Interface Synergistic Effect of NiFe-LDH/3D GA Composites on Efficient Electrocatalytic Water Oxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of NiFe-LDH
2.2. Synthesis of 3D NiFe-LDH/GA
2.3. Characterization
2.4. Electrochemical Measurements
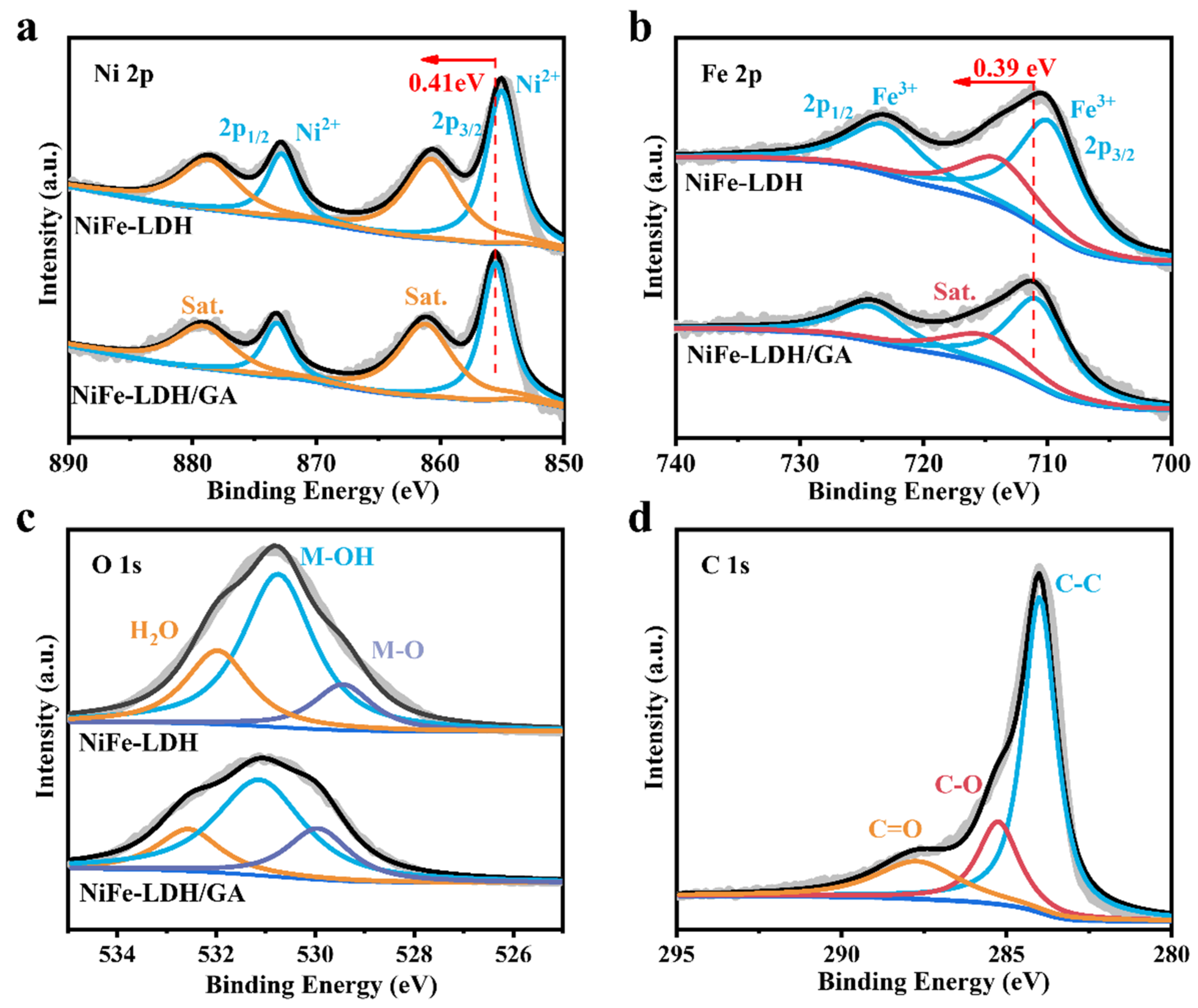
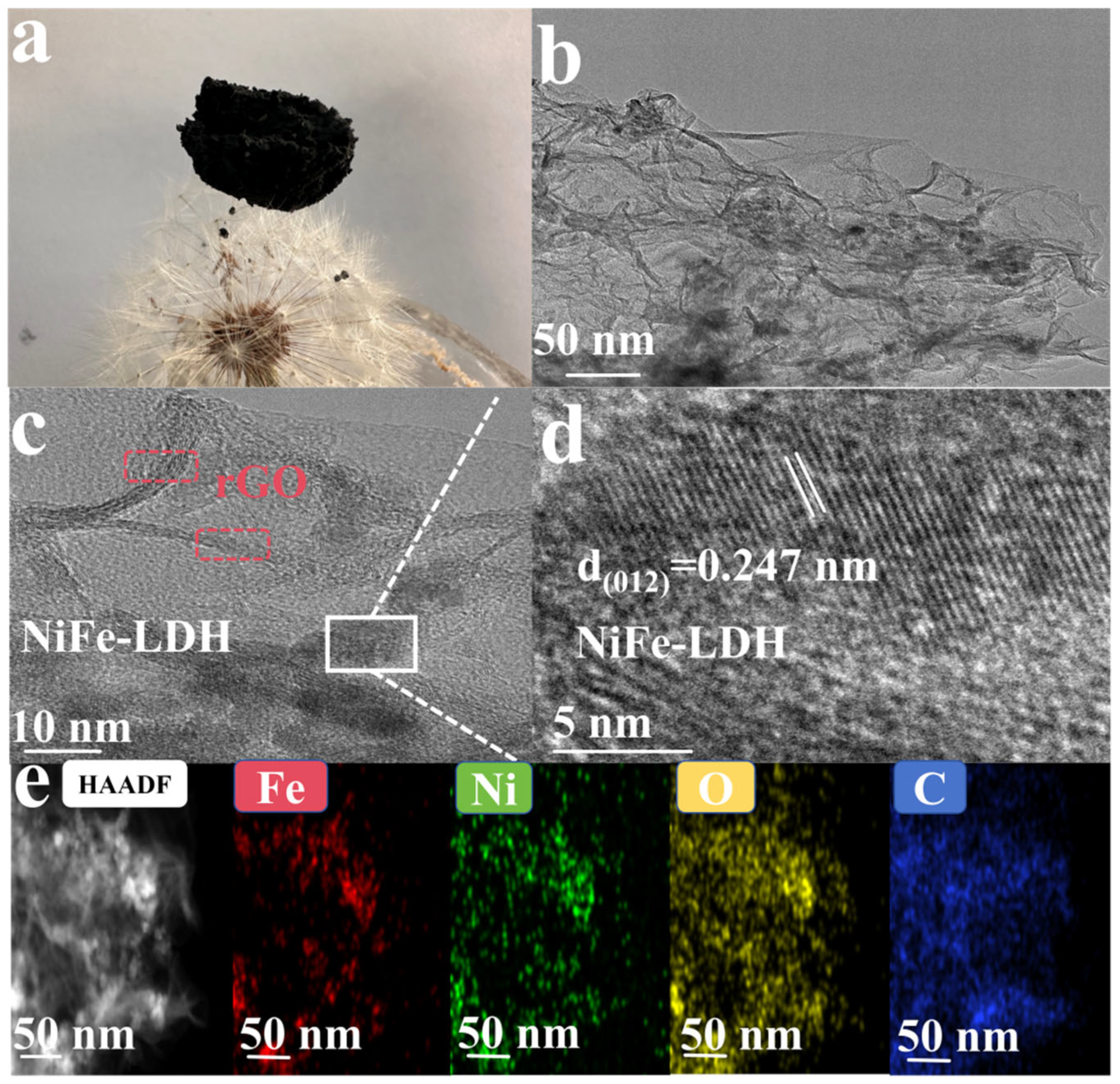
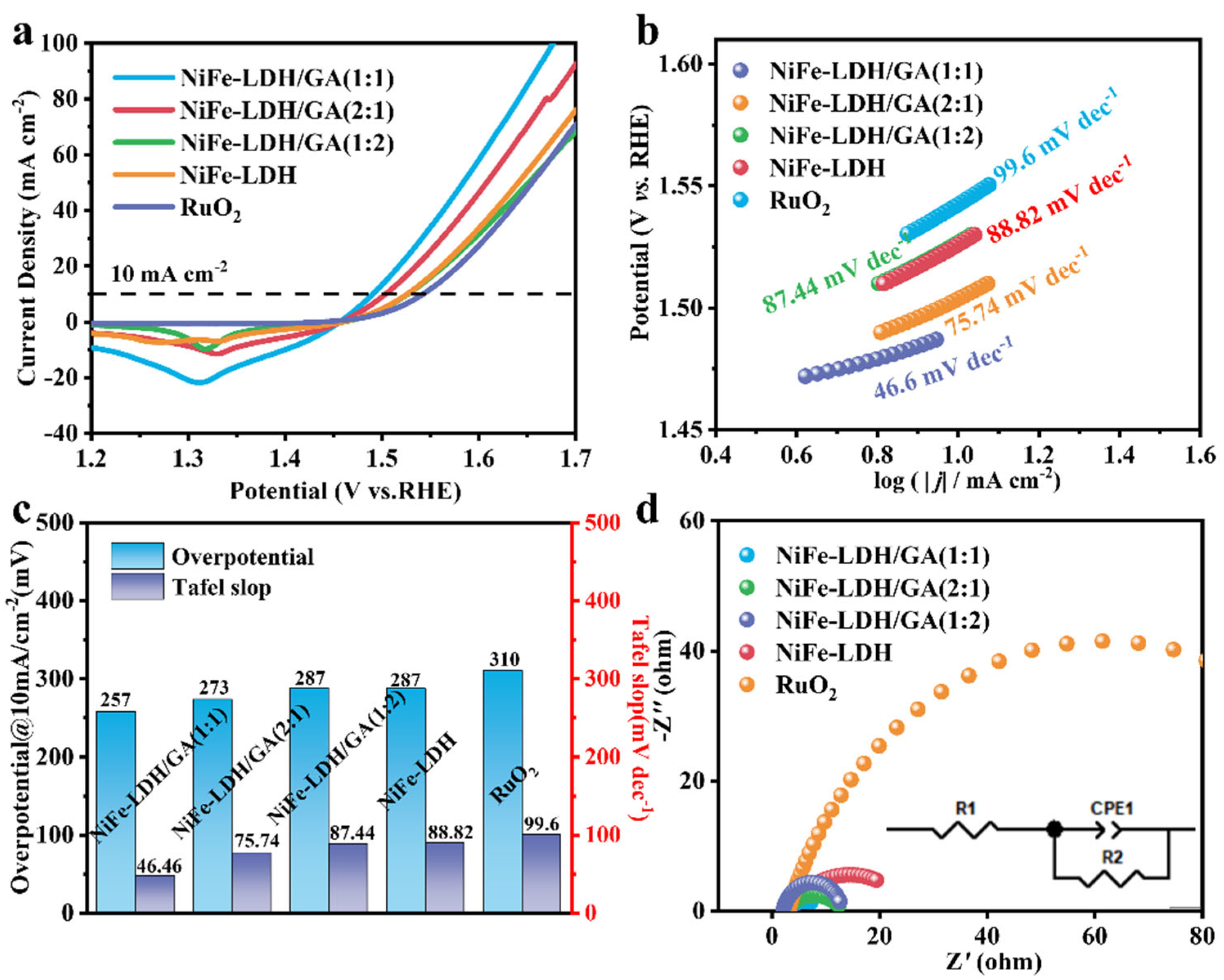
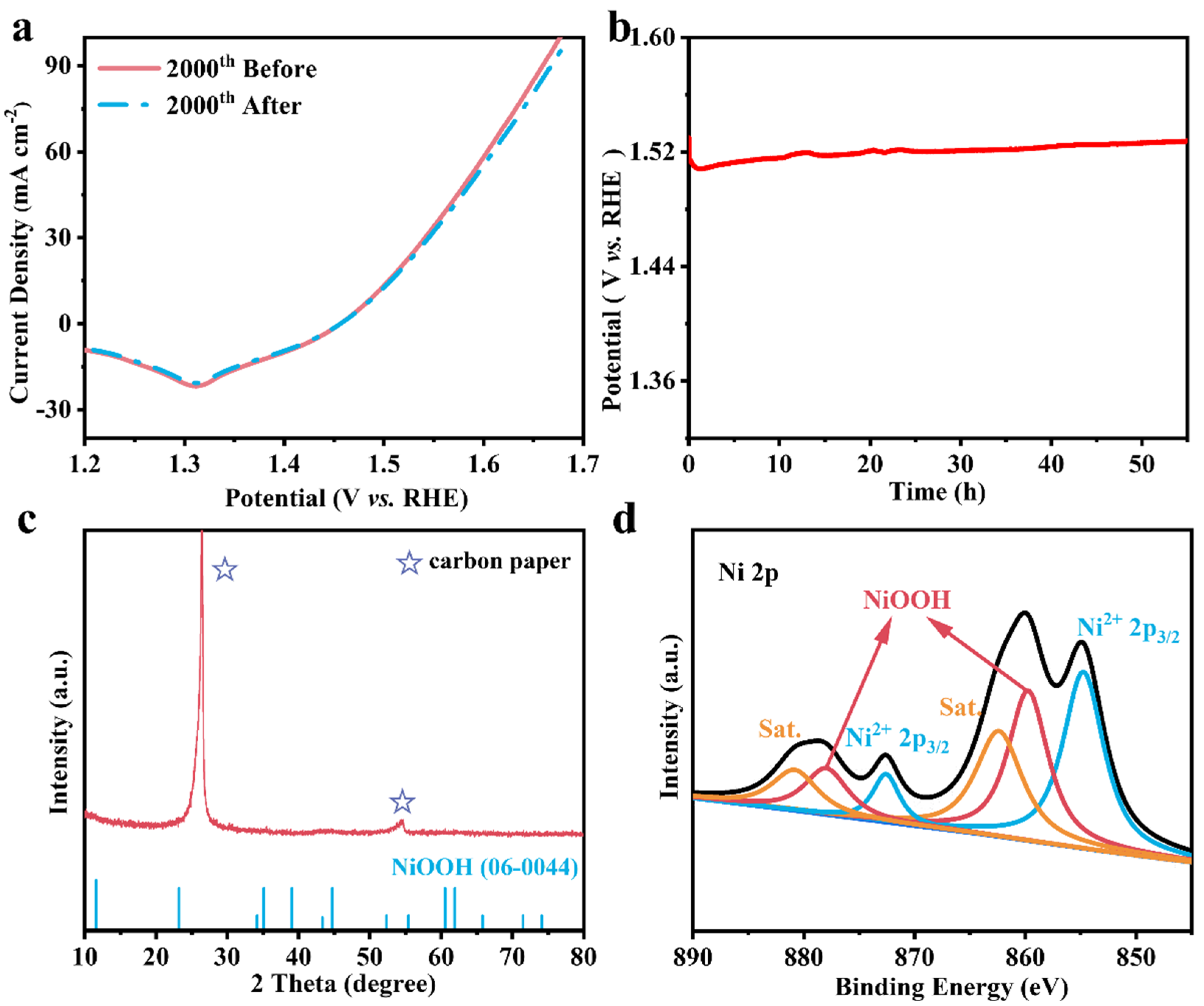
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiu, Y.; Rao, Y.F.; Zheng, Y.N.; Hu, H.; Zhang, W.H.; Guo, X.H. Activating ruthenium dioxide via compressive strain achieving efficient multifunctional electrocatalysis for Zn-air batteries and overall water splitting. Infomat 2022, 4, e12326. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.Y.; Guan, D.Q.; Chen, D.F.; Shao, Z.P.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Yao, H.; Zheng, Y.A.; Yue, S.L.; Hu, S.J.; Yuan, W.Y.; Guo, X.H. B-site substitution in NaCo1−2xFexNixF3 perovskites for efficient oxygen evolution. Inorg. Chem. Front. 2023, 10, 804–814. [Google Scholar] [CrossRef]
- Diao, J.X.; Qiu, Y.; Liu, S.Q.; Wang, W.T.; Chen, K.; Li, H.L.; Yuan, W.Y.; Qu, Y.T.; Guo, X.H. Interfacial Engineering of WN/WC Heterostructures Derived from Solid-State Synthesis: A Highly Efficient Trifunctional Electrocatalyst for ORR, OER, and HER. Adv. Mater. 2020, 32, 1905679. [Google Scholar] [CrossRef]
- Yu, M.Q.; Budiyanto, E.; Tüysüz, H. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zou, R.Q. Advanced Transition Metal-Based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Nam, D.; Kim, J. Development of NiO/CoO nanohybrids catalyst with oxygen vacancy for oxygen evolution reaction enhancement in alkaline solution. Int. J. Hydrogen Energy 2022, 47, 16900–16907. [Google Scholar] [CrossRef]
