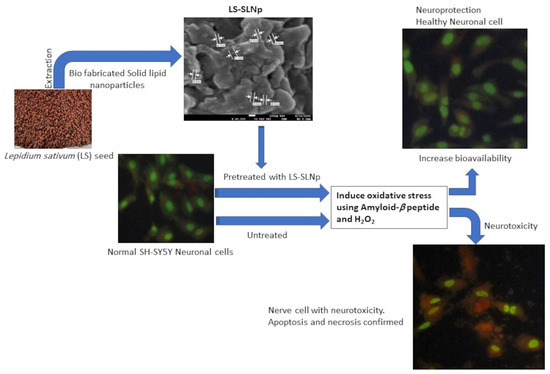
Neuroprotective Effect of Solid Lipid Nanoparticles Loaded with Lepidium sativum (L.) Seed Bioactive Components Enhance Bioavailability and Wnt/β-Catenin/Camk-II Signaling Cascade in SH-SY5Y Neuroblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Collection, Extraction of Lepidium sativum L. (Cress Seed) and Salvia hispanica. L. (Chia Seed)
2.3. Phytochemical Identification
2.4. Preparation of L. sativum Seed Extract Containing Solid Lipid Nanoparticle (LS-SLNp)
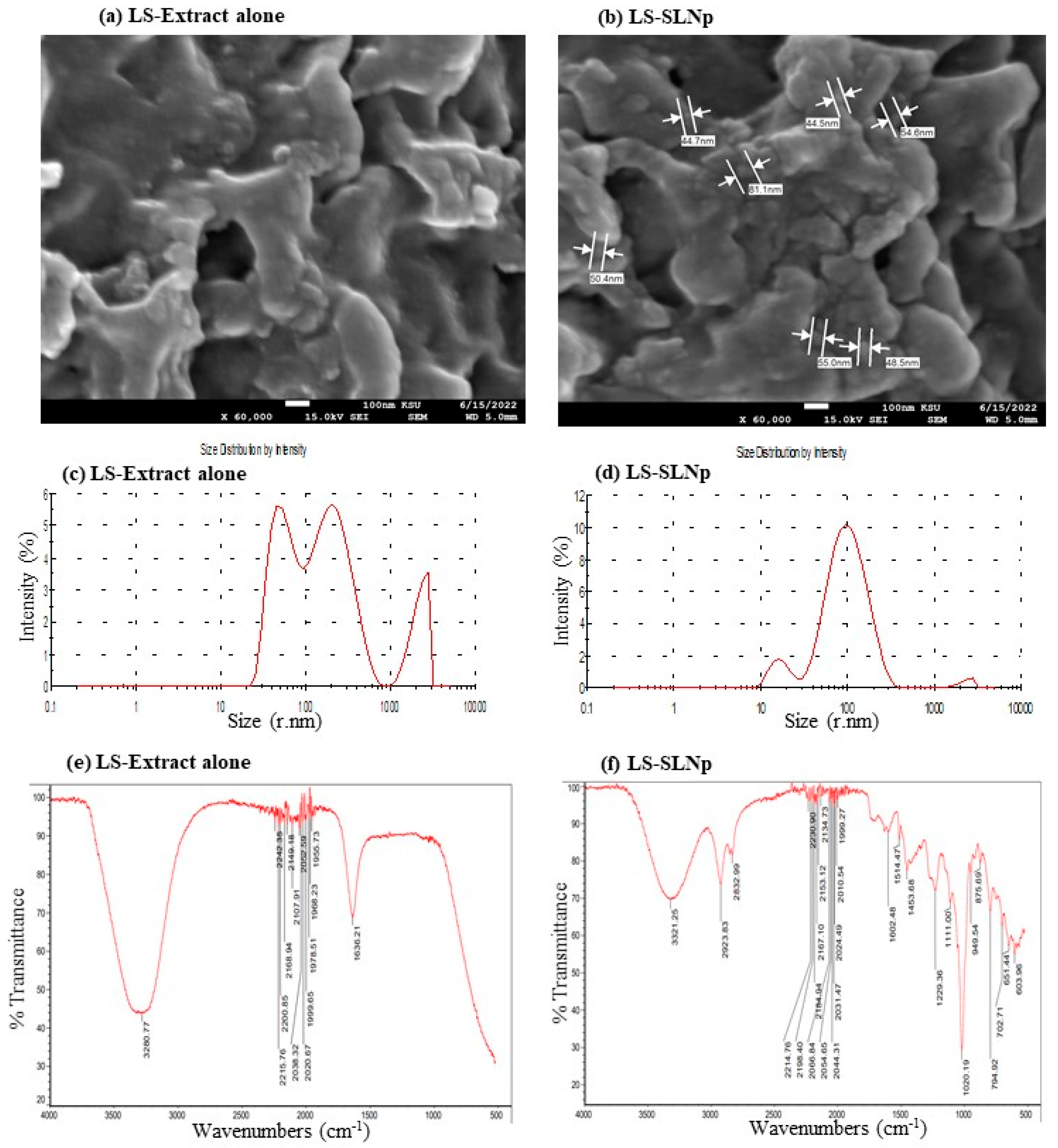
2.5. Characterization of Prepared LS-SLNp
2.6. Cell lines and Cell Culture
2.7. Preparation of Beta-Amyloid (Aβ,1-42) Peptides
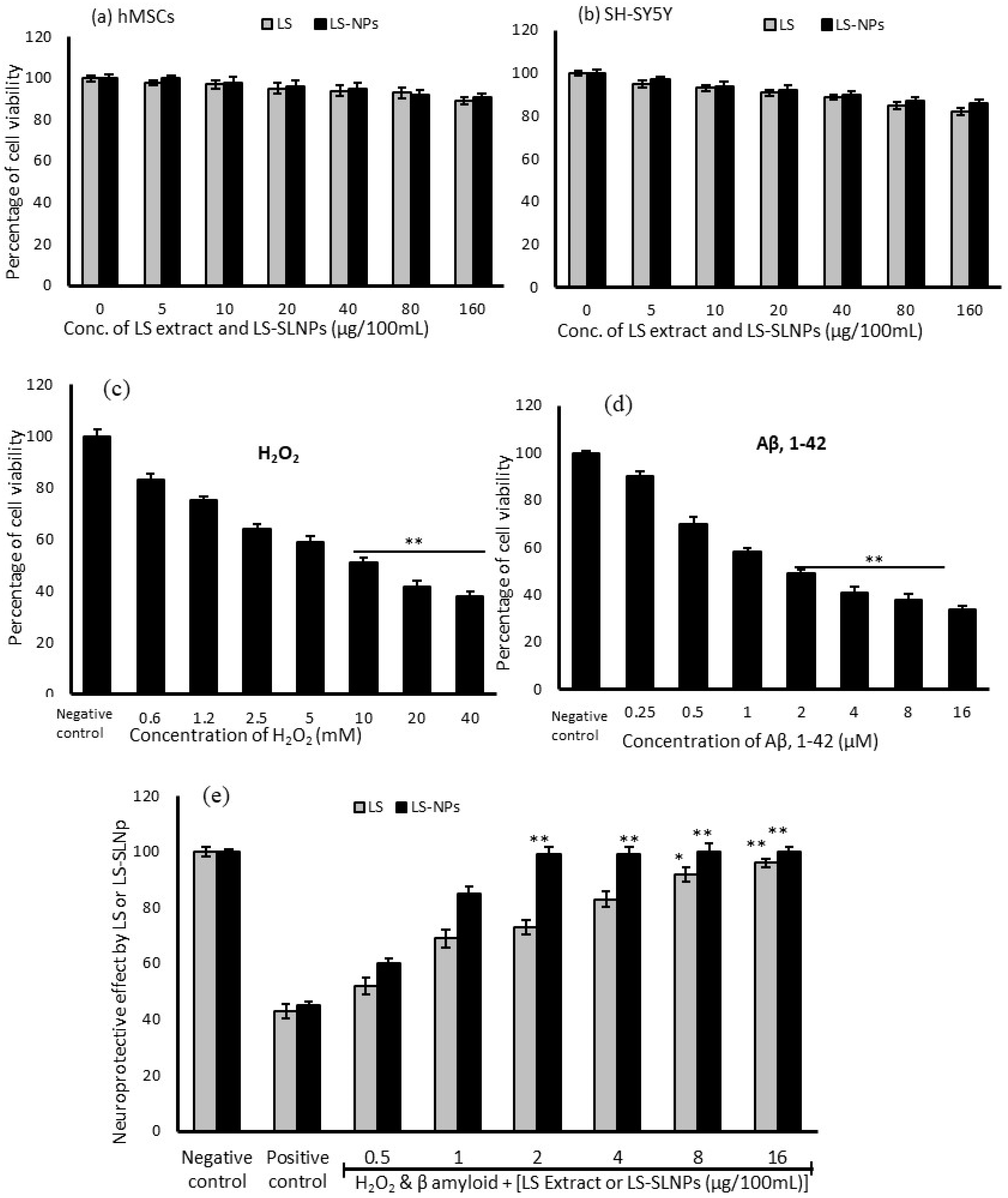
2.8. Biocompatibility Assessment of LS and LS-SLNp in hMSCs and SH-SY5Y Cells
2.9. Assessing the Toxic Dose for H2O2 and Aβ (1-42) Using MTT Assay
2.10. Neuroprotective Effect of LS-SLNp in H2O2 and Aβ (1-42) Induced SH-SY5Y Cells
3. Experimental Design
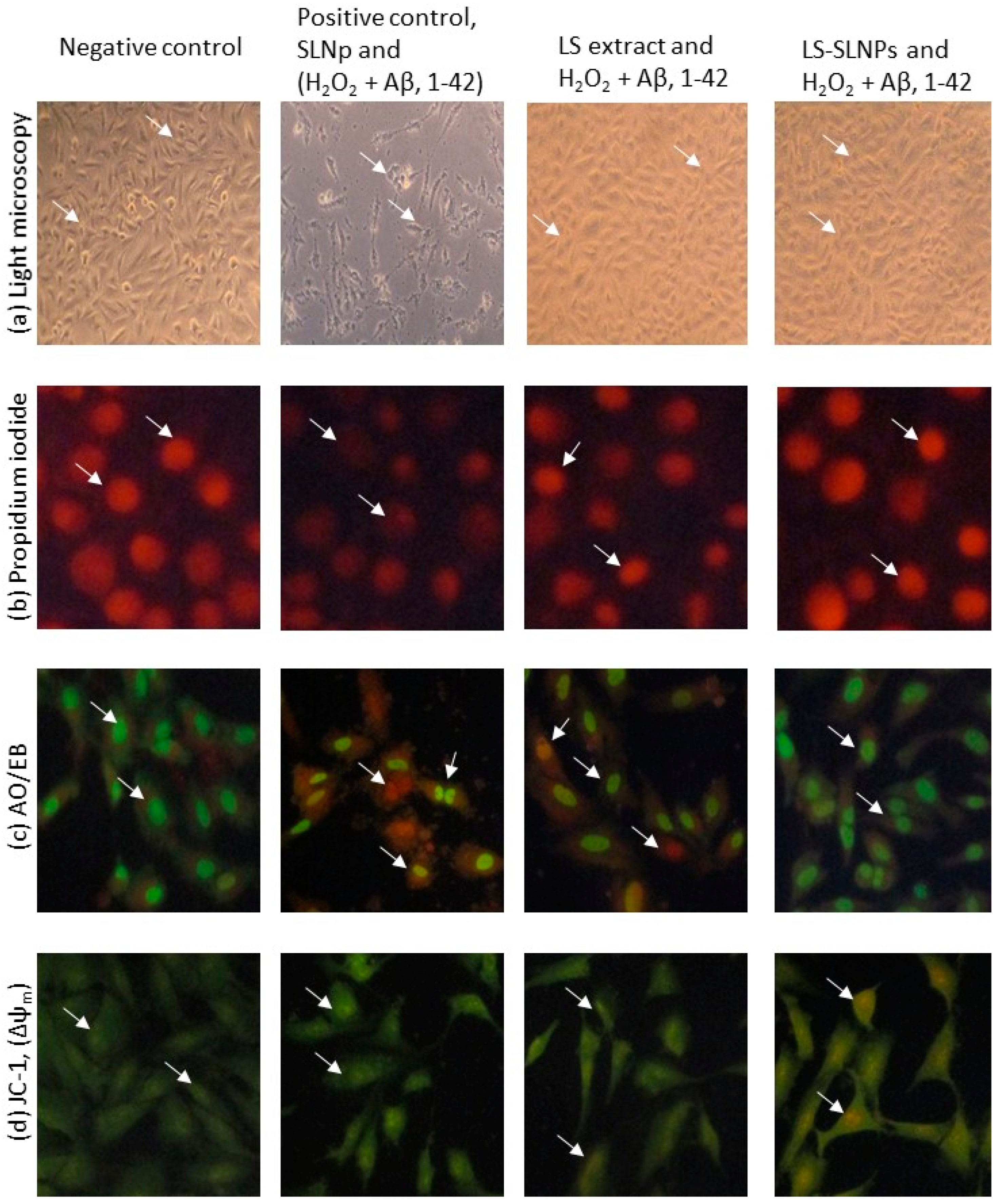
3.1. Cell and Nuclear Morphology
3.2. JC-1 Staining assay
3.3. Measurement of Pro-Oxidant and Antioxidant Levels in LS and LS-SLNp-Pretreated hMSCs and SH-SY5Y Cells Undergo Oxidative Stress
3.4. Gene Expression Analysis
3.5. Quantification of Protein Using ELISA
3.6. Statistical Analysis
4. Results
4.1. Characterization of LS-SLNp
4.2. Effect LS and LS-SLNp on hMSCs and SH-SY5Y Cell Proliferation
4.3. Cytotoxic Effect of H2O2 and β-Amyloid on SH-SY5Y Neuroblastoma Cells
4.4. Preventative Effect of LS-SLNp against H2O2 and Aβ,1-42 Induced Toxicity in SH-SY5Y Cells
4.5. Determination of Neuroprotective Potential via Cell and Nuclear Staining
4.6. Mitochondrial Membrane Potential (Δψm, JC-1)
4.7. Changes in Pro-Oxidant and Antioxidant Activity
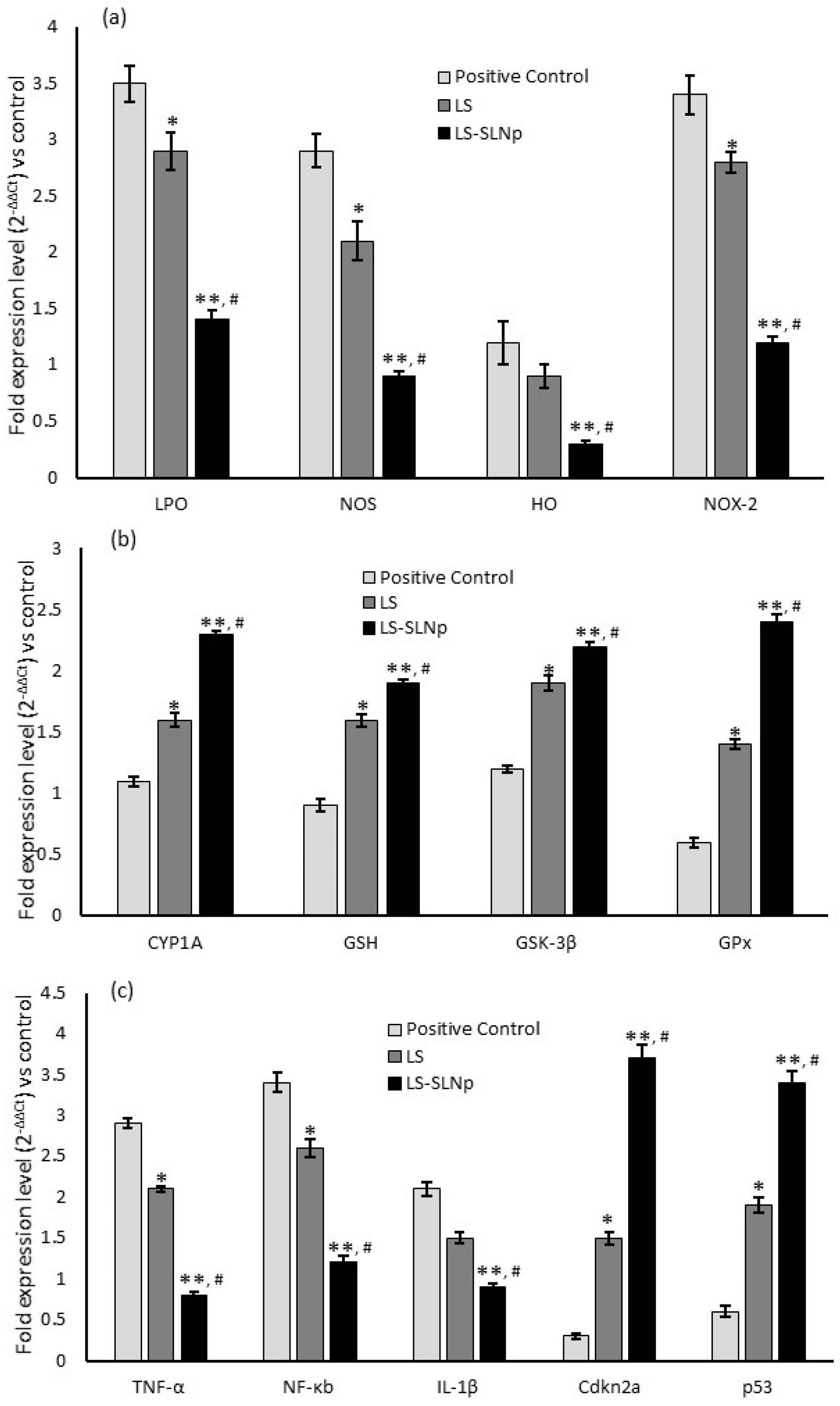
4.8. Alterations in Gene Expression Levels in hMSCs Pretreated with LS-SLNp
