Twisted Nanographenes with Robust Conformational Stability
Abstract
:1. Introduction
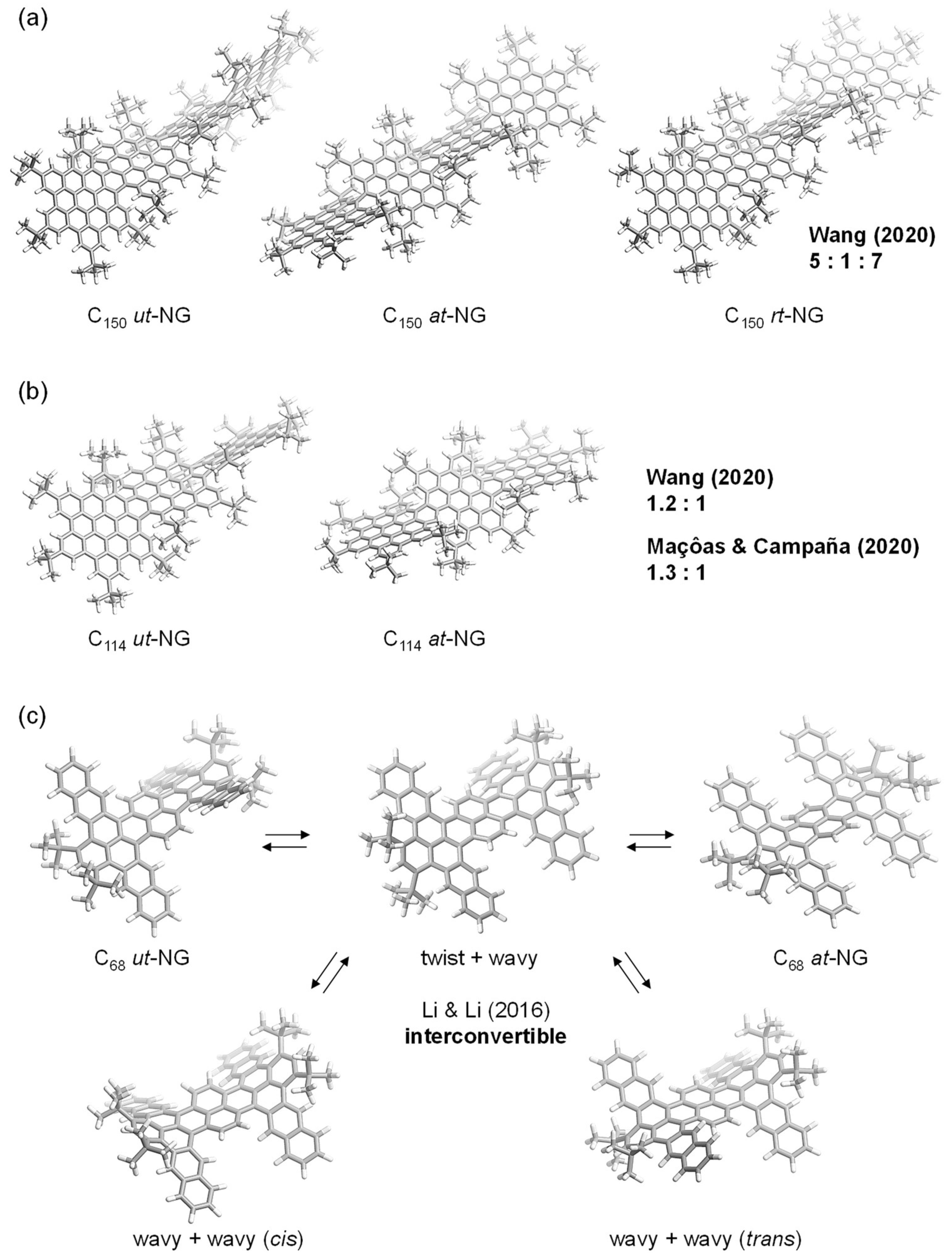
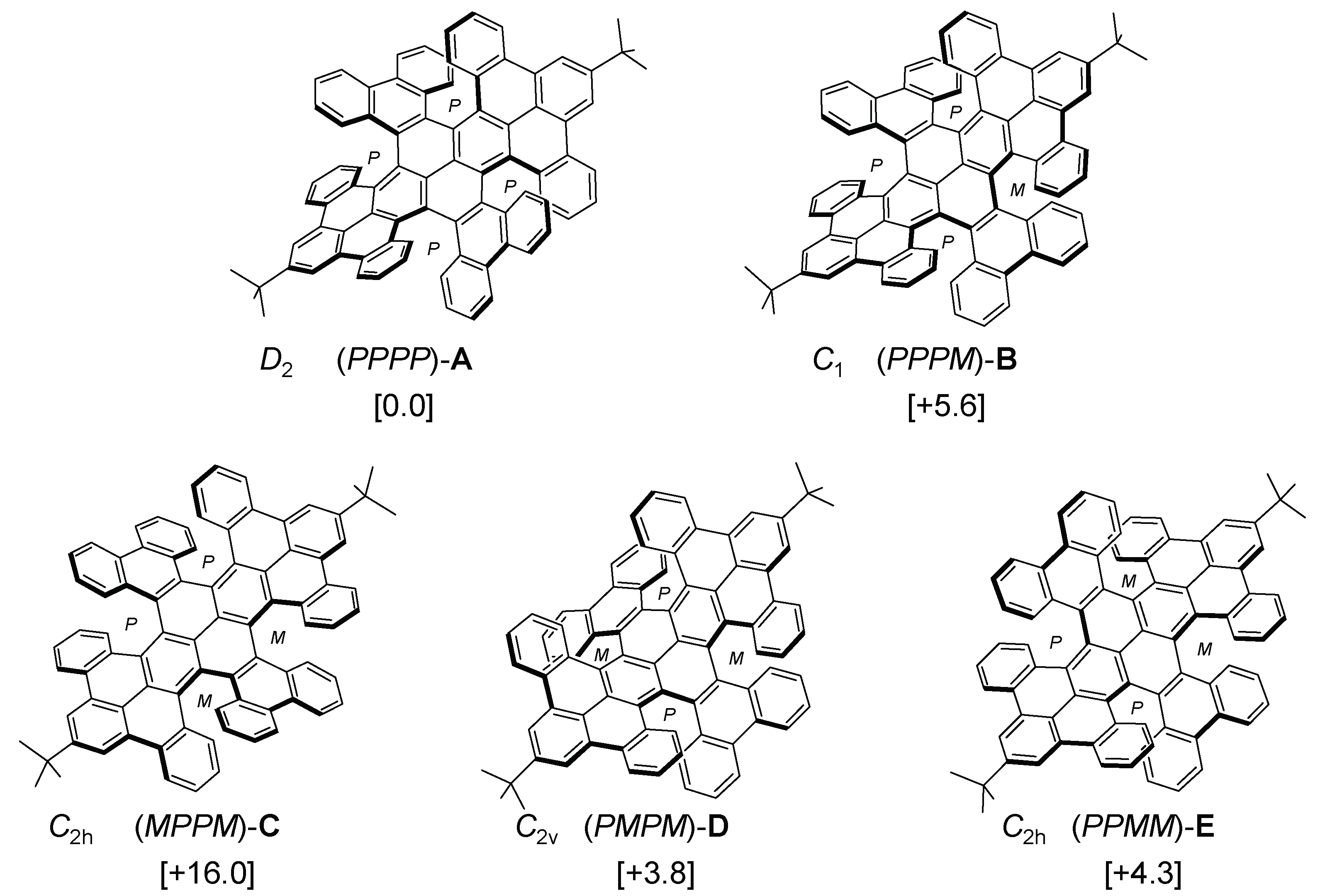
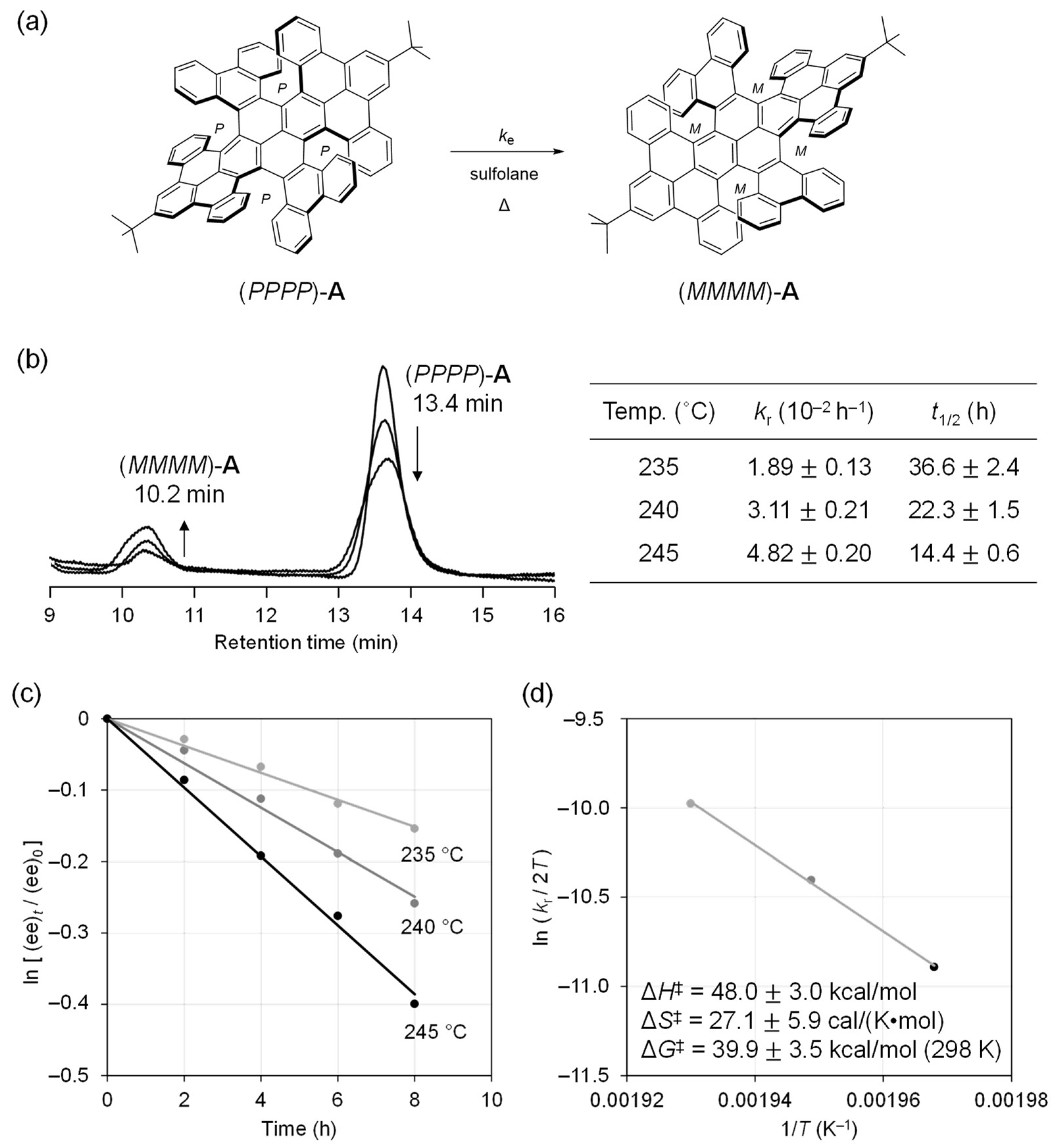
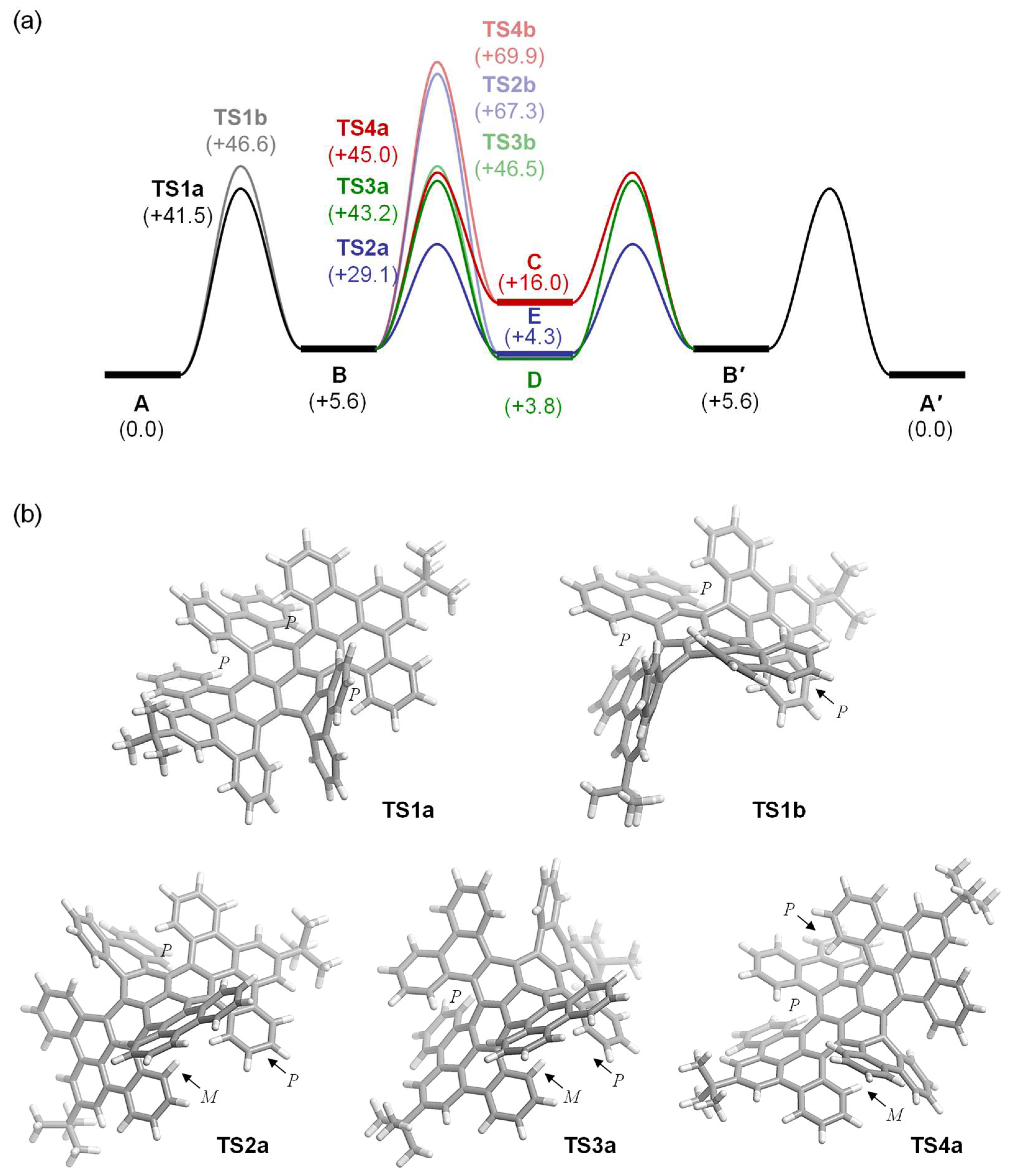
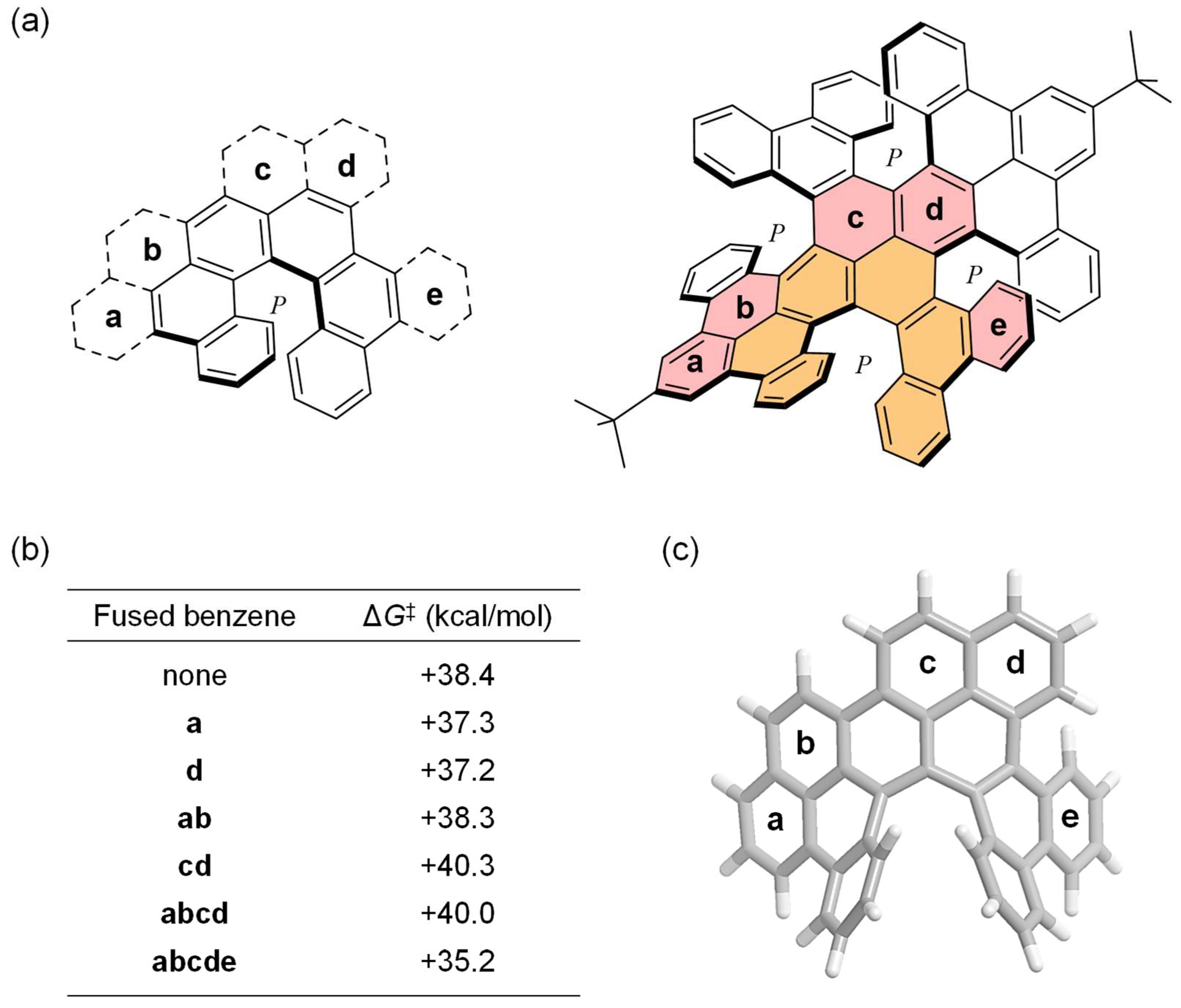
2. Results and Discussion
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Narita, A.; Wang, X.Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.; Zhong, Y.; Wu, Y.; Schenck, C.; Ng, F.; Steigerwald, M.; Xiao, S.; Nuckolls, C. Contorted Polycyclic Aromatics. Acc. Chem. Res. 2015, 48, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Ito, H.; Itami, K. Structurally uniform and atomically precise carbon nanostructures. Nat. Rev. Mater. 2016, 1, 15002. [Google Scholar] [CrossRef]
- Rickhaus, M.; Mayor, M.; Juríček, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46, 1643–1660. [Google Scholar] [CrossRef]
