Hierarchical Heterojunctions of Metal Sulfide WS2 Nanosheets/Metal Oxide In2O3 Nanofibers for an Efficient Detection of Formaldehyde
Abstract
:1. Introduction
2. Experimental Section
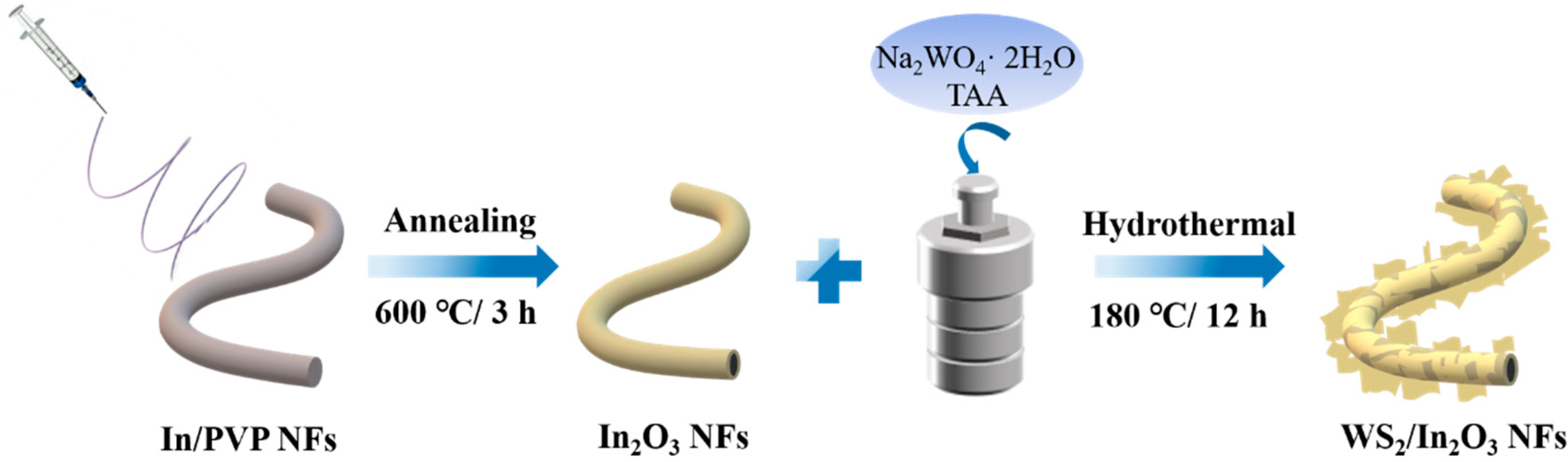
2.1. Synthesis of Pristine In2O3 Nanofibers (NFs)
2.2. Synthesis of WS2/In2O3 Nanofibers (NFs)
2.3. Characterizations
2.4. Device Fabrication and Gas Sensing Property Tests
3. Results and Discussion
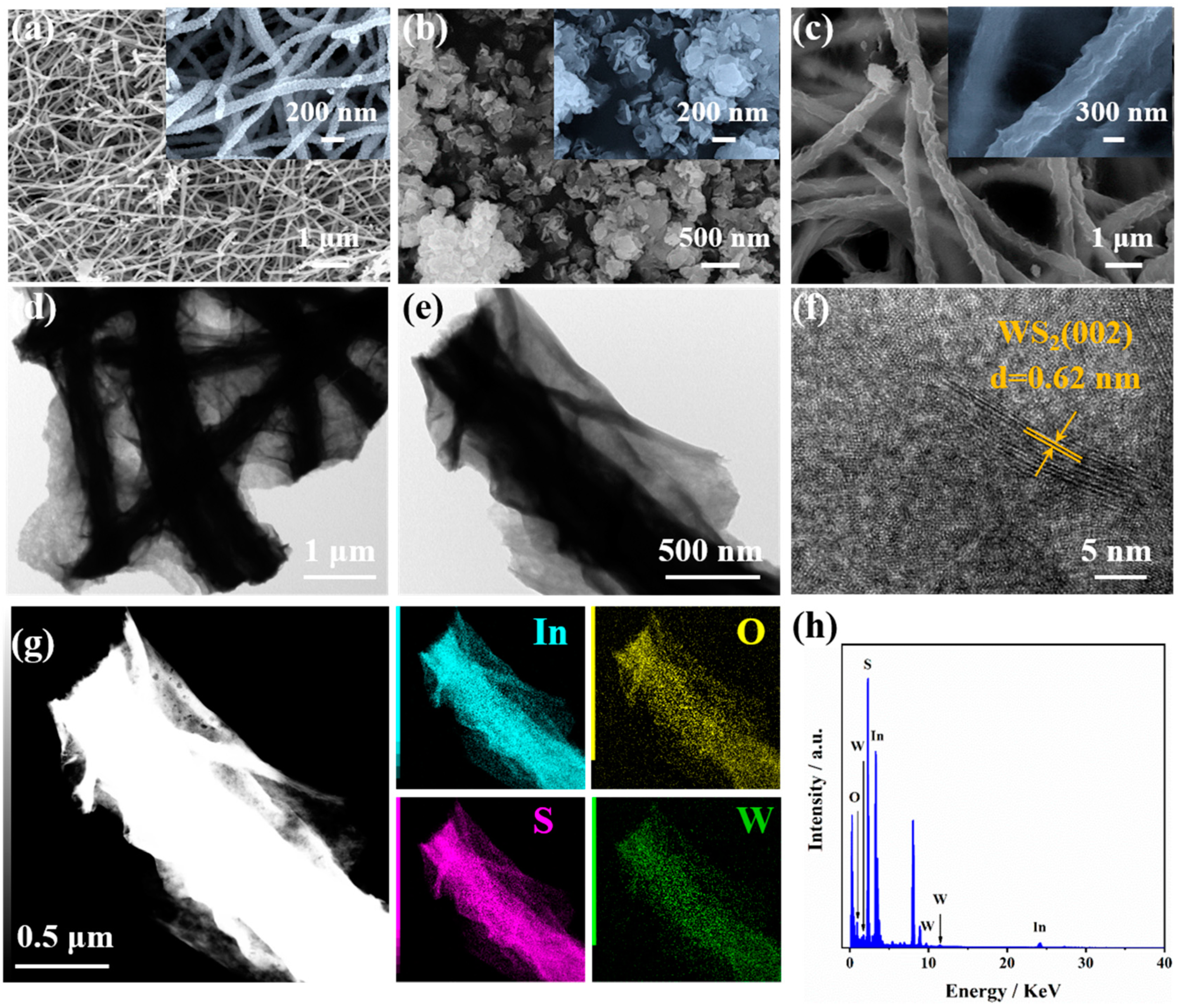
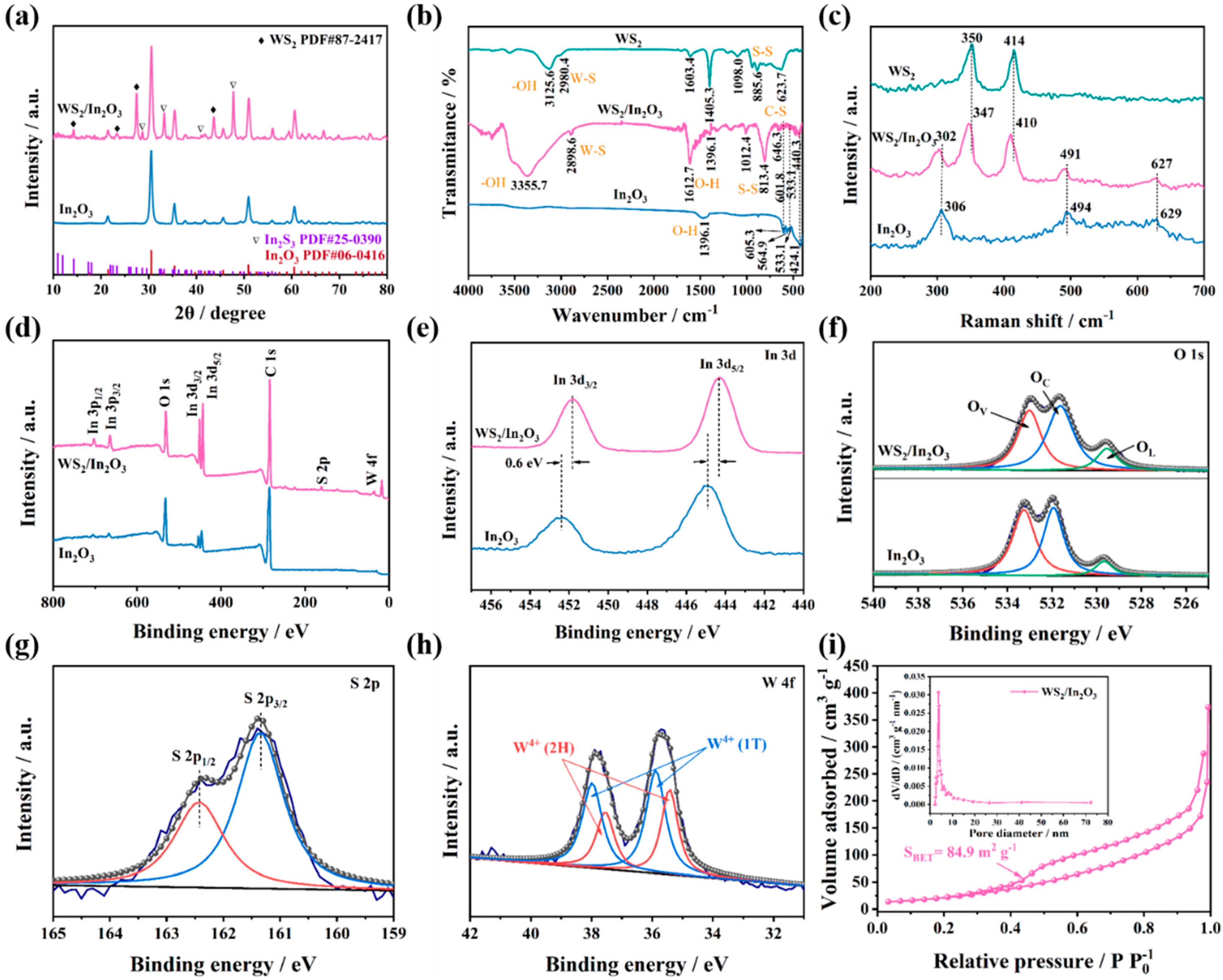
3.1. Morphology Characterization and Phase Composition
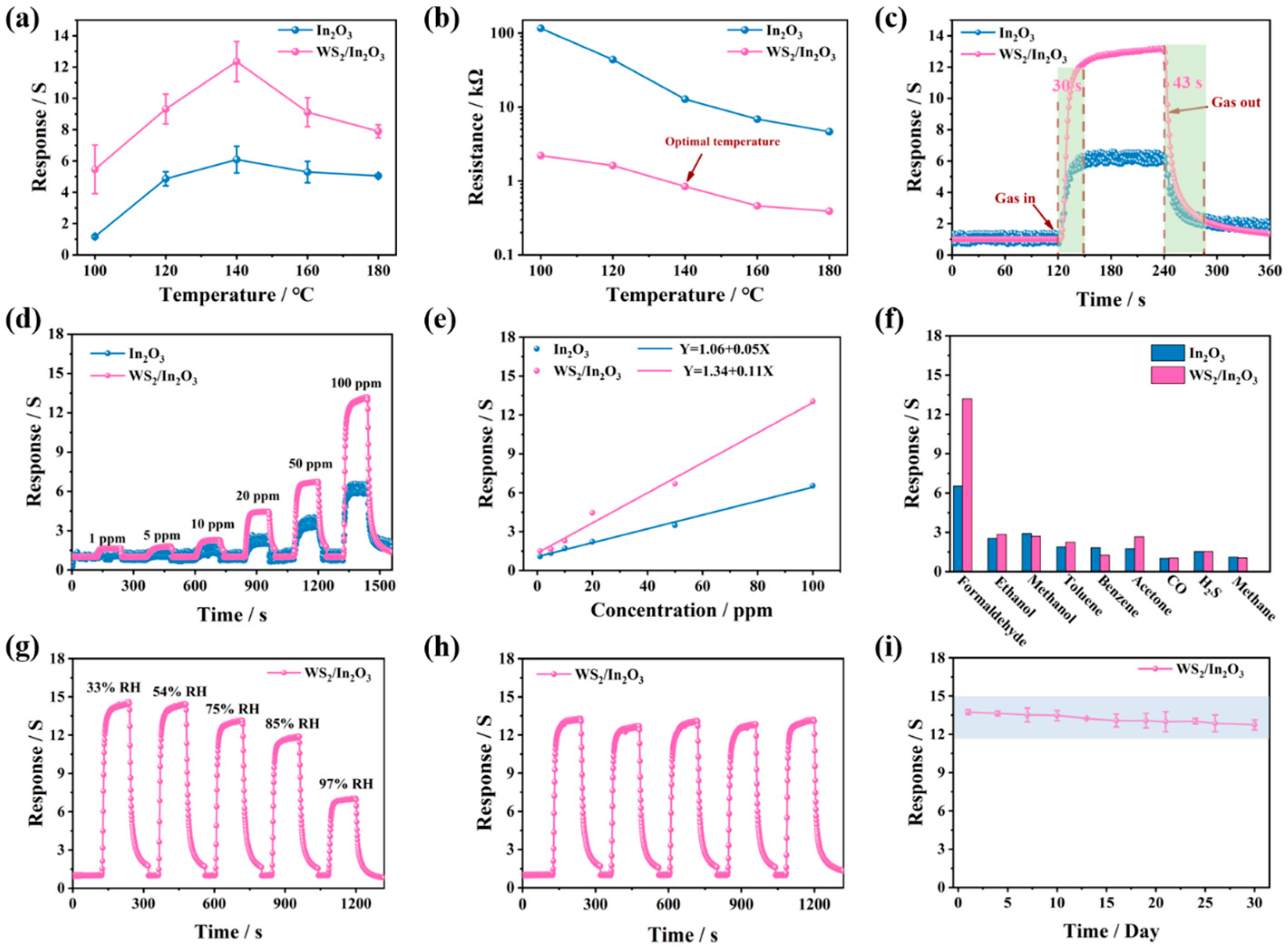
3.2. Gas Sensing Performance
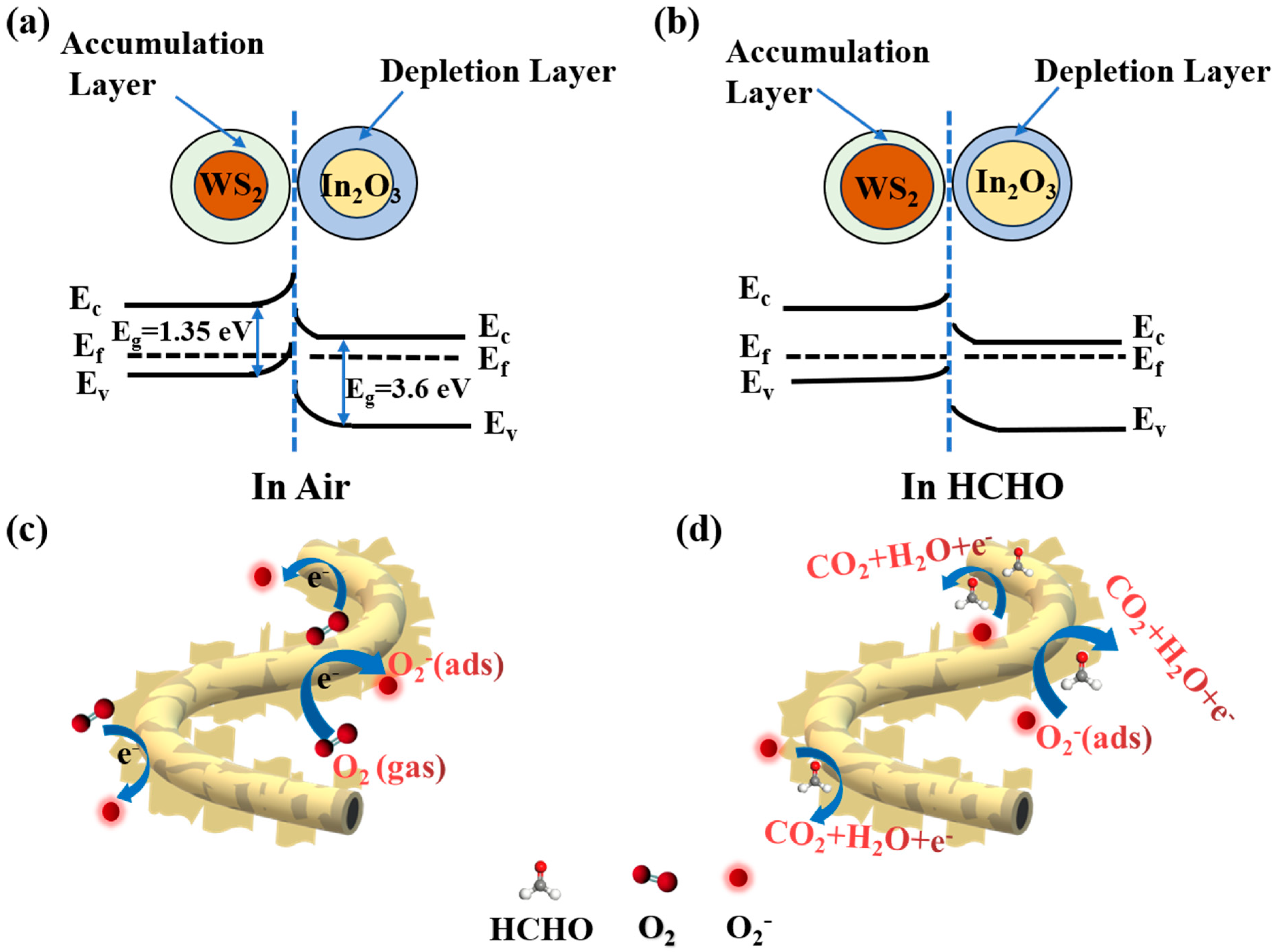
3.3. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, D.; Li, X.; Wang, H.; Li, M.; Xi, Y.; Chen, Y.; Wang, J.; Rumyntseva, M.N.; Gaskov, A.M. Light enhanced VOCs sensing of WS2 microflakes based chemiresistive sensors powered by triboelectronic nangenerators. Sens. Actuators B 2018, 256, 992–1000. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wu, J.; Wang, X.; Lv, X.; Liu, G.; Li, B.; Zhou, J.; Xie, E.; Zhang, Z. Electrospun Nb-doped CeO2 nanofibers for humidity independent acetone sensing. Appl. Surf. Sci. 2022, 602, 154303. [Google Scholar] [CrossRef]
- Zhao, Z.; Lv, Z.; Chen, Z.; Zhou, B.; Shao, Z. α-Fe2O3/TiO2/Ti3C2Tx Nanocomposites for Enhanced Acetone Gas Sensors. Sensors 2024, 24, 2604. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Wang, D.; Peng, L.; Lin, Y.; Li, X.; Xie, T. Visible-light-induced photoelectric gas sensing to formaldehyde based on CdS nanoparticles/ZnO heterostructures. Sens. Actuators B 2010, 147, 234–240. [Google Scholar] [CrossRef]
- Souri, M.; Salar Amoli, H.; Yamini, Y. Three-dimensionally ordered porous In-doped SmFeO3 perovskite gas sensor for highly sensitive and selective detection of formaldehyde. Sens. Actuators B 2024, 404, 135213. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.J.; Lee, W. Synergetic crystal phases of SnO2/NiO heterostructure in an interconnected morphology for chemiresistive formaldehyde sensors. Sens. Actuators B 2024, 404, 135257. [Google Scholar] [CrossRef]
