Effects of ZrO2 Nano-Particles’ Incorporation into SnAgCu Solder Alloys: An Experimental and Theoretical Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
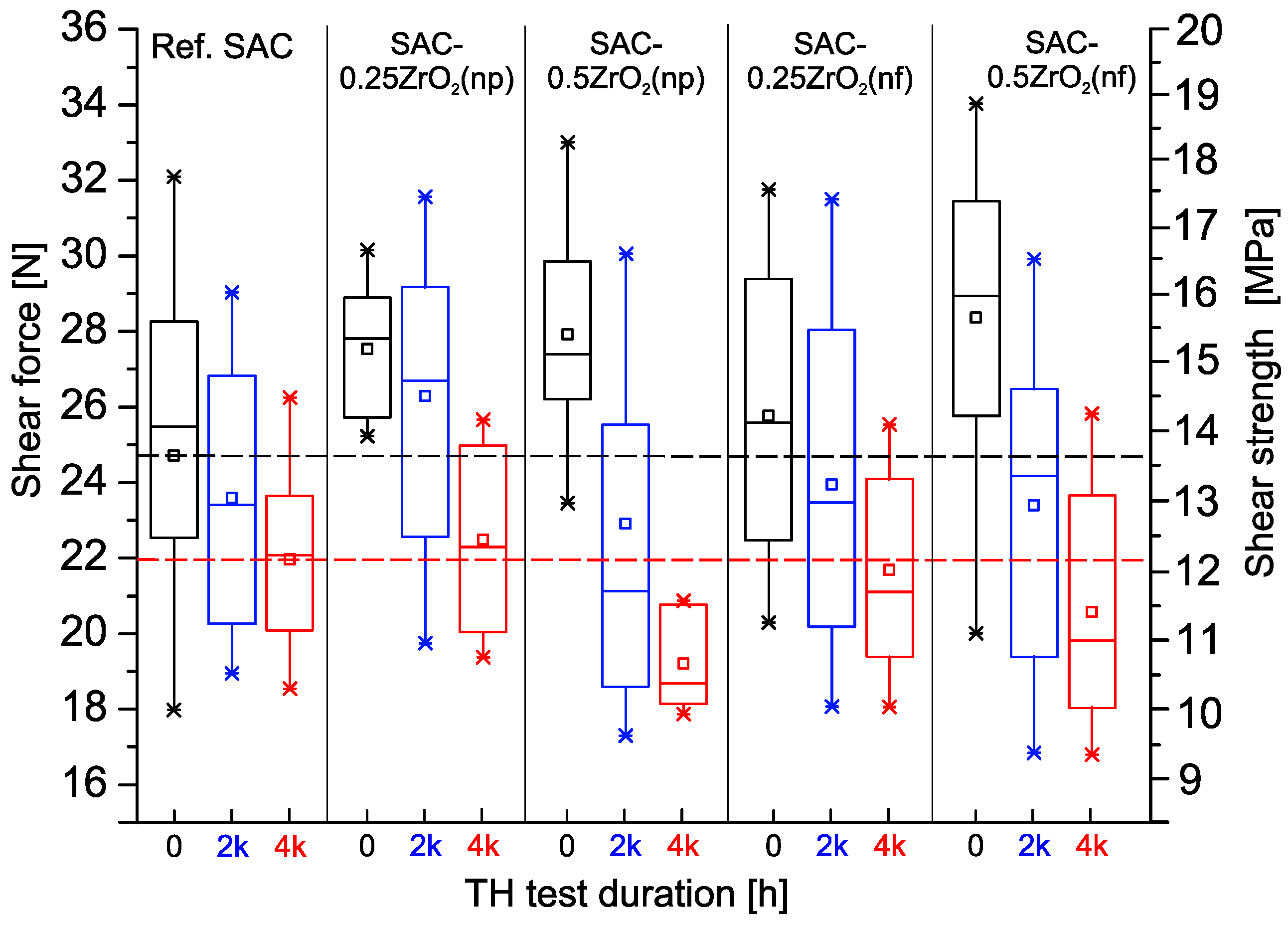
3.1. Shear Forces of the Solder Joints
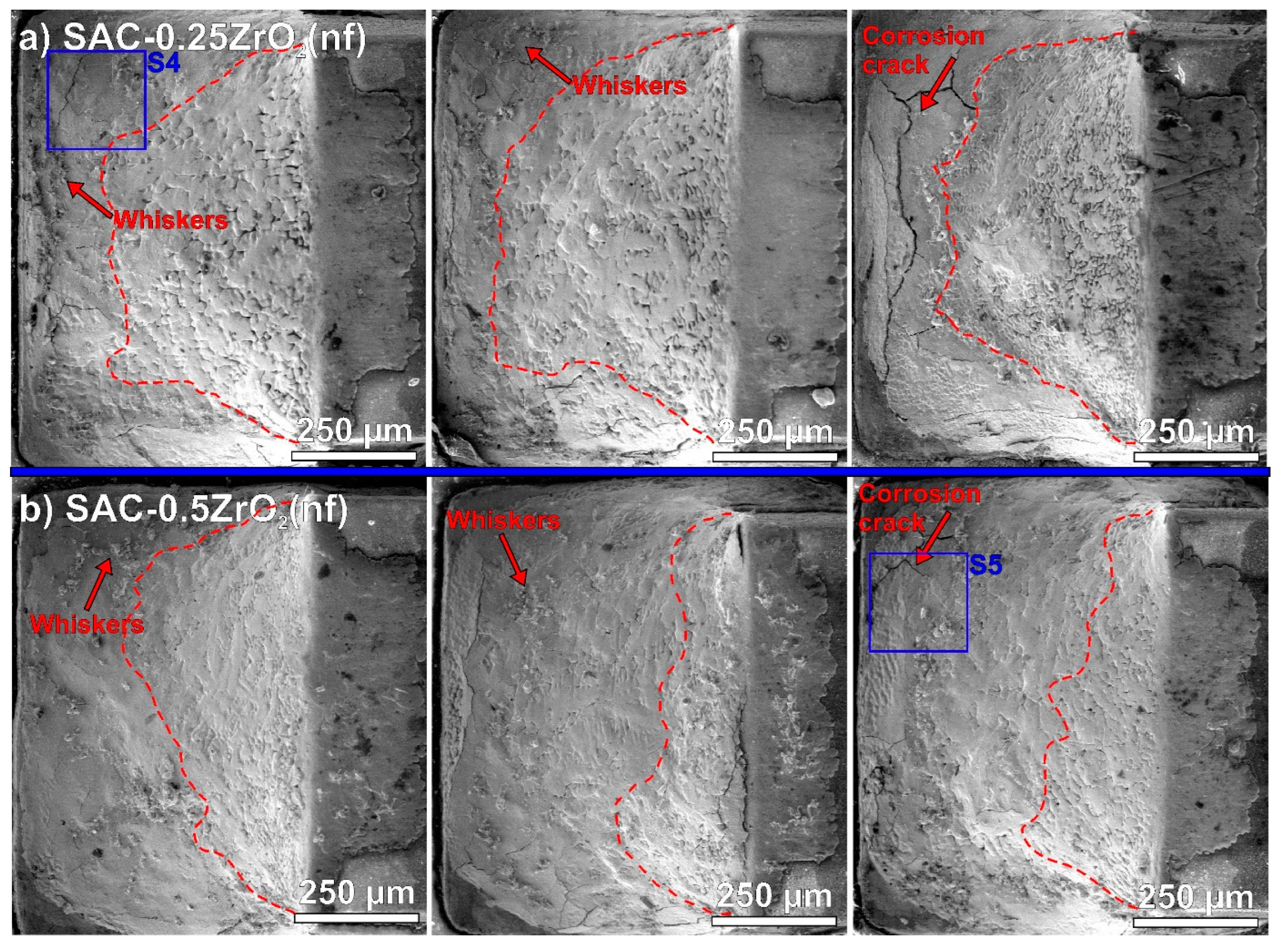
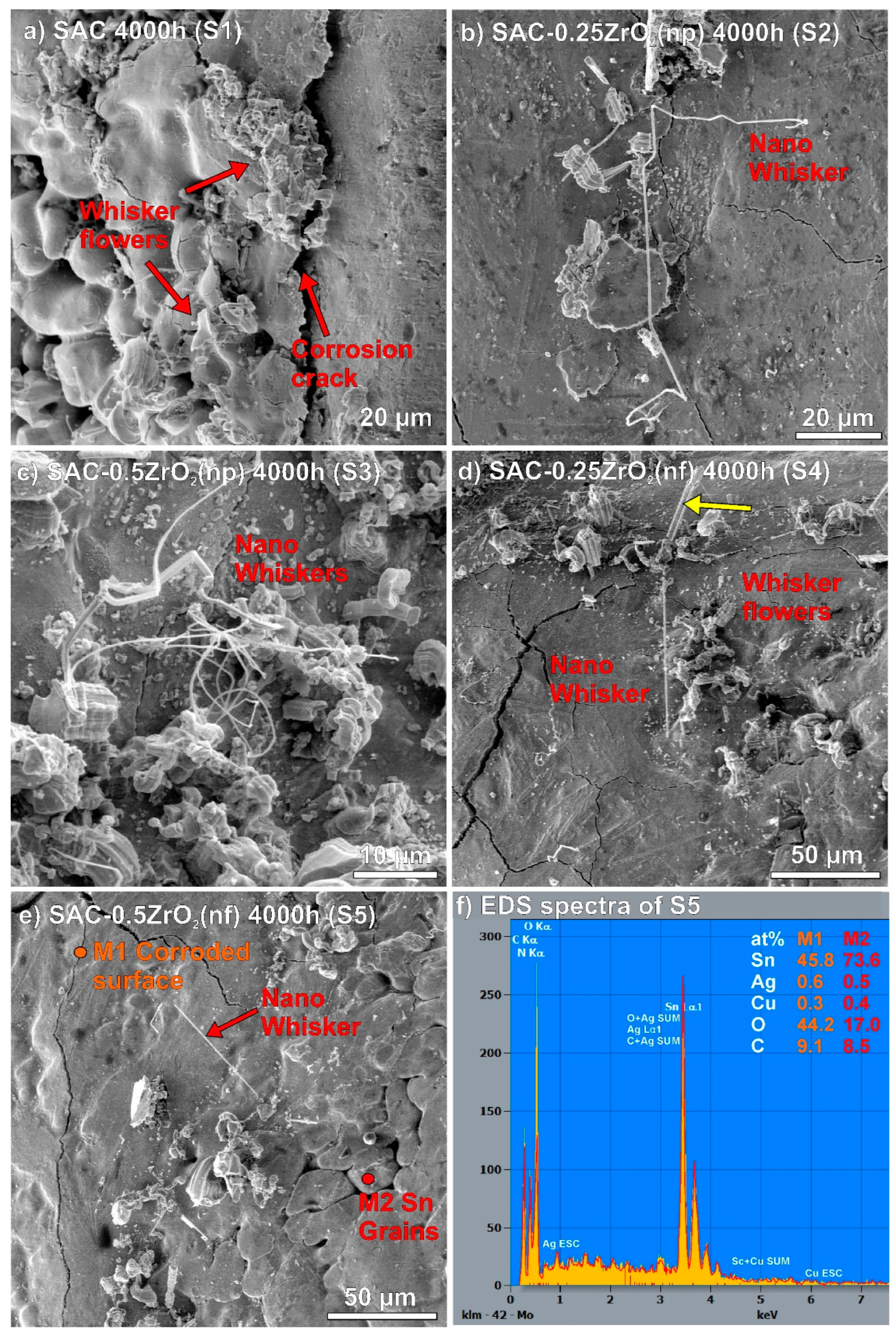
3.2. Surface Defects on the Solder Joints
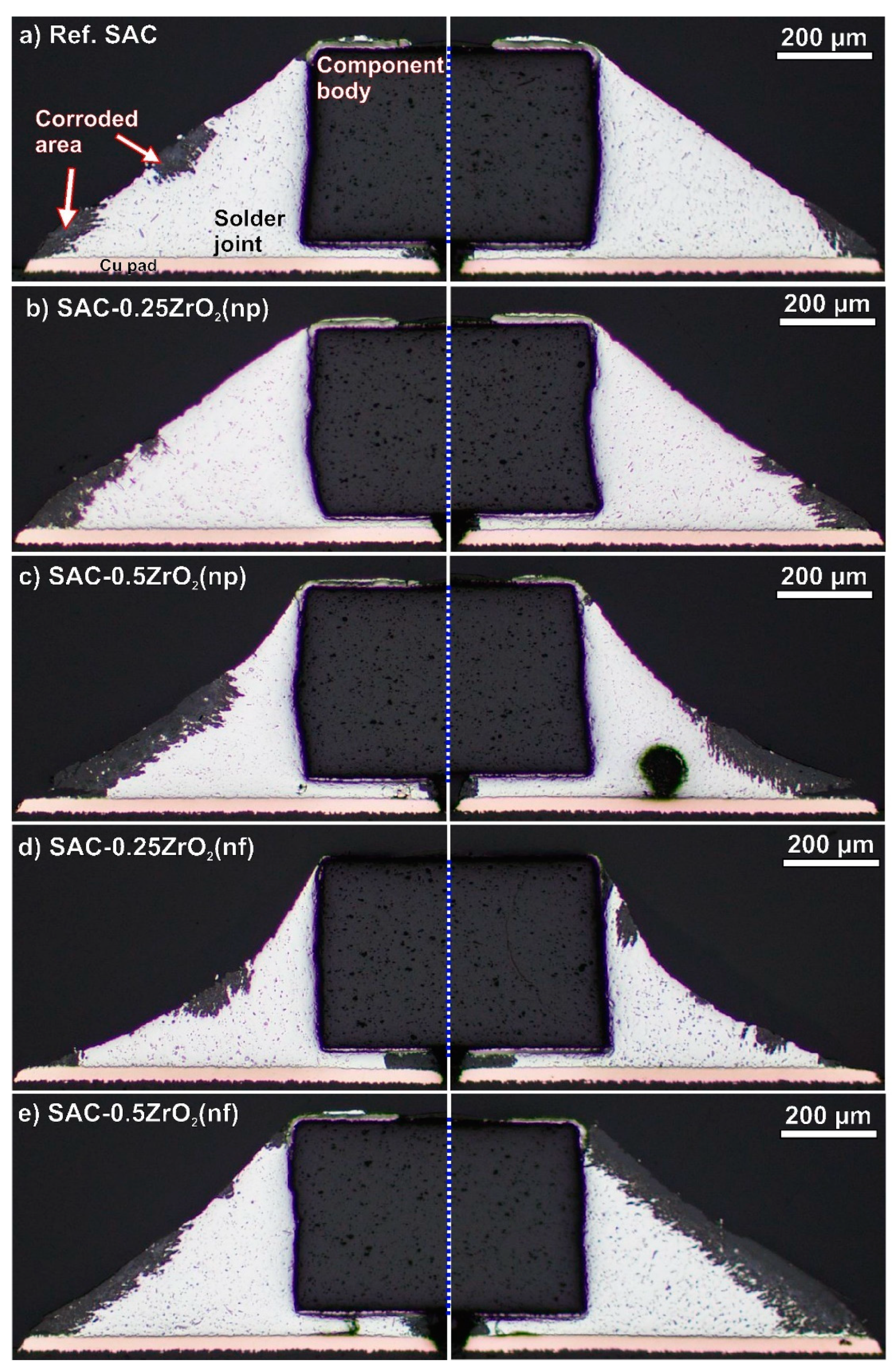