- Pun, S.H.; Miao, Q. Toward Negatively Curved Carbons. Acc. Chem. Res. 2018, 51, 1630–1642. [Google Scholar] [CrossRef]
- Majewski, M.A.; Stępień, M. Bowls, Hoops, and Saddles: Synthetic Approaches to Curved Aromatic Molecules. Angew. Chem. Int. Ed. 2019, 58, 86–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Pun, S.; Miao, Q. The Scholl Reaction as a Powerful Tool for the Synthesis of Curved Polycyclic Aromatics. Chem. Rev. 2022, 122, 14554–14593. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Z.; Müllen, K. Nanographenes and Graphene Nanoribbons as Multitalents of Present and Future Materials Science. J. Am. Chem. Soc. 2022, 144, 11499–11524. [Google Scholar] [CrossRef]
- Hashikawa, Y.; Okamoto, S.; Murata, Y. Synthesis of Inter-[60]Fullerene Conjugates with Inherent Chirality. Nat. Commun. 2024, 15, 514. [Google Scholar] [CrossRef]
- Anderson, H.V.; Gois, N.D.; Chalifoux, W.A. New advances in chiral nanographene chemistry. Org. Chem. Front. 2023, 10, 4167–4197. [Google Scholar] [CrossRef]
- Fernández-García, J.M.; Evans, P.J.; Filippone, S.; Herranz, M.Á.; Martín, N. Chiral Molecular Carbon Nanostructures. Acc. Chem. Res. 2019, 52, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Zhang, L.; Zhang, Q.; Xie, S.Y.; Zhang, L.S. Multiple [n]Helicenes with Various Aromatic Cores. Org. Chem. Front. 2022, 9, 4726–4743. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J. Helical Synthetic Nanographenes with Atomic Precision. Acc. Chem. Res. 2023, 56, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, R.; Zhang, Y.-K.; Wang, S.; Ma, B.; Zhang, B.; An, P. BINOL-like atropisomeric chiral nanographene. Chem. Sci. 2023, 14, 3286–3292. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, P.; Fernández-García, J.M.; Fernandez, I.; Perles, J.; Martín, N. Helically Arranged Chiral Molecular Nanographenes. J. Am. Chem. Soc. 2021, 143, 11864–11870. [Google Scholar] [CrossRef]