- Bu, W.; Liu, N.; Zhang, Y.; Han, W.; Chuai, X.; Zhou, Z.; Hu, C.; Lu, G. Atomically dispersed Pt on MOF-derived In2O3 for chemiresistive formaldehyde gas sensing. Sens. Actuators B 2024, 404, 135260. [Google Scholar] [CrossRef]
- Kong, D.L.; Wu, W.J.; Hong, B.; Xu, J.C.; Peng, X.L.; Ge, H.L.; Li, J.; Zeng, Y.X.; Wang, X.Q. MIL-68 derived In2O3 microtubes and Co3O4/In2O3 heterostructures for high sensitive formaldehyde gas sensors. Ceram. Int. 2024, 50, 6995–7005. [Google Scholar] [CrossRef]
- Zhou, L.; Chang, X.; Zheng, W.; Liu, X.; Zhang, J. Single atom Rh-sensitized SnO2 via atomic layer deposition for efficient formaldehyde detection. Chem. Eng. J. 2023, 475, 146300. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Zhou, T.; Song, Z.; Deng, Z.; Zi, B.; Zhang, J.; Zhao, J.; Liu, Q.; Hu, G. Nonstoichiometric Doping of La0.9FexSn1–xO3 Hollow Microspheres for an Ultrasensitive Formaldehyde Sensor. ACS Sens. 2023, 8, 4334–4343. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Choi, M.S.; Huh, J.-S. Formaldehyde Gas Sensing Characteristics of ZnO-TiO2 Gas Sensors. Chemosensors 2023, 11, 140. [Google Scholar] [CrossRef]
- Li, Y.; DiStefano, J.G.; Murthy, A.A.; Cain, J.D.; Hanson, E.D.; Li, Q.; Castro, F.C.; Chen, X.; Dravid, V.P. Superior Plasmonic Photodetectors Based on Au@MoS2 Core-Shell Heterostructures. ACS Nano 2017, 11, 10321–10329. [Google Scholar] [CrossRef]
- Suh, J.M.; Kwon, K.C.; Lee, T.H.; Kim, C.; Lee, C.W.; Song, Y.G.; Choi, M.-J.; Choi, S.; Cho, S.H.; Kim, S.; et al. Edge-exposed WS2 on 1D nanostructures for highly selective NO2 sensor at room temperature. Sens. Actuators B 2021, 333, 129566. [Google Scholar] [CrossRef]
- Wei, C.; Zhu, M.; Zhou, Z.; Zhao, S.; Mao, J.; Yin, D.; Li, J.; Wang, Y.; Hao, J. Two-dimensional Bi2O2S based high-sensitivity and rapid-response humidity sensor for respiratory monitoring and Human-Machine Interaction. Chem. Eng. J. 2024, 485, 149805. [Google Scholar] [CrossRef]