4.9. Neuroprotection-Related Gene Expression Levels in SH-SY5Y Cells Pretreated with LS-SLNp
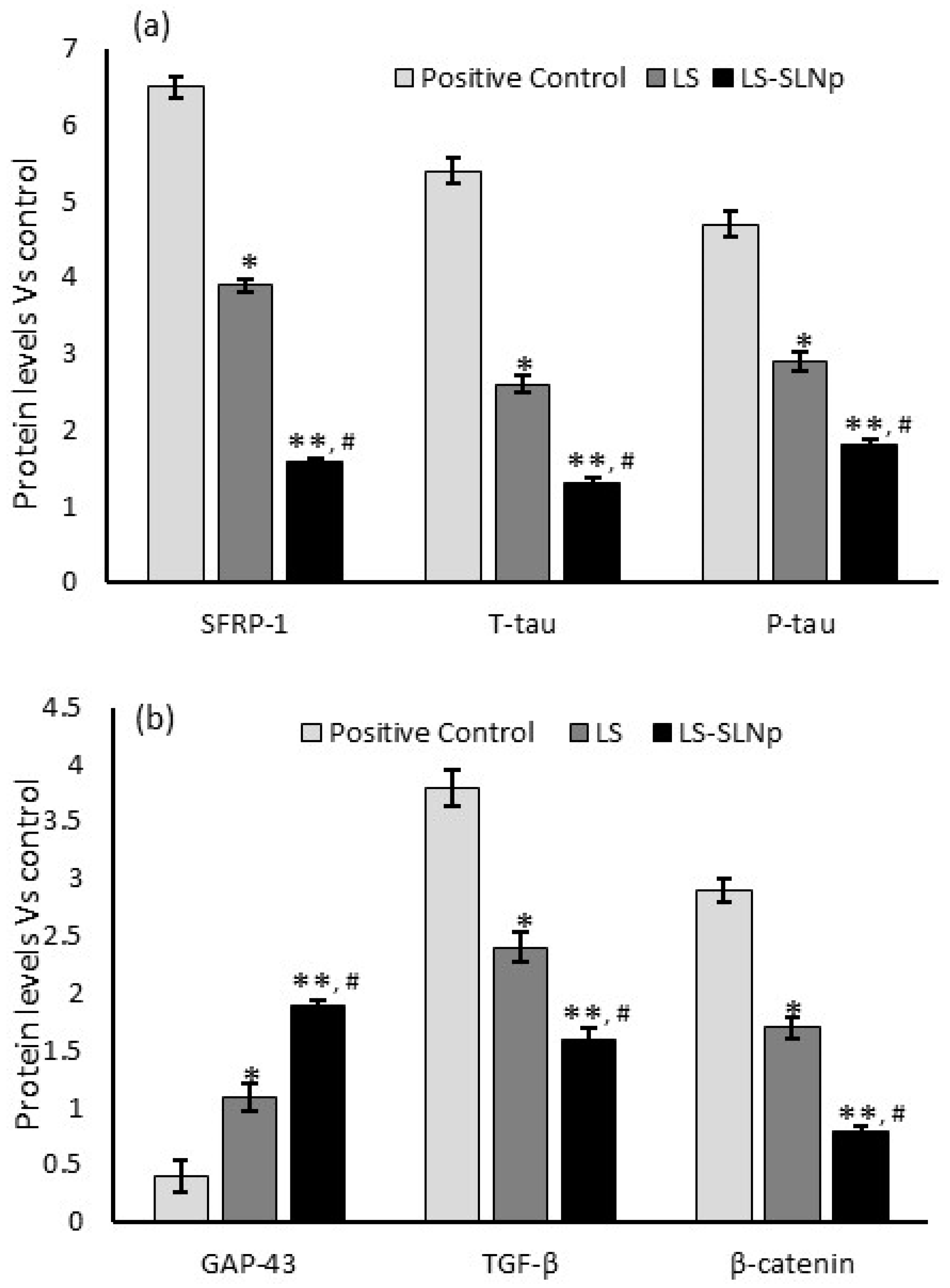
4.10. Protein Levels
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Mansor, N.I.; Ntimi, C.M.; Abdul-Aziz, N.M.; Ling, K.H.; Adam, A.; Rosli, R.; Hassan, Z.; Nordin, N. Asymptomatic neurotoxicity of amyloid β-peptides (Aβ1-42 and Aβ25-35) on mouse embryonic stem cell-derived neural cells. Bosn. J. Basic Med. Sci. 2021, 21, 98–110. [Google Scholar] [CrossRef]
- Wells, C.; Brennan, S.E.; Keon, M.; Saksena, N.K. Prionoid Proteins in the Pathogenesis of Neurodegenerative Diseases. Front. Mol. Neurosci. 2019, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia-inducible factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Tanokashira, D.; Mamada, N.; Yamamoto, F.; Taniguchi, K.; Tamaoka, A.; Lakshmana, M.K.; Araki, W. The neurotoxicity of amyloid β-protein oligomers is reversible in a primary neuron model. Mol. Brain 2017, 10, 4. [Google Scholar] [CrossRef]
- Zhaliazka, K.; Matveyenka, M.; Kurouski, D. Lipids uniquely alter the secondary structure and toxicity of amyloid beta 1–42 aggregates. FEBS J. 2023, 290, 3203–3220. [Google Scholar] [CrossRef]
- Ahmed, A.; Subaiea, M.; Eid, A.; Li, L.; Seeram, P.; Zawia, H. Pomegranate extract modulates procesh of amyloid-β precursor protein in an aged Alzheimer’s Disease animal model. Curr. Alzheimer Res. 2014, 11, 834–843. [Google Scholar] [CrossRef]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramaswamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4875–4883. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guo, M.S.; Zhang, Y.; Yu, L.; Wu, J.M.; Tang, Y.; Ai, W.; Zhu, F.D.; Law, B.Y.K.; Chen, Q.; et al. Dietary plant polyphenols as the potential drugs in neurodegenerative diseases: Current evidence, advances, and opportunities. Oxidative Med. Cell. Longev. 2022, 2022, 5288698. [Google Scholar] [CrossRef] [PubMed]