- Zhou, D.J.; Li, P.S.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.M.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: Identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Liu, Z.B.; Corva, M.; Amin, H.M.A.; Blanc, N.; Linnemann, J.; Tschulik, K. Single Co3O4 Nanocubes Electrocatalyzing the Oxygen Evolution Reaction: Nano-Impact Insights into Intrinsic Activity and Support Effects. Int. J. Mol. Sci. 2021, 22, 13137. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.-K. Metal–Organic Framework-Derived Hollow CoS Nanoarray Coupled with NiFe Layered Double Hydroxides as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Small 2022, 18, 2200586. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, W.W.; Shao, W.J.; Bai, M.R.; Zhou, M.; Li, S.; Ma, T.; Ma, L.; Cheng, C.; Liu, X.K. Synthesis and Electronic Modulation of Nanostructured Layered Double Hydroxides for Efficient Electrochemical Oxygen Evolution. Chemsuschem 2021, 14, 5112–5134. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.G.; Wang, H.L.; Liang, Y.Y.; Wu, J.Z.; Zhou, J.G.; Wang, J.; Regier, T.; Wei, F.; Dai, H.J. An Advanced Ni-Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.D.; Luo, G.; Wang, L.G.; Liu, X.K.; Qu, Y.T.; Zhou, Y.S.; Zhou, F.Y.; Li, Z.J.; Li, Y.F.; Yao, T.; et al. Single Ru Atoms Stabilized by Hybrid Amorphous/Crystalline FeCoNi Layered Double Hydroxide for Ultraefficient Oxygen Evolution. Adv. Energy Mater. 2021, 11, 2002816. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.Y.; Zhou, Y.S.; Li, R.K.; Lu, W.P.; Wang, K.K.; Liu, W.K. In situ growth of NiCoFe-layered double hydroxide through etching Ni foam matrix for highly enhanced oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 23644–23652. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.C.; Yu, Z.H.; Guo, S.S.; Yuan, M.W.; Yao, H.Q.; Liang, Z.P.; Lu, Y.R.; Chan, T.S.; Li, C.; et al. Self-supported NiFe-LDH@CoS nanosheet arrays grown on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 419, 129512. [Google Scholar] [CrossRef]