- Wu, R.; Mao, J.; Li, H.; Yang, Y.; Hao, W.; Wang, Y.; Hao, J. Revealing the relationship of NO2 sensing with energy level in 2D van der Waals SnS1−xSex alloys. Chem. Eng. J. 2023, 469, 144018. [Google Scholar] [CrossRef]
- Kim, Y.; Sohn, I.; Shin, D.; Yoo, J.; Lee, S.; Yoon, H.; Park, J.; Chung, S.-m.; Kim, H. Recent Advances in Functionalization and Hybridization of Two-Dimensional Transition Metal Dichalcogenide for Gas Sensor. Adv. Eng. Mater. 2023, 26, 2301063. [Google Scholar] [CrossRef]
- Sonwal, S.; Ranjith, K.S.; Han, S.; Han, Y.-K.; Oh, M.-H.; Huh, Y.S. Live-tracking of beef freshness by sub-ppb level ammonia detection using WS2/rGO nanoflakes incorporating edge site-enriched acidic sulfur. J. Mater. Chem. A 2024, 12, 11004–11019. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, B.; Liu, S.; Zhao, Z.; Zhang, W.; Zhang, W.; Suematsu, K.; Hu, J. WS2 quantum dots modified In2O3 hollow hexagonal prisms for conductometric NO2 sensing at room-temperature. Sens. Actuators B 2023, 380, 133341. [Google Scholar] [CrossRef]
- Minezaki, T.; Krüger, P.; Annanouch, F.E.; Casanova-Cháfer, J.; Alagh, A.; Villar-Garcia, I.J.; Pérez-Dieste, V.; Llobet, E.; Bittencourt, C. Hydrogen Sensing Mechanism of WS2 Gas Sensors Analyzed with DFT and NAP-XPS. Sensors 2023, 23, 4623. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, H.; Yang, K.; Shen, Y.; Qin, K.; Yuan, P.; Li, E. Highly Sensitive and Selective Defect WS2 Chemical Sensor for Detecting HCHO Toxic Gases. Sensors 2024, 24, 762. [Google Scholar] [CrossRef]
- Qin, Z.; Ouyang, C.; Zhang, J.; Wan, L.; Wang, S.; Xie, C.; Zeng, D. 2D WS2 nanosheets with TiO2 quantum dots decoration for high-performance ammonia gas sensing at room temperature. Sens. Actuators B 2017, 253, 1034–1042. [Google Scholar] [CrossRef]