- Shivananjegowda, M.G.; Hani, U.; Osmani, R.A.M.; Alamri, A.H.; Ghazwani, M.; Alhamhoom, Y.; Rahamathulla, M.; Paranthaman, S.; Gowda, D.V.; Siddiqui, A. Development and evaluation of solid lipid nanoparticles for the clearance of Aβ in Alzheimer’s disease. Pharmaceutics 2023, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya, M.A.; Mossa, J.S.; Ageel, A.M.; Rafatullah, S. Pharmacological and safety evaluation studies on Lepidium sativum L., Seeds. Phytomedicine 1994, 1, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Al-Suwaydani, A.; Alam, M.A.; Raish, M.; ABin Jardan, Y.; Ahad, A.; IAl-Jenoobi, F. Effect of C. cyminum and L. sativum on Pharmacokinetics and Pharmacodynamics of Antidiabetic Drug Gliclazide. Curr. Drug Metab. 2022, 23, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Vazifeh, S.; Kananpour, P.; Khalilpour, M.; Eisalou, S.V.; Hamblin, M.R. Anti-inflammatory and immunomodulatory properties of Lepidium sativum. BioMed Res. Int. 2022, 2022, 3645038. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, L.; Yang, M.X.; Zhang, W.; Li, X.M.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int. J. Nanomed. 2015, 10, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death—Study in a model of UV radiation of the leukemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 139–146. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Kim, H.-Y. Analysis of variance (ANOVA) comparing means of more than two groups. Restor. Dent. Endod. 2014, 39, 74–77. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry on Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. July 2005. Available online: www.fda.gov (accessed on 25 October 2023).