- Lai, F.L.; Miao, Y.E.; Zuo, L.Z.; Lu, H.Y.; Huang, Y.P.; Liu, T.X. Biomass-Derived Nitrogen-Doped Carbon Nanofiber Network: A Facile Template for Decoration of Ultrathin Nickel-Cobalt Layered Double Hydroxide Nanosheets as High-Performance Asymmetric Supercapacitor Electrode. Small 2016, 12, 3235–3244. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.Z.; Gao, G.P.; Chen, H.; Wang, B.; Zhou, J.Z.; Soo, M.T.; Hong, M.; Yan, X.C.; Qian, G.R.; et al. A Heterostructure Coupling of Exfoliated Ni-Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Meng, W.; He, H.Y.; Yang, L.; Jiang, Q.G.; Yuliarto, B.; Yamauchi, Y.; Xu, X.T.; Huang, H.J. 1D-2D hybridization: Nanoarchitectonics for grain boundary-rich platinum nanowires coupled with MXene nanosheets as efficient methanol oxidation electrocatalysts. Chem. Eng. J. 2022, 450, 137932. [Google Scholar] [CrossRef]
- Yang, C.Z.; Huang, H.J.; He, H.Y.; Yang, L.; Jiang, Q.G.; Li, W.H. Recent advances in MXene-based nanoarchitectures as electrode materials for future energy generation and conversion applications. Coordin. Chem. Rev. 2021, 435, 213806. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.C.; Li, J.; Luo, J.; Fu, L.; Chen, S.L. NiFe LDH nanodots anchored on 3D macro/mesoporous carbon as a high-performance ORR/OER bifunctional electrocatalyst. J. Mater. Chem. A 2018, 6, 14299–14306. [Google Scholar] [CrossRef]
- Rinawati, M.; Wang, Y.X.; Huang, W.H.; Wu, Y.T.; Cheng, Y.S.; Kurniawan, D.; Haw, S.C.; Chiang, W.H.; Su, W.N.; Yeh, M.H. Unraveling the efficiency of heteroatom-doped graphene quantum dots incorporated MOF-derived bimetallic layered double hydroxide towards oxygen evolution reaction. Carbon 2022, 200, 437–447. [Google Scholar] [CrossRef]
