Microporous Polymer-Modified Glassy Carbon Electrodes for the Electrochemical Detection of Metronidazole: Experimental and Theoretical Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. General Procedure for Electrochemical Polymerization and Characterization
2.2.1. Determination of the Optimal Potential Range for Electropolymerization
2.2.2. Electrochemical Stability, Reversibility, and Adherence of Deposited Films
2.2.3. Spectroscopy and Microscopy Characterization of Polymer Films
2.3. Sensing Application
2.3.1. Metronidazole Purification
2.3.2. The Modification of Glassy Carbon Electrodes and the Electrochemical Detection of MNZ
2.4. Computational Approach
3. Results and Discussion
3.1. Electrochemical Polymerization and Characterization
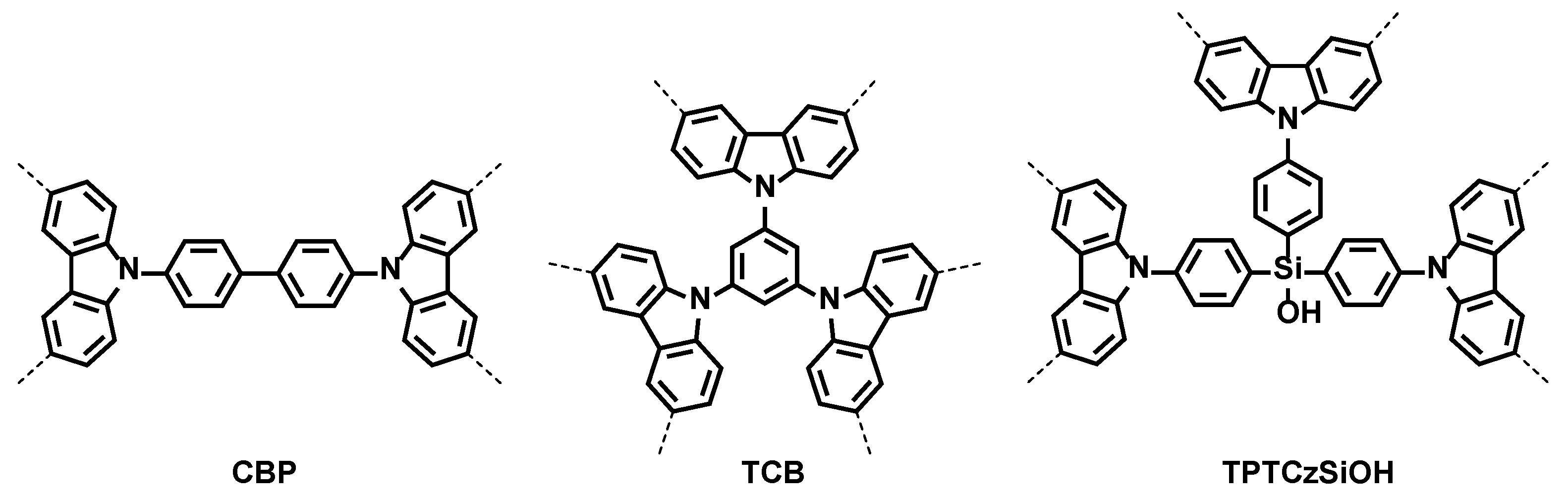
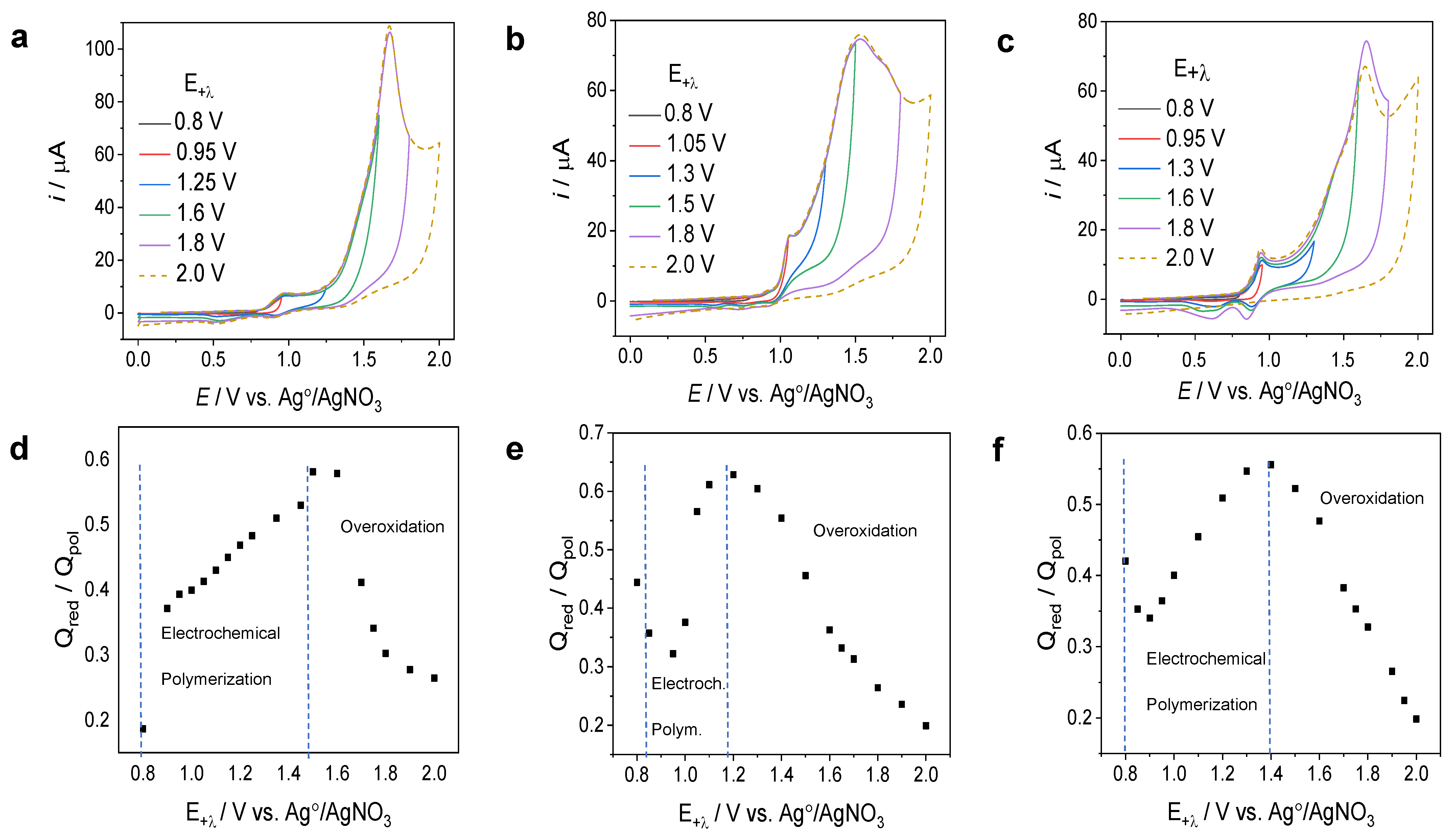
3.1.1. The Determination of the Anodic Potential Range for the Electropolymerization of CBP, TCB, and TPTCzSiOH Monomers
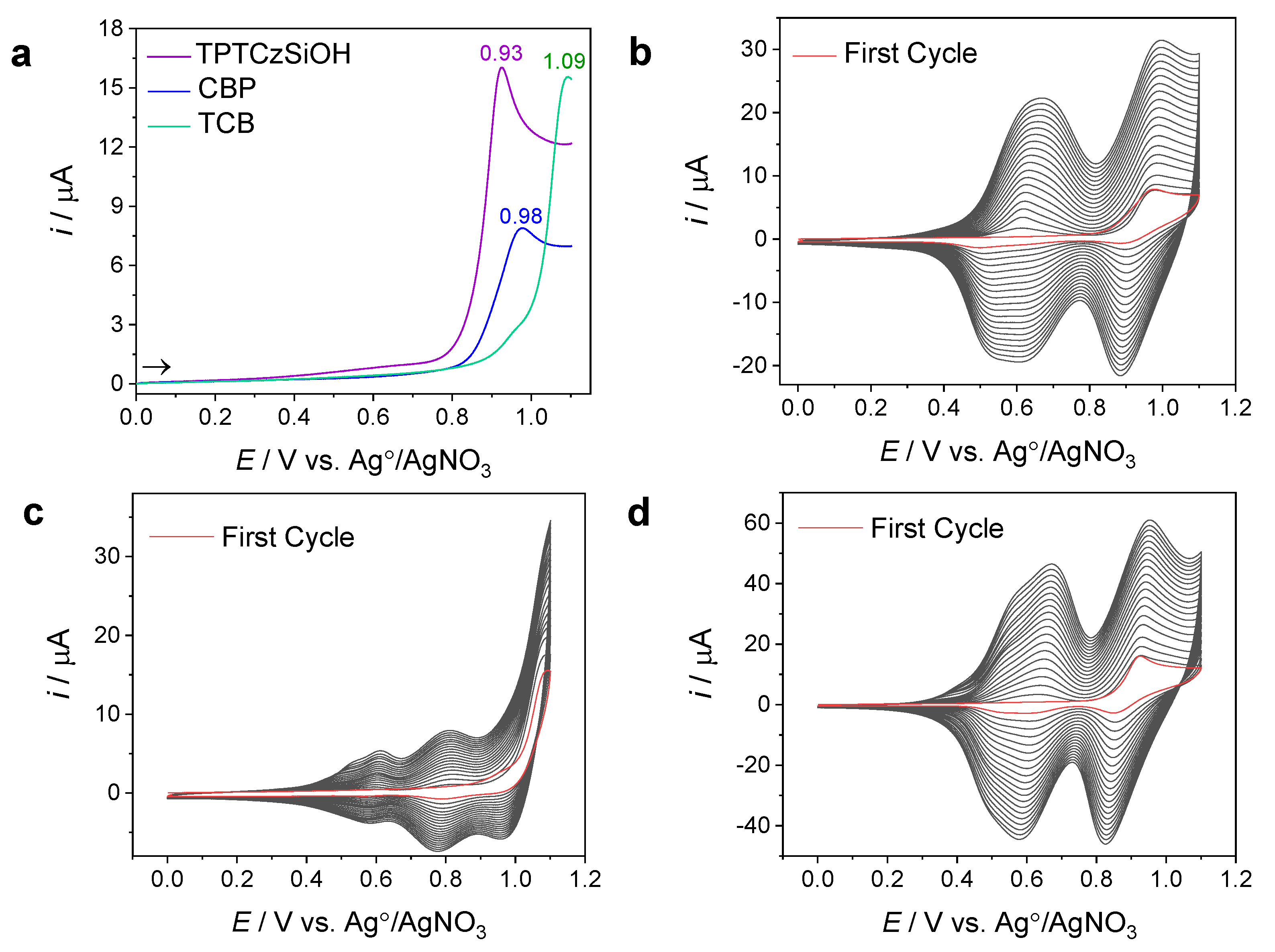
3.1.2. Electrochemical Polymerization of the CBP, TCB, and TPTCzSiOH Monomers
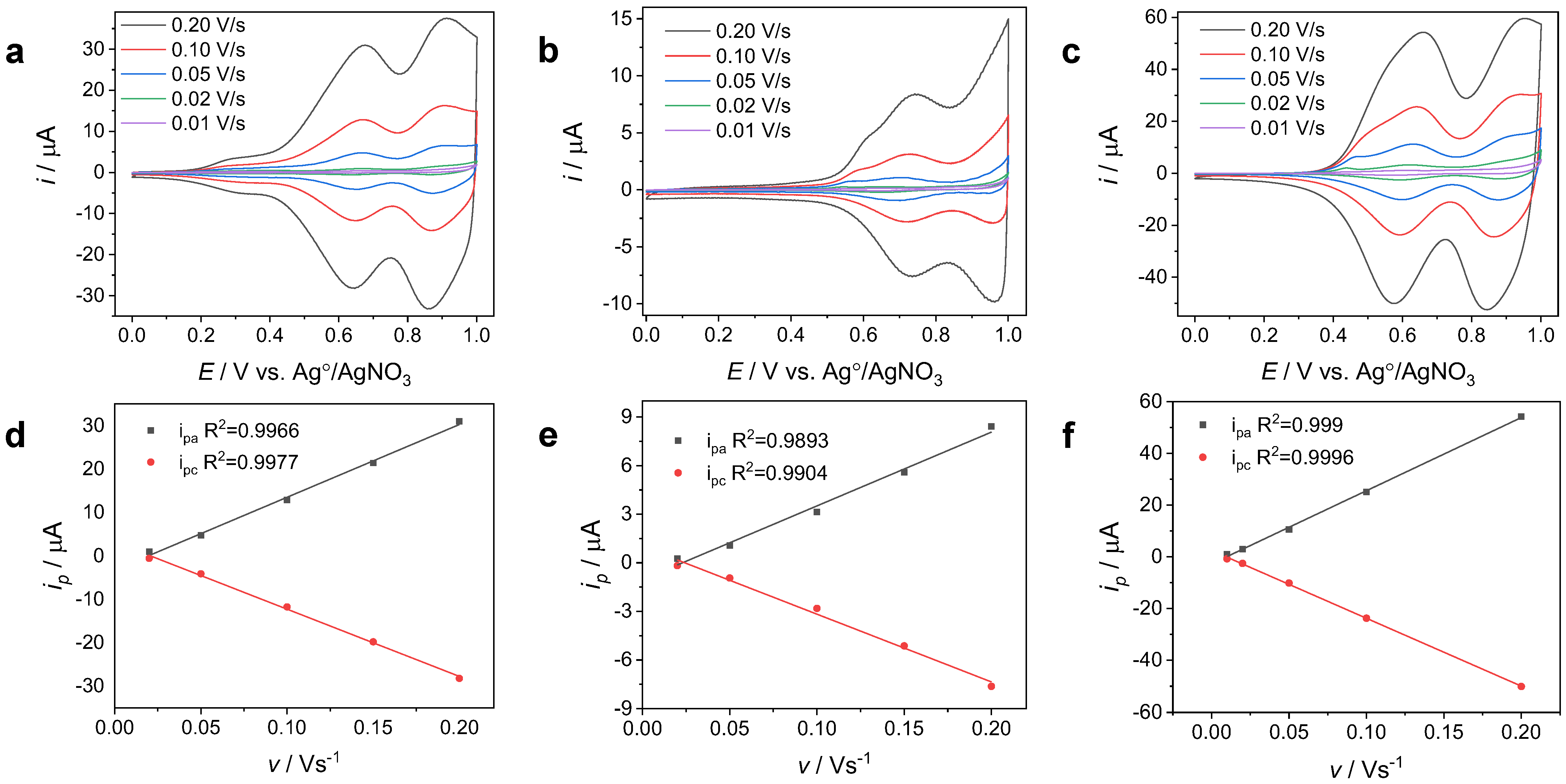
3.1.3. Electrochemical Stability and Reversibility and the Adherence of the PCBP, PTCB, and PTPTCzSiOH Films on GCEs
3.2. Superficial Characterization of MPN Films
3.2.1. Surface Chemical Analysis of MPN Films
3.2.2. Surface Morphology of the MPN Films
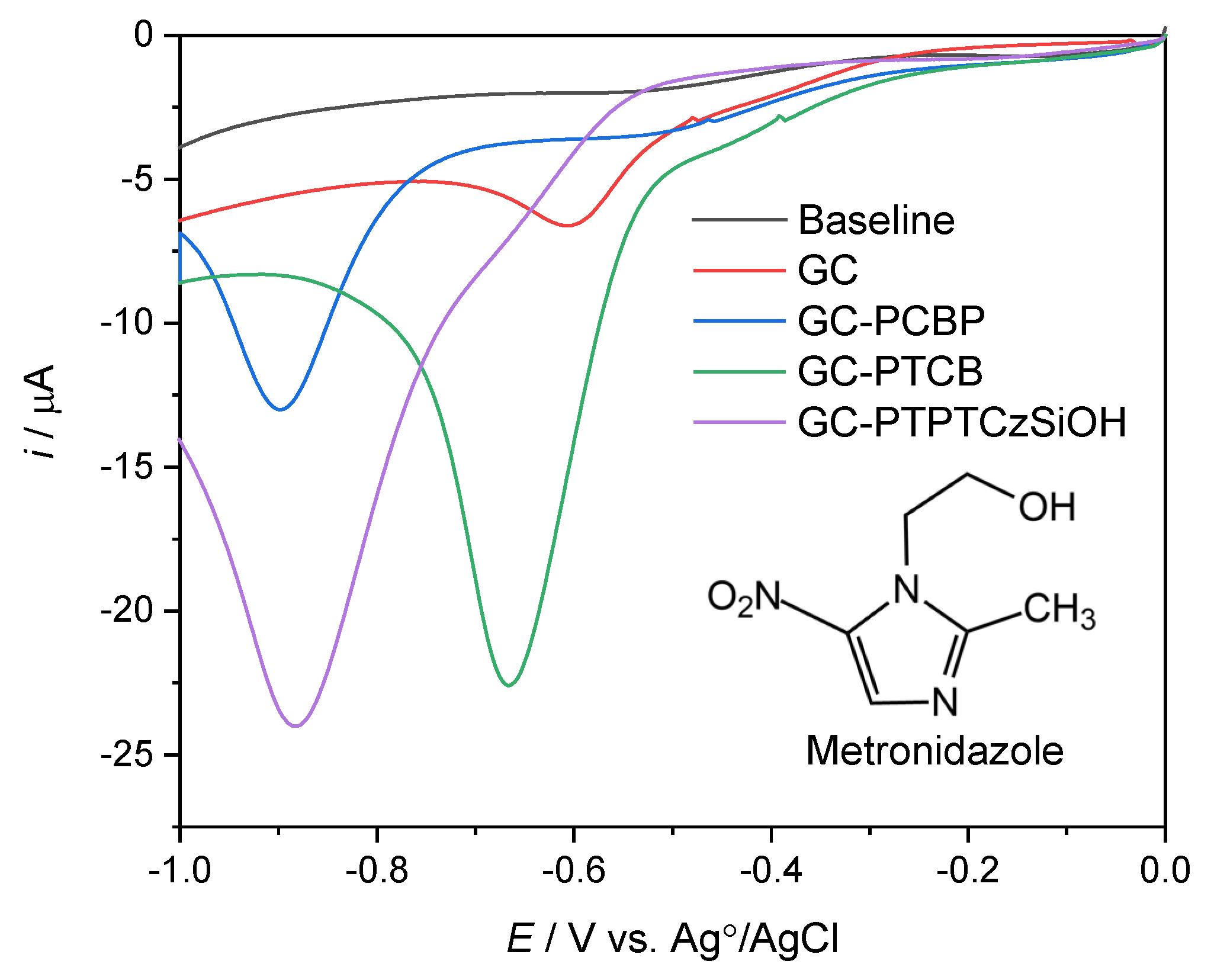
3.3. The Electrochemical Detection of Metronidazole
3.4. Computational Approach
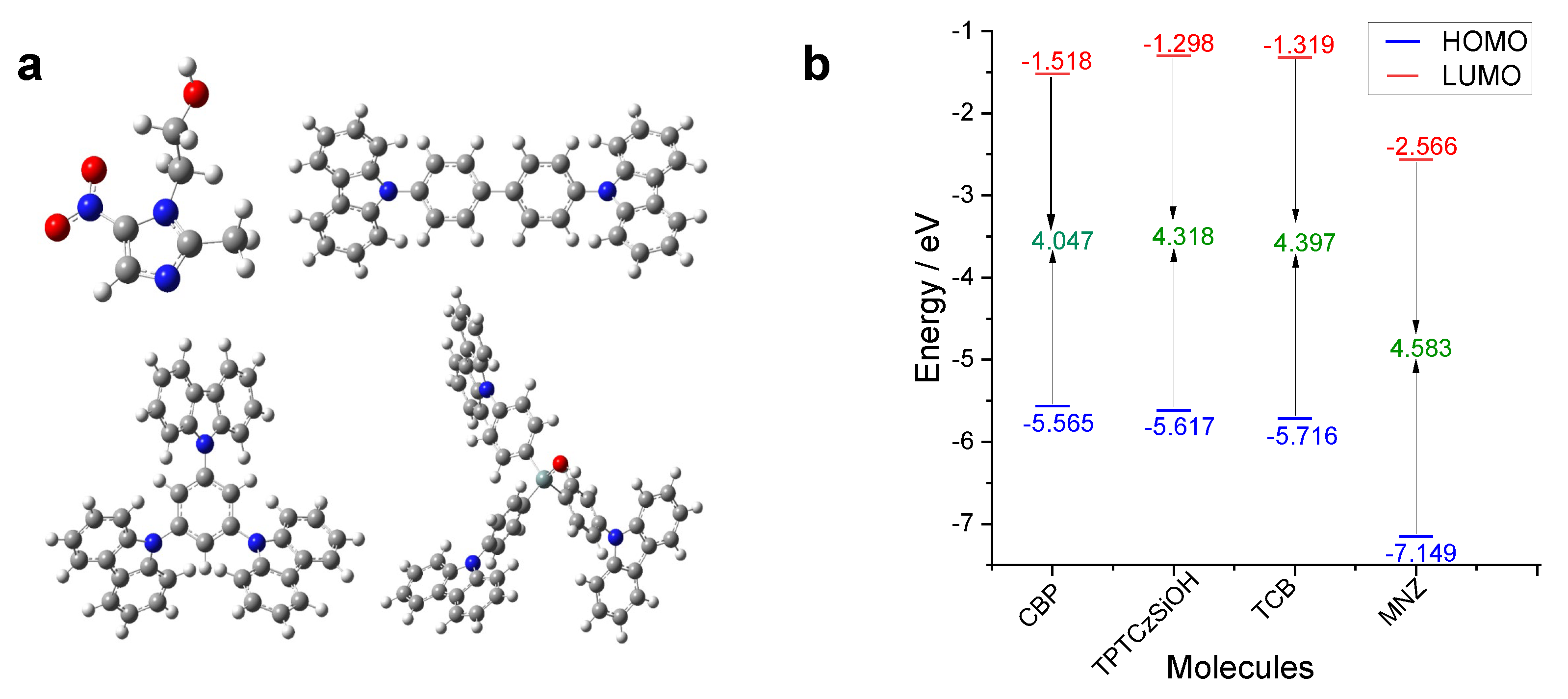
3.4.1. Ground State Geometric Optimization and Frontier Molecular Orbitals (FMOs)
3.4.2. Molecular Electrostatic Potential (MEP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frontana Uribe, B.A.; Palma-Cando, A. Conducting Polymers. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2022; pp. 1–49. [Google Scholar]
- Liu, H.; Li, M.; Kaner, R.B.; Chen, S.; Pei, Q. Monolithically Integrated Self-Charging Power Pack Consisting of a Silicon Nanowire Array/Conductive Polymer Hybrid Solar Cell and a Laser-Scribed Graphene Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 15609–15615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Liu, X.; Mao, E.; Wang, M.; Li, G.; Fu, L.; Li, Z.; Eng, A.Y.S.; Seh, Z.W.; et al. Engineering Stable Electrode-Separator Interfaces with Ultrathin Conductive Polymer Layer for High-Energy-Density Li-S Batteries. Energy Storage Mater. 2019, 23, 261–268. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive Polymers for Stretchable Supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Omelchenko, O.D.; Tameev, A.R.; Lypenko, D.A.; Nekrasov, A.A.; Posudievskii, O.Y.; Koshechko, V.G.; Vannikov, A.V. The Specific Effect of Graphene Additives in Polyaniline-Based Nanocomposite Layers on Performance Characteristics of Electroluminescent and Photovoltaic Devices. High Energy Chem. 2016, 50, 134–138. [Google Scholar] [CrossRef]