- Siegrist, S.; Cörek, E.; Detampel, P.; Sandström, J.; Wick, P.; Huwyler, J. Preclinical hazard evaluation strategy for nanomedicines. Nanotoxicolog 2019, 13, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Reigner, B.G.; Blesch, K.S. Estimating the starting dose for entry into humans: Principles and practice. Eur. J. Clin. Pharmacol. 2002, 57, 835–845. [Google Scholar] [CrossRef]
- Li, S.; Dong, L.; Pan, Z.; Yang, G. Targeting the neural stem cells in subventricular zone for the treatment of glioblastoma: An update from preclinical evidence to clinical interventions. Stem Cell Res. Ther. 2023, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.; Ribeiro, I.; Pinto, L. Frontiers in Neurogenesis. Cells 2022, 11, 3567. [Google Scholar] [CrossRef] [PubMed]
- Ay, H.F.; Yesilkir-Baydar, S.; Cakir-Koc, R. Synthesis characterisation and neuroprotectivity of Silybum marianum extract loaded chitosan nanoparticles. J. Microencapsul. 2023, 40, 29–36. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer’s disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Lee, R.-L.; Funk, K.E. Imaging blood-brain barrier disruption in neuroinflammation and Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1144036. [Google Scholar] [CrossRef]
- Pereira, I.; Lopez-Martinez, M.J.; Villasante, A.; Introna, C.; Tornero, D.; Canals, J.M.; Samitier, J. Hyaluronic acid-based bioink improves the differentiation and network formation of neural progenitor cells. Front. Bioeng. Biotechnol. 2023, 11, 1110547. [Google Scholar] [CrossRef]
- Alhasan, M.A.; Tomokiyo, A.; Hamano, S.; Sugii, H.; Ono, T.; Ipposhi, K.; Yamashita, K.; Mardini, B.; Minowa, F.; Maeda, H. Hyaluronic Acid Induction Promotes the Differentiation of Human Neural Crest-like Cells into Periodontal Ligament Stem-like Cells. Cells 2023, 12, 2743. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Zhang, H.-J.; Wu, F.; Chen, Y.-H.; Ma, X.