- Song, W.Q.; Ren, Z.; Chen, S.Y.; Meng, Y.T.; Biswas, S.; Nandi, P.; Elsen, H.A.; Gao, P.X.; Suib, S.L. Ni- and Mn-Promoted Mesoporous Co3O4: A Stable Bifunctional Catalyst with Surface-Structure-Dependent Activity for Oxygen Reduction Reaction and Oxygen Evolution Reaction. ACS Appl. Mater. Inter. 2016, 8, 20802–20813. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Baltruschat, H.; Wittmaier, D.; Friedrich, K.A. A Highly Efficient Bifunctional Catalyst for Alkaline Air-Electrodes Based on a Ag and Co3O4 Hybrid: RRDE and Online DEMS Insights. Electrochim. Acta 2015, 151, 332–339. [Google Scholar] [CrossRef]
- Zhi, D.D.; Li, T.; Li, J.Z.; Ren, H.S.; Meng, F.B. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B 2021, 211, 108642. [Google Scholar] [CrossRef]
- Dang, A.L.; Liu, X.; Wang, Y.J.; Liu, Y.H.; Cheng, T.; Zada, A.; Ye, F.; Deng, W.B.; Sun, Y.T.; Zhao, T.K.; et al. High-efficient adsorption for versatile adsorbates by elastic reduced graphene oxide/Fe3O4 magnetic aerogels mediated by carbon nanotubes. J. Hazard. Mater. 2023, 457, 131846. [Google Scholar] [CrossRef]
- Huang, Y.L.; Wei, Q.L.; Wang, Y.Y.; Dai, L.Y. Three-dimensional amine-terminated ionic liquid functionalized graphene/Pd composite aerogel as highly efficient and recyclable catalyst for the Suzuki cross-coupling reactions. Carbon 2018, 136, 150–159. [Google Scholar] [CrossRef]
- Zheng, Z.C.; Wu, D.; Chen, G.; Zhang, N.; Wan, H.; Liu, X.H.; Ma, R.Z. Microcrystallization and lattice contraction of NiFe LDHs for enhancing water electrocatalytic oxidation. Carbon Energy 2022, 4, 901–913. [Google Scholar] [CrossRef]
- Chen, W.Y.; Han, B.; Xie, Y.L.; Liang, S.J.; Deng, H.; Lin, Z. Ultrathin Co-Co LDHs nanosheets assembled vertically on MXene: 3D nanoarrays for boosted visible-light-driven CO reduction. Chem. Eng. J. 2020, 391, 123519. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, Y.I.; Lee, S.Y.; Lee, Y.S.; Kim, S.K.; Kim, Y.A.; Yang, C.M. Ni-Co layered double hydroxide coated on microsphere nanocomposite of graphene oxide and single-walled carbon nanohorns as supercapacitor electrode material. Int. J. Energy Res. 2022, 46, 23564–23577. [Google Scholar] [CrossRef]
