Graphene Oxide (GO) for the Treatment of Bone Cancer: A Systematic Review and Bibliometric Analysis
Abstract
1. Introduction
2. Methodology
Design of the Search Strategy and Eligibility Criteria for the Systematic Review
3. Results and Discussion
3.1. Review of Bone Cancer and the Use of GO for Biomedical Applications
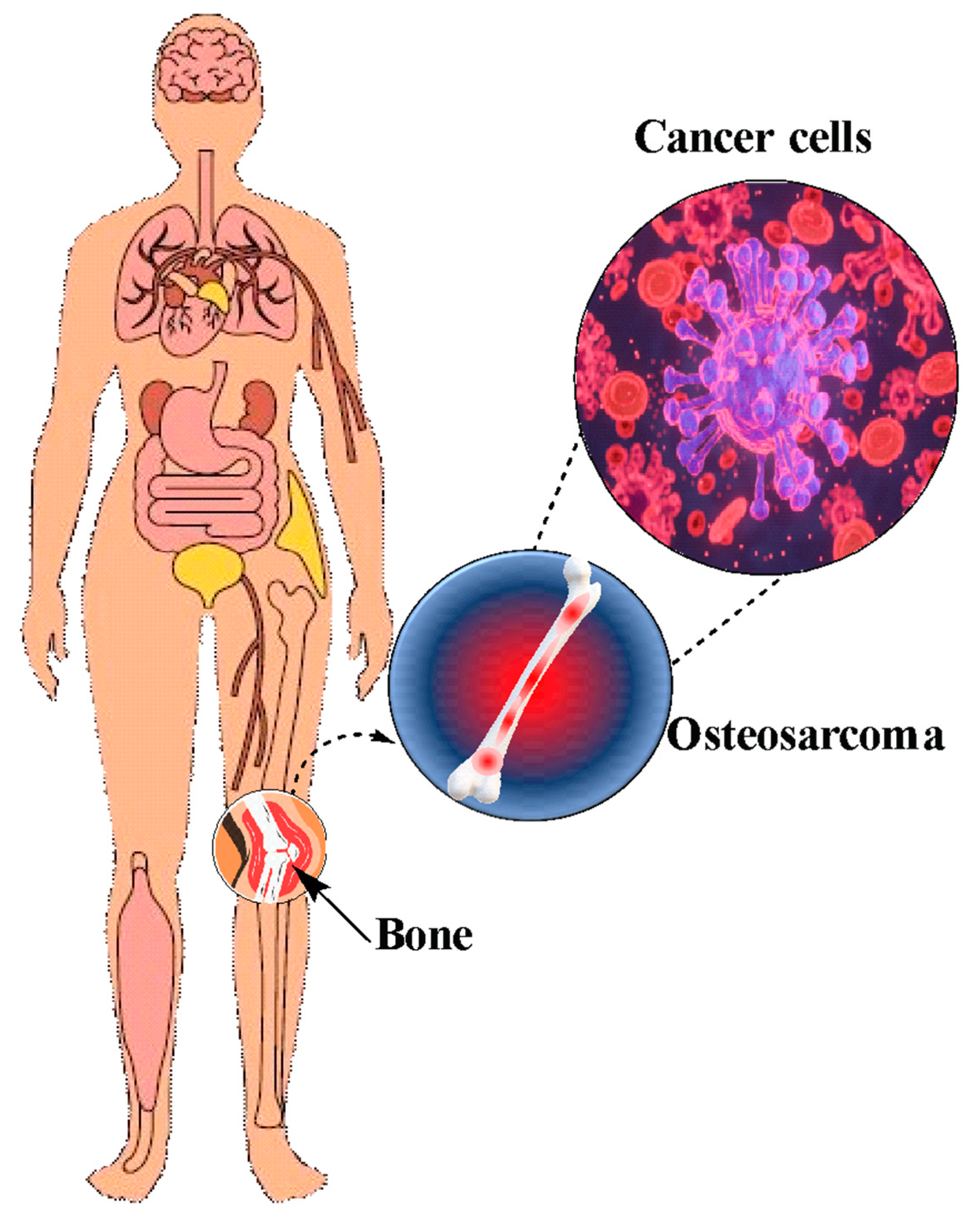
3.1.1. Osteosarcoma
3.1.2. Conventional Treatments and the Application of GO in the Treatment of Bone Cancer
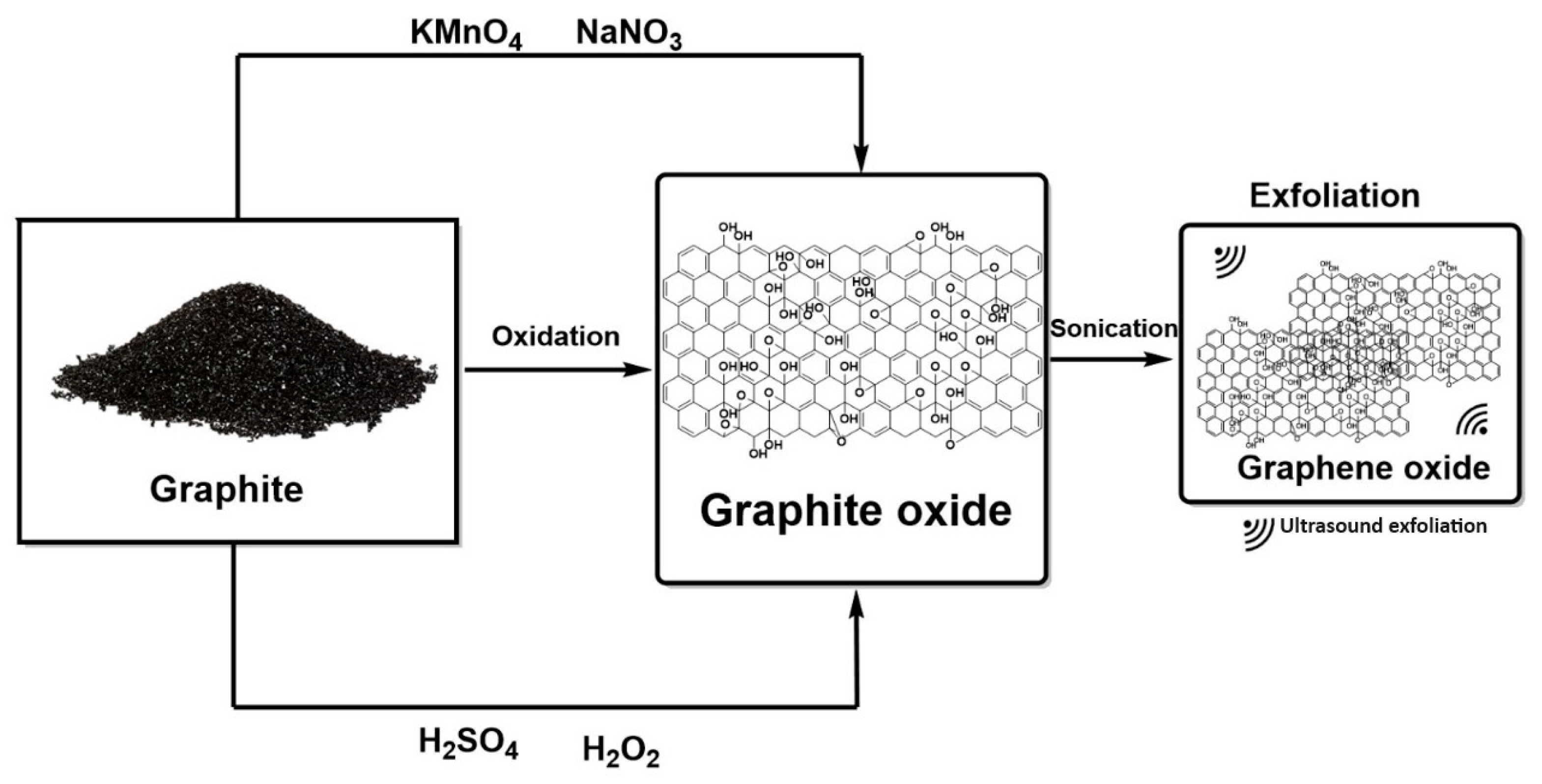
3.1.3. GO and rGO Synthesis
3.1.4. Action Mechanisms
3.1.5. GO’s Anti-Cancer Applications
In Vitro Studies
In Vivo Studies
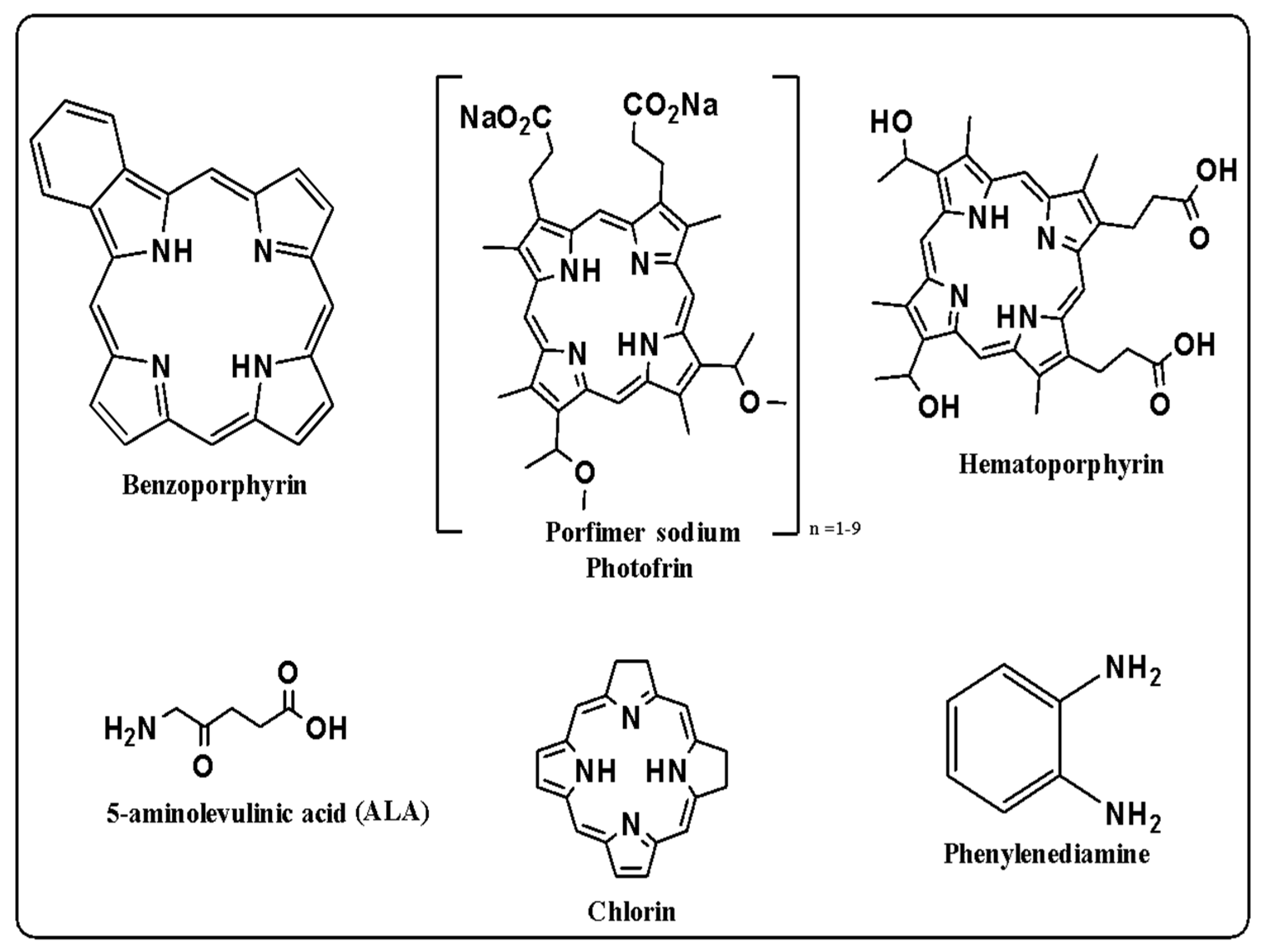
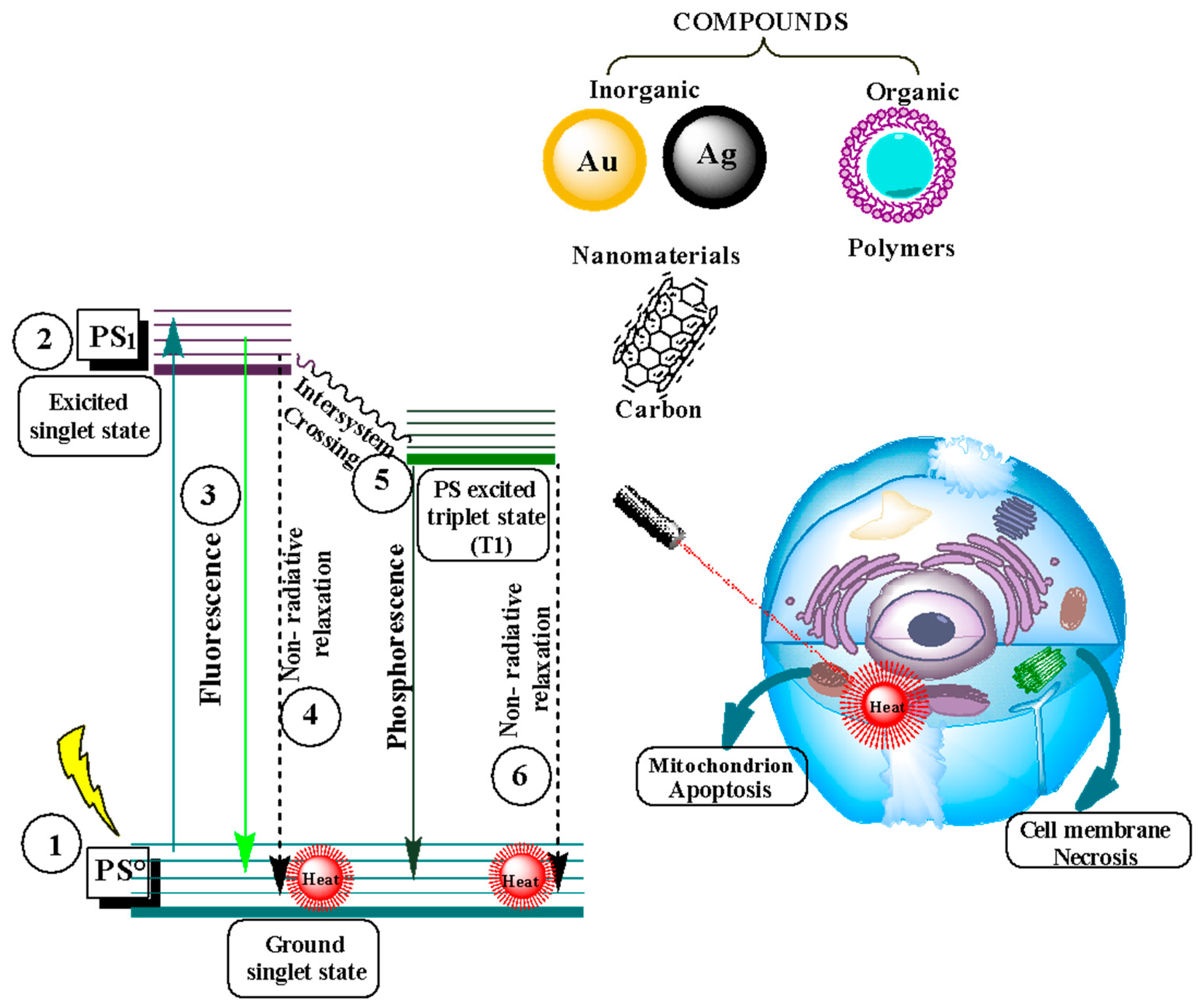
3.2. Photodynamic Therapy
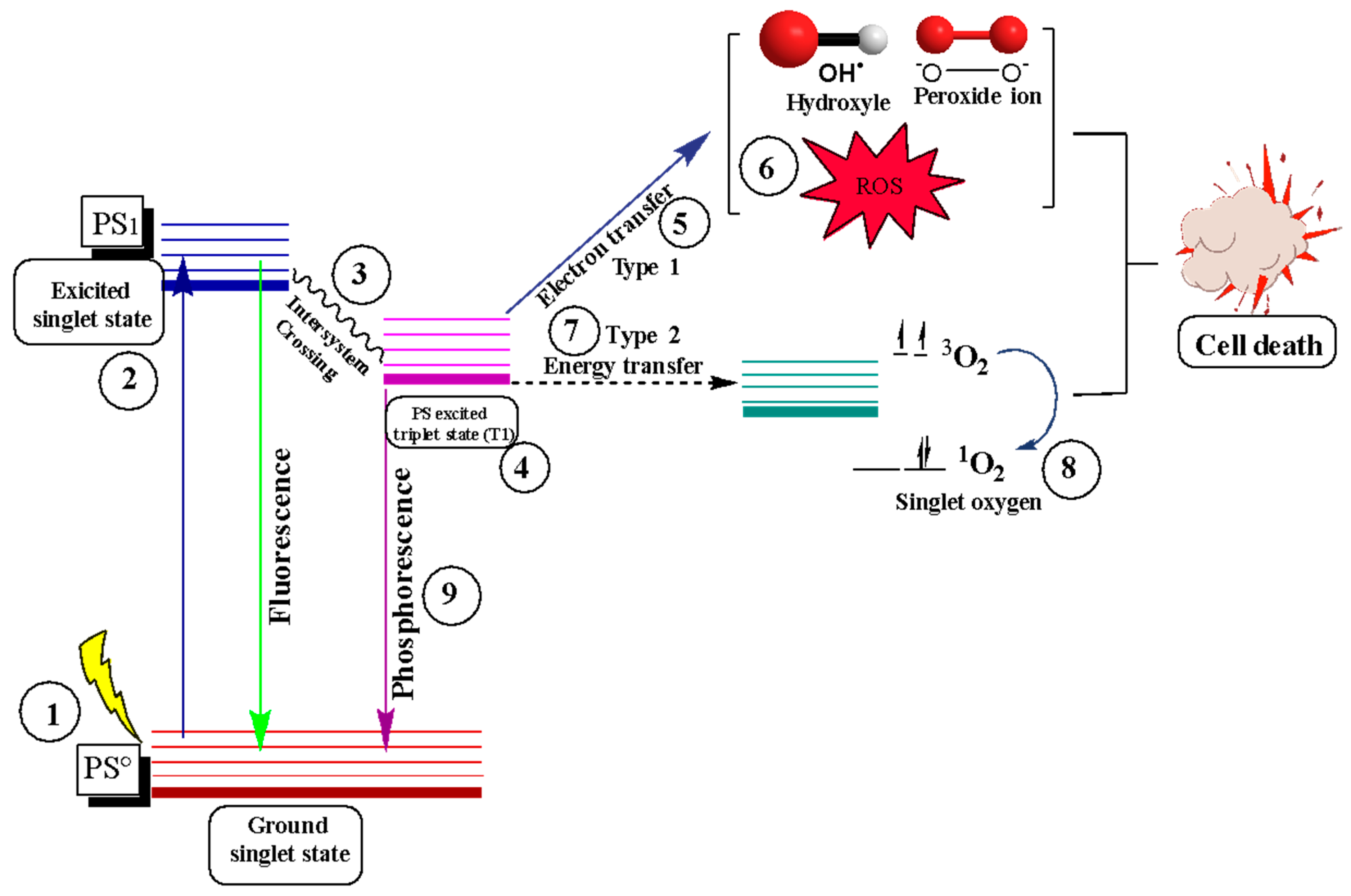
Photodynamic Therapy as a Cancer Treatment
3.3. Photothermal Therapy
Photothermal Therapy as a Cancer Treatment
| PTT System | * PTA | Type of Study | Analytical Method of Osteosarcoma Anticancer Activity | Results | Ref. |
|---|---|---|---|---|---|
| Nanomaterial of SiO2 @PDA/Fe3+ | PDA | In vivo In vitro |
| The burden percentages of doxorubicin and cisplatin were 21.25% and 23.80%, respectively. The system saw a considerable increase in the photothermal conversion efficiency of nanomaterials by 57.63% and photothermal conversion at low temperatures of 42–43 °C. | [170] |
| Scaffolds with DOX-gelatin/SrCuSi4O10-β-TCP core/ shell filaments. | CS | In vitro |
| Hyperthermia increased the release of DOX caused by NIR-II by irradiating the HtCP/2SC-DOX scaffolds with a 1064 nm laser for five cycles of 5 and 10 min. A synergistic effect was achieved in the combination of photothermal therapy and chemotherapy. | [171] |
| Multifunctional nano-hydroxyapatite/graphene oxide/chitosan (nHA/GO/CS) scaffold | GO | In vitro |
| The 30% nHA in GO increased biocompatibility and the photothermal effect to eliminate HOS cells. Excellent in vitro performance of the nHA/GO/CS scaffolding and efficient operation with NIR for osteosarcoma cell removal and tissue regeneration were achieved. | [169] |
| Methotrexate-loaded polydopamine (pDA)-based ZIF-8-based nanoparticles (pDA/MTX@ZIF-8) | pDA | In vitro |
| The in vitro study demonstrated excellent antitumor efficacy by inducing apoptosis in MG63 cells. In addition, pDA/MTX@ZIF-8 nanoparticles showed good biocompatibility and an exceptional ability to release methotrexate as a function of pH, with 93% and 94.5% release in 12 h and 3 days, respectively. pDA/MTX@ZIF-8 nanoparticles exhibited a synergistic chemo-photothermal effect for cancer therapy. | [172] |
| Indocyanine green-loaded membrane-coated silica nanoparticles of cancer cells (CM/SLN/ICG) | ICG | In vitro In vivo |
| It was shown that the photothermal conversion efficiency of CM/SLN/ICG and ICG was 57.93% and 57.21%, respectively, indicating that ICG generates higher protected photothermal conversion. In addition, it was found that the release at pH 5.5 and 7.4 was 74.41% and 32.96%, respectively. It was found that the anticancer efficacy was superior in modified CM, CM/SLN/ICG, and could specifically target 143B cells, enhancing its promise as a drug manager in TTP. | [173] |
