Layer-Dependent Sensing Performance of WS2-Based Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
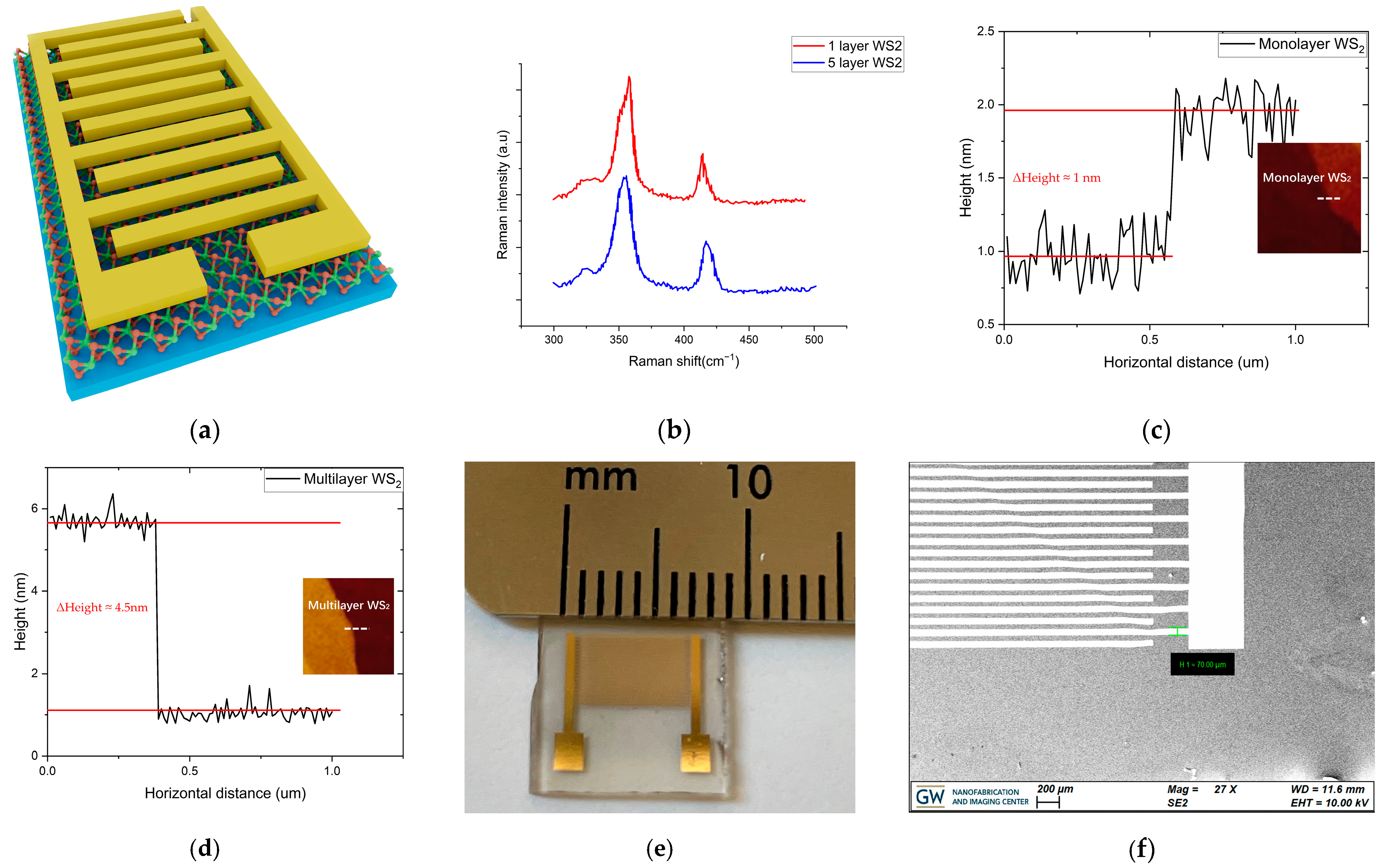
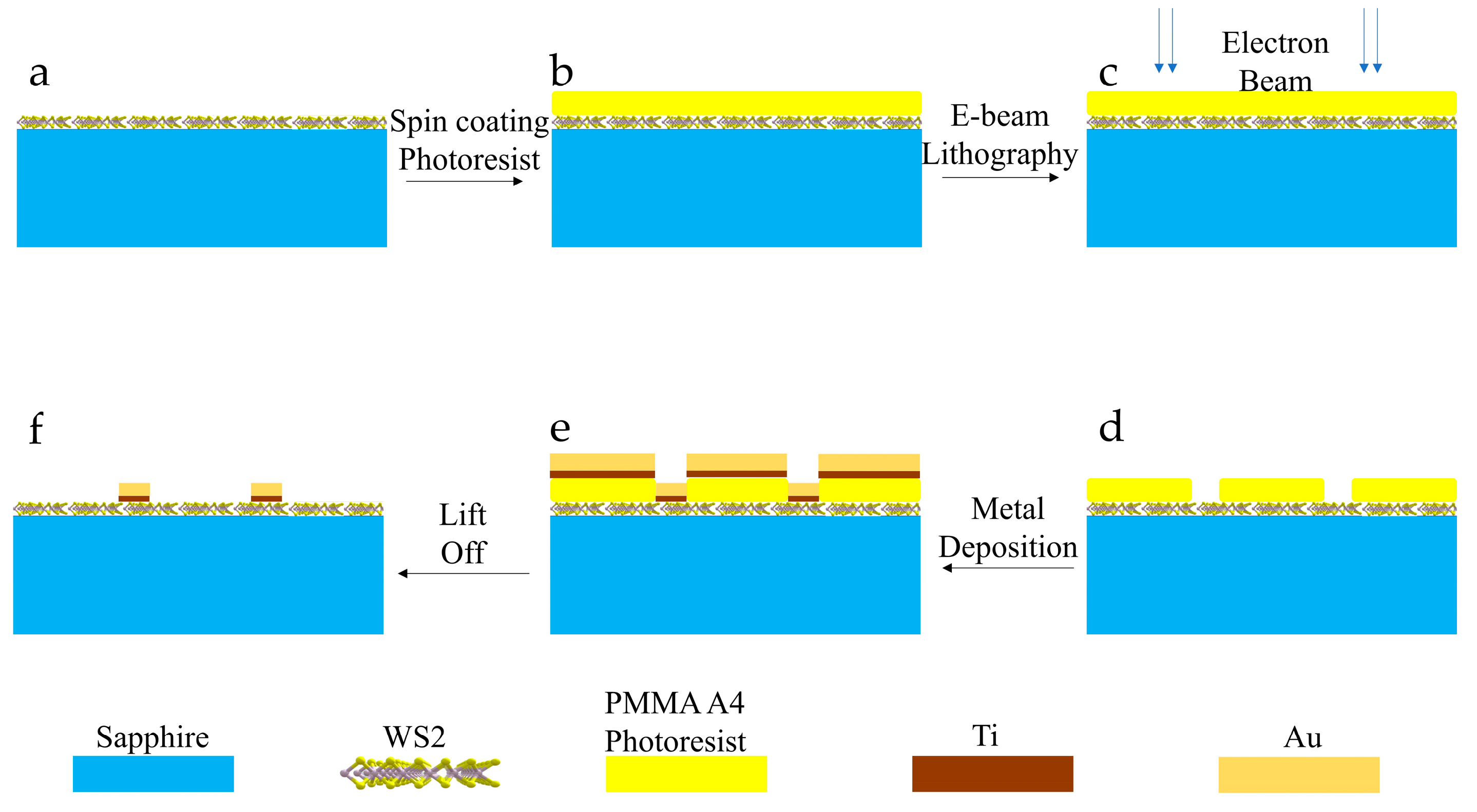
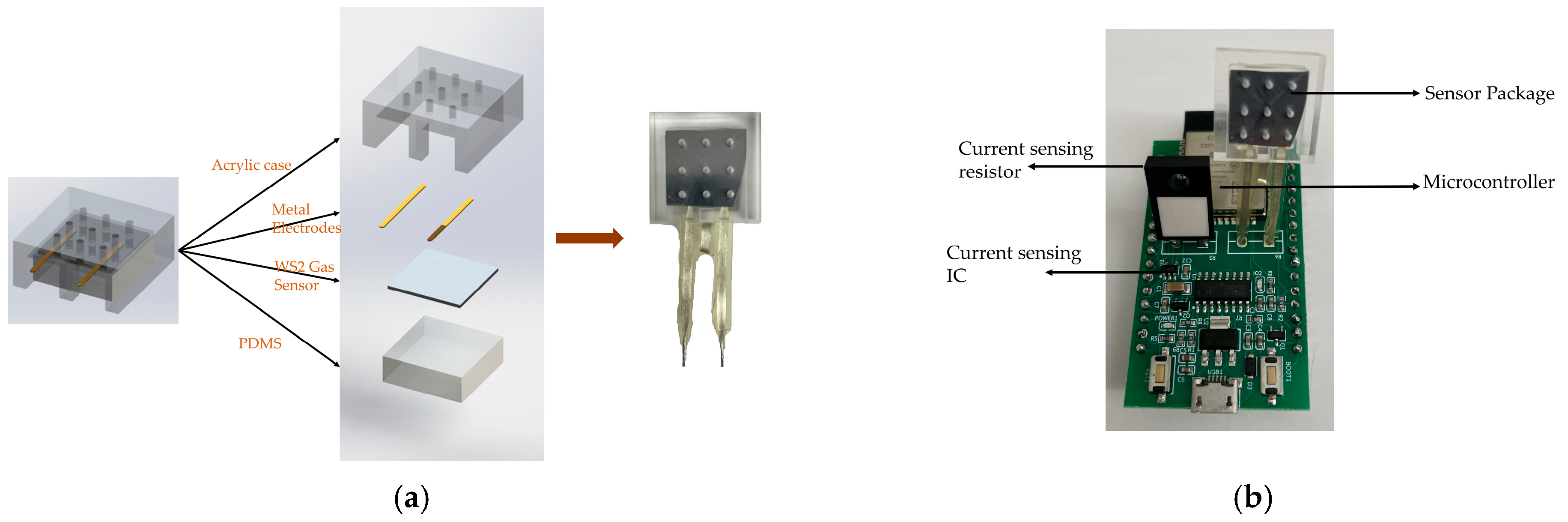
2.1. Sample Preparation and Characterization
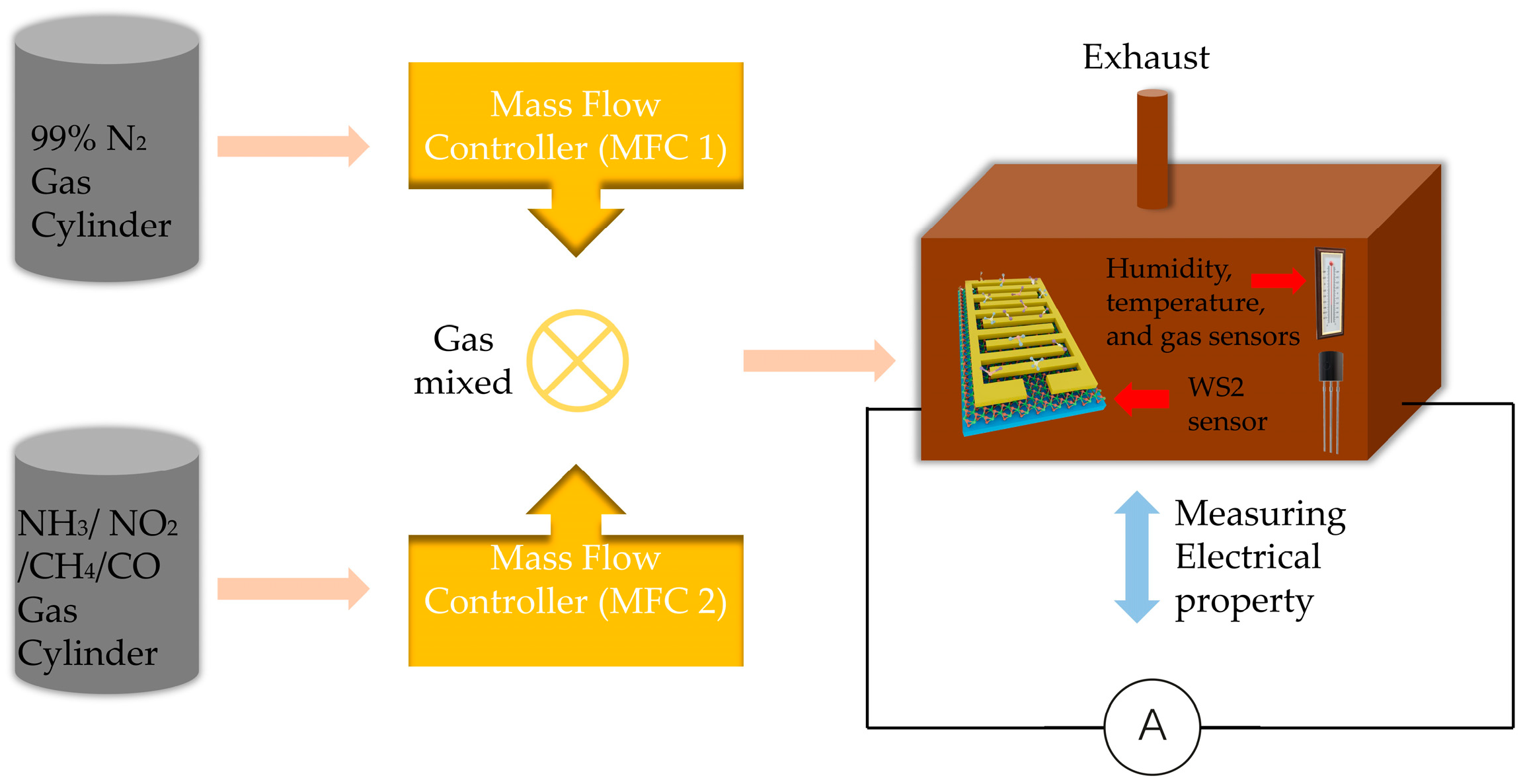
2.2. Experimental Setup
3. Results
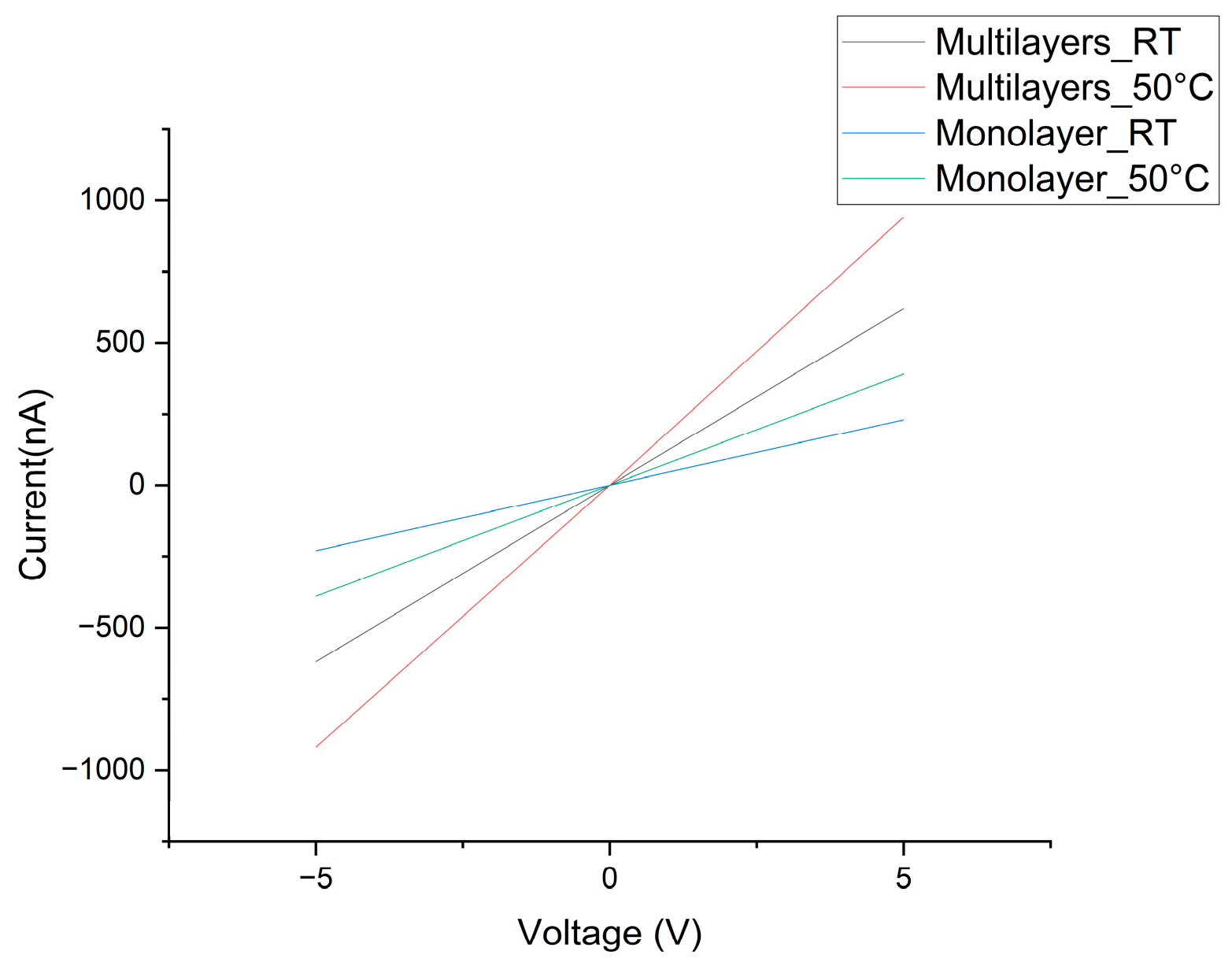
3.1. Current–Voltage Characterization
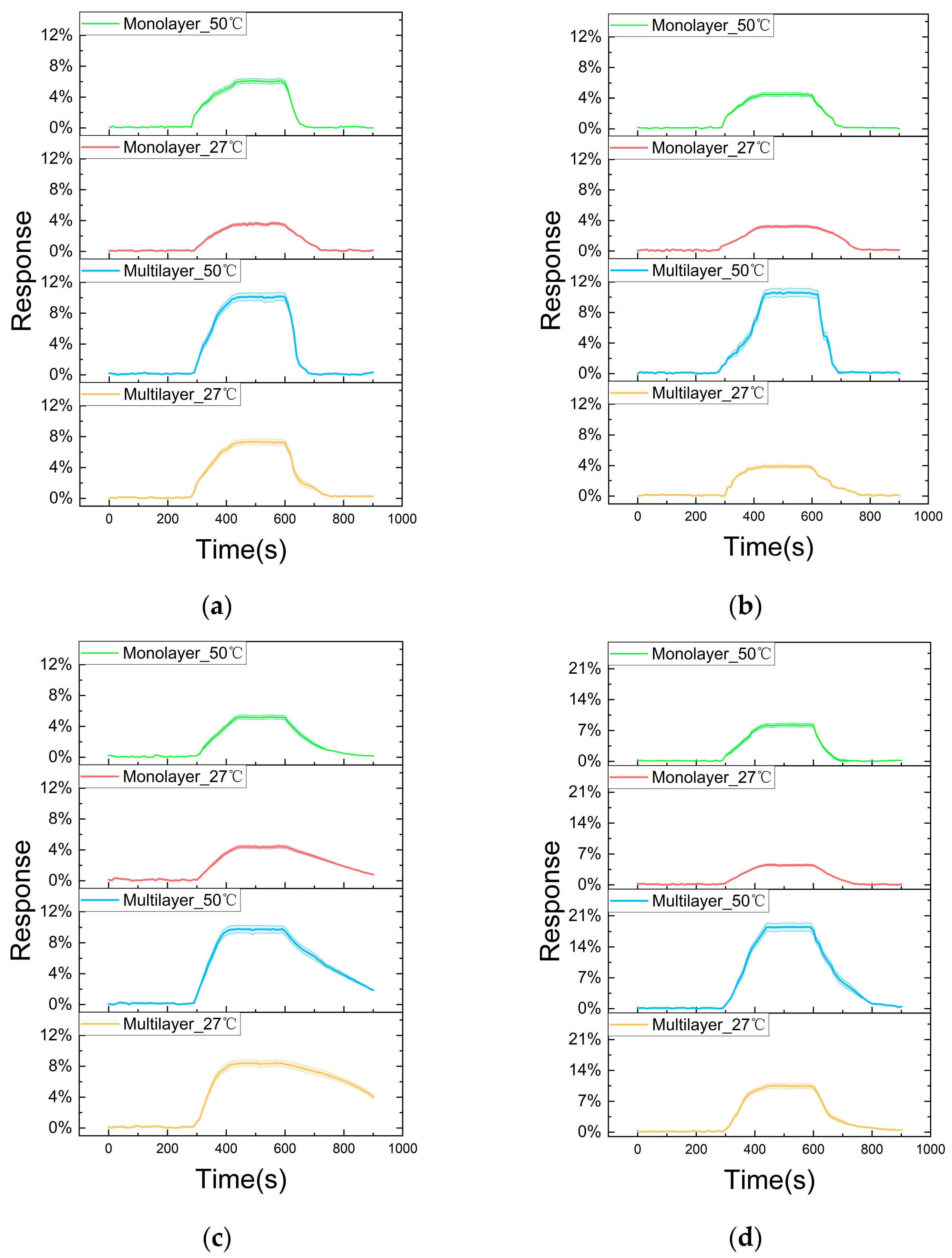
3.2. Sensing Performance Tests
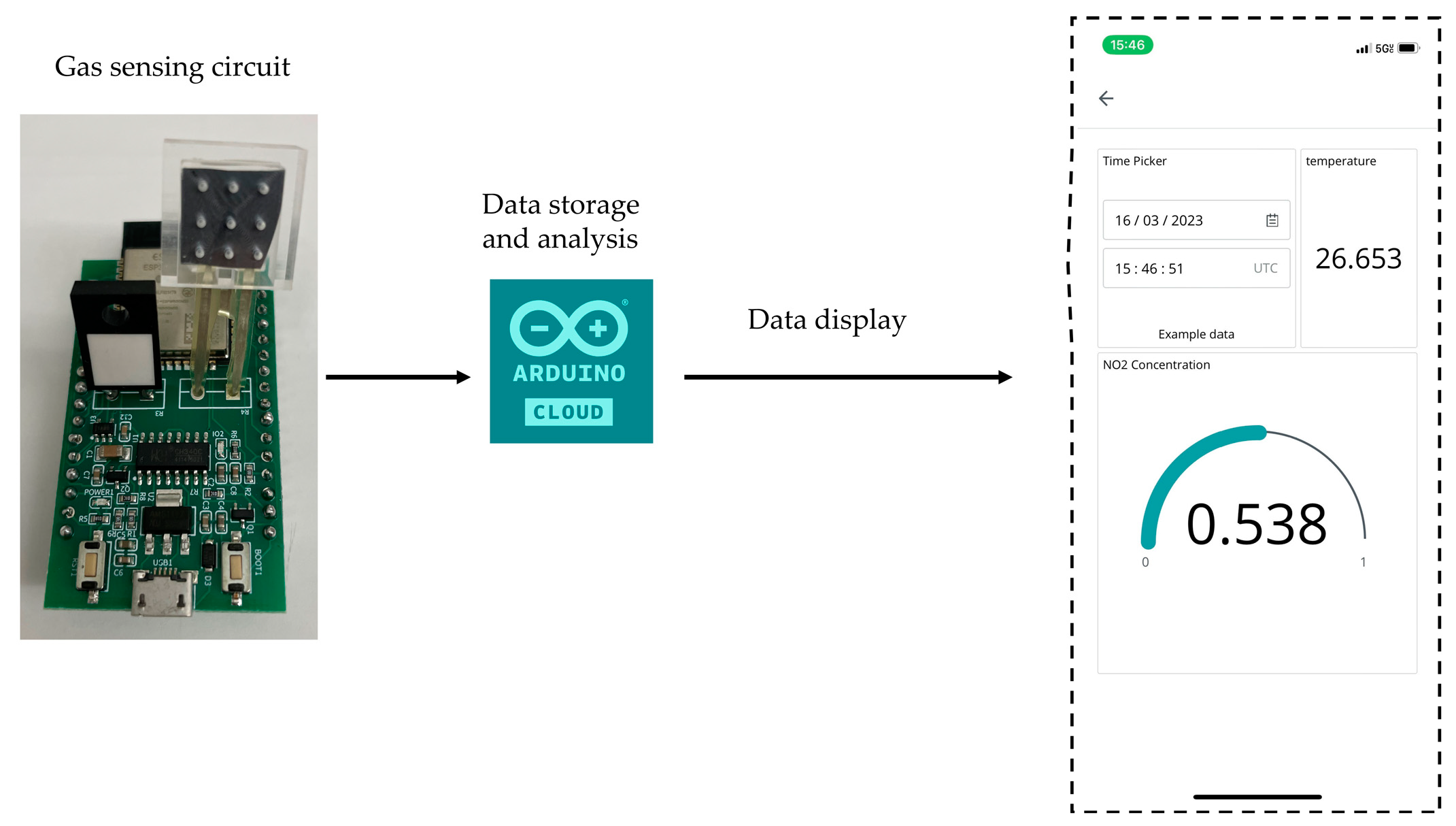
3.3. Gas Monitoring System
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of Environment Pollution on the Ocular Surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- Israt, A. Analysis of Air Pollution Levels Due to Methane (Ch4) Emissions at Final Disposal Site. J. Asian Multicult. Res. Med. Health Sci. Study 2022, 3, 10–15. [Google Scholar] [CrossRef]
- Hasnain, M.G.; Garcia-Esperon, C.; Tomari, Y.K.; Walker, R.; Saluja, T.; Rahman, M.M.; Boyle, A.; Levi, C.R.; Naidu, R.; Filippelli, G.; et al. Effect of Short-Term Exposure to Air Pollution on Daily Cardio- and Cerebrovascular Hospitalisations in Areas with a Low Level of Air Pollution. Env. Sci. Pollut. Res. 2023, 30, 102438–102445. [Google Scholar] [CrossRef]