- Tang, H.; Kumar, P.; Zhang, S.; Yi, Z.; De Crescenzo, G.; Santato, C.; Soavi, F.; Cicoira, F. Conducting Polymer Transistors Making Use of Activated Carbon Gate Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.Y.; Hu, C.W.; Chang, L.C.; Ho, K.C. A Complementary Electrochromic Device Based on Carbon Nanotubes/Conducting Polymers. Sol. Energy Mater. Sol. Cells 2012, 98, 294–299. [Google Scholar] [CrossRef]
- Rendón-Enríquez, I.; Palma-Cando, A.; Körber, F.; Niebisch, F.; Forster, M.; Tausch, M.W.; Scherf, U. Thin Polymer Films by Oxidative or Reductive Electropolymerization and Their Application in Electrochromic Windows and Thin-Film Sensors. Molecules 2023, 28, 883. [Google Scholar] [CrossRef]
- García-Gallegos, J.C.; Martín-Gullón, I.; Conesa, J.A.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J. The Effect of Carbon Nanofillers on the Performance of Electromechanical Polyaniline-Based Composite Actuators. Nanotechnology 2015, 27, 15501. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Developments in Conducting Polymers: Applications for Electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Woitassek, D.; Brunklaus, G.; Scherf, U. Luminescent Tetraphenylethene-Cored, Carbazole- and Thiophene-Based Microporous Polymer Films for the Chemosensing of Nitroaromatic Analytes. Mater. Chem. Front. 2017, 1, 1118–1124. [Google Scholar] [CrossRef]
- Räupke, A.; Palma-Cando, A.; Shkura, E.; Teckhausen, P.; Polywka, A.; Görrn, P.; Scherf, U.; Riedl, T. Highly Sensitive Gas-Phase Explosive Detection by Luminescent Microporous Polymer Networks. Sci. Rep. 2016, 6, 29118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Borah, P.; Devi, P. Chapter 3—Priority and Emerging Pollutants in Water. In Inorganic Pollutants in Water; Devi, P., Singh, P., Kansal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–49. ISBN 978-0-12-818965-8. [Google Scholar]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Aarab, N.; Hsini, A.; Essekri, A.; Laabd, M.; Lakhmiri, R.; Albourine, A. Removal of an Emerging Pharmaceutical Pollutant (Metronidazole) Using PPY-PANi Copolymer: Kinetics, Equilibrium and DFT Identification of Adsorption Mechanism. Groundw. Sustain. Dev. 2020, 11, 100416. [Google Scholar] [CrossRef]