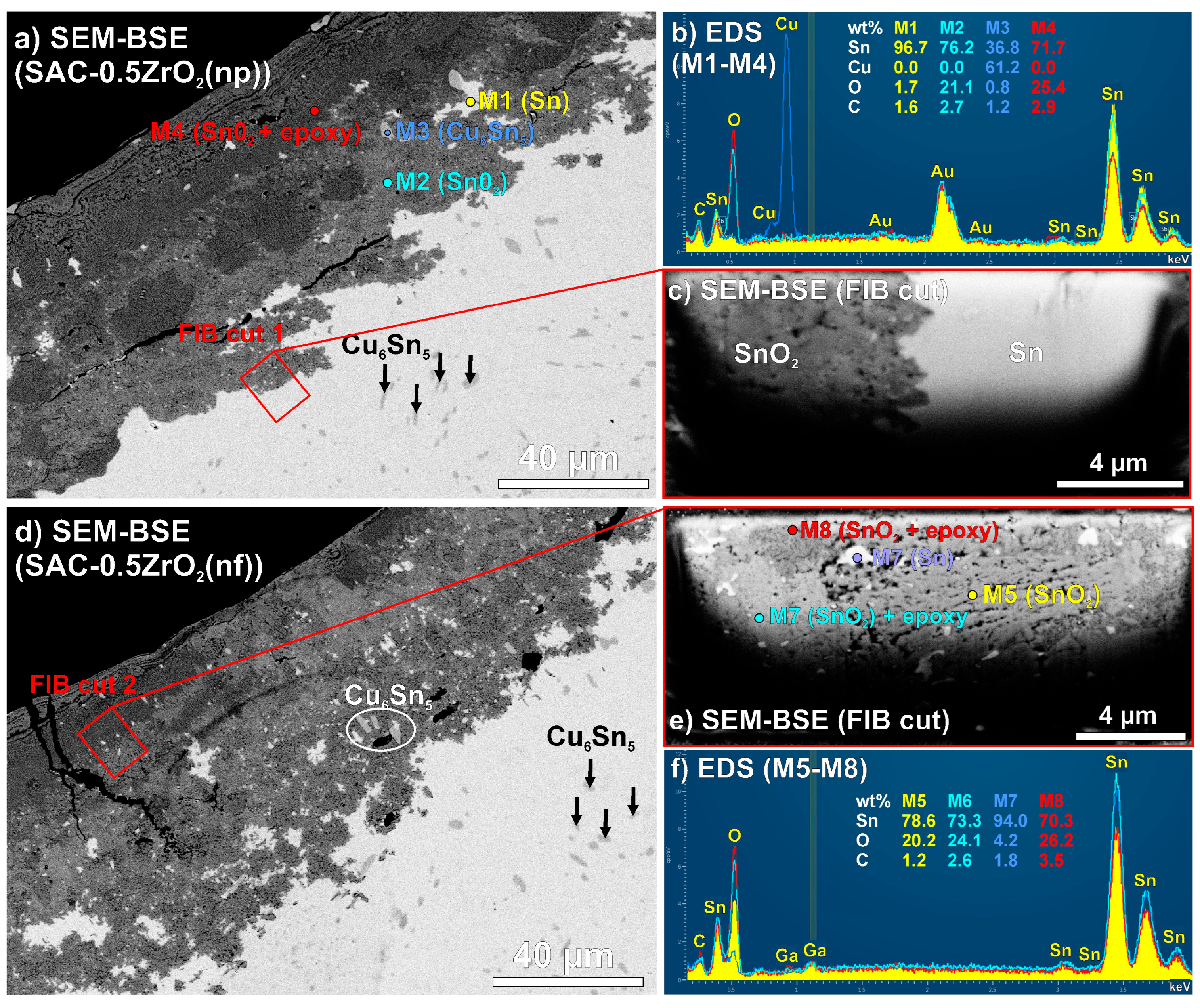
3.3. Corrosion Depth in the Solder Joints
4. Discussion
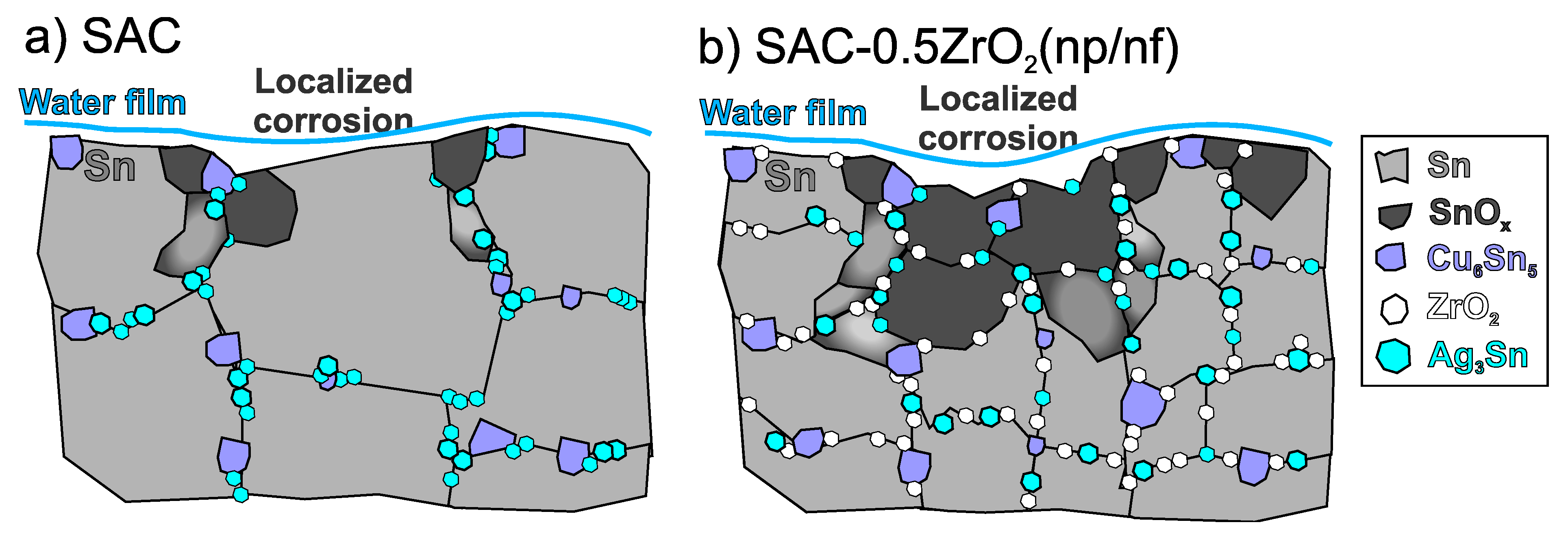
4.1. Microstructural Differences of the Composite Solder Joints
4.2. DFT Calculations of the Sn Cluster on the ZrO2
4.3. Corrosion Process of the Composite Solder Joints
5. Conclusions
- The application of ZrO2 NPs increased the initial shear force of the composite solder joints by 4–13.2%. During the 4000 h 85 °C/85% RH test, the shear force of the composite joints with 0.5 wt% ZrO2 NPs decreased by 29–35%, while the rest of the solder joints (reference and composite) lost only 12–12.5%.
- The composite solder joints with 0.5 wt% ZrO2 corroded twice more seriously than the rest of the samples. The intensive corrosion initiated the growth of numerous Sn whiskers. Interestingly, the correlation between the intensity of whiskering and the size of the corroded areas was weaker than initially expected. The ZrO2 composite solder joints produced a bit longer but not more whiskers than the reference SAC0307 joints.
- DFT simulations showed that Sn can bond to the ZrO2, but only with weak binding energy, which does not result in a stable system. ZrO2 nano-particles refined the microstructure of the solder joints, the β-Sn grain size was reduced, and the intermetallic compounds were more dispersed. This resulted in improved mechanical properties by dispersion strengthening but may have also reduced the corrosion resistance of the ZrO2 composite solder joints.