- Linghu, W.S.; Yang, H.; Sun, Y.X.; Sheng, G.D.; Huang, Y.Y. One-Pot Synthesis of LDH/GO Composites as Highly Effective Adsorbents for Decontamination of U(VI). ACS Sustain. Chem. Eng. 2017, 5, 5608–5616. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Attia, M.; Tetzlaff, D.; Apfel, U.-P. Tailoring the Electrocatalytic Activity of Pentlandite FexNi9-XS8 Nanoparticles via Variation of the Fe : Ni Ratio for Enhanced Water Oxidation. ChemElectroChem 2021, 8, 3863–3874. [Google Scholar] [CrossRef]
- Wu, Y.J.; Song, M.L.; Huang, Y.C.; Dong, C.L.; Li, Y.Y.; Lu, Y.X.; Zhou, B.; Wang, D.D.; Jia, J.F.; Wang, S.Y.; et al. Promoting surface reconstruction of NiFe layered double hydroxides via intercalating [Cr(C2O4)3]3-for enhanced oxygen evolution. J. Energy Chem. 2022, 74, 140–148. [Google Scholar] [CrossRef]
- Si, S.; Hu, H.S.; Liu, R.J.; Xu, Z.X.; Wang, C.B.; Feng, Y.Y. Co-NiFe layered double hydroxide nanosheets as an efficient electrocatalyst for the electrochemical evolution of oxygen. Int. J. Hydrogen Energy 2020, 45, 9368–9379. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, J.; Liu, D.P.; Wu, L.; Li, T.Z.; Yan, S.C.; Fan, Q.; Zhu, K.; Zou, Z.G. Ultrafast Fenton-like reaction route to FeOOH/NiFe-LDH heterojunction electrode for efficient oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 21785–21791. [Google Scholar] [CrossRef]
- Yan, L.; Du, Z.P.; Lai, X.Y.; Lan, J.Y.; Liu, X.J.; Liao, J.Y.; Feng, Y.F.; Li, H. Synergistically modulating the electronic structure of Cr-doped FeNi LDH nanoarrays by O-vacancy and coupling of MXene for enhanced oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 1892–1903. [Google Scholar] [CrossRef]
- Yin, P.Q.; Wu, G.; Wang, X.Q.; Liu, S.J.; Zhou, F.Y.; Dai, L.; Wang, X.; Yang, B.; Yu, H.Q. NiCo-LDH nanosheets strongly coupled with GO-CNTs as a hybrid electrocatalyst for oxygen evolution reaction. Nano Res. 2021, 14, 4783–4788. [Google Scholar] [CrossRef]