| UiO-66 nanoparticles, polydopamine-coated with perfluorotributylamine/thyrazamine (TPZ/PFA@UiO66@PDA) | PDA | In vitro In vivo |
| Tumor cell destruction was achieved by NIR irradiation and effective synergy between hypoxia-activated bioreducing prodrug therapy and TTP. The photothermal effect in vivo and in vitro at a temperature of 60.27 ± 3.02 showed that tumor size was significantly reduced, demonstrating an excellent antitumor capacity of TPZ/PFA@UiO66@PDA nanoparticles. | [174] |
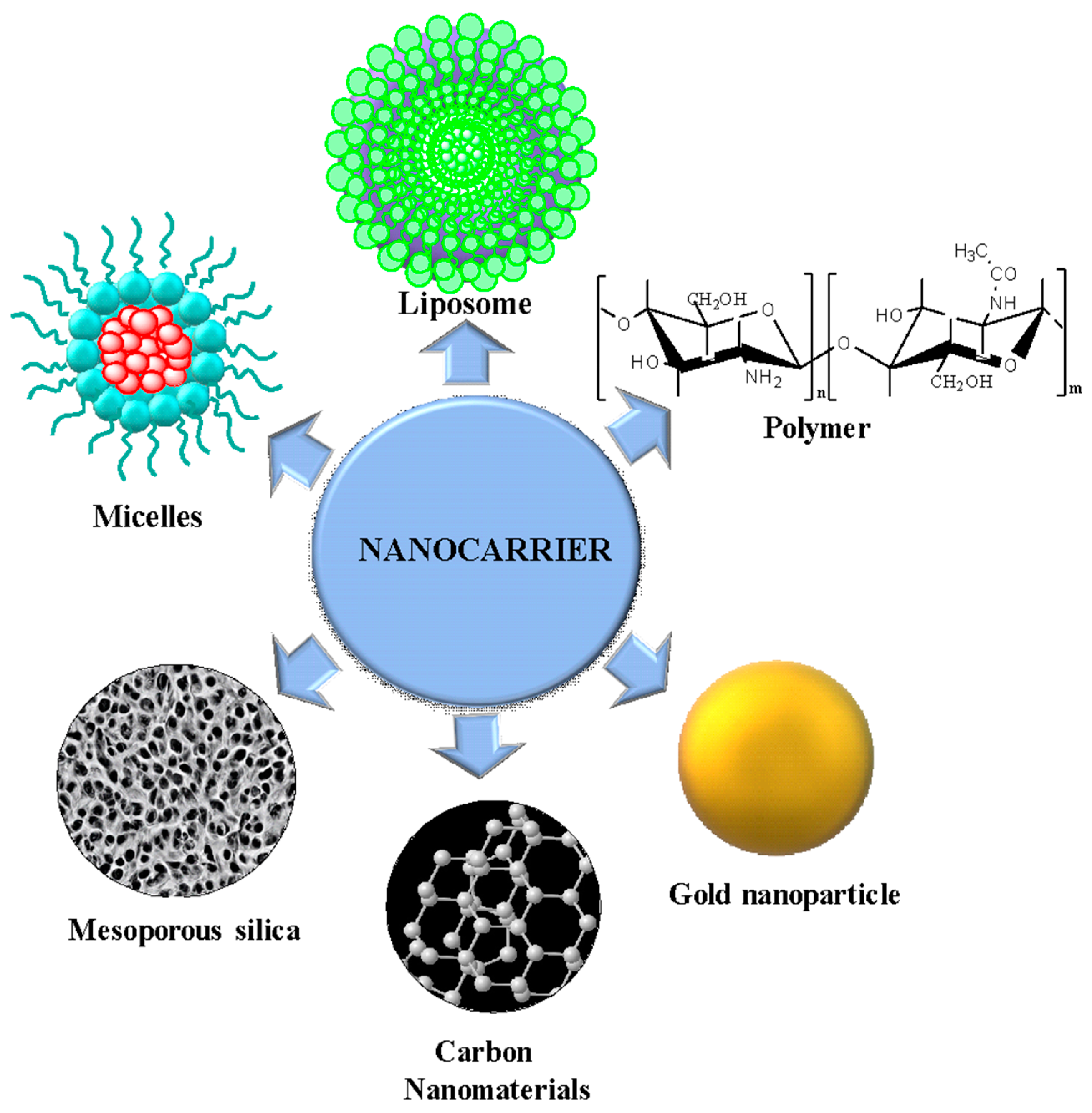
3.4. Graphene Oxide in Drug Delivery
3.5. Nanocarriers in Cancer Treatment
4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Byler, S.; Goldgar, S.; Heerboth, S.; Leary, M.; Housman, G.; Moulton, K.; Sarkar, S. Genetic and Epigenetic Aspects of Breast Cancer Progression and Therapy. Anticancer Res. 2014, 34, 1071–1077. [Google Scholar] [PubMed]
- Kanwal, R.; Gupta, K.; Gupta, S. Cancer Epigenetics: An Introduction. In Cancer Epigenetics; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1238, pp. 3–25. [Google Scholar] [CrossRef]
- Weinberg, R.A. How Cancer Arises. Sci. Am. 1996, 275, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Cancer Overtakes CVD to Become Leading Cause of Death in High Income Countries. BMJ 2019, 366, l5368. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Andreou, D.; Hardes, J.; Gosheger, G.; Henrichs, M.-P.; Nottrott, M.; Streitbürger, A. Interdisciplinary diagnostic and treatment of bone sarcomas of the extremities and trunk. Handchir. Mikrochir. Plast. Chir. 2015, 47, 90–99. [Google Scholar] [CrossRef]
- Ferguson, J.L.; Turner, S.P. Bone Cancer: Diagnosis and Treatment Principles. Am. Fam. Physician 2018, 98, 205–213. [Google Scholar]
- Marques, C.; Ferreira, J.M.F.; Andronescu, E.; Ficai, D.; Sonmez, M.; Ficai, A. Multifunctional Materials for Bone Cancer Treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar] [CrossRef]
- Alexandrino, E.M.; Ritz, S.; Marsico, F.; Baier, G.; Mailänder, V.; Landfester, K.; Wurm, F.R. Paclitaxel-Loaded Polyphosphate Nanoparticles: A Potential Strategy for Bone Cancer Treatment. J. Mater. Chem. B 2014, 2, 1298. [Google Scholar] [CrossRef]
- Illouz, F.; Braun, D.; Briet, C.; Schweizer, U.; Rodien, P. ENDOCRINE SIDE-EFFECTS OF ANTI-CANCER DRUGS: Thyroid Effects of Tyrosine Kinase Inhibitors. Eur. J. Endocrinol. 2014, 171, R91–R99. [Google Scholar] [CrossRef]
- Fauzee, N.J.; Dong, Z.; Wang, Y.L. Taxanes: Promising Anti-Cancer Drugs. Asian Pac. J. Cancer Prev. 2011, 12, 837–851. [Google Scholar] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sahoo, N.G.; Li, L. The Application of Graphene Oxide in Drug Delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Iravani, S.; Varma, R.S. Graphene and Graphene Oxide with Anticancer Applications: Challenges and Future Perspectives. MedComm 2022, 3, e118. [Google Scholar] [CrossRef]