- Duval, B.D.; Martin, J.; Marsalis, M.A. The Effect of Variable Fertilizer and Irrigation Treatments on Greenhouse Gas Fluxes from Aridland Sorghum. Agronomy 2022, 12, 3109. [Google Scholar] [CrossRef]
- Fadlilah, A.; Jayadi, M.; Nathan, M. Methane Gas Emissions (CH4) in Paddy Fields in Minasatene District, Pangkep Regency: Microbial Abundance against Increased Methane Gas. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012035. [Google Scholar] [CrossRef]
- Bayer-Oglesby, L.; Grize, L.; Gassner, M.; Takken-Sahli, K.; Sennhauser, F.H.; Neu, U.; Schindler, C.; Braun-Fahrländer, C. Decline of Ambient Air Pollution Levels and Improved Respiratory Health in Swiss Children. Environ. Health Perspect. 2005, 113, 1632–1637. [Google Scholar] [CrossRef]
- Ho, A.F.W.; Hu, Z.; Woo, T.Z.C.; Tan, K.B.K.; Lim, J.H.; Woo, M.; Liu, N.; Morgan, G.G.; Ong, M.E.H.; Aik, J. Ambient Air Quality and Emergency Hospital Admissions in Singapore: A Time-Series Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13336. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Fisher, S.; Suk, W.A.; Sly, P.; Chiles, T.C.; Bose-O’Reilly, S. Pollution and Children’s Health. Sci. Total Environ. 