- The shape of the nano-particles did not have a major effect on the composite solder joints. While ZrO2 nano-particles improved the solder joint mechanical properties, their use is recommended only in non-corrosive environments, such as microelectronics for space applications. In corrosive climates, the weight fraction of ZrO2 NPs should not exceed 0.25 wt% to avoid reliability problems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhavan, J.S.; Pazhani, A.; Robi, P.S.; Ambi, A.; Tg, U.; Kc, V. EBSD characterization of graphene nano sheet reinforced Sn–Ag solder alloy composites. J. Mater. Res. Technol. 2024, 30, 2768–2780. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y. Main application limitations of lead-free composite solder doped with foreign reinforcements. J. Mater. Sci. Mater. Electron. 2021, 32, 24644–24660. [Google Scholar] [CrossRef]
- Sarkar, M.; Gulshan, F.; Rashid, A.R.M.H.; Hasanuzzaman, M. A Review of TiO2-nanoparticle Reinforced Lead-Free Solder Composites Used in Electronic Components Soldering. Encycl. Mater. Electron. 2023, 3, 456–463. [Google Scholar]
- El-Daly, A.A.; Fawzy, A.; Mansour, S.F.; Younis, M.J. Thermal analysis and mechanical properties of Sn–1.0 Ag–0.5 Cu solder alloy after modification with SiC nano-sized particles. J. Mater. Sci. Mater. Electron. 2013, 24, 2976–2988. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Microstructure, mechanical and electrical performances of zirconia nanoparticles-doped tin-silver-copper solder alloys. J. Mater. Sci. Mater. Electron. 2016, 27, 7524–7533. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, K.; Wang, B.; Hou, R.; Wang, H.; Gao, Y. Study on the properties of low silver Sn1.0Ag0.5Cu composite solder reinforced with nickel-plated zinc oxide and its soldering joint. Mater. Today Commun. 2023, 37, 107040. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.; Pan, Y. Effects of TiO2 nanoparticles addition on microstructure, microhardness and tensile properties of Sn–3.0Ag–0.5Cu–xTiO2 composite solder. Mater. Des. 2014, 55, 574–582. [Google Scholar] [CrossRef]
- Tamizi, M.; Movahedi, M.; Kokabi, A.H.; Miyashita, Y. Cobalt-Graphene NanoSheets enhanced Sn–0.3Ag–0.7Cu composite solder: Study on microstructure, crystal orientation relations and mechanical properties. Mater. Sci. Eng. A 2024, 894, 146199. [Google Scholar] [CrossRef]
- Wu, C.J.; Zhang, L.; Chen, C.; Lu, X. Thermal, microstructural, wettability and mechanical properties of Sn1.0Ag0.5Cu composite solder modified with Ti nanoparticles. Mater. Char. 2024, 212, 113927. [Google Scholar] [CrossRef]
- Tian, R.; Gao, Y.; Wen, J.; Lin, P.; Xu, S.; Tian, Y. Improved shear property of Sn-3.0Ag-0.5Cu/Ni micro solder joints under thermal shock between 77 K and 423 K by adding TiO2 nanoparticles. J. Mater. Res. Technol. 2024, 29, 5034–5047. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.; Wang, J.; Xue, P.; Zhong, S.; Long, W. Effect of Nanoparticles Addition on the Microstructure and Properties of Lead-Free Solders: A Review. App. Sci. 2019, 9, 2044. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Guo, Y.H.; Wang, X.; Li, M.L.; Gao, L.L.; Zhao, M. Microstructure evolution and thermal, wetting, mechanical properties of SiC nanowires reinforced SAC105 composite solder. Intermetallics 2023, 154, 107816. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.C.; Han, Y.J.; Tian, Y.M.; Gao, H.X. Strengthening Effects of ZrO2 Nanoparticles on the Microstructure and Microhardness of Sn-3.5Ag Lead-Free Solder. J. Electron. Mater. 2006, 35, 1672–1679. [Google Scholar] [CrossRef]
- Shen, J.; Chan, Y.C. Effects of ZrO2 nanoparticles on the mechanical properties of Sn–Zn solder joints on Au/Ni/Cu pads. J. Alloys Compd. 2009, 477, 552–559. [Google Scholar] [CrossRef]
- Gain, A.K.; Fouzder, T.; Chan, Y.C.; Yung, W.K.C. Microstructure, kinetic analysis and hardness of Sn–Ag–Cu–1 wt% nano-ZrO2 composite solder on OSP-Cu pads. J. Alloys Compd. 2011, 509, 3319–3325. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Yung, W.K.C. Effect of additions of ZrO2 nano-particles on the microstructure and shear strength of Sn–Ag–Cu solder on Au/Ni metallized Cu pads. Microelectron. Reliab. 2011, 51, 2306–2313. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C. Growth mechanism of intermetallic compounds and damping properties of Sn–Ag–Cu-1 wt% nano-ZrO2 composite solders. Microelectron. Reliab. 2014, 54, 945–955. [Google Scholar] [CrossRef]