- Romero, M.P.; Buzza, H.H.; Stringasci, M.D.; Estevão, B.M.; Silva, C.C.C.; Pereira-Da-silva, M.A.; Inada, N.M.; Bagnato, V.S. Graphene Oxide Theranostic Effect: Conjugation of Photothermal and Photodynamic Therapies Based on an in Vivo Demonstration. Int. J. Nanomed. 2021, 16, 1601–1616. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qiao, G.; Han, Y.; Shen, E.; Alfranca, G.; Tan, H.; Wang, L.; Pan, S.; Ma, L.; Xiong, W.; et al. Targeted Theranostics of Lung Cancer: PD-L1-Guided Delivery of Gold Nanoprisms with Chlorin E6 for Enhanced Imaging and Photothermal/Photodynamic Therapy. Acta Biomater. 2020, 117, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhang, Q.; Shi, J.; Li, J.; Chen, Z.; Wang, B. Co-Loading of Photothermal Agents and Anticancer Drugs into Porous Silicon Nanoparticles with Enhanced Chemo-Photothermal Therapeutic Efficacy to Kill Multidrug-Resistant Cancer Cells. Colloids Surf. B Biointerfaces 2018, 164, 291–298. [Google Scholar] [CrossRef]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for next Generation of Liver Cancer Nanodrug Modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef]
- Lara-Vega, I.; Vega-López, A. Combinational Photodynamic and Photothermal-Based Therapies for Melanoma in Mouse Models. Photodiagnosis Photodyn. Ther. 2023, 43, 103596. [Google Scholar] [CrossRef]
- Tang, P.; Xing, M.; Xing, X.; Tao, Q.; Cheng, W.; Liu, S.; Lu, X.; Zhong, L. Receptor-Mediated Photothermal/Photodynamic Synergistic Anticancer Nanodrugs with SERS Tracing Function. Colloids Surf. B Biointerfaces 2021, 199, 111550. [Google Scholar] [CrossRef]
- Zeng, W.-N.; Yu, Q.-P.; Wang, D.; Liu, J.-L.; Yang, Q.-J.; Zhou, Z.-K.; Zeng, Y.-P. Mitochondria-Targeting Graphene Oxide Nanocomposites for Fluorescence Imaging-Guided Synergistic Phototherapy of Drug-Resistant Osteosarcoma. J. Nanobiotechnol. 2021, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Wu, Y.F.; Lan, T.J.; Chen, Y.; Su, S.H. Codelivery of Anticancer Drug and Photosensitizer by PEGylated Graphene Oxide and Cell Penetrating Peptide Enhanced Tumor-Suppressing Effect on Osteosarcoma. Front. Mol. Biosci. 2021, 7, 618896. [Google Scholar] [CrossRef] [PubMed]
- Oz, Y.; Barras, A.; Sanyal, R.; Boukherroub, R.; Szunerits, S.; Sanyal, A. Functionalization of Reduced Graphene Oxide via Thiol–Maleimide “Click” Chemistry: Facile Fabrication of Targeted Drug Delivery Vehicles. ACS Appl. Mater. Interfaces 2017, 9, 34194–34203. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Elsevier, S. Scopus Content Coverage Guide; Elsevier BV: Amsterdam, The Netherlands, 2016. [Google Scholar]
- García, J.A.; Rodriguez-Sánchez, R.; Fdez-Valdivia, J. Ranking of the Subject Areas of Scopus. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 2013–2023. [Google Scholar] [CrossRef]
- Sweileh, W.M. Research Trends on Human Trafficking: A Bibliometric Analysis Using Scopus Database. Global. Health 2018, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Canese, K.; Weis, S. PubMed: The Bibliographic Database. In The NCBI Handbook [Internet], 2nd ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK153385/ (accessed on 29 November 2023).