2019, 650, 2389–2394. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx Sensors Based on Semiconducting Metal Oxide Nanostructures: Progress and Perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, H.W. One-Dimensional Oxide Nanostructures as Gas-Sensing Materials: Review and Issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef]
- Qin, Z.; Zeng, D.; Zhang, J.; Wu, C.; Wen, Y.; Shan, B.; Xie, C. Effect of Layer Number on Recovery Rate of WS2 Nanosheets for Ammonia Detection at Room Temperature. Appl. Surf. Sci. 2017, 414, 244–250. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, G.; Liao, J.; Lv, S.; Zhu, Z.; Li, Z. Vertically Aligned MoS2/ZnO Nanowires Nanostructures with Highly Enhanced NO2 Sensing Activities. Appl. Surf. Sci. 2018, 456, 808–816. [Google Scholar] [CrossRef]
- Choi, G.J.; Mishra, R.K.; Gwag, J.S. 2D Layered MoS2 Based Gas Sensor for Indoor Pollutant Formaldehyde Gas Sensing Applications. Mater. Lett. 2020, 264, 127385. [Google Scholar] [CrossRef]
- Yoon, Y.; Ganapathi, K.; Salahuddin, S. How Good Can Monolayer MoS2 Transistors Be? Nano Lett. 2011, 11, 3768–3773. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Q.; Wang, X.; Zhang, Q.; Yu, H.-D.; Huang, X.; Huang, W. Recent Development of Gas Sensing Platforms Based on 2D Atomic Crystals. Research 2021, 2021, 9863038. [Google Scholar] [CrossRef]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics Based on Two-Dimensional Materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Dumcenco, D.; Ovchinnikov, D.; Marinov, K.; Lazić, P.; Gibertini, M.; Marzari, N.; Sanchez, O.L.; Kung, Y.-C.; Krasnozhon, D.; Chen, M.-W.; et al. Large-Area Epitaxial Monolayer MoS2. ACS Nano 2015, 9, 4611–4620. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Chen, L.; Ji, L.; Zhu, H.; Sun, Q. Gas Sensing Devices Based on Two-Dimensional Materials: A Review. Nanotechnology 2022, 33, 252001. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Naumis, G.G.; Barraza-Lopez, S.; Oliva-Leyva, M.; Terrones, H. Electronic and Optical Properties of Strained Graphene and Other Strained 2D Materials: A Review. Rep. Prog. Phys. 2017, 80, 096501. [Google Scholar] [CrossRef]