- Shen, B.F.; Feng, Y.; Wang, Y.; Sun, P.Y.; Yang, L.; Jiang, Q.G.; He, H.Y.; Huang, H.J. Holey MXene nanosheets intimately coupled with ultrathin Ni-Fe layered double hydroxides for boosted hydrogen and oxygen evolution reactions. Carbon 2023, 212, 118141. [Google Scholar] [CrossRef]
- Wan, C.W.; Jin, J.; Wei, X.Y.; Chen, S.Z.; Zhang, Y.; Zhu, T.L.; Qu, H.X. Inducing the SnO based electron transport layer into NiFe LDH/NF as efficient catalyst for OER and methanol oxidation reaction. J. Mater. Sci. Technol. 2022, 124, 102–108. [Google Scholar] [CrossRef]
- Fan, K.; Chen, H.; Ji, Y.F.; Huang, H.; Claesson, P.M.; Daniel, Q.; Philippe, B.; Rensmo, H.; Li, F.S.; Luo, Y.; et al. Nickel-vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Yu, W.L.; Cao, Y.N.; Zhao, J.; Dong, B.; Ma, Y.; Wang, F.L.; Fan, R.Y.; Zhou, Y.L.; Chai, Y.M. S-doped nickel-iron hydroxides synthesized by room-temperature electrochemical activation for efficient oxygen evolution. Appl. Catal. B 2021, 292, 120150. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, P.; Sun, J.M. Promoting electrocatalytic water oxidation through tungsten-modulated oxygen vacancies on hierarchical FeNi-layered double hydroxide. Nano Energy 2021, 80, 105540. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, F.; Liu, X.; Wu, N.; Che, S.; Li, Y. Phosphorus doped nickel-molybdenum aerogel for efficient overall water splitting. Appl. Catal. B Environ. 2021, 298, 120494. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, Q.; Wang, X.; Shao, Z.; Yao, L.; Zeng, H.; Wu, X.; Chen, J.; Huang, K.; Feng, S. Formation and Stabilization of NiOOH by Introducing α-FeOOH in LDH: Composite Electrocatalyst for Oxygen Evolution and Urea Oxidation Reactions. Adv. Mater. 2023, 35, 2209338. