Sustainable Scalable Mechanochemical Synthesis of CdS/Bi2S3 Nanocomposites for Efficient Hydrogen Evolution
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Mechanochemical Synthesis of CdS Nanoparticles
2.3. Preparation of Bi2S3 NPs via Mechanical Activation with Further Annealing
2.4. Fabrication of CdS/Bi2S3 Nanocomposite
2.5. Photocatalytic Hydrogen Evolution Test
2.6. Instruments and Characterization
3. Results and Discussions
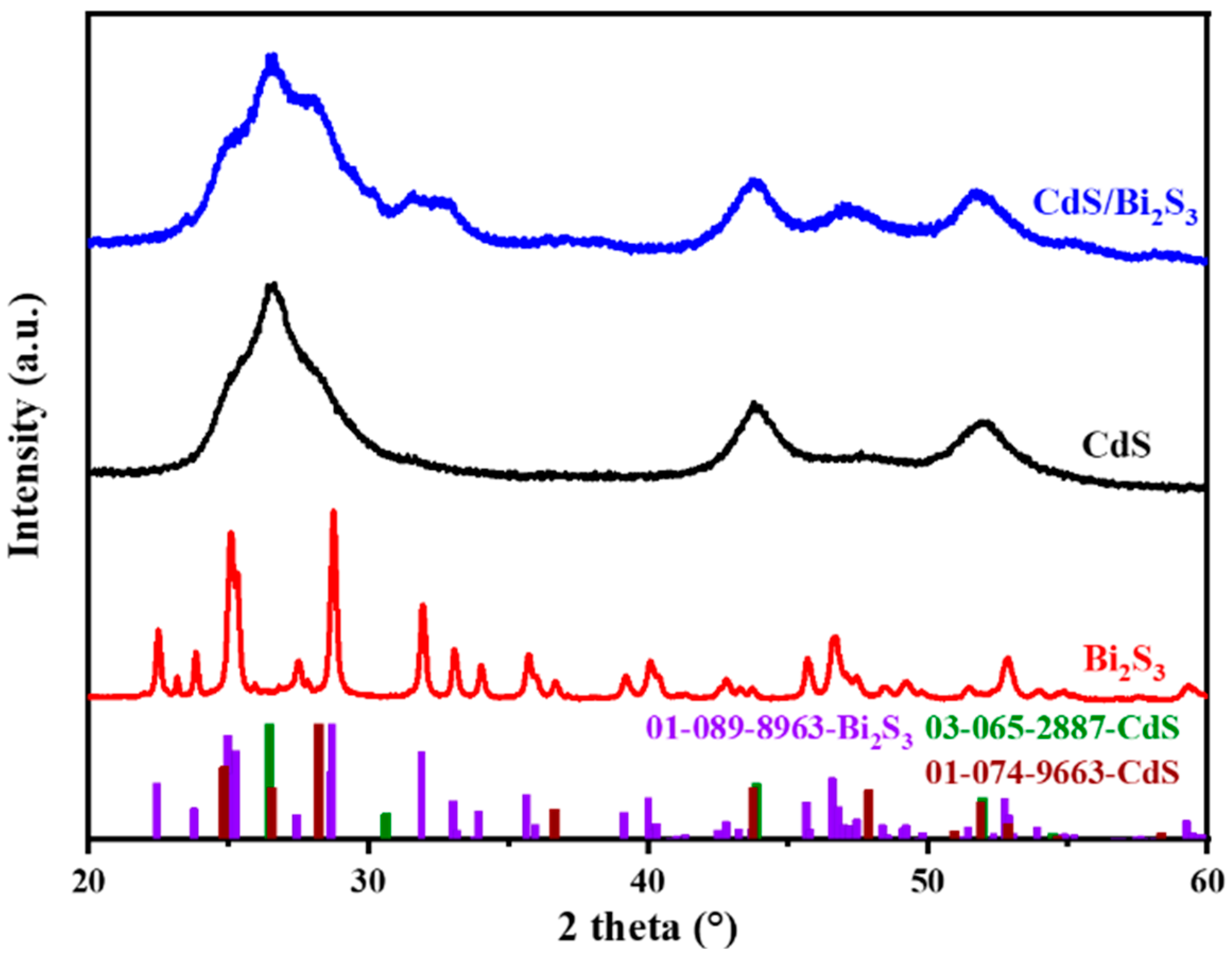
3.1. XRD Analysis
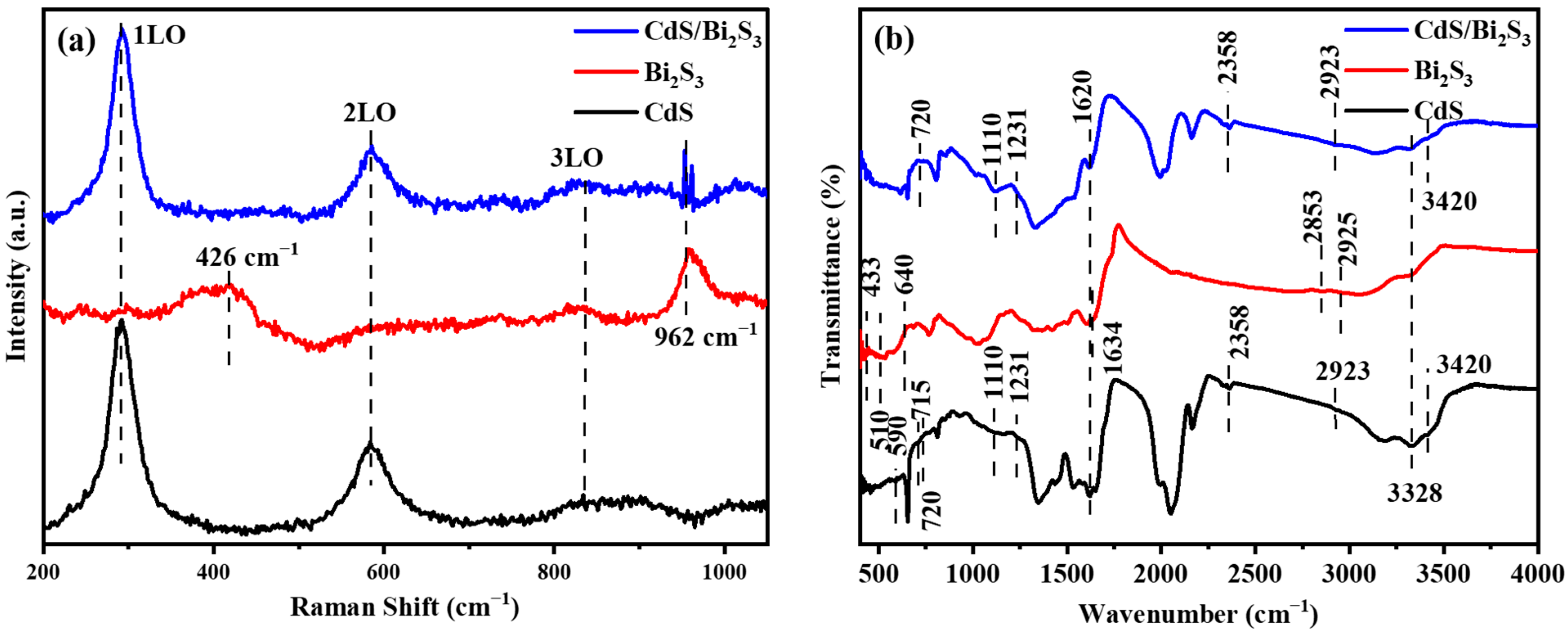
3.2. Raman Spectroscopy
3.3. FTIR Spectroscopy
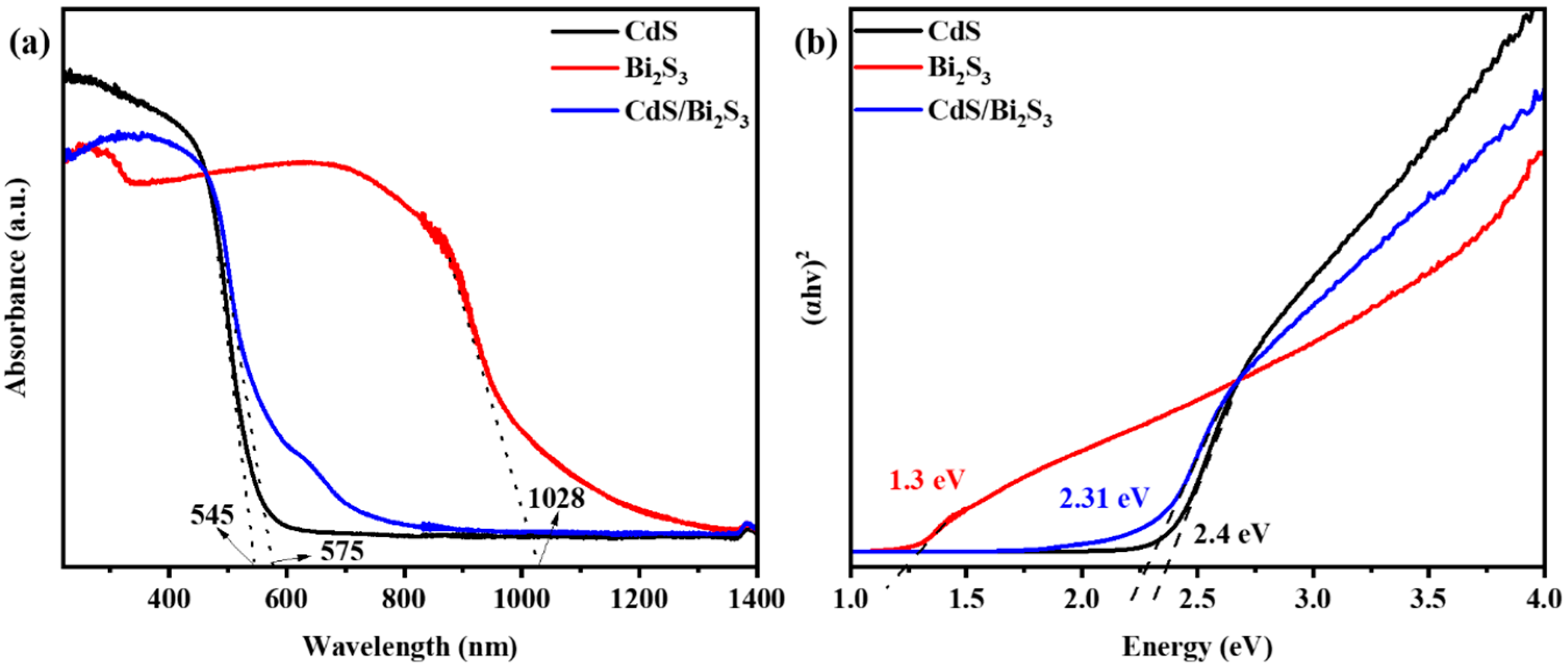
3.4. UV–Visible Spectroscopy
3.5. XPS Analysis
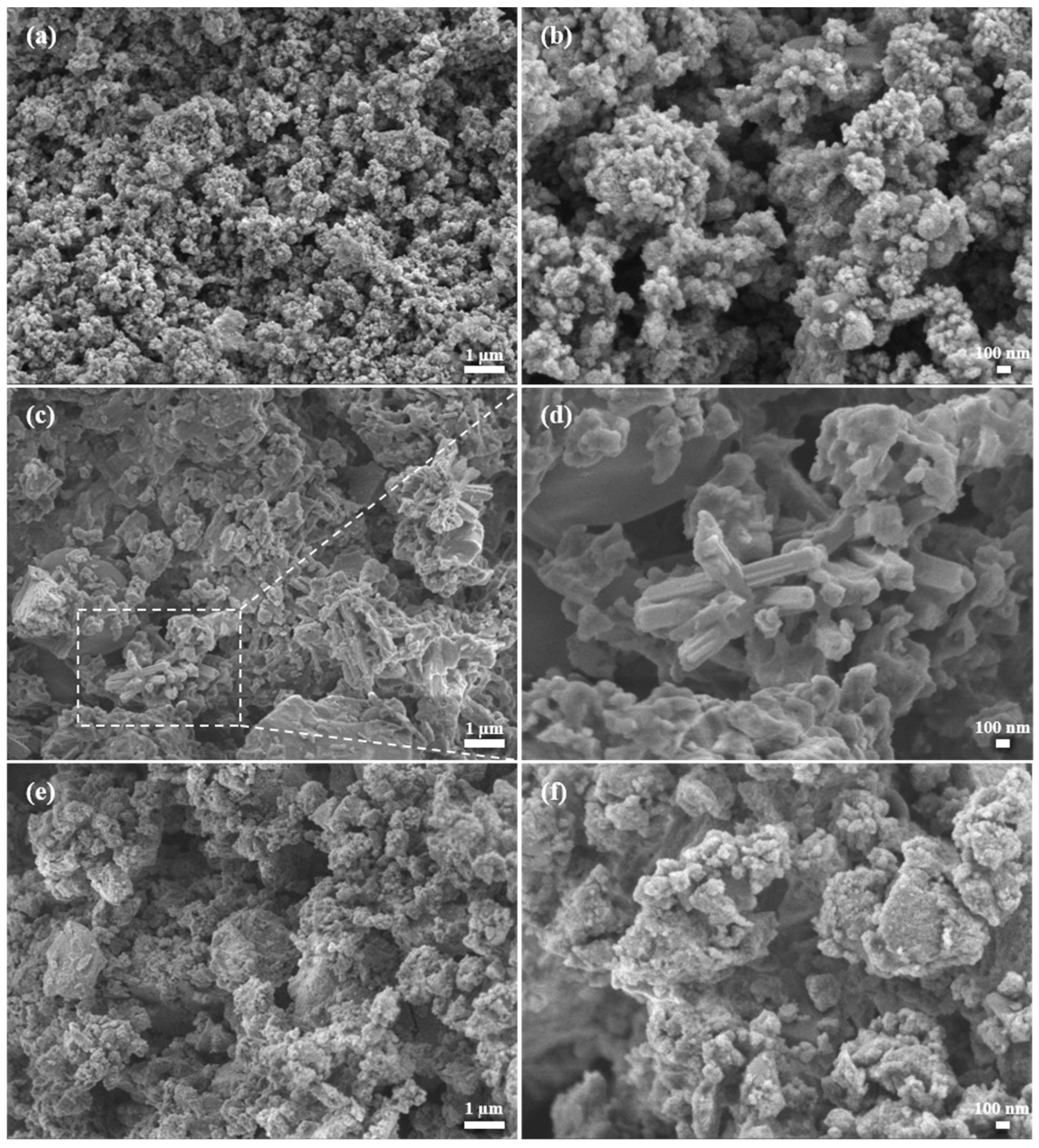
3.6. SEM Analysis
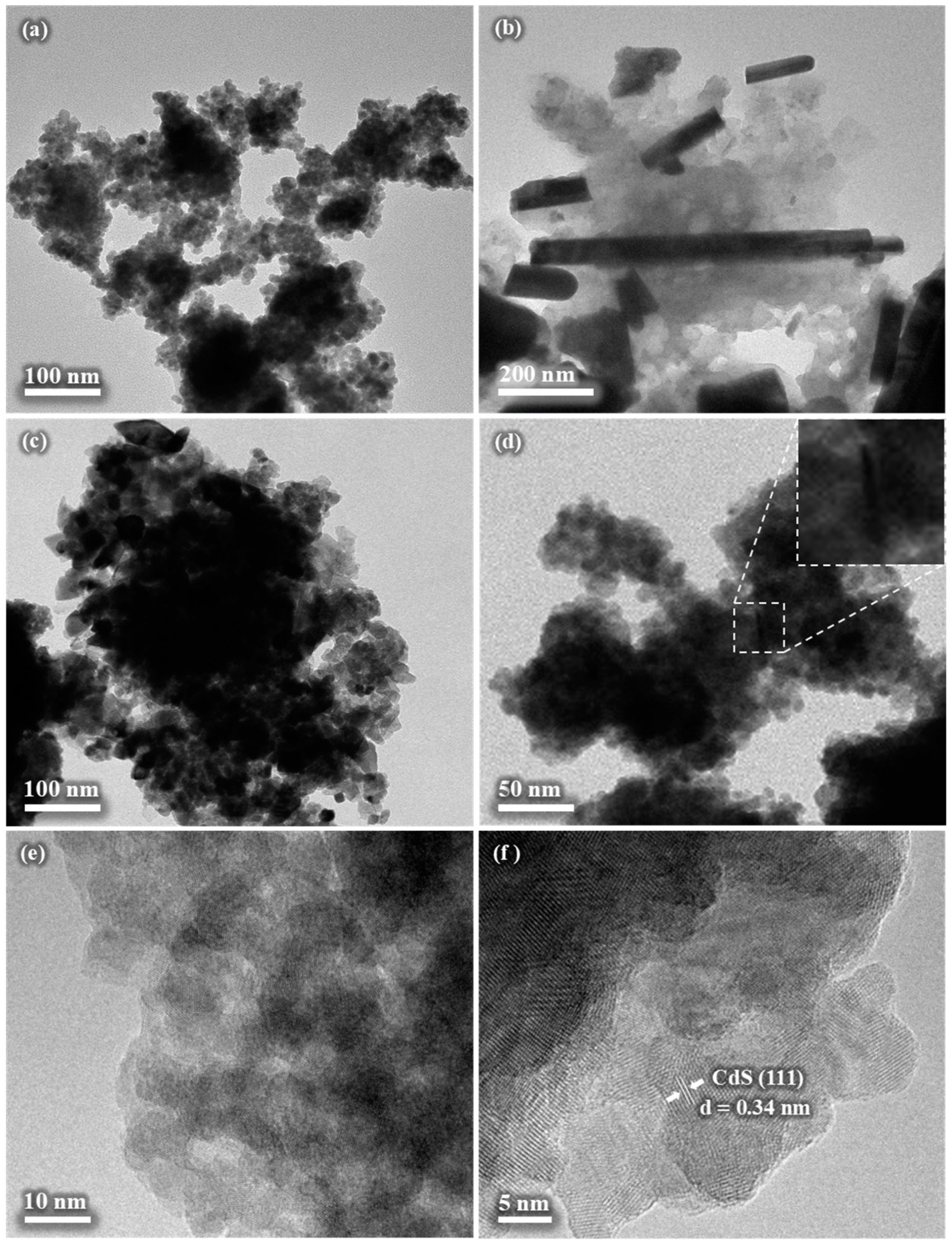
3.7. TEM Analysis
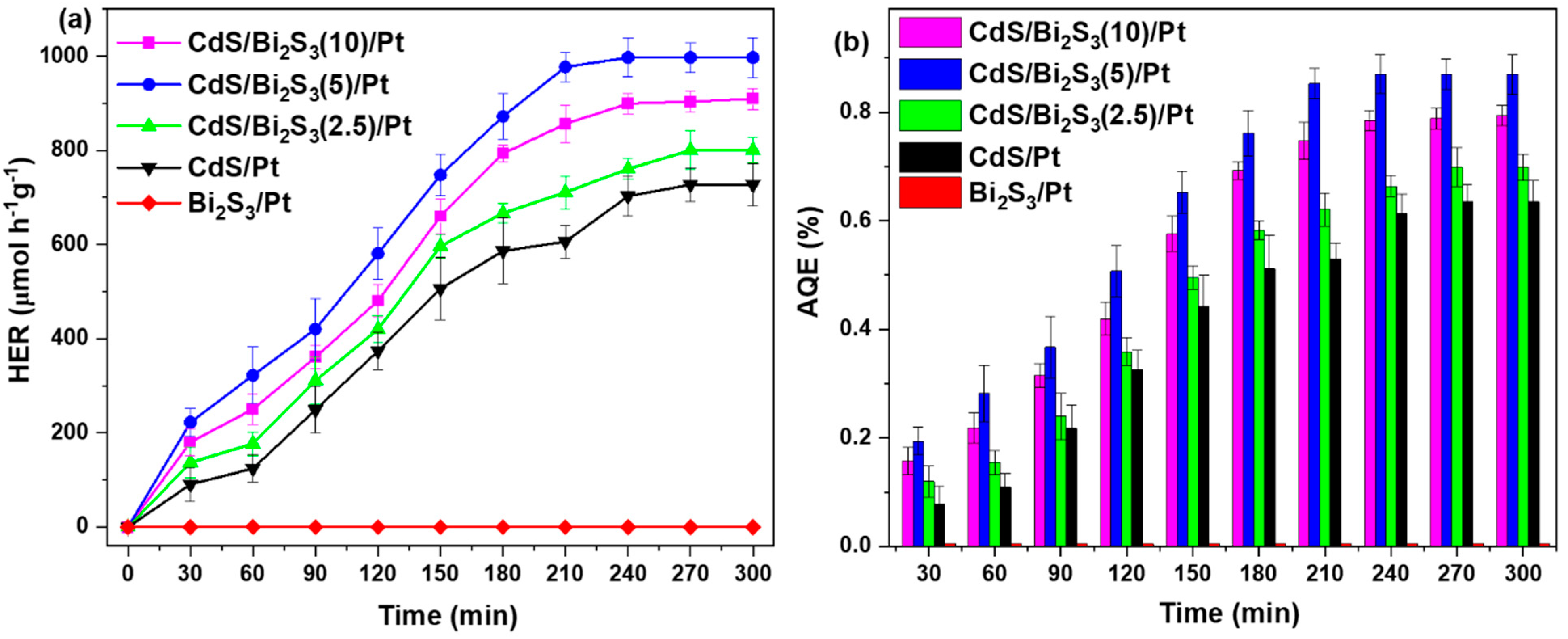
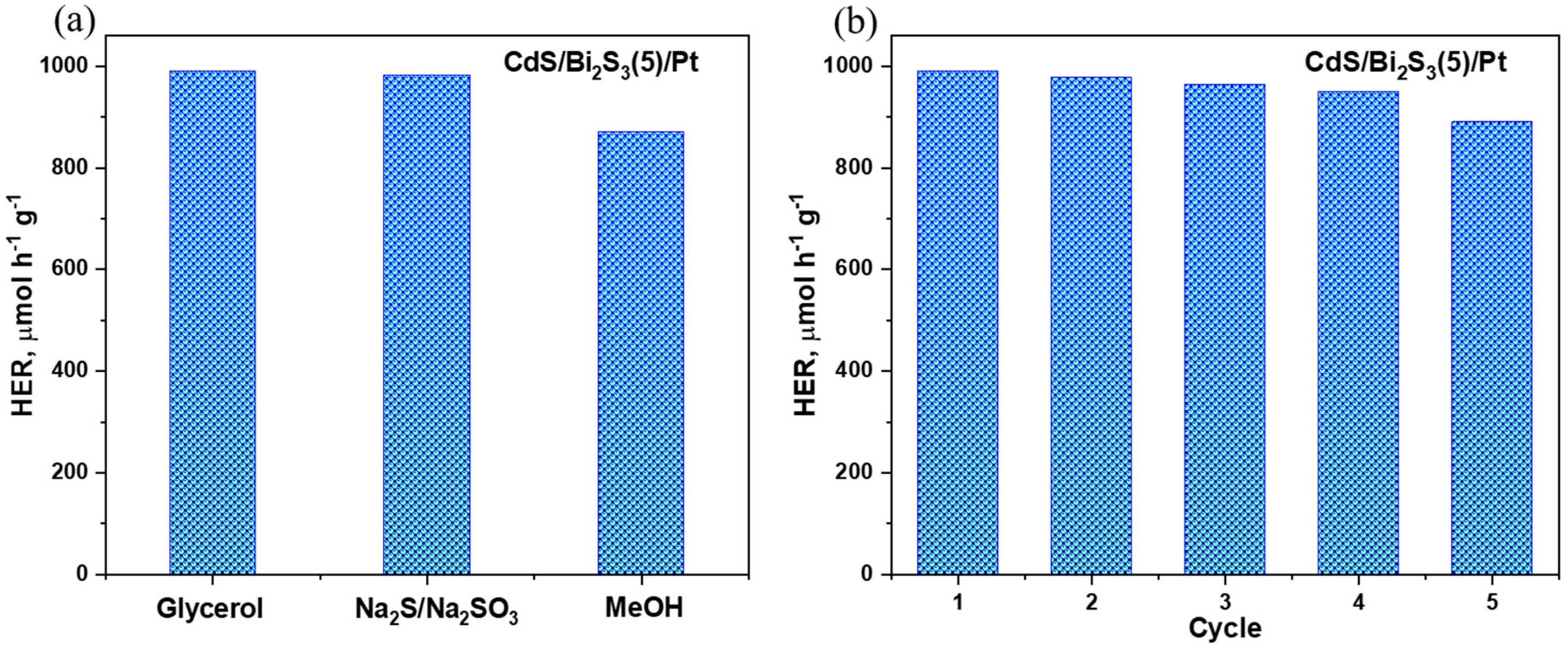