- Gusakova, J.; Wang, X.; Shiau, L.L.; Krivosheeva, A.; Shaposhnikov, V.; Borisenko, V.; Gusakov, V.; Tay, B.K. Electronic Properties of Bulk and Monolayer TMDs: Theoretical Study Within DFT Framework (GVJ-2e Method). Phys. Status Solidi (a) 2017, 214, 1700218. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.-Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh Sensitivity and Layer-Dependent Sensing Performance of Phosphorene-Based Gas Sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef]
- Varghese, S.S.; Varghese, S.H.; Swaminathan, S.; Singh, K.K.; Mittal, V. Two-Dimensional Materials for Sensing: Graphene and Beyond. Electronics 2015, 4, 651–687. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Gas Sensing with Heterostructures Based on Two-Dimensional Nanostructured Materials: A Review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Pham, P.V.; Bodepudi, S.C.; Shehzad, K.; Liu, Y.; Xu, Y.; Yu, B.; Duan, X. 2D Heterostructures for Ubiquitous Electronics and Optoelectronics: Principles, Opportunities, and Challenges. Chem. Rev. 2022, 122, 6514–6613. [Google Scholar] [CrossRef]
- Mathew, M.; Shinde, P.V.; Samal, R.; Rout, C.S. A Review on Mechanisms and Recent Developments in P-n Heterojunctions of 2D Materials for Gas Sensing Applications. J. Mater. Sci. 2021, 56, 9575–9604. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Mathew, M.; Sekhar Rout, C. Schottky Diodes Based on 2D Materials for Environmental Gas Monitoring: A Review on Emerging Trends, Recent Developments and Future Perspectives. J. Mater. Chem. C 2021, 9, 395–416. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition Metal Dichalcogenides-Based Flexible Gas Sensors. Sens. Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A Review on Chemiresistive Room Temperature Gas Sensors Based on Metal Oxide Nanostructures, Graphene and 2D Transition Metal Dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Alene Asres, G.; Baldoví, J.J.; Dombovari, A.; Järvinen, T.; Lorite, G.S.; Mohl, M.; Shchukarev, A.; Pérez Paz, A.; Xian, L.; Mikkola, J.-P.; et al. Ultrasensitive H2S Gas Sensors Based on P-Type WS2 Hybrid Materials. Nano Res. 2018, 11, 4215–4224. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Acharyya, D.; Dutta, K. Resistive and Capacitive Measurement of Nano-Structured Gas Sensors. In Environmental Nanotechnology: Volume 2; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2019; pp. 25–62. ISBN 978-3-319-98708-8. [Google Scholar]
- Bhattacharyya, P.; Acharyya, D. Impact of Device Configurations on Sensing Performance of WS2-Based Gas Sensors: A Review. IEEE Sens. J. 2021, 21, 22414–22425. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, G.-B.; Dai, J.; Yan, Y.; Zhu, B.; He, R.; Xie, L.; Xu, S.; Chen, X.; Yao, W.; et al. Optical Signature of Symmetry Variations and Spin-Valley Coupling in Atomically Thin Tungsten Dichalcogenides. Sci. Rep. 2013, 3, 1608. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ji, Q.; Ju, J.; Yuan, H.; Shi, J.; Gao, T.; Ma, D.; Liu, M.; Chen, Y.; et al. Controlled Growth of High-Quality Monolayer WS2 Layers on Sapphire and Imaging Its Grain Boundary. ACS Nano 2013, 7, 8963–8971. [Google Scholar] [CrossRef]
- PS1-NO2-50 SGX Sensortech Gas Sensor—Solid Polymer Sensor|SGX. Available online: https://sgx.cdistore.com/products/detail/ps1no250-sgx-sensortech/741050/ (accessed on 15 January 2024).
- FECS42-20: Gas Sensors & Modules—Products—Figaro Engineering Inc. Available online: https://www.figarosensor.com/product/entry/fecs42-20.html (accessed on 15 January 2024).
- AGSM-NO2-5. Available online: https://www.k-allsensing.com/products/agsm-no2-5 (accessed on 15 January 2024).


| Gas Sensor | Measurement Range | Limit of Detection | Response Time (T90) |
|---|---|---|---|