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.Z.; Liu, L.; Qin, W.J.; Liu, S.J.; Tu, J.P.; Qin, Y.P.; Liu, J.F.; Wu, H.Y.; Zhang, D.Y.; et al. Mesoporous Single Crystals with Fe-Rich Skin for Ultralow Overpotential in Oxygen Evolution Catalysis. Adv. Mater. 2022, 34, 2200088. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wang, H.; Peng, C.K.; Bu, L.M.; Chiang, C.L.; Tian, K.; Zhao, Y.; Zhao, J.Q.; Lin, Y.G.; Lee, J.M.; et al. Co-Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2020, 16, 2002426. [Google Scholar] [CrossRef] [PubMed]
- Han, X.T.; Li, N.N.; Kang, Y.B.; Dou, Q.Y.; Xiong, P.X.; Liu, Q.; Lee, J.Y.; Dai, L.M.; Park, H.S. Unveiling Trifunctional Active Sites of a Heteronanosheet Electrocatalyst for Integrated Cascade Battery/Electrolyzer Systems. ACS Energy Lett. 2021, 6, 2460–2468. [Google Scholar] [CrossRef]
- Meng, H.Y.; Xi, W.; Ren, Z.Y.; Du, S.C.; Wu, J.; Zhao, L.; Liu, B.W.; Fu, H.G. Solar-boosted electrocatalytic oxygen evolution via catalytic site remodelling of CoCr layered double hydroxide. Appl. Catal. B 2021, 284, 119707. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Wang, C.X.; Wang, Z.M.; Fan, C.C.; Bai, G.; Wang, G.; Yu, F. Modification of NiFe layered double hydroxide by lanthanum doping for boosting water splitting. Electrochim. Acta 2021, 390, 138824. [Google Scholar] [CrossRef]
- Liu, W.X.; Zheng, D.; Deng, T.Q.; Chen, Q.L.; Zhu, C.Z.; Pei, C.J.; Li, H.; Wu, F.F.; Shi, W.H.; Yang, S.W.; et al. Boosting Electrocatalytic Activity of 3d-Block Metal (Hydro)oxides by Ligand-Induced Conversion. Angew. Chem. Int. Edit 2021, 60, 10614–10619. [Google Scholar] [CrossRef]
- Han, M.Y.; Zhang, X.W.; Gao, H.Y.; Chen, S.Y.; Cheng, P.; Wang, P.; Zhao, Z.Y.; Dang, R.; Wang, G. In situ semi-sacrificial template-assisted growth of ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution. Chem. Eng. J. 2021, 426, 131348. [Google Scholar] [CrossRef]
- Sun, H.C.A.; Zhang, W.; Li, J.G.; Li, Z.S.; Ao, X.; Xue, K.H.; Ostrikov, K.K.; Tang, J.; Wang, C.D. Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl. Catal. B 2021, 284, 119740. [Google Scholar] [CrossRef]