- Yang, M.; Guo, M.; Feng, Y.; Lei, Y.; Cao, Y.; Zhu, D.; Yu, Y.; Ding, L. Sensitive Voltammetric Detection of Metronidazole Based on Three-Dimensional Graphene-Like Carbon Architecture/Polythionine Modified Glassy Carbon Electrode. J. Electrochem. Soc. 2018, 165, B530–B535. [Google Scholar] [CrossRef]
- Zoubir, J.; Bougdour, N.; El Hayaoui, W.; Radaa, C.; Idlahcen, A.; Assabbane, A.; Bakas, I. Electrochemical Detection of Metronidazole Using Silver Nanoparticle-Modified Carbon Paste Electrode. Electrocatalysis 2022, 13, 386–401. [Google Scholar] [CrossRef]
- Pandiyan, R.; Vinothkumar, V.; Chen, S.-M.; Sangili, A.; Kim, T.H. Integrated LaFeO3/RGO Nanocomposite for the Sensitive Electrochemical Detection of Antibiotic Drug Metronidazole in Urine and Milk Samples. Appl. Surf. Sci. 2023, 635, 157672. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-Based Microporous Polymer Networks via Chemical or Electrochemical Oxidative Coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef]
- Salimi, A.; Izadi, M.; Hallaj, R.; Rashidi, M. Simultaneous Determination of Ranitidine and Metronidazole at Glassy Carbon Electrode Modified with Single Wall Carbon Nanotubes. Electroanalysis 2007, 19, 1668–1676. [Google Scholar] [CrossRef]
- Zhai, H.; Liang, Z.; Chen, Z.; Wang, H.; Liu, Z.; Su, Z.; Zhou, Q. Simultaneous Detection of Metronidazole and Chloramphenicol by Differential Pulse Stripping Voltammetry Using a Silver Nanoparticles/Sulfonate Functionalized Graphene Modified Glassy Carbon Electrode. Electrochim. Acta 2015, 171, 105–113. [Google Scholar] [CrossRef]
- Ahmad, K.; Raza, W.; Alsulmi, A.; Kim, H. Fabrication of Electrochemical Sensor for Metronidazole Using MoS2/Graphite-like Carbon Nitride Composite Modified Glassy Carbon Electrode. Diam. Relat. Mater. 2023, 138, 110178. [Google Scholar] [CrossRef]
- Dawit, M.; Turbale, M.; Moges, A.; Amare, M. Poly(Alizarin Red S) Modified Glassy Carbon Electrode for Square Wave Adsorptive Stripping Voltammetric Determination of Metronidazole in Tablet Formulation. PLoS ONE 2020, 15, e0244115. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, S.; Rama, R.; Pandian, K.; Gopinath, S.C.B. Modified Electrodes for Electrochemical Determination of Metronidazole in Drug Formulations and Biological Samples: An Overview. Microchem. J. 2021, 165, 106151. [Google Scholar] [CrossRef]