3.8. Photocatalytic Hydrogen Evolution Results
| Synthetic Method | Catalyst | Co-Catalyst | Precursors | Experimental Conditions | Light Source | HER, mmol g−1h−1 | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Synthesis Time | Temperature, t °C | |||||||
| Biomolecule-assisted | CdS | - | Cd(NO3)2∙4H2O, glutathione (GSH) | 24 h | 120 | Simulated sunlight | 0.085 | [77] |
| Hydrothermal | CdS | - | CdCl2, Na3PO4·12H2O, Na2S∙9H2O, H2PtCl6·6H2O | 12 h | 180 | Simulated sunlight | 0.0051 | [78] |
| CdS | Pt | 0.122 | ||||||
| Mechanochemical | CdS | NiS | Cd(NO3)2∙4H2O, CS(NH2)2, NaOH, NaCl, Ni(NO3)2, Na2S∙9H2O | 10 min | RT | Simulated sunlight | 0.192 | [42] |
| Hydrothermal | Bi2S3 | - | Bi(NO3)3∙5H2O, CO(NH2)2, Na2S∙9H2O | 12 h | 120 | Simulated sunlight | 0.271 | [79] |
| Hydrothermal | CdS/Bi2S3 | - | Bi(NO3)3∙5H2O, Cd(NO3)2∙4H2O, CS(NH2)2 | 10 h | 150 | Simulated sunlight | 0.38 | [30] |