-Q.; Du, J.-Q.; Zhou, Z.-L.; Li, J.-Y.; Nan, F.-J.; Li, J. Isoquinoline-1,3,4-trione and its derivatives attenuate β-amyloid-induced apoptosis of neuronal cells. FEBS J. 2006, 273, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.N.; Wang, C.J.; Lin, C.L.; Yen, A.T.; Li, H.H.; Peng, C.H. Abelmoschus esculentus subfractions attenuate beta amyloid-induced neuron apoptosis by regulating DPP-4 with improving insulin resistance signals. PLoS ONE 2018, 14, e0217400. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kundu, M.; Chakrabarti, S.; Patel, D.R.; Pahan, K. Oleamide, a sleep-inducing supplement, upregulates doublecortin in hippocampal progenitor cells via PPARα. J. Alzheimer’s Dis. 2021, 84, 1539–1554. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.G.; Jeong, J.H.; Lee, K.M.; Jeong, K.H.; Yang, H.; Kim, M.; Jung, H.; Lee, S. Nanostructured lipid carrier-loaded hyaluronic acid microneedles for controlled dermal delivery of a lipophilic molecule. Int. J. Nanomed. 2013, 8, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.; Prabhuswaminath, S.C.; Reddy, P.; Srinivasa, S.M.; Shati, A.A.; Alfaifi, M.Y.; Eldin, I.; Elbehairi, S.; Achar, R.R.; Silina, E.; et al. Anticholinesterase activity of Areca Catechu: In vitro and in silico green synthesis approach in search for therapeutic agents against Alzheimer’s disease. Front. Pharmacol. 2022, 13, 1044248. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Yang, Y.J.; Qin, Z.; Liu, X.W.; Li, S.H.; Bai, L.X.; Li, J.Y. Protective activity of aspirin eugenol ester on paraquat-induced cell damage in SH-SY5Y cells. Oxidative Med. Cell. Longev. 2021, 2021, 6697872. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, S.B.; Valenzuela-Bezanilla, D.; Santibanez, S.H.; Varela-Nallar, L. Wnt signaling in the adult hippocampal neurogenic niche. Stem Cells 2022, 40, E198–E206. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.; Salinas, P.C. Wnt-Frizzled Signaling Regulates Activity-Mediated Synapse Formation. Front. Mol. Neurosci. 2021, 14, 683035. [Google Scholar] [CrossRef]