- Li, J.; Grimsdale, A.C. Carbazole-Based Polymers for Organic Photovoltaic Devices. Chem. Soc. Rev. 2010, 39, 2399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, M.; Hammershøj; Zhou, D.; Han, Y.; Laursen, B.W.; Yan, C.G.; Han, B.H. Microporous Polycarbazole with High Specific Surface Area for Gas Storage and Separation. J. Am. Chem. Soc. 2012, 134, 6084–6087. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Preis, E.; Scherf, U. Silicon- or Carbon-Cored Multifunctional Carbazolyl Monomers for the Electrochemical Generation of Microporous Polymer Films. Macromolecules 2016, 49, 8041–8047. [Google Scholar] [CrossRef]
- Chen, Q.; Han, B.H. Microporous Polycarbazole Materials: From Preparation and Properties to Applications. Macromol. Rapid Commun. 2018, 39, 1800040. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrochemically Generated Thin Films of Microporous Polymer Networks: Synthesis, Properties, and Applications. Macromol. Chem. Phys. 2016, 217, 827–841. [Google Scholar] [CrossRef]
- Sotzing, G.A.; Reddinger, J.L.; Katritzky, A.R.; Soloducho, J.; Musgrave, R.; Reynolds, J.R.; Steel, P.J. Multiply Colored Electrochromic Carbazole-Based Polymers. Chem. Mater. 1997, 9, 1578–1587. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrogenerated Thin Films of Microporous Polymer Networks with Remarkably Increased Electrochemical Response to Nitroaromatic Analytes. ACS Appl. Mater. Interfaces 2015, 7, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Palma-Cando, A.; Rendón-Enríquez, I.; Tausch, M.; Scherf, U. Thin Functional Polymer Films by Electropolymerization. Nanomaterials 2019, 9, 1125. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-XTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-binding Quantum Chemistry Methods. WIREs Comput. Mol. Sci. 2020, 11, e1493. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient Evaluation of Electrostatic Potential with Computerized Optimized Code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Salinas, G.; Del-Oso, J.A.; Espinoza-Montero, P.J.; Heinze, J.; Frontana-Uribe, B.A. Electrochemical Polymerization, Characterization and in-Situ Conductivity Studies of Poly-3,4-Ortho-Xylendioxythiophene (PXDOT). Synth. Met. 2018, 245, 135–143. [Google Scholar] [CrossRef]
- Skompska, M.; Chmielewski, M.J.; Tarajko, A. Poly(1,8-Diaminocarbazole)—A Novel Conducting Polymer for Sensor Applications. Electrochem. Commun. 2007, 9, 540–544. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Nelson, R.F. Anodic Oxidation Pathways of Carbazoles I. Carbazole and N-Substituted Derivatives. J. Electrochem. Soc. 1968, 115, 1159–1164. [Google Scholar] [CrossRef]