| CdSQDs/Bi2S3 | - | Cd(NO3)2∙4H2O, EG, DMF, C2H4NS, Bi(NO3)3∙5H2O | 9 h | 200 | Simulated sunlight | 41.67 | [80] | |
| Intermediate-assisted chemical route | CdS/Bi2S3 | - | Bi(NO3)3∙5H2O, KOH, CS(NH2)2, CH3OH, Zn(CH3COO)2⋅2H2O, Cd(CH3COO)2·2H2O | 4 h 10 min | 200 | Simulated sunlight | 3.85 | [31] |
| Sonochemical | Bi2S3/CdS | - | Cd(CH3COO)2∙2H2O, Bi(NO3)3∙5H2O, CH3CSNH2 | 1 h | 60 | Visible light | 5.5 | [23] |
| Mechanochemical | CdS/Bi2S3 | Pt | Cd(NO3)2∙4H2O, PtCl2, Bi(NO3)3∙5H2O, CS(NH2)2 | 2 h 10 min | 275 | Simulated sunlight | 0.997 | this work |
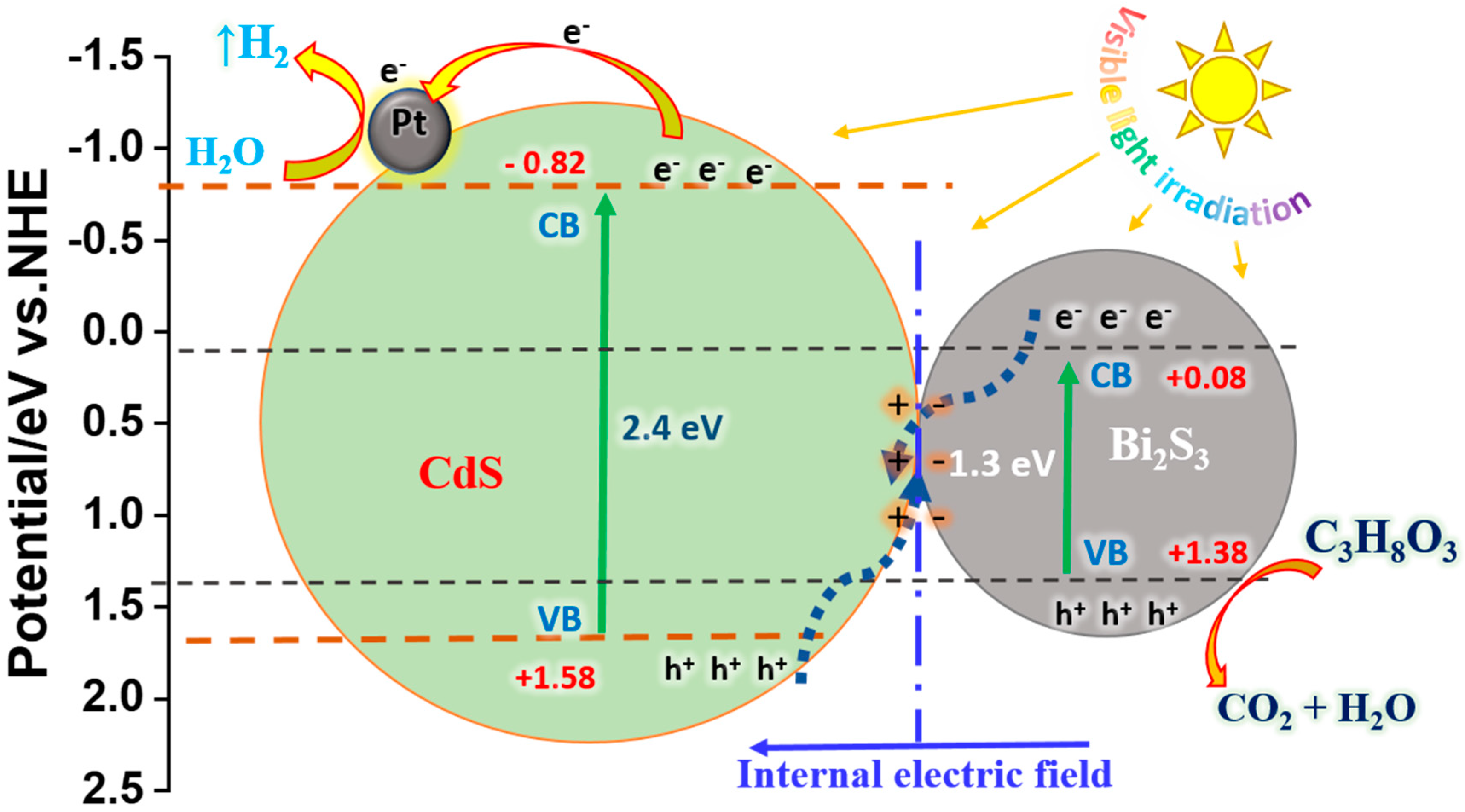
The Mechanism of Photocatalytic Hydrogen Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winter, C.-J. Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Oruc, O.; Dincer, I. Assessing the potential of thermo-chemical water splitting cycles: A bridge towards clean and sustainable hydrogen generation. Fuel 2021, 286, 119325. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Shanmugam, S.; Sekar, M.; Mathimani, T.; Incharoensakdi, A.; Kim, S.-H.; Parthiban, A.; Geo, V.E.; Brindhadevi, K.; Pugazhendhi, A. Insights on biological hydrogen production routes and potential microorganisms for high hydrogen yield. Fuel 2021, 291, 120136. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A review of hydrogen production processes by photocatalytic water splitting—From atomistic catalysis design to optimal reactor engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A critical review of renewable hydrogen production methods: Factors affecting their scale-up and its role in future energy generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Reddy, N.L.; Rao, V.N.; Kumari, M.M.; Kakarla, R.R.; Ravi, P.; Sathish, M.; Karthik, M.; Venkatakrishnan, S.M.; Inamuddin. Nanostructured semiconducting materials for efficient hydrogen generation. Environ. Chem. Lett. 2018, 16, 765–796. [Google Scholar] [CrossRef]
- Khan, N.; Burashev, G.; Kadylbekova, A.; Atabaev, T.S.; Bakenov, Z.; Sultanov, F.; Mentbayeva, A.; Tatykayev, B. Sustainable scalable solid-state synthesis of highly efficient synergetic 2D/0D micro/nanostructured g-C3N4/CdS photocatalysts for hydrogen production and water purification. Sustain. Mater. Technol. 2024, 41, e01063. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Zhu, H.; Gou, L.; Li, C.; Fu, X.; Weng, Y.; Chen, L.; Fang, B.; Shuai, L.; Liao, G. Dual interfacial electric fields in black phosphorus/MXene/MBene enhance broad-spectrum carrier migration efficiency of photocatalytic devices. Device 2024, 2, 100283. [Google Scholar] [CrossRef]