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.A.S.; Rose, P.S.; Folpe, A.L.; Laack, N.N. Common Musculoskeletal Tumors of Childhood and Adolescence. Mayo Clin. Proc. 2012, 87, 475–487. [Google Scholar] [CrossRef]
- Rosenberg, A.E.; Cleton-Jansen, A.M.; De Pinieux, G.; Deyrup, A.T.; Hauben, E.; Squire, J. Conventional Osteosarcoma. In WHO Classification of Tumours of Soft Tissue and Bone; WHO: Geneva, Switzerland, 2013; pp. 282–288. [Google Scholar]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- Hui, J.Y.C. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef]
- Le Vu, B.; de Vathaire, F.; Shamsaldin, A.; Hawkins, M.M.; Grimaud, E.; Hardiman, C.; Diallo, I.; Vassal, G.; Bessa, E.; Campbell, S.; et al. Radiation Dose, Chemotherapy and Risk of Osteosarcoma after Solid Tumours during Childhood. Int. J. Cancer 1998, 77, 370–377. [Google Scholar] [CrossRef]
- Overholtzer, M.; Rao, P.H.; Favis, R.; Lu, X.-Y.; Elowitz, M.B.; Barany, F.; Ladanyi, M.; Gorlick, R.; Levine, A.J. The Presence of P53 Mutations in Human Osteosarcomas Correlates with High Levels of Genomic Instability. Proc. Natl. Acad. Sci. USA 2003, 100, 11547–11552. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Song, W.-X.; Luo, J.; Haydon, R.C.; He, T.-C. Osteosarcoma Development and Stem Cell Differentiation. Clin. Orthop. Relat. Res. 2008, 466, 2114–2130. [Google Scholar] [CrossRef] [PubMed]
- Ehnman, M.; Chaabane, W.; Haglund, F.; Tsagkozis, P. The Tumor Microenvironment of Pediatric Sarcoma: Mesenchymal Mechanisms Regulating Cell Migration and Metastasis. Curr. Oncol. Rep. 2019, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.; Abarrategi, A.; Garcia-Castro, J.; Martinez-Cruzado, L.; Suarez, C.; Tornin, J.; Santos, L.; Astudillo, A.; Colmenero, I.; Mulero, F.; et al. Bone Environment Is Essential for Osteosarcoma Development from Transformed Mesenchymal Stem Cells. Stem Cells 2014, 32, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Mohseny, A.B.; Szuhai, K.; Romeo, S.; Buddingh, E.P.; Briaire-de Bruijn, I.; de Jong, D.; van Pel, M.; Cleton-Jansen, A.; Hogendoorn, P.C.W. Osteosarcoma Originates from Mesenchymal Stem Cells in Consequence of Aneuploidization and Genomic Loss of Cdkn2. J. Pathol. 2009, 219, 294–305. [Google Scholar] [CrossRef] [PubMed]
- He, J.-P.; Hao, Y.; Wang, X.-L.; Yang, X.-J.; Shao, J.-F.; Guo, F.-J.; Feng, J.-X. Review of the Molecular Pathogenesis of Osteosarcoma. Asian Pac. J. Cancer Prev. 2014, 15, 5967–5976. [Google Scholar] [CrossRef]
- Broadhead, M.L.; Clark, J.C.M.; Myers, D.E.; Dass, C.R.; Choong, P.F.M. The Molecular Pathogenesis of Osteosarcoma: A Review. Sarcoma 2011, 2011, 959248. [Google Scholar] [CrossRef]
- Denduluri, S.K.; Wang, Z.; Yan, Z.; Wang, J.; Wei, Q.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; He, T.-C. Molecular Pathogenesis and Therapeutic Strategies of Human Osteosarcoma. J. Biomed. Res. 2016, 30, 5–18. [Google Scholar] [CrossRef]
- Kansara, M.; Thomas, D.M. Molecular Pathogenesis of Osteosarcoma. DNA Cell Biol. 2007, 26, 1–18. [Google Scholar] [CrossRef]
- Gerrand, C.H.; Rankin, K. A System for the Surgical Staging of Musculoskeletal Sarcoma. In Classic Papers in Orthopaedics; Springer: London, UK, 2014; Volume 153, pp. 487–488. ISBN 9781447154518. [Google Scholar]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of TheExtremities or Trunk: An Analysis of 1,702 Patients Treatedon Neoadjuvant Cooperative Osteosarcoma Study GroupProtocols. J. Clin. Oncol. 2023, 41, 4323–4337. [Google Scholar] [CrossRef] [PubMed]
- Pekarek, L.; De la Torre-Escuredo, B.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cobo-Prieto, D.; Guijarro, L.G.; Saz, J.V.; De Castro-Martinez, P.; Torres-Carranza, D.; et al. Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations. Int. J. Mol. Sci. 2022, 23, 14939. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Flege, S.; Kevric, M.; Lindner, N.; Maas, R.; Delling, G.; Schwarz, R.; von Hochstetter, A.R.; Salzer-Kuntschik, M.; Berdel, W.E.; et al. Osteosarcoma of the Pelvis: Experience of the Cooperative Osteosarcoma Study Group. J. Clin. Oncol. 2003, 21, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Franzius, C.; Bielack, S.; Flege, S.; Eckardt, J.; Sciuk, J.; Jürgens, H.; Schober, O. High-Activity Samarium-153-EDTMP Therapy Followed by Autologous Peripheral Blood Stem Cell Support in Unresectable Osteosarcoma. Nuklearmedizin 2001, 40, 215–220. [Google Scholar] [CrossRef]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2021, 68, e28352. [Google Scholar] [CrossRef]
- Hundsdoerfer, P.; Albrecht, M.; Rühl, U.; Fengler, R.; Kulozik, A.E.; Henze, G. Long-Term Outcome after Polychemotherapy and Intensive Local Radiation Therapy of High-Grade Osteosarcoma. Eur. J. Cancer 2009, 45, 2447–2451. [Google Scholar] [CrossRef]
- Aran, V.; Devalle, S.; Meohas, W.; Heringer, M.; Cunha Caruso, A.; Pinheiro Aguiar, D.; Leite Duarte, M.E.; Moura Neto, V. Osteosarcoma, Chondrosarcoma and Ewing Sarcoma: Clinical Aspects, Biomarker Discovery and Liquid Biopsy. Crit. Rev. Oncol. Hematol. 2021, 162, 103340. [Google Scholar] [CrossRef]
- Hasanzade, Z.; Raissi, H. Density Functional Theory Calculations and Molecular Dynamics Simulations of the Adsorption of Ellipticine Anticancer Drug on Graphene Oxide Surface in Aqueous Medium as Well as under Controlled PH Conditions. J. Mol. Liq. 2018, 255, 269–278. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Zaboli, M.; Raissi, H.; Moghaddam, N.R.; Farzad, F. Probing the Adsorption and Release Mechanisms of Cytarabine Anticancer Drug on/from Dopamine Functionalized Graphene Oxide as a Highly Efficient Drug Delivery System. J. Mol. Liq. 2020, 301, 112458. [Google Scholar] [CrossRef]
- Safdari, F.; Raissi, H.; Shahabi, M.; Zaboli, M. DFT Calculations and Molecular Dynamics Simulation Study on the Adsorption of 5-Fluorouracil Anticancer Drug on Graphene Oxide Nanosheet as a Drug Delivery Vehicle. J. Inorg. Organomet. Polym. Mater. 2017, 27, 805–817. [Google Scholar] [CrossRef]
- Hasanzade, Z.; Raissi, H. Solvent/Co-Solvent Effects on the Electronic Properties and Adsorption Mechanism of Anticancer Drug Thioguanine on Graphene Oxide Surface as a Nanocarrier: Density Functional Theory Investigation and a Molecular Dynamics. Appl. Surf. Sci. 2017, 422, 1030–1041. [Google Scholar] [CrossRef]
- Ge, Y.-W.; Liu, X.-L.; Yu, D.-G.; Zhu, Z.-A.; Ke, Q.-F.; Mao, Y.-Q.; Guo, Y.-P.; Zhang, J.-W. Graphene-Modified CePO4 Nanorods Effectively Treat Breast Cancer-Induced Bone Metastases and Regulate Macrophage Polarization to Improve Osteo-Inductive Ability. J. Nanobiotechnol. 2021, 19, 11. [Google Scholar] [CrossRef]
- Boran, G.; Tavakoli, S.; Dierking, I.; Kamali, A.R.; Ege, D. Synergistic Effect of Graphene Oxide and Zoledronic Acid for Osteoporosis and Cancer Treatment. Sci. Rep. 2020, 10, 7827. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C., XIII. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren Zur Darstellung Der Graphitsäure. Berichte Dtsch. Chem. Ges. 1899, 32, 1394–1399. [Google Scholar] [CrossRef]
- Hummers, W.S.J.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Pendolino, F.; Armata, N. (Eds.) Synthesis, Characterization and Models of Graphene Oxide; Springer International Publishing: Cham, Switzerland, 2017; pp. 5–21. ISBN 978-3-319-60429-9. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Gao, W. The Chemistry of Graphene Oxide. In Graphene Oxide; Gao, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–95. ISBN 978-3-319-15500-5. [Google Scholar]
- Wang, Y.; Wang, H.; Liu, D.; Song, S.; Wang, X.; Zhang, H. Graphene Oxide Covalently Grafted Upconversion Nanoparticles for Combined NIR Mediated Imaging and Photothermal/Photodynamic Cancer Therapy. Biomaterials 2013, 34, 7715–7724. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Bartolo, P. The Effect of Graphene and Graphene Oxide Induced Reactive Oxygen Species on Polycaprolactone Scaffolds for Bone Cancer Applications. Mater. Today Bio. 2024, 24, 100886. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, D.P.; Muñoz, R.; Amami, M.; Singh, R.K.; Singh, S.; Kumar, V. Graphene-Based Materials for Biotechnological and Biomedical Applications: Drug Delivery, Bioimaging and Biosensing. Mater. Today Chem. 2023, 33, 101750. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Barar, J.; Aghanejad, A.; Davaran, S. Recent Advances and Trends in Nanoparticles Based Photothermal and Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 37, 102697. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, L.; Pérez-Davila, S.; López-Álvarez, M.; Chiussi, S.; Serra, J.; González, P. Review Article Laser-Induced Hyperthermia on Graphene Oxide Composites. J. Nanobiotechnol. 2023, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Weng, J.; Fu, X.; Lin, J.; Fan, W.; Lu, N.; Qu, J.; Chen, S.; Wang, T.; Huang, P. Black Phosphorus Nanosheets for Mild Hyperthermia-Enhanced Chemotherapy and Chemo-Photothermal Combination Therapy. Nanotheranostics 2017, 1, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Brace, C. Thermal Tumor Ablation in Clinical Use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Liu, J.; Zhai, G. Recent Developments of Phototherapy Based on Graphene Family Nanomaterials. Curr. Med. Chem. 2017, 24, 268–291. [Google Scholar] [CrossRef]
- Oh, J.; Yoon, H.; Park, J.-H. Nanoparticle Platforms for Combined Photothermal and Photodynamic Therapy. Biomed. Eng. Lett. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Balaji, A.; Zhang, J. Electrochemical and Optical Biosensors for Early-Stage Cancer Diagnosis by Using Graphene and Graphene Oxide. Cancer Nanotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, L.; Yang, Z.; Liu, Z.; Gu, J.; Bai, B.; Liu, J.; Xu, J.; Yang, H. Mechanisms of Oxidative Stress, Apoptosis, and Autophagy Involved in Graphene Oxide Nanomaterial Anti-Osteosarcoma Effect. Int. J. Nanomed. 2018, 13, 2907–2919. [Google Scholar] [CrossRef]