- Yu, T.; Wu, X.; Lv, Y.; Liu, L.; Du, L.; Zhou, J.; Xie, Z.; Ma, Y. An Electrochemically Deposited Film as an Interface Layer to Improve the Performance of Polymer Light-Emitting Diodes. J. Mater. Chem. C Mater. 2014, 2, 4117–4120. [Google Scholar] [CrossRef]
- Skompska, M.; Vorotyntsev, M.A.; Refczynska, M.; Goux, J.; Lesniewska, E.; Boni, G.; Moise, C. Electrosynthesis and Properties of Poly(3,4-Ethylenedioxythiophene) Films Functionalized with Titanocene Dichloride Complex. Electrochim. Acta 2006, 51, 2108–2119. [Google Scholar] [CrossRef]
- Cosnier, S.; Karyakin, A. Electropolymerization: Concepts, Materials and Applications; Wiley-VCH: Weinheim, Germany, 2010; p. 280. ISBN 9783527324743. [Google Scholar]
- Mothika, V.S.; Räupke, A.; Brinkmann, K.O.; Riedl, T.; Brunklaus, G.; Scherf, U. Nanometer-Thick Conjugated Microporous Polymer Films for Selective and Sensitive Vapor-Phase TNT Detection. ACS Appl. Nano Mater. 2018, 1, 6483–6492. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Casalta, M.; Pousson, E.; Roullier, L.; Boni, G.; Moise, C. Redox Properties of Titanocene-Pyrrole Derivative and Its Electropolymerization. Electrochim. Acta 2001, 46, 4017–4033. [Google Scholar] [CrossRef]
- Salinas, G.; Ibanez, J.G.; Vásquez-Medrano, R.; Frontana-Uribe, B.A. Electrochemical Behavior of Poly-Bithiophene, Poly-3,4-Ethylendioxythiophene and Poly-3,4-Ortho-Xylendioxythiophene in EtOH/H2O (1:1) Mixture. Synth. Met. 2018, 237, 65–72. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.H.; Innis, P.C.; Wallace, G.G.; MacFarlane, D.R.; Forsyth, M. Conducting Polymer Electrochemistry in Ionic Liquids. Synth. Met. 2003, 135–136, 31–32. [Google Scholar] [CrossRef]
- He, Y.; Tan, Q.; Lu, L.; Sokolowski, J.; Wu, G. Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction in PEM Fuel Cells: Self-Template Synthesis Approach to Enhancing Catalytic Activity and Stability. Electrochem. Energy Rev. 2019, 2, 231–251. [Google Scholar] [CrossRef]
- Innis, P.C.; Mazurkiewicz, J.; Nguyen, T.; Wallace, G.G.; MacFarlane, D. Enhanced Electrochemical Stability of Polyaniline in Ionic Liquids. Curr. Appl. Phys. 2004, 4, 389–393. [Google Scholar] [CrossRef]
- Wei, Y.; Chan, C.C.; Tian, J.; Jang, G.W.; Hsueh, K.F. Electrochemical Polymerization of Thiophenes in the Presence of Bithiophene or Terthiophene: Kinetics and Mechanism of the Polymerization. Chem. Mater. 1991, 3, 888–897. [Google Scholar] [CrossRef]
- Cui, P.; Qin, B.; Martinez, A.M.; Haarberg, G.M. Removal and Recovery of Indium from ITO Coated Glass and Scraps Using Molten Salt Electrolysis. J. Electrochem. Soc. 2019, 166, D810–D815. [Google Scholar] [CrossRef]