- Cui, S.; Wen, Z.; Huang, X.; Chang, J.; Chen, J. Stabilizing MoS2 Nanosheets through SnO2 Nanocrystal Decoration for High-Performance Gas Sensing in Air. Small 2015, 11, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ferlazzo, A.; Hussain, M.; Fazio, E.; Corsaro, C.; Mezzasalma, A.M.; Neri, G. Ethanol-Gas-Sensing Performances of Built-in ZrO2/Co3O4 Hybrid Nanostructures. Sensors 2023, 23, 9578. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Mirzaei, A.; Kim, J.-H. Effect of ZnO thickness on gas sensing behavior of WS2-ZnO p-n heterojunction nanosheets towards reducing gases. J. Alloys Compd. 2024, 984, 173967. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Cao, Y.H.; Yang, Z.M.; Wu, J.F. Nanoheterostructure construction and DFT study of Ni-doped In2O3 nanocubes/WS2 hexagon nanosheets for formaldehyde sensing at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 11979–11989. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Wang, Z.; Sun, S.; Li, M.; Yan, W. ZIF-L(Co) derived cobalt doped In2O3 hollow nanofibers with high surface activity for efficient formaldehyde gas sensing. Sens. Actuators B 2024, 403, 135129. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hou, C.L.; De, Q.M.; Gu, F.B.; Han, D.M. One-step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens. 2018, 3, 468–475. [Google Scholar] [CrossRef]
- Scarfiello, R.; Mazzotta, E.; Altamura, D.; Nobile, C.; Mastria, R.; Rella, S.; Giannini, C.; Cozzoli, P.D.; Rizzo, A.; Malitesta, C. An Insight into Chemistry and Structure of Colloidal 2D-WS2 Nanoflakes: Combined XPS and XRD Study. Nanomaterials 2021, 11, 1969. [Google Scholar] [CrossRef]
- Wang, S.K.; Wang, A.C.; Zhang, C.Y.; Liu, Q.Y.; Cheng, J.D.; Wang, Y.C.; Gao, X.P.; Xie, Q.F.; Zhang, Z.X.; Sun, G.Z.; et al. Sandwich-Structured In2S3/In2O3/In2S3 Hollow Nanofibers as Sensing Materials for Ethanol Detection. ACS Appl. Nano Mater. 2023, 6, 2625–2635. [Google Scholar] [CrossRef]
- Zhao, F.; Lu, Q.; Liu, S.; Wang, C. In2O3/ZnO heterostructured nanotubes: Electrospinning fabrication, characterization, and highly enhanced photocatalytic properties. J. Sol-Gel Sci. Technol. 2014, 72, 137–143. [Google Scholar] [CrossRef]