- Gao, W. Synthesis, Structure, and Characterizations. In Graphene Oxide; Gao, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–28. ISBN 978-3-319-15500-5. [Google Scholar]
- Agarwal, V.; Zetterlund, P.B. Strategies for Reduction of Graphene Oxide—A Comprehensive Review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hamishehkar, H.; Dolatabadi, J.E.N.; Arsalani, N. Nano Graphene Oxide: A Novel Carrier for Oral Delivery of Flavonoids. Colloids Surf. B Biointerfaces 2014, 123, 331–338. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene Oxide Is Degraded by Neutrophils and the Degradation Products Are Non-Genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Tewari, C.; Rana, A.; Pandey, N.; Basak, S.; Sahoo, N.G. Functionalized Graphene Oxide as a Vehicle for Targeted Drug Delivery and Bioimaging Applications. J. Mater. Chem. B 2020, 8, 8116–8148. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mallela, J.; Garapati, U.S.; Ravi, S.; Chinnasamy, V.; Girard, Y.; Howell, M.; Mohapatra, S. A Chitosan-Modified Graphene Nanogel for Noninvasive Controlled Drug Release. Nanomedicine 2013, 9, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene Oxide: A New Direction in Dentistry. Appl. Mater. Today 2020, 19, 100576. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Xu, C.; Ma, B.; Peng, J.; Gao, L.; Xu, Y.; Huan, Z.; Chang, J. Tricalcium Silicate/Graphene Oxide Bone Cement with Photothermal Properties for Tumor Ablation. J. Mater. Chem. B 2019, 7, 2808–2818. [Google Scholar] [CrossRef]
- Li, D.; Nie, W.; Chen, L.; McCoul, D.; Liu, D.; Zhang, X.; Ji, Y.; Yu, B.; He, C. Self-Assembled Hydroxyapatite-Graphene Scaffold for Photothermal Cancer Therapy and Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 2003–2017. [Google Scholar] [CrossRef]
- Ricci, R.; Leite, N.C.S.; da-Silva, N.S.; Pacheco-Soares, C.; Canevari, R.A.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Graphene Oxide Nanoribbons as Nanomaterial for Bone Regeneration: Effects on Cytotoxicity, Gene Expression and Bactericidal Effect. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 78, 341–348. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Cai, H.; Chen, Z.; Wang, T.; Jia, L.; Wang, J.; Wan, Q.; Pei, X. Osteogenic Activity and Antibacterial Effect of Zinc Oxide/Carboxylated Graphene Oxide Nanocomposites: Preparation and in Vitro Evaluation. Colloids Surf. B. Biointerfaces 2016, 147, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of New Biocompatible 3D Printed Graphene Oxide-Based Scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef] [PubMed]
- Jodati, H.; Yilmaz, B.; Evis, Z. In Vitro and in Vivo Properties of Graphene-Incorporated Scaffolds for Bone Defect Repair. Ceram. Int. 2021, 47, 29535–29549. [Google Scholar] [CrossRef]
- Vakili, B.; Karami-Darehnaranji, M.; Mirzaei, E.; Hosseini, F.; Nezafat, N. Graphene Oxide as Novel Vaccine Adjuvant. Int. Immunopharmacol. 2023, 125, 111062. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Yousefi, B.; Qujeq, D.; Marjani, A.; Asadi, J.; Wang, Z.; Mir, S.M. Melatonin and Doxorubicin Co-Delivered via a Functionalized Graphene-Dendrimeric System Enhances Apoptosis of Osteosarcoma Cells. Mater. Sci. Eng. C 2021, 119, 111554. [Google Scholar] [CrossRef] [PubMed]
- Saravanabhavan, S.S.; Rethinasabapathy, M.; Zsolt, S.; Kalambettu, A.B.; Elumalai, S.; Janakiraman, M.; Huh, Y.S.; Natesan, B. Graphene Oxide Functionalized with Chitosan Based Nanoparticles as a Carrier of SiRNA in Regulating Bcl-2 Expression on Saos-2 & MG-63 Cancer Cells and Its Inflammatory Response on Bone Marrow Derived Cells from Mice. Mater. Sci. Eng. C 2019, 99, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Taheriazam, A.; Abad, G.G.Y.; Hajimazdarany, S.; Imani, M.H.; Ziaolhagh, S.; Zandieh, M.A.; Bayanzadeh, S.D.; Mirzaei, S.; Hamblin, M.R.; Entezari, M.; et al. Graphene Oxide Nanoarchitectures in Cancer Biology: Nano-Modulators of Autophagy and Apoptosis. J. Control. Release 2023, 354, 503–522. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Li, L.; Luo, C.; Song, Z.; Reyes-Vargas, E.; Clayton, F.; Huang, J.; Jensen, P.; Chen, X. Association of Anti-HER2 Antibody with Graphene Oxide for Curative Treatment of Osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 581–593. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Ma, J.; Lin, J.; Lin, H.; Su, G.; Chen, D.; Ye, S.; Chen, X.; Zhu, X.; et al. Chemotherapeutic Drug-Photothermal Agent Co-Self-Assembling Nanoparticles for near-Infrared Fluorescence and Photoacoustic Dual-Modal Imaging-Guided Chemo-Photothermal Synergistic Therapy. J. Control. Release Off. J. Control. Release Soc. 2017, 258, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, W.; Bártolo, P. Novel Poly(ε-Caprolactone)/Graphene Scaffolds for Bone Cancer Treatment and Bone Regeneration. 3D Print. Addit. Manuf. 2020, 7, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-L.; Wang, Y.-H.; Liu, G.-F.; Wang, L.; Li, Y.; Guo, Z.-Y.; Cheng, C. Graphene Oxide Nanoparticle–Loaded Ginsenoside Rg3 Improves Photodynamic Therapy in Inhibiting Malignant Progression and Stemness of Osteosarcoma. Front. Mol. Biosci. 2021, 8, 663089. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Silva, V.S.; Hortigüela, M.J.; Matesanz, M.C.; Vila, M.; Portolés, M.T. MC3T3-E1 Pre-Osteoblast Response and Differentiation after Graphene Oxide Nanosheet Uptake. Colloids Surf. B Biointerfaces 2017, 158, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, C.; Rui, Q.; Sheng, Y.; Cai, W.; Li, J.; Kong, Y. A Tri-Responsive Dual-Drug Delivery System Based on Mesoporous Silica Nanoparticles@polydopamine@graphene Oxide Nanosheets for Chemo-Photothermal Therapy of Osteosarcoma. J. Saudi Chem. Soc. 2023, 27, 101655. [Google Scholar] [CrossRef]
- Burnett, M.; Abuetabh, Y.; Wronski, A.; Shen, F.; Persad, S.; Leng, R.; Eisenstat, D.; Sergi, C. Graphene Oxide Nanoparticles Induce Apoptosis in Wild-Type and CRISPR/Cas9-IGF/IGFBP3 Knocked-out Osteosarcoma Cells. J. Cancer 2020, 11, 5007–5023. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Hu, J.J.; Lei, Q.; Zhang, X.Z. Recent Advances in Photonanomedicines for Enhanced Cancer Photodynamic Therapy. Prog. Mater. Sci. 2020, 114, 100685. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.P. Tailoring Photosensitive ROS for Advanced Photodynamic Therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic Therapy: Illuminating the Road from Cell Death towards Anti-Tumour Immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Hasrat, K.; Wang, X.; Li, Y.; Qi, Z. A Viscosity-Sensitive and Mitochondria-Targeted AIEgen Effectuated Fatty Liver Imaging and Cancer Photodynamic Therapy. Sens. Actuators B Chem. 2023, 392, 134053. [Google Scholar] [CrossRef]
- Piksa, M.; Fortuna, W.; Lian, C.; Gacka, M.; Samuel, I.D.W.; Matczyszyn, K.; Pawlik, K.J. Treatment of Antibiotic-Resistant Bacteria Colonizing Diabetic Foot Ulcers by OLED Induced Antimicrobial Photodynamic Therapy. Sci. Rep. 2023, 13, 14087. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef]
- Del Grande, M.P.; Miyake, A.M.; Nagamine, M.K.; Leite, J.V.P.; da Fonseca, I.I.M.; Massoco, C.; de, O.; Dagli, M.L.Z. Methylene Blue and Photodynamic Therapy for Melanomas: Inducing Different Rates of Cell Death (Necrosis and Apoptosis) in B16-F10 Melanoma Cells According to Methylene Blue Concentration and Energy Dose. Photodiagnosis Photodyn. Ther. 2022, 37, 102635. [Google Scholar] [CrossRef]
- Mao, W.; Chen, J.; Wang, Y.; Fang, Y.; Wu, H.; He, P. Combination of Carboplatin and Photodynamic Therapy with 9-Hydroxypheophorbide ɑ Enhances Mitochondrial and Endoplasmic Reticulum Apoptotic Effect in AMC-HN-3 Laryngeal Cancer Cells. Photodiagnosis Photodyn. Ther. 2022, 40, 103135. [Google Scholar] [CrossRef]
- Ahmetali, E.; Sen, P.; Süer, N.C.; Nyokong, T.; Eren, T.; Şener, M.K. Photodynamic Therapy Activities of Phthalocyanine-Based Macromolecular Photosensitizers on MCF-7 Breast Cancer Cells. J. Macromol. Sci. Part A Pure Appl. Chem. 2021, 58, 748–757. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, Z.; Zhang, J.; Yang, S. Photodynamic Therapy Enhances Skin Cancer Chemotherapy Effects through Autophagy Regulation. Photodiagnosis Photodyn. Ther. 2019, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Luan, S.; Zhou, J.; Xiao, X.; Yang, Y.; Mao, C.; Fang, P.; Chen, L.; Zeng, X.; et al. The Development and Progress of Nanomedicine for Esophageal Cancer Diagnosis and Treatment. Semin. Cancer Biol. 2022, 86, 873–885. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, Z.; Guan, Z.; Shen, Y.; Jiang, Y.; Xu, X.; Huang, Z.; Zhao, C. A Light-Triggered Self-Reinforced Nanoagent for Targeted Chemo-Photodynamic Therapy of Breast Cancer Bone Metastases via ER Stress and Mitochondria Mediated Apoptotic Pathways. J. Control. Release 2020, 319, 119–134. [Google Scholar] [CrossRef]
- Liao, J.; Han, R.; Wu, Y.; Qian, Z. Review of a New Bone Tumor Therapy Strategy Based on Bifunctional Biomaterials. Bone Res. 2021, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photodynamic Therapy Using Pheophorbide and 670 Nm LEDs Exhibits Anti-Cancer Effects in-Vitro in Androgen Dependent Prostate Cancer. Photodiagnosis Photodyn. Ther. 2018, 21, 130–137. [Google Scholar] [CrossRef]