| SGX Solid Polymer Electrolyte Gas Sensor (PS1-NO2-50) | 0~50 ppm | 1 ppm | 30 s |
| FIGARO Solid Electrolyte Electrochemical Gas Sensor (FECS42-20) | 0~20 ppm | Approximately 0.5 ppm | 25 s |
| Allsensing Gas Sensor (AGSM-NO2-5) | 0~5 ppm | 0.1 ppm | 150 s |
| Multilayer WS2 Gas Sensor | 0~10 ppm | 0.2 ppm | 95 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, S.; Xin, S.; Sayin, S.; Yi, Z.; Li, Z.; Zaghloul, M. Layer-Dependent Sensing Performance of WS2-Based Gas Sensors. Nanomaterials 2024, 14, 235. https://doi.org/10.3390/nano14020235
Zhou Y, Wang S, Xin S, Sayin S, Yi Z, Li Z, Zaghloul M. Layer-Dependent Sensing Performance of WS2-Based Gas Sensors. Nanomaterials. 2024; 14(2):235. https://doi.org/10.3390/nano14020235
Chicago/Turabian StyleZhou, You, Sheng Wang, Sichen Xin, Sezin Sayin, Zhiqiang Yi, Zhenyu Li, and Mona Zaghloul. 2024. "Layer-Dependent Sensing Performance of WS2-Based Gas Sensors" Nanomaterials 14, no. 2: 235. https://doi.org/10.3390/nano14020235
APA StyleZhou, Y., Wang, S., Xin, S., Sayin, S., Yi, Z., Li, Z., & Zaghloul, M. (2024). Layer-Dependent Sensing Performance of WS2-Based Gas Sensors. Nanomaterials, 14(2), 235. https://doi.org/10.3390/nano14020235