- Elouali, S.; Bloor, L.G.; Binions, R.; Parkin, I.P.; Carmalt, C.J.; Darr, J.A. Gas sensing with nano-indium oxides (In2O3) prepared via continuous hydrothermal flow synthesis. Langmuir 2012, 28, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Wang, S.; Wu, Z.; Zhang, Z. Moisture-Resistant and Highly Selective NH3 Sensor Based on CdS/WS2 Composite Heterojunction. Langmuir 2023, 39, 10352–10366. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, B.; Song, Y.; Song, Y.; Sun, J. Highly conductive ZIF-67 derived La-doped hollow structure for H2S detection. Sens. Actuators B 2023, 379, 133139. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Wang, M.; Han, J. Mesoporous In2O3 nanorods/In2S3 nanosheets hierarchical heterojunctions toward highly efficient visible light photocatalysis. J. Alloys Compd. 2022, 921, 165973. [Google Scholar] [CrossRef]
- Peng, Z.; Kobayashi, H.; Lu, N.; Zhang, J.; Sui, J.; Yan, X. Direct Z-Scheme In2O3/In2S3 Heterojunction for Oxygen-Mediated Photocatalytic Hydrogen Production. Energy Fuels 2022, 36, 15100–15111. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Wang, S.; Liu, Y.; Zhang, J.; Chen, L.; Wang, J.; Liu, Y.; Shen, Q.; Qu, P.; et al. Hierarchically porous hydrangea-like In2S3/In2O3 heterostructures for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 587, 876–882. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Dong, X.; Zheng, N.; Ma, H.; Zhang, X. Fabrication of In2O3/In2S3 microsphere heterostructures for efficient and stable photocatalytic nitrogen fixation. Appl. Catal. B 2019, 257, 117932. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Chen, T.; Lu, Q.; Ur Rehman, S.; Zhu, L. Rationally designed mesoporous In2O3 nanofibers functionalized Pt catalysts for high-performance acetone gas sensors. Sens. Actuators B 2019, 298, 126871. [Google Scholar] [CrossRef]
