0.25 | 0.99759 |
| BG-A | 42.9 | 76.6 | 0.22 | 0.99697 |
| BG-B | 56.4 | 74.1 | 0.26 | 0.99867 |
| Mix | Maximum Heat [mW/g] | Loss in Comparison to C [%] | Peak Occurrence [h] | Loss in Comparison to C [%] |
|---|---|---|---|---|
| Control | 2.37 | - | 12 h 54 min | - |
| BG | 1.53 | −35% | 22 h 02 min | −71% |
| BG-A | 1.95 | −17% | 14 h 52 min | −15% |
| BG-B | 1.85 | −21% | 14 h 44 min | −14% |
| Mix | 1 Day | 2 Days | 7 Days | 28 Days | ||||
|---|---|---|---|---|---|---|---|---|
| Fc | SD | Fc | SD | Fc | SD | Fc | SD | |
| Control | 17.3 | 0.60 | 35.0 | 2.73 | 54.7 | 0.97 | 58.1 | 1.91 |
| BG | 3.1 | 0.11 | 23.5 | 1.07 | 44.0 | 1.83 | 59.0 | 0.58 |
| BG-A | 9.3 | 0.47 | 29,3 | 0.56 | 49.2 | 1.95 | 61.7 | 1.15 |
| BG-B | 7.8 | 0.43 | 27.7 | 0.51 | 46.5 | 0.97 | 58.2 | 2.45 |
| Mix | Total Porosity [%] | Total Surface Area [m2/g] |
|---|---|---|
| Control | 19.74 | 16.85 |
| BG | 22.63 | 25.61 |
| BG-A | 21.15 | 21.41 |
| BG-B | 20.87 | 21.75 |
| Energy (MeV) | μ (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| Control | BG | IMF% a | BG-A | IMF % b | BG-B | IMF% c | |
| 0.080 | 0.8645 | 1.8325 | 112.0 | 1.9693 | 7.5 | 2.2344 | 13.5 |
| 0.238 | 0.38175 | 0.6279 | 64.5 | 0.6634 | 5.7 | 0.7348 | 10.8 |
| 0.356 | 0.245 | 0.3835 | 56.5 | 0.4053 | 5.7 | 0.4489 | 10.8 |
| 0.662 | 0.21075 | 0.2358 | 11.9 | 0.2489 | 5.5 | 0.2753 | 10.6 |
| 1.173 | 0.1679 | 0.1679 | 5.5 | 0.1771 | 5.5 | 0.1955 | 5.0 |
| 1.325 | 0.15626 | 0.1563 | 5.5 | 0.1648 | 5.5 | 0.1822 | 4.6 |
| 2.614 | 0.11222 | 0.1122 | 5.7 | 0.1186 | 5.7 | 0.1310 | 5.1 |
| Parameter | Control | BG | IMF% a | BG-A | IMF% b | BG-B | IMF% c |
|---|---|---|---|---|---|---|---|
| Σs (cm−1) | 0.0722 | 0.0760 | 5.2 | 0.0820 | 7.9 | 0.1024 | 34.8 |
| HVL (cm) | 9.60 | 9.12 | −4.9 | 8.45 | −7.4 | 6.77 | −25.8 |
| MFP (cm) | 13.85 | 13.16 | −4.9 | 12.19 | −7.4 | 9.76 | −25.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cendrowski, K.; Federowicz, K.; Techman, M.; Chougan, M.; El-Khayatt, A.M.; Saudi, H.A.; Kędzierski, T.; Mijowska, E.; Strzałkowski, J.; Sibera, D.; et al. Functional Bi2O3/Gd2O3 Silica-Coated Structures for Improvement of Early Age and Radiation Shielding Performance of Cement Pastes. Nanomaterials 2024, 14, 168. https://doi.org/10.3390/nano14020168
Cendrowski K, Federowicz K, Techman M, Chougan M, El-Khayatt AM, Saudi HA, Kędzierski T, Mijowska E, Strzałkowski J, Sibera D, et al. Functional Bi2O3/Gd2O3 Silica-Coated Structures for Improvement of Early Age and Radiation Shielding Performance of Cement Pastes. Nanomaterials. 2024; 14(2):168. https://doi.org/10.3390/nano14020168
Chicago/Turabian StyleCendrowski, Krzysztof, Karol Federowicz, Mateusz Techman, Mehdi Chougan, Ahmed M. El-Khayatt, H. A. Saudi, Tomasz Kędzierski, Ewa Mijowska, Jarosław Strzałkowski, Daniel Sibera, and et al. 2024. "Functional Bi2O3/Gd2O3 Silica-Coated Structures for Improvement of Early Age and Radiation Shielding Performance of Cement Pastes" Nanomaterials 14, no. 2: 168. https://doi.org/10.3390/nano14020168