- Li, Y.Y.; Luo, W.; Wu, D.J.; Wang, Q.; Yin, J.; Xi, P.X.; Qu, Y.Q.; Gu, M.; Zhang, X.Y.; Lu, Z.G.; et al. Atomic-level correlation between the electrochemical performance of an oxygen-evolving catalyst and the effects of CeO functionalization. Nano Res. 2022, 15, 2994–3000. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W. Hierarchical Hollow Nanoprisms Based on Ultrathin Ni-Fe Layered Double Hydroxide Nanosheets with Enhanced Electrocatalytic Activity towards Oxygen Evolution. Angew. Chem. Int. Edit 2018, 57, 172–176. [Google Scholar] [CrossRef]
- Yin, X.; Hua, Y.N.; Hao, W.B.; Yang, J.; Gao, Z. Hierarchical nanocomposites of nickel/iron-layered double hydroxide ultrathin nanosheets strong-coupled with nanocarbon networks for enhanced oxygen evolution reaction. Electrochim. Acta 2022, 420, 140455. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cao, Q.; Yu, X.; Yao, H.; Su, B.; Guo, X. Interface Synergistic Effect of NiFe-LDH/3D GA Composites on Efficient Electrocatalytic Water Oxidation. Nanomaterials 2024, 14, 1661. https://doi.org/10.3390/nano14201661
Zhang J, Cao Q, Yu X, Yao H, Su B, Guo X. Interface Synergistic Effect of NiFe-LDH/3D GA Composites on Efficient Electrocatalytic Water Oxidation. Nanomaterials. 2024; 14(20):1661. https://doi.org/10.3390/nano14201661
Chicago/Turabian StyleZhang, Jiangcheng, Qiuhan Cao, Xin Yu, Hu Yao, Baolian Su, and Xiaohui Guo. 2024. "Interface Synergistic Effect of NiFe-LDH/3D GA Composites on Efficient Electrocatalytic Water Oxidation" Nanomaterials 14, no. 20: 1661. https://doi.org/10.3390/nano14201661
APA StyleZhang, J., Cao, Q., Yu, X., Yao, H., Su, B., & Guo, X. (2024). Interface Synergistic Effect of NiFe-LDH/3D GA Composites on Efficient Electrocatalytic Water Oxidation. Nanomaterials, 14(20), 1661. https://doi.org/10.3390/nano14201661