- Zhou, M.; Xie, X.; Liu, Q.; Zhang, M.; Peng, C.; Li, F.; Liu, Q.; Song, Y.; Wu, J.; Qiao, Z. Spherical In2S3 anchored on g-C3N4 nanosheets for efficient elemental mercury removal in the wide temperature range. Chem. Eng. J. 2022, 430, 132857. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, B.C. 2D/2D Nanostructured System Based on WO3/WS2 for Acetone Sensor and Breath Analyzer. ACS Appl. Nano Mater. 2023, 6, 5493–5507. [Google Scholar] [CrossRef]
- Yang, D.-H.; Kim, J.-H.; Mirzaei, A.; Woo Kim, H.; Sub Kim, S. Co-decoration of WS2 nanosheets with both Au and In2O3-nanoparticles for attaining the CO sensing in self-heating mode. Appl. Surf. Sci. 2023, 635, 157790. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, S.; Wang, X.; Cui, H.; Wang, X.; Tian, J. The metallic 1T-phase WS2 nanosheets as cocatalysts for enhancing the photocatalytic hydrogen evolution of g-C3N4 nanotubes. Appl. Catal. B 2020, 274, 119114. [Google Scholar] [CrossRef]
- Sui, C.; Zhang, M.; Li, Y.; Wang, Y.; Liu, Y.; Liu, Z.; Bai, J.; Liu, F.; Lu, G. Pd@Pt Core–Shell Nanocrystal-Decorated ZnO Nanosheets for ppt-Level NO2 Detection. ACS Sens. 2024, 9, 1967–1977. [Google Scholar] [CrossRef]
- Wang, B.-R.; Liu, L.-Y.; Guo, G.-C.; Bai, Y.-J.; Tu, J.-C.; Wang, R.-Z. Interface enhancement effect of hierarchical In2S3/In2O3 nanoflower heterostructures on NO2 gas sensitivity. Appl. Surf. Sci. 2022, 584, 152669. [Google Scholar] [CrossRef]
- Wang, P.; Tang, C.; Song, H.; Zhang, L.; Lu, Y.; Huang, F. 1D/2D Heterostructured WS2@PANI Composite for Highly Sensitive, Flexible, and Room Temperature Ammonia Gas Sensor. ACS Appl. Mater. Interfaces 2024, 16, 14082–14092. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Chen, X.; Xu, Z.; Ma, Q.; Wang, Z.; Liang, J.; Li, S.; Yan, W. Designing highly sensitive formaldehyde sensors via A-site cation deficiency in LaFeO3 hollow nanofibers. Appl. Surf. Sci. 2022, 590, 153085. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.N.; Liu, J.W.; Nasir, M.S.; Zhu, J.W.; Li, S.S.; Liang, J.D.; Yan, W. Smart formaldehyde detection enabled by metal organic framework-derived doped electrospun hollow nanofibers. Sens. Actuators B 2021, 326, 128819. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Cheng, Z.; Yang, J.; Li, Y. A Sensitive Acetone Sensor Based on WS2/WO3 Nanosheets with p-n Heterojunctions. ACS Appl. Nano Mater. 2022, 5, 12592–12599. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, R.; Zhang, W.; Yang, J.; Jiang, H.; Hu, Z.; Zhang, Y. High response to formaldehyde gas and ppb-level detectability of nanoparticle Cr2O3/porous cubic ZnSnO3 composites p-n heterojunction gas sensor. Ceram. Int. 2024, 50, 6950–6960. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Chen, X.; Li, B.; Jiang, W.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; et al. Hierarchical WS2–WO3 Nanohybrids with P–N Heterojunctions for NO2 Detection. ACS Appl. Nano Mater. 2021, 4, 1626–1634. [Google Scholar] [CrossRef]
- Mao, J.; Yin, D.; Lu, W.; Wang, Y.; Zhou, Z.; Hao, W.; Chen, X.; Hao, J. SnSe2/Ag2Se heterostructures with an accumulation layer for rapid and sensitive detection of NO2. New J. Chem. 2023, 47, 15631–15637. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Xu, Z.; Li, B.; Yang, S. rGO/In2O3 heterostructures based gas sensor for efficient ppb-level n-butanol detection. J. Alloys Compd. 2024, 986, 174154. [Google Scholar] [CrossRef]
- Xavier, J.R. Evaluation of reduced graphene oxide/WO3/WS2 hybrids for high performance supercapacitor electrode. J. Alloys Compd. 2023, 947, 169483. [Google Scholar] [CrossRef]
- Singh, A.K.; Yen, C.-C.; Wuu, D.-S. Structural and photodetector characteristics of Zn and Al incorporated ZnGa2O4 films via co-sputtering. Results Phys. 2022, 33, 105206. [Google Scholar] [CrossRef]
- Lou, C.; Huang, Q.; Li, Z.; Lei, G.; Liu, X.; Zhang, J. Fe2O3-sensitized SnO2 nanosheets via atomic layer deposition for sensitive formaldehyde detection. Sens. Actuators B 2021, 345, 130429. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Bai, S.; Fu, H.; Guo, J.