- Zheng, D.; Gao, Z.; He, X.; Zhang, F.; Liu, L. Surface and Interface Analysis for Copper Phthalocyanine (CuPc) and Indium-Tin-Oxide (ITO) Using X-ray Photoelectron Spectroscopy (XPS). Appl. Surf. Sci. 2003, 211, 24–30. [Google Scholar] [CrossRef]
- Landoulsi, J.; Genet, M.J.; Fleith, S.; Touré, Y.; Liascukiene, I.; Méthivier, C.; Rouxhet, P.G. Organic Adlayer on Inorganic Materials: XPS Analysis Selectivity to Cope with Adventitious Contamination. Appl. Surf. Sci. 2016, 383, 71–83. [Google Scholar] [CrossRef]
- Peñafiel-Vicuña, S.; Rendón-Enríquez, I.; Vega-Poot, A.; López-Téllez, G.; Palma-Cando, A.; Frontana-Uribe, B.A. Recovering Indium Tin Oxide Electrodes for the Fabrication of Hematite Photoelectrodes. J. Electrochem. Soc. 2020, 167, 126512. [Google Scholar] [CrossRef]
- Bernede, J.C.; Taoudi, H.; Bonnet, A.; Molinie, P.; Morsli, M.; De Valle, M.A.; Diaz, F. XPS and ESR Studies of Electrochemically Synthesized Polycarbazole Post-Doped with Iodine. J. Appl. Polym. Sci. 1999, 71, 115–124. [Google Scholar] [CrossRef]
- Abé, S.Y.; Bernède, J.C.; Ugalde, L.; Trégouët, Y.; Del Valle, M.A. Electrochemical Deposition of Polycarbazole Thin Films onto Tin Oxide Coated Glass: Physicochemical and Optoelectronic Characterizations. J. Appl. Polym. Sci. 2007, 106, 1568–1575. [Google Scholar] [CrossRef]
- Urquhart, S.G.; Turci, C.C.; Tyliszczak, T.; Brook, M.A.; Hitchcock, A.P. Core Excitation Spectroscopy of Phenyl- and Methyl-Substituted Silanol, Disiloxane, and Disilane Compounds: Evidence for π-Delocalization across the Si—Cphenyl Bond. Organometallics 1997, 16, 2080–2088. [Google Scholar] [CrossRef]
- Sonnenfeld, A.; Tun, T.M.; Zajíčková, L.; Kozlov, K.V.; Wagner, H.E.; Behnke, J.F.; Hippler, R. Deposition Process Based on Organosilicon Precursors in Dielectric Barrier Discharges at Atmospheric Pressure—A Comparison. Plasmas Polym. 2001, 6, 237–266. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS Studies of Metal Oxides, Hydroxides and Peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Donley, C.; Dunphy, D.; Paine, D.; Carter, C.; Nebesny, K.; Lee, P.; Alloway, D.; Armstrong, N.R. Characterization of Indium−Tin Oxide Interfaces Using X-ray Photoelectron Spectroscopy and Redox Processes of a Chemisorbed Probe Molecule: Effect of Surface Pretreatment Conditions. Langmuir 2002, 18, 450–457. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights from a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Fernandez, V.; Fairley, N.; Baltrusaitis, J. Unraveling Spectral Shapes of Adventitious Carbon on Gold Using a Time-Resolved High-Resolution X-ray Photoelectron Spectroscopy and Principal Component Analysis. Appl. Surf. Sci. 2021, 538, 148031. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database (Beamson, G.; Briggs, D.). J. Chem. Educ. 1993, 70, A25. [Google Scholar] [CrossRef]
- Kato, T.; Yamada, Y.; Nishikawa, Y.; Otomo, T.; Sato, H.; Sato, S. Origins of Peaks of Graphitic and Pyrrolic Nitrogen in N1s X-ray Photoelectron Spectra of Carbon Materials: Quaternary Nitrogen, Tertiary Amine, or Secondary Amine? J. Mater. Sci. 2021, 56, 15798–15811. [Google Scholar] [CrossRef]
- Olteanu, L.; Ion, R.M.; Bucurică, I.A.; Gurgu, I.V.; Dulamă, I.D.; Teodorescu, S. Ito and Fto Coated Glass Characterization Using Sem and Afm Techniques. Bull. Transilv. Univ. Brasov. Ser. I—Eng. Sci. 2020, 12, 41–46. [Google Scholar] [CrossRef]
- Liu, L.; Yellinek, S.; Valdinger, I.; Donval, A.; Mandler, D. Important Implications of the Electrochemical Reduction of ITO. Electrochim. Acta 2015, 176, 1374–1381. [Google Scholar] [CrossRef]
- Ye, W.; Yang, B.; Cao, G.; Duan, L.; Wang, C. Electrocatalytic Oxidation of Hydrazine Compound on Electroplated Pd/WO3 Film. Thin Solid Films 2008, 516, 2957–2961. [Google Scholar] [CrossRef]
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in Surface Morphologies, Properties, and Electrochemical Responses to Nitro-Analyte by Controlled Electropolymerization of Thiophene Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Y.; Zhang, M.; Zhao, F.; Zeng, B. A Highly Selective Electrochemical Sensor Based on Surface Molecularly Imprinted Copolymer for the Detection of 5-Hydroxytryptamine. Microchem. J. 2021, 160, 105748. [Google Scholar] [CrossRef]
- Tamang, S.; Thapa, A.; Chettri, K.; Datta, B.; Biswas, J. Analysis of Dipyridine Dipyrrole Based Molecules for Solar Cell Application Using Computational Approach. J. Comput. Electron. 2022, 21, 94–105. [Google Scholar] [CrossRef]
- Shao, S.; Shi, J.; Murtaza, I.; Xu, P.; He, Y.; Ghosh, S.; Zhu, X.; Perepichka, I.F.; Meng, H. Exploring the Electrochromic Properties of Poly(Thieno[3,2-: B] Thiophene)s Decorated with Electron-Deficient Side Groups. Polym. Chem. 2017, 8, 769–784. [Google Scholar] [CrossRef]
- El Azzouzi, M.; Aouniti, A.; Herrag, L.; Chetouani, A.; Elmsellem, H.; Hammouti, B. Investigation of Isomers of Hydroxyphenylamino Propane Nitrile as Mild Steel Corrosion Inhibitors in 1M HCl. Der Pharma Chem. 2015, 7, 12–24. [Google Scholar]