- Garapati, C.; Boddu, S.H.; Jacob, S.; Ranch, K.M.; Patel, C.; Babu, R.J.; Tiwari, A.K.; Yasin, H. Photodynamic Therapy: A Special Emphasis on Nanocarrier-Mediated Delivery of Photosensitizers in Antimicrobial Therapy. Arab. J. Chem. 2023, 16, 104583. [Google Scholar] [CrossRef]
- Lv, H.; Suo, Y.; Sun, Q.; Fan, J.; Wang, Q. Study on the Effect of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy Combined with Cisplatin on Human Ovarian Cancer OVCAR-3 Cells. Adv. Med. Sci. 2023, 68, 147–156. [Google Scholar] [CrossRef]
- Jiang, Y.; He, K. Nanobiotechnological Approaches in Osteosarcoma Therapy: Versatile (Nano)Platforms for Theranostic Applications. Environ. Res. 2023, 229, 115939. [Google Scholar] [CrossRef]
- Makhadmeh, G.N.; Abuelsamen, A.; Al-Akhras, M.A.H.; Aziz, A.A. Silica Nanoparticles Encapsulated Cichorium Pumilum as a Promising Photosensitizer for Osteosarcoma Photodynamic Therapy: In-Vitro Study. Photodiagnosis Photodyn. Ther. 2022, 38, 102801. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Zhu, J.; Jin, L.; Liu, B.; Xia, K.; Wang, J.; Gao, J.; Liang, C.; Tao, H. Autophagy Inhibitor Enhance ZnPc/BSA Nanoparticle Induced Photodynamic Therapy by Suppressing PD-L1 Expression in Osteosarcoma Immunotherapy. Biomaterials 2019, 192, 128–139. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Wu, W.; Yang, W.; Zhong, B.; Qing, X.; Shao, Z. Delivery of MutT Homolog 1 Inhibitor by Functionalized Graphene Oxide Nanoparticles for Enhanced Chemo-Photodynamic Therapy Triggers Cell Death in Osteosarcoma. Acta Biomater. 2020, 109, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zhao, G.; Zhang, Y.; Zhan, F.; Chen, Z.; He, T.; Cao, Y.; Hao, L.; Wang, Z.; et al. Correction to: Homologous Targeting Nanoparticles for Enhanced PDT against Osteosarcoma HOS Cells and the Related Molecular Mechanisms. J. Nanobiotechnol. 2022, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ye, M.; Zhu, J.; Wang, Y.; Liang, C.; Tang, J.; Tao, H.; Shen, Y. Zinc Phthalocyanine Encapsulated in Polymer Micelles as a Potent Photosensitizer for the Photodynamic Therapy of Osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, G.Q.; Ding, D. Design of Superior Phototheranostic Agents Guided by Jablonski Diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef]
- Ouyang, R.; Zhang, Q.; Cao, P.; Yang, Y.; Zhao, Y.; Liu, B.; Miao, Y.; Zhou, S. Efficient Improvement in Chemo/Photothermal Synergistic Therapy against Lung Cancer Using Bi@Au Nano-Acanthospheres. Colloids Surf. B Biointerfaces 2023, 222, 113116. [Google Scholar] [CrossRef]
- Chu, Z.; Tian, T.; Tao, Z.; Yang, J.; Chen, B.; Chen, H.; Wang, W.; Yin, P.; Xia, X.; Wang, H.; et al. Upconversion Nanoparticles@AgBiS2 Core-Shell Nanoparticles with Cancer-Cell-Specific Cytotoxicity for Combined Photothermal and Photodynamic Therapy of Cancers. Bioact. Mater. 2022, 17, 71–80. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, A.; Zhai, X.; Ji, H.; Chen, Z.; Chen, X.; Yu, X. The Cellular Immunotherapy of Integrated Photothermal Anti-Oxidation Pd–Se Nanoparticles in Inhibition of the Macrophage Inflammatory Response in Rheumatoid Arthritis. Acta Pharm. Sin. B 2021, 11, 1993–2003. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Li, Y.; Hu, T.; Fan, L.; Xi, J.; Han, J.; Guo, R. Yolk-Shell Fe3O4@Carbon@Platinum-Chlorin E6 Nanozyme for MRI-Assisted Synergistic Catalytic-Photodynamic-Photothermal Tumor Therapy. J. Colloid Interface Sci. 2022, 628, 1033–1043. [Google Scholar] [CrossRef]
- Naief, M.F.; Mohammed, S.N.; Mayouf, H.J.; Mohammed, A.M. A Review of the Role of Carbon Nanotubes for Cancer Treatment Based on Photothermal and Photodynamic Therapy Techniques. J. Organomet. Chem. 2023, 999, 122819. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold Nanoparticles-Mediated Photothermal Therapy and Immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Zhao, D.; Zhang, C.; Liu, K.; He, Y.; Guan, F.; Yao, M. PMN-Incorporated Multifunctional Chitosan Hydrogel for Postoperative Synergistic Photothermal Melanoma Therapy and Skin Regeneration. Int. J. Biol. Macromol. 2023, 253, 126854. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Q.; Song, Y.; Feng, X.; Zeng, L.; Liu, Z.; Hu, X.; Tao, C.; Wang, L.; Qi, Y.; et al. Cubosomes-Assisted Transdermal Delivery of Doxorubicin and Indocyanine Green for Chemo-Photothermal Combination Therapy of Melanoma. Biomed. Pharmacother. 2023, 166, 115316. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, E.; Wang, Y.; Fu, J.; Liu, T.; Gou, R.; Shi, S.; Gu, C. Albumin-Stabilized Polydopamine Nanoparticles for Chemo-Photothermal Synergistic Therapy of Melanoma. J. Drug Deliv. Sci. Technol. 2023, 87, 104759. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent Advances in Selective Photothermal Therapy of Tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yong, T.; Wei, Z.; Bie, N.; Zhang, X.; Zhan, G.; Li, J.; Qin, J.; Yu, J.; Zhang, B.; et al. Reversing Insufficient Photothermal Therapy-Induced Tumor Relapse and Metastasis by Regulating Cancer-Associated Fibroblasts. Nat. Commun. 2022, 13, 2794. [Google Scholar] [CrossRef]
- Ying-Yan, M.; Meng, L.; Jin-Da, W.; Kai-Jie, W.; Jing-Shang, Z.; Shu-Ying, C.; Xu, L.; Qing-Feng, L.; Fei, G.; Xiu-Hua, W. NIR-Triggered Drug Delivery System for Chemo-Photothermal Therapy of Posterior Capsule Opacification. J. Control. Release 2021, 339, 391–402. [Google Scholar] [CrossRef]
- Qu, W.Q.; Fan, J.X.; Zheng, D.W.; Gu, H.Y.; Yu, Y.F.; Yan, X.; Zhao, K.; Hu, Z.B.; Qi, B.W.; Zhang, X.Z.; et al. Deep-Penetration Functionalized Cuttlefish Ink Nanoparticles for Combating Wound Infections with Synergetic Photothermal-Immunologic Therapy. Biomaterials 2023, 301, 122231. [Google Scholar] [CrossRef]
- Cheng, S.; Pan, M.; Hu, D.; Han, R.; Li, L.; Bei, Z.; Li, Y.; Sun, A.; Qian, Z. Adhesive Chitosan-Based Hydrogel Assisted with Photothermal Antibacterial Property to Prompt Mice Infected Skin Wound Healing. Chin. Chem. Lett. 2023, 34, 108276. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D Printing of Hydrogel Scaffolds for Future Application in Photothermal Therapy of Breast Cancer and Tissue Repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-Photothermal Therapy Combination Elicits Anti-Tumor Immunity against Advanced Metastatic Cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, X.; Qi, T.; Wu, Y.; Xie, X.; Chen, F.; Shao, D.; Liao, J. Biomedical Applications and Prospects of Temperature-orchestrated Photothermal Therapy. MedComm—Biomater. Appl. 2022, 1, e25. [Google Scholar] [CrossRef]
- Ting, C.W.; Chou, Y.H.; Huang, S.Y.; Chiang, W.H. Indocyanine Green-Carrying Polymeric Nanoparticles with Acid-Triggered Detachable PEG Coating and Drug Release for Boosting Cancer Photothermal Therapy. Colloids Surf. B Biointerfaces 2021, 208, 112048. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging: Via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal Enhancement of Chemotherapy in Breast Cancer by Visible Irradiation of Gold Nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.C. Photothermally Triggerable Solid Lipid Nanoparticles Containing Gold Nanospheres. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 441–448. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Kwok, H.F. Nanotechnology-Based Approaches Overcome Lung Cancer Drug Resistance through Diagnosis and Treatment. Drug Resist. Updat. 2023, 66, 100904. [Google Scholar] [CrossRef]
- Kannampuzha, S.; Gopalakrishnan, A.V. Cancer Chemoresistance and Its Mechanisms: Associated Molecular Factors and Its Regulatory Role. Med. Oncol. 2023, 40, 264. [Google Scholar] [CrossRef]
- Elfadadny, A.; El-Husseiny, H.M.; Abugomaa, A.; Ragab, R.F.; Mady, E.A.; Aboubakr, M.; Samir, H.; Mandour, A.S.; El-Mleeh, A.; El-Far, A.H.; et al. Role of Multidrug Resistance-Associated Proteins in Cancer Therapeutics: Past, Present, and Future Perspectives. Environ. Sci. Pollut. Res. 2021, 28, 49447–49466. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, X.; Hou, Z.; Ji, J.; Li, Z.; Luan, Y. Tailored Graphene Oxide-Doxorubicin Nanovehicles via near-Infrared Dye-Lactobionic Acid Conjugates for Chemo-Photothermal Therapy. J. Colloid Interface Sci. 2019, 545, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Volkova, T.V.; Drozd, K.V.; Surov, A.O. Effect of Polymers and Cyclodextrins on Solubility, Permeability and Distribution of Enzalutamide and Apalutamide Antiandrogens. J. Mol. Liq. 2021, 322, 114937. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.A.; Han, H.; Santos, H.A. Nanoparticles-Based Phototherapy Systems for Cancer Treatment: Current Status and Clinical Potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Elahi, M.; Ali, S.; Tahir, H.M.; Mushtaq, R.; Bhatti, M.F. Sericin and Fibroin Nanoparticles—Natural Product for Cancer Therapy: A Comprehensive Review. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 256–269. [Google Scholar] [CrossRef]
- Bellier, N.; Baipaywad, P.; Ryu, N.; Lee, J.Y.; Park, H. Recent Biomedical Advancements in Graphene Oxide- and Reduced Graphene Oxide-Based Nanocomposite Nanocarriers. Biomater. Res. 2022, 26, 65. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Nader, N.D.; Aghanejad, A. Targeting Mitochondria in Cancer Therapy: Insight into Photodynamic and Photothermal Therapies. Life Sci. 2022, 307, 120898. [Google Scholar] [CrossRef]
- Işıklan, N.; Hussien, N.A.; Türk, M. Hydroxypropyl Cellulose Functionalized Magnetite Graphene Oxide Nanobiocomposite for Chemo/Photothermal Therapy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130322. [Google Scholar] [CrossRef]
- Laraba, S.R.; Luo, W.; Rezzoug, A.; Zahra, Q.U.A.; Zhang, S.; Wu, B.; Chen, W.; Xiao, L.; Yang, Y.; Wei, J.; et al. Graphene-Based Composites for Biomedical Applications. Green Chem. Lett. Rev. 2022, 15, 724–748. [Google Scholar] [CrossRef]