- Hu, X.; Chan, Y.C. Reinforced solder joint performance by incorporation of ZrO2 nanoparticles in electroless Ni–P composite layer. J. Mater. Res. 2014, 29, 2657–2666. [Google Scholar] [CrossRef]
- Sharma, A.; Xu, D.E.; Chow, J.; Mayer, M.; Sohn, H.R.; Jung, J.P. Electromigration of Composite Sn-Ag-Cu Solder Bumps. Electron. Mater. Lett. 2015, 11, 1072–1077. [Google Scholar] [CrossRef]
- Yakymovych, A.; Plevachuk, Y.; Švec, P., Sr.; Švec, P.; Janičkovič, D.; Šebo, P.; Beronská, N.; Roshanghias, A. Morphology and Shear Strength of Lead-Free Solder Joints with Sn3.0Ag0.5Cu Solder Paste Reinforced with Ceramic Nanoparticles. J. Electron. Mater. 2016, 45, 6143–6149. [Google Scholar] [CrossRef]
- Rajendran, S.H.; Hwang, S.J.; Jung, J.P. Shear Strength and Aging Characteristics of Sn-3.0Ag-0.5Cu/Cu Solder Joint Reinforced with ZrO2 Nanoparticles. Metals 2010, 10, 1295. [Google Scholar] [CrossRef]
- Wodak, I.; Yakymovych, A.P.; Orovcik, L.; Khatibil, G. Hybrid solder joints: The effect of nanosized ZrO2 particles on morphology of as-reflowed and thermally aged Sn–3.5Ag solder joints. App. Nanosci. 2023, 13, 7379–7385. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.; Jin, Z.; Han, D.L.; Li, J.; Zhang, K.; Nishikawa, H. Novel interface regulation of Sn1.0Ag0.5Cu composite solders reinforced with modified ZrO2: Microstructure and mechanical properties. J. Mater. Sci. Technol. 2022, 125, 157–170. [Google Scholar] [CrossRef]
- Mousa, M.M.; Mohammed, M.M.; El-Kady, O.A.; Mohamed, H.S. Microstructure, hardness, electrical, and thermal conductivity of SZCN solder reinforced with TiO2 and ZrO2 nanoparticles fabricated by powder metallurgy method. J. Mater. Sci. Mater. Electron. 2024, 35, 1133. [Google Scholar] [CrossRef]
- Mohamed, H.S.; Mostafa, M.M.; Mahmoud, N.M.; Mousa, M.M. Evaluation of the microstructure, thermal, and mechanical characteristics of Sn-9Zn reinforced with Ni and ZrO2 nanoparticles via vacuum melting process. Phys. Scr. 2023, 98, 105912. [Google Scholar] [CrossRef]
- Nitta, S.; Tatsumi, H.; Nishikawa, H. Strength-enhanced Sn–In low-temperature alloy with surface-modified ZrO2 nanoparticle addition. J. Mater. Sci. Mater. Electron. 2023, 34, 2066. [Google Scholar] [CrossRef]
- Singh, A.; Choo, H.L.; Tan, W.H.; Durkairaj, R. Effect of Mo and ZrO2 nanoparticles addition on interfacial properties an shear strength of Sn58Bi/Cu solder joint. Trans. Nonferrous Met. Soc. China 2024, 34, 2619–2628. [Google Scholar] [CrossRef]
- Chuang, T.H.; Wu, M.W.; Chang, S.Y.; Ping, S.F.; Tsao, L.C. Strengthening mechanism of nano-Al2O3 particles reinforced Sn3.5Ag0.5Cu lead-free solder. J. Mater. Sci. Mater. Electron. 2011, 22, 1021–1027. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Wang, H. Effects of Ni modified MWCNTs on the microstructural evolution and shear strength of Sn-3.0Ag-0.5Cu composite solder joints. Mater. Char. 2020, 163, 110287. [Google Scholar]
- Illés, B.; Choi, H.; Hurtony, T.; Skwarek, A.; Dusek, K.; Busek, D. Suppression of Sn whisker growth from SnAgCu solder alloy with TiO2 and ZnO reinforcement nano-particles by increasing the corrosion resistance of the composite alloy. J. Mater. Res. Technol. 2022, 20, 4231–4240. [Google Scholar] [CrossRef]
- Choi, H.; Illés, B.; Hurtony, T.; Byun, J.; Géczy, A.; Skwarek, A. Corrosion problems of SAC-SiC composite solder alloys. Corr. Sci. 2023, 224, 111488. [Google Scholar] [CrossRef]
- JESD-201; Environmental Acceptance Requirements for Tin Whisker Susceptibility of Tin and Tin Alloy Surface Finishes. JEDEC Solid State Technology Association: Arlington County, VA, USA, 2006.
- Illés, B.; Hurtony, T.; Medgyes, B. Effect of current load on corrosion induced tin whisker growth from SnAgCu solder alloys. Corr. Sci. 2015, 99, 313–319. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Xie, X.; Gu, Y.; Liu, Y. Effects of nano-SiO2 particles addition on the microstructure, wettability, joint shear force and the interfacial IMC growth of Sn3.0Ag0.5Cu solder. J. Mater. Sci. Mater. Electron. 2015, 26, 9387–9395. [Google Scholar] [CrossRef]
- Henning, C.; Schumann, R.; Bauer, R. Influences of SMD sample preparation and examination conditions on shear test results. In Proceedings of the 40th International Spring Seminar on Electronics Technology (ISSE), Sofia, Bulgaria, 10–14 May 2017. [Google Scholar] [CrossRef]