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Pyrolyzing Co/Zn bimetallic organic framework to form p-n heterojunction of Co3O4/ZnO for detection of formaldehyde. Sens. Actuators B 2019, 285, 291–301. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Li, J.; Zhang, J.; Yang, Z.; Wang, Q. Facile approach to prepare hierarchical Au-loaded In2O3 porous nanocubes and their enhanced sensing performance towards formaldehyde. Sens. Actuators B 2017, 241, 1130–1138. [Google Scholar] [CrossRef]
- Gao, X.; Li, F.; Wang, R.; Zhang, T. A formaldehyde sensor: Significant role of p-n heterojunction in gas-sensitive core-shell nanofibers. Sens. Actuators B 2018, 258, 1230–1241. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Y.P.; Zhao, J.; Dong, W.W.; Qiao, X.Q.; Hou, D.F.; Bu, X.; Li, D.S. Efficient Gas-Sensing for Formaldehyde with 3D Hierarchical Co3O4 Derived from Co5-Based MOF Microcrystals. Inorg. Chem. 2017, 56, 14111–14117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhang, J.; Wang, J.; Liu, J.; Yan, W. Hierarchical Heterojunctions of Metal Sulfide WS2 Nanosheets/Metal Oxide In2O3 Nanofibers for an Efficient Detection of Formaldehyde. Nanomaterials 2024, 14, 1702. https://doi.org/10.3390/nano14211702
Zhu L, Zhang J, Wang J, Liu J, Yan W. Hierarchical Heterojunctions of Metal Sulfide WS2 Nanosheets/Metal Oxide In2O3 Nanofibers for an Efficient Detection of Formaldehyde. Nanomaterials. 2024; 14(21):1702. https://doi.org/10.3390/nano14211702
Chicago/Turabian StyleZhu, Lei, Jiaxin Zhang, Jianan Wang, Jianwei Liu, and Wei Yan. 2024. "Hierarchical Heterojunctions of Metal Sulfide WS2 Nanosheets/Metal Oxide In2O3 Nanofibers for an Efficient Detection of Formaldehyde" Nanomaterials 14, no. 21: 1702. https://doi.org/10.3390/nano14211702
APA StyleZhu, L., Zhang, J., Wang, J., Liu, J., & Yan, W. (2024). Hierarchical Heterojunctions of Metal Sulfide WS2 Nanosheets/Metal Oxide In2O3 Nanofibers for an Efficient Detection of Formaldehyde. Nanomaterials, 14(21), 1702. https://doi.org/10.3390/nano14211702