- Dash, B.S.; Lu, Y.-J.; Pejrprim, P.; Lan, Y.-H.; Chen, J.-P. Hyaluronic Acid-Modified, IR780-Conjugated and Doxorubicin-Loaded Reduced Graphene Oxide for Targeted Cancer Chemo/Photothermal/Photodynamic Therapy. Biomater. Adv. 2022, 136, 212764. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A Novel Photothermally Controlled Multifunctional Scaffold for Clinical Treatment of Osteosarcoma and Tissue Regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Yang, W.; Hu, H.; Pan, Q.; Deng, X.; Zhang, Y.; Shao, Z. Iron-Polydopamine Coated Multifunctional Nanoparticle SiO2@PDA/Fe3+-FA Mediated Low Temperature Photothermal for Chemodynamic Therapy of Cisplatin-Insensitive Osteosarcoma. Mater. Des. 2023, 227, 111785. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Dong, C.; Wang, J.; Zhang, T.; Huang, L.; Ni, D.; Luo, Y. 3D Printed Hydrogel/Bioceramics Core/Shell Scaffold with NIR-II Triggered Drug Release for Chemo-Photothermal Therapy of Bone Tumors and Enhanced Bone Repair. Chem. Eng. J. 2023, 461, 141855. [Google Scholar] [CrossRef]
- Yin, X.; Ran, S.; Cheng, H.; Zhang, M.; Sun, W.; Wan, Y.; Shao, C.; Zhu, Z. Polydopamine-Modified ZIF-8 Nanoparticles as a Drug Carrier for Combined Chemo-Photothermal Osteosarcoma Therapy. Colloids Surf. B Biointerfaces 2022, 216, 112507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, Y.; Ni, W.; Xiao, H.; Zhang, J. Cancer Cell Membrane Coated Silica Nanoparticles Loaded with ICG for Tumour Specific Photothermal Therapy of Osteosarcoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2298–2305. [Google Scholar] [CrossRef]
- Chen, H.; Fu, Y.; Feng, K.; Zhou, Y.; Wang, X.; Huang, H.; Chen, Y.; Wang, W.; Xu, Y.; Tian, H.; et al. Polydopamine-Coated UiO-66 Nanoparticles Loaded with Perfluorotributylamine/Tirapazamine for Hypoxia-Activated Osteosarcoma Therapy. J. Nanobiotechnol. 2021, 19, 298. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef]
- Lawal, A.T. Recent Progress in Graphene Based Polymer Nanocomposites. Cogent Chem. 2020, 6, 1833476. [Google Scholar] [CrossRef]
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.T.; Pinto, L.A.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef]
- Rajaei, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M.; Rahdar, A.; Díez-Pascual, A.M. Chitosan/Agarose/Graphene Oxide Nanohydrogel as Drug Delivery System of 5-Fluorouracil in Breast Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104307. [Google Scholar] [CrossRef]
- Li, S.; Gan, Y.; Lin, C.; Lin, K.; Hu, P.; Liu, L.; Yu, S.; Zhao, S.; Shi, J. NIR-/PH-Responsive Nanocarriers Based on Mesoporous Hollow Polydopamine for Codelivery of Hydrophilic/Hydrophobic Drugs and Photothermal Synergetic Therapy. ACS Appl. Bio Mater. 2021, 4, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-Delivery of Doxorubicin and Paclitaxel by Reduction/PH Dual Responsive Nanocarriers for Osteosarcoma Therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef]
- Sheng, Y.; Cao, C.; Liang, Z.; Yin, Z.Z.; Gao, J.; Cai, W.; Kong, Y. Construction of a Dual-Drug Delivery System Based on Oxidized Alginate and Carboxymethyl Chitosan for Chemo-Photothermal Synergistic Therapy of Osteosarcoma. Eur. Polym. J. 2022, 174, 111331. [Google Scholar] [CrossRef]
- Yadav, P.; Jain, J.; Sherje, A.P. Recent Advances in Nanocarriers-Based Drug Delivery for Cancer Therapeutics: A Review. React. Funct. Polym. 2021, 165, 104970. [Google Scholar] [CrossRef]
- Li, M.; Yu, B.; Wang, S.; Zhou, F.; Cui, J.; Su, J. Microenvironment-Responsive Nanocarriers for Targeted Bone Disease Therapy. Nano Today 2023, 50, 101838. [Google Scholar] [CrossRef]
- Wang, J.; Ni, Q.; Wang, Y.; Zhang, Y.; He, H.; Gao, D.; Ma, X.; Liang, X.J. Nanoscale Drug Delivery Systems for Controllable Drug Behaviors by Multi-Stage Barrier Penetration. J. Control. Release 2021, 331, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Almurshedi, A.S.; Radwan, M.; Omar, S.; Alaiya, A.A.; Badran, M.M.; Elsaghire, H.; Saleem, I.Y.; Hutcheon, G.A. A Novel PH-Sensitive Liposome to Trigger Delivery of Afatinib to Cancer Cells: Impact on Lung Cancer Therapy. J. Mol. Liq. 2018, 259, 154–166. [Google Scholar] [CrossRef]
- Avedian, N.; Zaaeri, F.; Daryasari, M.P.; Akbari Javar, H.; Khoobi, M. PH-Sensitive Biocompatible Mesoporous Magnetic Nanoparticles Labeled with Folic Acid as an Efficient Carrier for Controlled Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 323–332. [Google Scholar] [CrossRef]
- Narmani, A.; Ganji, S.; Amirishoar, M.; Jahedi, R.; Kharazmi, M.S.; Jafari, S.M. Smart Chitosan-PLGA Nanocarriers Functionalized with Surface Folic Acid Ligands against Lung Cancer Cells. Int. J. Biol. Macromol. 2023, 245, 125554. [Google Scholar] [CrossRef]
- De Oliveira Silva, J.; Fernandes, R.S.; Ramos Oda, C.M.; Ferreira, T.H.; Machado Botelho, A.F.; Martins Melo, M.; de Miranda, M.C.; Assis Gomes, D.; Dantas Cassali, G.; Townsend, D.M.; et al. Folate-Coated, Long-Circulating and PH-Sensitive Liposomes Enhance Doxorubicin Antitumor Effect in a Breast Cancer Animal Model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar] [CrossRef]
- Lamey, C.A.; Moussa, N.; Helmy, M.W.; Haroun, M.; Sabra, S.A. Simultaneous Encapsulation of Dasatinib and Celecoxib into Caseinate Micelles towards Improved in Vivo Anti-Breast Cancer Efficacy with Reduced Drug Toxicity. J. Drug Deliv. Sci. Technol. 2023, 87, 104807. [Google Scholar] [CrossRef]
- Kheyrandish, M.; Bazi, Z.; Sheikh Arabi, M. Aptamer Grafted Dendrimer-Silver Nanocarrier for Specific Delivery of CALML5 SiRNA: A 2D and 3D Study in Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2023, 84, 104514. [Google Scholar] [CrossRef]
- Singh, S.P.; Mishra, A.; Shyanti, R.K.; Singh, R.P.; Acharya, A. Silver Nanoparticles Synthesized Using Carica Papaya Leaf Extract (AgNPs-PLE) Causes Cell Cycle Arrest and Apoptosis in Human Prostate (DU145) Cancer Cells. Biol. Trace Elem. Res. 2021, 199, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jang, E.K.; Ki, M.R.; Son, R.G.; Kim, S.; Choe, Y.; Pack, S.P.; Chung, S. PH-Responsive Phototherapeutic Poly(Acrylic Acid)-Calcium Phosphate Passivated TiO2 Nanoparticle-Based Drug Delivery System for Cancer Treatment Applications. J. Ind. Eng. Chem. 2022, 112, 258–270. [Google Scholar] [CrossRef]
- Kazemi, S.; Pourmadadi, M.; Yazdian, F.; Ghadami, A. The Synthesis and Characterization of Targeted Delivery Curcumin Using Chitosan-Magnetite-Reduced Graphene Oxide as Nano-Carrier. Int. J. Biol. Macromol. 2021, 186, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zoulikha, M.; Qiu, M.; Teng, C.; Lin, C.; Li, X.; Sallam, M.A.; Xu, Q.; He, W. The Effects of Protein Corona on in Vivo Fate of Nanocarriers. Adv. Drug Deliv. Rev. 2022, 186, 114356. [Google Scholar] [CrossRef]
- Grilli, F.; Hajimohammadi Gohari, P.; Zou, S. Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6802. [Google Scholar] [CrossRef]
- Ortega, M.; Vilhena, J.G.; Rubio-Pereda, P.; Serena, P.A.; Pérez, R. Assessing the Accuracy of Different Solvation Models To Describe Protein Adsorption. J. Chem. Theory Comput. 2019, 15, 2548–2560. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Tiwari, S.; Purkait, M.K. A Comprehensive Review on Graphene Oxide-Based Nanocarriers: Synthesis, Functionalization and Biomedical Applications. FlatChem 2023, 38, 100484. [Google Scholar] [CrossRef]
- Rahimi, S.; Chen, Y.; Zareian, M.; Pandit, S.; Mijakovic, I. Cellular and Subcellular Interactions of Graphene-Based Materials with Cancerous and Non-Cancerous Cells. Adv. Drug Deliv. Rev. 2022, 189, 114467. [Google Scholar] [CrossRef]
- Zoghi, M.; Pourmadadi, M.; Yazdian, F.; Nigjeh, M.N.; Rashedi, H.; Sahraeian, R. Synthesis and Characterization of Chitosan/Carbon Quantum Dots/Fe2O3 Nanocomposite Comprising Curcumin for Targeted Drug Delivery in Breast Cancer Therapy. Int. J. Biol. Macromol. 2023, 249, 125788. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, S.; Pourmadadi, M.; Abdouss, M.; Pourmousavi, S.A.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Carboxymethyl Cellulose/Starch/Reduced Graphene Oxide Composite as a PH-Sensitive Nanocarrier for Curcumin Drug Delivery. Int. J. Biol. Macromol. 2023, 241, 124566. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, Q.; Cao, J.; Gao, Y.; Han, S.; Liang, Y.; Zhang, T.; Song, Y.; Sun, Y. Recent Progress of Graphene Oxide-Based Multifunctional Nanomaterials for Cancer Treatment. Cancer Nanotechnol. 2021, 12, 18. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Chithra Sekhar, V.; Athira, V.S. Graphene Oxide Based Functionalized Chitosan Polyelectrolyte Nanocomposite for Targeted and PH Responsive Drug Delivery. Int. J. Biol. Macromol. 2020, 150, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Jeshvaghani, P.A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Khoshmaram, K.; Nigjeh, M.N. Synthesis and Characterization of a Novel, PH-Responsive Sustained Release Nanocarrier Using Polyethylene Glycol, Graphene Oxide, and Natural Silk Fibroin Protein by a Green Nano Emulsification Method to Enhance Cancer Treatment. Int. J. Biol. Macromol. 2023, 226, 1100–1115. [Google Scholar] [CrossRef]