- Bernhardt, A.; Cavlovic, D.; Mayländer, M.; Blacque, O.; Cruz, C.M.; Richert, S.; Juríček, M. π-Radical Cascade to a Chiral Saddle-Shaped Peropyrene. Angew. Chem. Int. Ed. 2024, 63, e202318254. [Google Scholar] [CrossRef]
- Penty, S.E.; Orton, G.R.F.; Black, D.J.; Pal, R.; Zwijnenburg, M.A.; Barendt, T.A. A Chirally Locked Bis-perylene Diimide Macrocycle: Consequences for Chiral Self-Assembly and Circularly Polarized Luminescence. J. Am. Chem. Soc. 2024, 146, 5470–5479. [Google Scholar] [CrossRef]
- Eichelmann, R.; Jeudy, P.; Schneider, L.; Zerhoch, J.; Mayer, P.R.; Ballmann, J.; Deschler, F.; Gade, L.H. Chiral Bay-Alkynylated Tetraazaperylenes: Photophysics and Chiroptical Properties. Org. Lett. 2024, 26, 1172–1177. [Google Scholar] [CrossRef]
- Bam, R.; Yang, W.; Longhi, G.; Abbate, S.; Lucotti, A.; Tommasini, M.; Franzini, R.; Villani, C.; Catalano, V.J.; Olmstead, M.M.; et al. Chiral Teropyrenes: Synthesis, Structure, and Spectroscopic Studies. Angew. Chem. Int. Ed. 2024, 63, e202404849. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Wang, M.-W.; Chan, J.; Liu, G.; Wang, Z.; Jiang, W. Highly Luminescent Chiral Double π-Helical Nanoribbons. J. Am. Chem. Soc. 2024, 146, 5295–5304. [Google Scholar] [CrossRef]
- Dubey, R.K.; Melle-Franco, M.; Mateo-Alonso, A. Inducing Single-Handed Helicity in a Twisted Molecular Nanoribbon. J. Am. Chem. Soc. 2022, 144, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.; Radacki, K.; Braunschweig, H.; Ravat, P. Helically twisted nanoribbons via stereospecific annulative π-extension reaction employing [7]helicene as a molecular wrench. Chem. Sci. 2024, 15, 11737–11747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, Z.; Qiu, F.; Guo, Y.; Chen, Y.; Sun, Z.; Zhang, L. Hexabenzoheptacene: A Longitudinally Multihelicene Nanocarbon with Local Aromaticity and Enhanced Stability. Angew. Chem. Int. Ed. 2024, 63, e202407547. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gu, J.J.; Lin, C.J.; Luo, Z.X.; Zhu, Y.P.; Wang, J.B. Supertwistacene: A Helical Graphene Nanoribbon. J. Am. Chem. Soc. 2020, 142, 16887–16893. [Google Scholar] [CrossRef]
- Castro-Fernández, S.; Cruz, C.M.; Mariz, I.F.A.; Márquez, I.R.; Jiménez, V.G.; Palomino-Ruiz, L.; Cuerva, J.M.; Maçôas, E.; Campaña, A.G. Two-Photon Absorption Enhancement by the Inclusion of a Tropone Ring in Distorted Nanographene Ribbons. Angew. Chem. Int. Ed. 2020, 59, 7139–7145. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Xiao, S.; Liu, H.; Li, Y. A method for controlling the synthesis of stable twisted two-dimensional conjugated molecules. Nat. Commun. 2016, 7, 11637. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Hashikawa, Y.; Meng, H.; Bai, F.; Itami, K.; Chaolumen. A Double Twisted Nanographene with a Contorted Pyrene Core. Angew. Chem. Int. Ed. 2024, 63, e202406927. [Google Scholar] [CrossRef]
- Ravat, P. Carbo[n]helicenes Restricted to Enantiomerize: An Insight into the Design Process of Configurationally Stable Functional Chiral PAHs. Chem. Eur. J. 2021, 27, 3957–3966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, P.; Hashikawa, Y.; Chaolumen. Twisted Nanographenes with Robust Conformational Stability. Nanomaterials 2024, 14, 1737. https://doi.org/10.3390/nano14211737
Song P, Hashikawa Y, Chaolumen. Twisted Nanographenes with Robust Conformational Stability. Nanomaterials. 2024; 14(21):1737. https://doi.org/10.3390/nano14211737
Chicago/Turabian StyleSong, Penghui, Yoshifumi Hashikawa, and Chaolumen. 2024. "Twisted Nanographenes with Robust Conformational Stability" Nanomaterials 14, no. 21: 1737. https://doi.org/10.3390/nano14211737
APA StyleSong, P., Hashikawa, Y., & Chaolumen. (2024). Twisted Nanographenes with Robust Conformational Stability. Nanomaterials, 14(21), 1737. https://doi.org/10.3390/nano14211737