- Oliva, C.A.; Vargas, J.Y.; Inestrosa, N.C. Wnts in adult brain: From synaptic plasticity to cognitive deficiencies. Front. Cell. Neurosci. 2013, 7, 224. [Google Scholar] [CrossRef]
- Folke, J.; Pakkenberg, B.; Brudek, T. Impaired Wnt signaling in the prefrontal cortex of Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 956–965. [Google Scholar] [CrossRef]
- Lu, T.; Aron, L.; Zullo, J.; Pan, Y.; Kim, H.; Chen, Y.; Yang, T.H.; Kim, H.M.; Drake, D.; Liu, X.S.; et al. REST and stress resistance in aging and Alzheimer’s disease. Nature 2014, 507, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Li, A.; Liu, Z.; Ma, S.; Guo, T. Presynaptic membrane protein dysfunction occurs prior to neurodegeneration and predicts faster cognitive decline. Alzheimer’s Dement. 2023, 19, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Sandelius, A.; Portelius, E.; Källén, A.; Zetterberg, H.; Rot, U.; Olsson, B.; Toledo, J.B.; Shaw, L.M.; Lee, V.M.Y.; Irwin, D.J.; et al. Elevated CSF GAP-43 is Alzheimer’s disease-specific and associated with tau and amyloid pathology. Alzheimer’s Dement. 2019, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wray, J.; Hartmann, C. WNTing embryonic stem cells. Trends Cell Biol. 2012, 22, 159–168. [Google Scholar] [CrossRef]
- Nalci, O.B.; Nadaroglu, H.; Genc, S.; Hacimuftuoglu, A.; Alayli, A. The effects of MgS nanoparticles-Cisplatin-bio-conjugate on SH-SY5Y neuroblastoma cell line. Mol. Biol. Rep. 2020, 47, 9933–9940. [Google Scholar] [CrossRef]


| Primer | Forward Sequence (5′ to 3′) | Reverse Sequence (5′ to 3′) |
|---|---|---|
| Oxidative stress | ||
| LPO | CTGCCCTATGACAGCAAGAAGC | CGGTTATGCTCGCGGAGAAAGA |