- Guzel, M.; Celik, E.; Kart, S.O.; Tasli, P.T.; Karatas, E.; Ak, M. Experimental and Theoretical Investigation of the Substitution Effects on N-Substituted Carbazole Derivatives Functionalized with Azomethine Bonds. React. Funct. Polym. 2022, 172, 105180. [Google Scholar] [CrossRef]
- Jiang, J.X.; Su, F.; Trewin, A.; Wood, C.D.; Niu, H.; Jones, J.T.A.; Khimyak, Y.Z.; Cooper, A.I. Synthetic Control of the Pore Dimension and Surface Area in Conjugated Microporous Polymer and Copolymer Networks. J. Am. Chem. Soc. 2008, 130, 7710–7720. [Google Scholar] [CrossRef]
- Myhal, A.V.; Golovchenko, O.S.; Krutskikh, T.V.; Gubar, S.M.; Georgiyants, V.A. IR-Spectroscopy Research into the Structure of Products of Interaction between Metronidazole and Metal Salts. Der Pharma Chem. 2016, 8, 148–154. [Google Scholar]
- Szente, V.; Baska, F.; Zelkó, R.; Süvegh, K. Prediction of the Drug Release Stability of Different Polymeric Matrix Tablets Containing Metronidazole. J. Pharm. Biomed. Anal. 2011, 54, 730–734. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Felczak, A.; Checińska, L.; Zawadzka, K.; Patyna, E.; Lisowska, K.; Ochocki, J. Synthesis, Characterization and Antimicrobial Activity of Water-Soluble Silver(I) Complexes of Metronidazole Drug and Selected Counter-Ions. Dalton Trans. 2015, 44, 8178–8189. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz-Arturo, H.; Reinoso, C.; Scherf, U.; Palma-Cando, A. Microporous Polymer-Modified Glassy Carbon Electrodes for the Electrochemical Detection of Metronidazole: Experimental and Theoretical Insights. Nanomaterials 2024, 14, 180. https://doi.org/10.3390/nano14020180
Quiroz-Arturo H, Reinoso C, Scherf U, Palma-Cando A. Microporous Polymer-Modified Glassy Carbon Electrodes for the Electrochemical Detection of Metronidazole: Experimental and Theoretical Insights. Nanomaterials. 2024; 14(2):180. https://doi.org/10.3390/nano14020180
Chicago/Turabian StyleQuiroz-Arturo, Héctor, Carlos Reinoso, Ullrich Scherf, and Alex Palma-Cando. 2024. "Microporous Polymer-Modified Glassy Carbon Electrodes for the Electrochemical Detection of Metronidazole: Experimental and Theoretical Insights" Nanomaterials 14, no. 2: 180. https://doi.org/10.3390/nano14020180
APA StyleQuiroz-Arturo, H., Reinoso, C., Scherf, U., & Palma-Cando, A. (2024). Microporous Polymer-Modified Glassy Carbon Electrodes for the Electrochemical Detection of Metronidazole: Experimental and Theoretical Insights. Nanomaterials, 14(2), 180. https://doi.org/10.3390/nano14020180