- Targhazeh, N.; Maleki, M.; Alemi, F.; Yousefi, B. Enhanced Drug Loading Capacity of Graphene Oxide Nanoparticles by Polyglycerol Hyper Branching and Increasing the Sensitivity of Osteosarcoma Cancer Cells to Doxorubicin. J. Drug Deliv. Sci. Technol. 2023, 88, 104871. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A. Chemical Functionalization of Graphene Family Members. Phys. Sci. Rev. 2017, 2, 20160103. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, S.; Lv, X.; Gao, Y.; Ge, L. Global Trends and Prospects in Microplastics Research: A Bibliometric Analysis. J. Hazard. Mater. 2020, 400, 123110. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, W.; Zhao, R.; Wang, M.; Tang, J.; Wei, Y. Insight into the Interaction Between Microplastics and Microorganisms Based on a Bibliometric and Visualized Analysis. Bull. Environ. Contam. Toxicol. 2021, 107, 585–596. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in Mice: Ultrahigh in Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-Graphene in Biomedicine: Theranostic Applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and Graphene Oxide as New Nanocarriers for Drug Delivery Applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal Imaging Guided Photothermal Therapy Using Functionalized Graphene Nanosheets Anchored with Magnetic Nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic Effect of Chemo-Photothermal Therapy Using PEGylated Graphene Oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The Influence of Surface Chemistry and Size of Nanoscale Graphene Oxide on Photothermal Therapy of Cancer Using Ultra-Low Laser Power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.-S. Near-Infrared Light-Responsive Nanomaterials in Cancer Therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A Functionalized Graphene Oxide-Iron Oxide Nanocomposite for Magnetically Targeted Drug Delivery, Photothermal Therapy, and Magnetic Resonance Imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Feng, L.; Wu, L.; Qu, X. New Horizons for Diagnostics and Therapeutic Applications of Graphene and Graphene Oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced Chemotherapy Efficacy by Sequential Delivery of siRNA and Anticancer Drugs Using PEI-Grafted Graphene Oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Aghayee, S.; Fereydooni, Y.; Talebi, A. The Use of a Glucose-Reduced Graphene Oxide Suspension for Photothermal Cancer Therapy. J. Mater. Chem. 2012, 22, 13773. [Google Scholar] [CrossRef]
- Kim, H.; Namgung, R.; Singha, K.; Oh, I.-K.; Kim, W.J. Graphene Oxide–Polyethylenimine Nanoconstruct as a Gene Delivery Vector and Bioimaging Tool. Bioconjug. Chem. 2011, 22, 2558–2567. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Graphene Nanomesh Promises Extremely Efficient in Vivo Photothermal Therapy. Small 2013, 9, 3593–3601. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene Oxide Mediated Delivery of Methylene Blue for Combined Photodynamic and Photothermal Therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential Drug Release and Enhanced Photothermal and Photoacoustic Effect of Hybrid Reduced Graphene Oxide-Loaded Ultrasmall Gold Nanorod Vesicles for Cancer Therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials Formulations for Photothermal and Photodynamic Therapy of Cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]


| Human Osteosarcoma Cell Lines | Type of Study | Method of Analysis of Anti-Cancer Activity | Results | Ref |
|---|---|---|---|---|
| Saos-2 and MG-63 | In vitro |
| The GO nanocarrier loaded or functionalized with DOX inhibited cell proliferation depending on the dose (between 10 and 1280 µg/mL) after 12 h. Additionally, a synergistic effect was evidenced in the inhibition of cancer cells when MLT was combined with DOX due to the regulation of the X-linked inhibitor of apoptosis (XIAP) and the catalytic subunit of human telomerase (hTERT). | [93] |
| Saos-2 and MG-63 | In vitro |
| This study used GO as a nanocarrier functionalized with chitosan nanoparticles for siRNA delivery. The results of this study showed a controlled release of siRNA in the acidic pH prevailing at the tumor site. In addition, it inhibited the activity of the Bcl-2 oncogene (considered the main factor in multidrug resistance). This is interesting because it facilitates the treatment of the disease and prevents the infection from reappearing by inhibiting the overexpression of Bcl-2. | [94] |
| Saos-2 | In vitro |
| Osteosarcoma treatment using poly(ε-caprolactone)/graphene porous scaffolds obtained by liquid fusion with GO nanosheets demonstrated good mechanical properties and up to 83.5% inhibition of the disease. In addition, the results of this research show that the application of these scaffolds reduces the survival rate of cancer cells. | [100] |
| MG-63 | In vivo |
| The non-covalent stable interaction of trastuzumab (TRA) with GO (TRA-GO) demonstrated good binding to the Her2 antibody (a potential therapeutic target), causing the rapid inhibition of cancer cells. In addition, TRA-GO induced oxidative stress and Her2 signaling in target cells; this induced the rapid depletion of cellular inhibitors of apoptosis protein (cIAP) and caspase8, RIP1/RIPK3/MLKL necroptosome formation, and necroptosis of cancer cells, significantly enhancing the antitumor activity of TRA. | [98] |
| MG-63 | In vivo |
| The synthesis of GO-based nanoparticles doped with photosensitizers indocyanine green (ICG), folic acid (FA), and polyethylene glycol (PEG) and loaded with ginsenoside (Rg3: a significant component of ginseng), called PEG-GO-FA/ICG -Rg3, inhibited the proliferation, invasion, migration, and enhanced apoptosis and autophagy of cancer cells. This study demonstrated that PEG-GO-FA/ICG-Rg3 improves osteosarcoma cells’ tumor growth inhibition capacity, which presents a promising therapeutic strategy for treating osteosarcoma. | [101] |
| MC3T3-E1 | In vitro |
| Functionalization of GO with polyethylene glycol-amine (PEG) significantly decreased cancer cells’ proliferation and increased their apoptosis. This study suggests that the application of the GO-PEG nanomaterial is promising at a concentration of 40 µg/mL since, at this concentration, it does not affect the differentiation of healthy preosteoclasts. | [102] |
| Saos-2 | In vitro |
| A methotrexate (MTX) delivery system based on a mesoporous structure of @polidopamine@GO silica nanoparticles improved the drug delivery capacity and photothermal capacity for chemo-photothermal applications of osteosarcoma. | [103] |
| U2OS and Saos-2 | In vitro |
| Induction of apoptosis in cancer cells via GO and CRISPR-Cas9. This study targets the IGF1 and IGFBP3 signaling pathways, strengthening GO-related cytotoxicity. | [104] |
| PDT System | PS | Type of Study | Method of Analysis of Anti-Cancer Activity of Osteosarcoma | Results | Ref |
|---|---|---|---|---|---|
| Poly(lactic-co-glycolic acid) nanoparticles were concealed with human osteosarcoma cell membranes and encapsulated with the photosensitizer IR780 (MH-PLGA-IR780). | IR780 | In vivo, In vitro |
| Greater penetration into deeper tissues and apoptosis and ferroptosis induction were achieved. In addition, an absorption rate of more than 90% of MH-PLGA-IR780 generates a high affinity with the HOS cell line. Intracellular ROS percentages of 98.97% were obtained, improving PDT performance. | [130] |
| Bovine serum albumin nanoparticles—zinc phthalocyanine (BSA-ZnPc, BZ) | ZnPc | In vivo, In vitro |
| BSA as a ZnP carrier increased water solubility and enhanced PDT effects. An increase in tumor resection and a more significant effect of PDT were achieved without affecting healthy tissue. In addition, results were obtained on the cytotoxicity of BZ after irradiation and the reliability of cells when exposed to BZ without irradiation. | [128] |
| Chemophotodynamically functionalized graphene oxide (GO) nanoparticles (PEG-GO-FA/ICG). | ICG | In vitro, In vivo |
| It was found that the PEG-GO-FA/ICG had a more significant photothermal effect as the temperature increased over periods, remaining above 50 °C. A loading yield of 54% of the drugs and a stability of 30% of the in vitro release of the PEG-GO-FA/ICG nanoparticles (DOX + TH287) at an acidic pH were obtained. Cells with a chemo-photodynamic effect showed high calcium levels in the cytoplasm of osteosarcoma cells, showing more significant damage to the ER membrane. | [129] |
| Poly(ethylene glycol)-poly [2(methyl acryloyl)ethylnicotinate] nanoparticles with Zinc phthalocyanine (PEG-PMAN/ZnPc) (PPZ). | ZnPc | In vitro, In vivo |
| ROS production was achieved in MNNG/Hos osteosarcoma cells, in addition to obtaining a charge content of 8.2%, an encapsulation efficiency of 89.4%, and an extended absorption at a wavelength of 660 nm, favorable for PTD and improved proliferation inhibition in the in vitro study. Significant tumor growth inhibition was achieved after 14 days of 21.7 mm3, with PPZ being a therapeutic potential for PDT in osteosarcomas. | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Rosado, L.V.; Carrascal-Hernández, D.C.; Insuasty, D.; Grande-Tovar, C.D. Graphene Oxide (GO) for the Treatment of Bone Cancer: A Systematic Review and Bibliometric Analysis. Nanomaterials 2024, 14, 186. https://doi.org/10.3390/nano14020186
Barba-Rosado LV, Carrascal-Hernández DC, Insuasty D, Grande-Tovar CD. Graphene Oxide (GO) for the Treatment of Bone Cancer: A Systematic Review and Bibliometric Analysis. Nanomaterials. 2024; 14(2):186. https://doi.org/10.3390/nano14020186
Chicago/Turabian StyleBarba-Rosado, Lemy Vanessa, Domingo César Carrascal-Hernández, Daniel Insuasty, and Carlos David Grande-Tovar. 2024. "Graphene Oxide (GO) for the Treatment of Bone Cancer: A Systematic Review and Bibliometric Analysis" Nanomaterials 14, no. 2: 186. https://doi.org/10.3390/nano14020186
APA StyleBarba-Rosado, L. V., Carrascal-Hernández, D. C., Insuasty, D., & Grande-Tovar, C. D. (2024). Graphene Oxide (GO) for the Treatment of Bone Cancer: A Systematic Review and Bibliometric Analysis. Nanomaterials, 14(2), 186. https://doi.org/10.3390/nano14020186