| NOS | GCTCTACACCTCCAATGTGACC | CTGCCGAGATTTGAGCCTCATG |
| HO | TCACCTTCCCGAGCATCGAC | TCACCCTGTGCTTGACCTCG |
| NOX-2 | GGAGTTTCAAGATGCGTGGAAACTA | GCCAGACTCAGAGTTGGAGATGCT |
| GSH | CTGCCGAGATTTGAGCCTCATG | CGGAGTCGAGACAGGTACATGT |
| GPX | GTGCTCGGCTTCCCGTGCAAC | CTCGAAGAGCATGAAGTTGGGC |
| GSK-3β | GGAACTCCAACAAGGGAGCA | TTCGGGGTCGGAAGACCTT |
| CYP1A | GCTGACTTCATCCCTATTCTTCG | TTTTGTAGTGCTCCTTGACCATCT |
| Pro-inflammatory genes | ||
| IL1β | CCACAGACCTTCCAGGAGAATG | GTGCAGTTCAGTGATCGTACAGG |
| IL4 | CCGTAACAGACATCTTTGCTGCC | GAGTGTCCTTCTCATGGTGGCT |
| NF-Kb | GCGCTTCTCTGCCTTCCTTA | TCTTCAGGTTTGATGCCCCC |
| TNF-α | CTCTTCTGCCTGCTGCACTTTG | ATGGGCTACAGGCTTGTCACTC |
| p53 | CCTCAGCATCTTATCCGAGTGG | TGGATGGTGGTACAGTCAGAGC |
| PRb2 | CTCGTGCTGATGCTACTGAGGA | GGTCGGCGCAGTTGGGCTCC |
| Cdkn-2A | CCTTCCAATGACTCCCTCC | TCAGAAACCCTAGTTCAAAGGA |
| Nerve cell proliferation-related gene | ||
| GAP-43 | AGGGAGAAGGCACCACTACT | GGAGGACGGCGAGTTATCAG |
| Wnt-3a | TCTACGACGTGCACACCTG | CCTGCCTTCAGGTAGGAGTT |
| Wnt-5a | AGCAGACGTTTCGGCTACAG | TGCCCCCAGTTCATTCACAC |
| Wnt-7a | GCGTCTCGCACACTTGCAC | CCGCGCTTTCCGGTTCATAG |
| Camk-IIa | CATGGTTTGGGTTTGCAGGG | CCGGCTTTGATGCTGGTA |
| Camk-IIb | GAGGACGGAGCAGTGTCTAA | GACGCACGATGTTGGAATGC |
| TUBB3 | CCGAAGCCAGCAGTGTCTAA | AGGCCTGGAGCTGCAATAAG |
| FZD2 | TCCATCTGGTGGGTGATTCTG | CTCGTGGCCCCACTTCATT |
| FZD3 | GCCTATAGCGAGTGTTCAAAACTCA | TGGAAACCTACTGCACTCCATATCT |
| Housekeeping gene | ||
| β Actin | GATCTTGATCTTCATGGTGCTAGG | TTGTAACCAACTGGGACCATATGG |
| hMSCs | SH-SY5Y Cells | |||||
|---|---|---|---|---|---|---|
| Positive Control | LS | LS-SLNp | Positive Control | LS | LS-SLNp | |
| LPO | 0.9 ± 0.02 | 0.6 ± 0.01 * | 0.4 ± 0.02 *, # | 0.5 ± 0.01 | 0.3 ± 0.01 * | 0.06 ± 0.01 **, # |
| GR | 2.1 ± 0.01 | 3.2 ± 0.2 ** | 3.9 ± 0.01 * | 0.06 ± 0.03 | 1.3 ± 0.012 * | 2.05 ± 0.02 **, # |