- Straubinger, D.; Géczy, A.; Sipos, A.; Kiss, A.; Gyarmati, D.; Krammer, O.; Rigler, D.; Bušek, D.; Harsányi, G. Advances on high current load effects on lead-free solder joints of SMD chip-size components and BGAs. Circuit World 2019, 45, 37–44. [Google Scholar] [CrossRef]
- Lee, B.Z.; Lee, D.N. Spontaneous growth mechanism of tin whiskers. Acta Mater. 1998, 46, 3701–3714. [Google Scholar] [CrossRef]
- Krammer, O.; Illés, B.; Bátorfi, R.; Dušek, K. Automatic characterisation method for statistical evaluation of tin whisker growth. Microelectron. Reliab. 2017, 73, 14–21. [Google Scholar] [CrossRef]
- Hu, X.; Xu, H.; Chen, W.; Jiang, X. Effects of ultrasonic treatment on mechanical properties and microstructure evolution of the Cu/SAC305 solder joints. J. Manuf. Proc. 2021, 64, 648–654. [Google Scholar] [CrossRef]
- Bi, X.; Hu, X.; Li, O. Effect of Co addition into Ni film on shear strength of solder/Ni/Cu system: Experimental and theoretical investigations. Mater. Sci. Eng. A 2020, 788, 139589. [Google Scholar] [CrossRef]
- Chang, S.Y.; Tsao, L.C.; Wu, M.W.; Chen, C.W. The morphology and kinetic evolution of intermetallic compounds at Sn–Ag–Cu solder/Cu and Sn–Ag–Cu-0.5Al2O3 composite solder/Cu interface during soldering reaction. J. Mater. Sci. Mater. Electron. 2012, 23, 100–107. [Google Scholar] [CrossRef]
- Yi, P.; Dong, C.; Xiao, K.; Li, X. Study on corrosion behavior of β-Sn and intermetallic compounds phases in SAC305 alloy by in-situ EC-AFM and first-principles calculation. Corr. Sci. 2021, 181, 109244. [Google Scholar] [CrossRef]
- Horváth, B.; Shinohara, T.; Illés, B. Corrosion Properties of Tin-Copper Alloy Coatings in Aspect of Tin Whisker Growth. J. Alloys Compd. 2013, 577, 439–444. [Google Scholar] [CrossRef]
- Séby, F.; Potin-Gautier, M.; Giffaut, E.; Donard, O. A critical review of thermodynamic data for inorganic tin species. Geochim. Cosmochim. Acta 2001, 65, 3041–3053. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.H.; Yang, J.; Xu, X.L.; Lin, Q. Effect of micromorphology on corrosion and mechanical properties of SAC305 lead-free solders. Microelectron. Reliab. 2010, 108, 113634–113650. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, C. Temperature driven texture and grain boundary engineering of electrodeposited β-Sn coatings and its effect on the coating corrosion behaviour: Five-parameter grain boundary character distribution analysis study. Scripta. Mater. 2021, 196, 3763. [Google Scholar] [CrossRef]
- Tsuji, K. Roll of Grain-boundary Free Energy & Surface Free Energy for Tin Whisker Growth. In Proceedings of the IPC/JEDEC 4th International Conference on Lead-Free Electronic Components and Assemblies, Brussels, Belgium, 11–12 June 2003. [Google Scholar]
- Lala, S.R.F.; Jyotheender, K.S.; Gupta, A.; Arora, S.; MK, P.K.; Srivastava, C. Evolution of texture and strain in Sn coating with Cr addition and its effect on the coating corrosion behavior. Materialia 2020, 14, 100944. [Google Scholar] [CrossRef]




| Sample Name | Whisker Density [pcs./mm2] | Average Length [µm] | Maximum Length [µm] |
|---|---|---|---|
| Reference SAC | 396 | 11.2 ± 4.3 | 69 |
| SAC-0.25ZrO2(np) | 441 | 11.9 ± 5.1 | 94 |
| SAC-0.5ZrO2(np) | 487 | 13.1 ± 5.7 | 118 |
| SAC-0.25ZrO2(nf) | 372 | 11.6 ± 4.9 | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skwarek, A.; Choi, H.; Hurtony, T.; Byun, J.; Mohamad, A.A.; Bušek, D.; Dušek, K.; Illés, B. Effects of ZrO2 Nano-Particles’ Incorporation into SnAgCu Solder Alloys: An Experimental and Theoretical Study. Nanomaterials 2024, 14, 1636. https://doi.org/10.3390/nano14201636
Skwarek A, Choi H, Hurtony T, Byun J, Mohamad AA, Bušek D, Dušek K, Illés B. Effects of ZrO2 Nano-Particles’ Incorporation into SnAgCu Solder Alloys: An Experimental and Theoretical Study. Nanomaterials. 2024; 14(20):1636. https://doi.org/10.3390/nano14201636
Chicago/Turabian StyleSkwarek, Agata, Halim Choi, Tamás Hurtony, Jaeduk Byun, Ahmad Azmin Mohamad, David Bušek, Karel Dušek, and Balázs Illés. 2024. "Effects of ZrO2 Nano-Particles’ Incorporation into SnAgCu Solder Alloys: An Experimental and Theoretical Study" Nanomaterials 14, no. 20: 1636. https://doi.org/10.3390/nano14201636
APA StyleSkwarek, A., Choi, H., Hurtony, T., Byun, J., Mohamad, A. A., Bušek, D., Dušek, K., & Illés, B. (2024). Effects of ZrO2 Nano-Particles’ Incorporation into SnAgCu Solder Alloys: An Experimental and Theoretical Study. Nanomaterials, 14(20), 1636. https://doi.org/10.3390/nano14201636