| SOD | 1.5 ± 0.01 | 2.9 ± 0.15 * | 4.2 ± 0.03 **, # | 0.03 ± 0.01 | 0.9 ± 0.01 * | 1.5 ± 0.01 **, # |
| CAT | 1.7 ± 0.02 | 2.3 ± 0.09 * | 2.9 ± 0.01 *, # | 0.05 ± 0.01 | 1.3 ± 0.02 * | 2.6 ± 0.03 **, # |
| GPX | 1.6 ± 0.01 | 2.04 ± 0.11 * | 3.1 ± 0.012 **, # | 1.1 ± 0.02 | 2.3 ± 0.013 * | 2.9 ± 0.025 *, # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saran, N.; Subash-Babu, P.; Al-Harbi, L.N.; Alrfaei, B.M.; Alshatwi, A.A. Neuroprotective Effect of Solid Lipid Nanoparticles Loaded with Lepidium sativum (L.) Seed Bioactive Components Enhance Bioavailability and Wnt/β-Catenin/Camk-II Signaling Cascade in SH-SY5Y Neuroblastoma Cells. Nanomaterials 2024, 14, 199. https://doi.org/10.3390/nano14020199
Al-Saran N, Subash-Babu P, Al-Harbi LN, Alrfaei BM, Alshatwi AA. Neuroprotective Effect of Solid Lipid Nanoparticles Loaded with Lepidium sativum (L.) Seed Bioactive Components Enhance Bioavailability and Wnt/β-Catenin/Camk-II Signaling Cascade in SH-SY5Y Neuroblastoma Cells. Nanomaterials. 2024; 14(2):199. https://doi.org/10.3390/nano14020199
Chicago/Turabian StyleAl-Saran, Nada, Pandurangan Subash-Babu, Laila Naif Al-Harbi, Bahauddeen M. Alrfaei, and Ali A. Alshatwi. 2024. "Neuroprotective Effect of Solid Lipid Nanoparticles Loaded with Lepidium sativum (L.) Seed Bioactive Components Enhance Bioavailability and Wnt/β-Catenin/Camk-II Signaling Cascade in SH-SY5Y Neuroblastoma Cells" Nanomaterials 14, no. 2: 199. https://doi.org/10.3390/nano14020199
APA StyleAl-Saran, N., Subash-Babu, P., Al-Harbi, L. N., Alrfaei, B. M., & Alshatwi, A. A. (2024). Neuroprotective Effect of Solid Lipid Nanoparticles Loaded with Lepidium sativum (L.) Seed Bioactive Components Enhance Bioavailability and Wnt/β-Catenin/Camk-II Signaling Cascade in SH-SY5Y Neuroblastoma Cells. Nanomaterials, 14(2), 199. https://doi.org/10.3390/nano14020199