- Manohar, A.; Suvarna, T.; Chintagumpala, K.; Ubaidullah, M.; Mameda, N.; Kim, K.H. Exploring progress in binary and ternary nanocomposites for photoelectrochemical water splitting: A comprehensive review. Coord. Chem. Rev. 2025, 522, 216180. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Z.; Zhou, W. Semiconductor-Based Nanomaterials for Photocatalytic Hydrogen Generation. In Hydrogen Production Technologies; Scrivener Publishing: Austin, TX, USA, 2017; pp. 487–543. [Google Scholar]
- Yuan, Y.-J.; Chen, D.; Yu, Z.-T.; Zou, Z.-G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Bai, Y.; Lian, J.; Huang, H.; Li, Z.; Lei, Z.; Shangguan, W. Photochemical preparation of Cd/CdS photocatalysts and their efficient photocatalytic hydrogen production under visible light irradiation. Green Chem. 2014, 16, 2728–2735. [Google Scholar] [CrossRef]
- Zheng, L.; Teng, F.; Ye, X.; Zheng, H.; Fang, X. Photo/electrochemical applications of metal sulfide/TiO2 heterostructures. Adv. Energy Mater. 2020, 10, 1902355. [Google Scholar] [CrossRef]
- Rao, V.N.; Ravi, P.; Sathish, M.; Vijayakumar, M.; Sakar, M.; Karthik, M.; Balakumar, S.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; et al. Metal chalcogenide-based core/shell photocatalysts for solar hydrogen production: Recent advances, properties and technology challenges. J. Hazard. Mater. 2021, 415, 125588. [Google Scholar]
- Vattikuti, S.P.; Shim, J.; Byon, C. Synthesis, characterization, and optical properties of visible light-driven Bi2S3 nanorod photocatalysts. J. Mater. Sci. Mater. Electron. 2017, 28, 14282–14292. [Google Scholar] [CrossRef]
- Hao, L.-X.; Chen, G.; Yu, Y.-G.; Zhou, Y.-S.; Han, Z.-H.; Liu, Y. Sonochemistry synthesis of Bi2S3/CdS heterostructure with enhanced performance for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 14479–14486. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Z.; Liu, D.; Tan, Z.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. S-scheme Bi2S3/CdS nanorod heterojunction photocatalysts with improved carrier separation and redox capacity for pollutant removal. ACS Appl. Nano Mater. 2022, 5, 5448–5458. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Ranjith, K.S.; Park, B.; Hwang, S.-K.; Hosseini, R.; Behjatmanesh-Ardakani, R.; Pourmortazavi, S.M.; Lee, H.U.; Son, B.; Mirsadeghi, S.; et al. Full-spectrum-responsive Bi2S3@CdS S-scheme heterostructure with intimated ultrathin RGO toward photocatalytic Cr(VI) reduction and H2O2 production: Experimental and DFT studies. Chem. Eng. J. 2021, 419, 129530. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.; Wang, C.; You, B.; Li, B.; Han, J.; Jiang, S.; Zhang, C.; He, S. Zeolitic imidazolate framework-67 and its derivatives for photocatalytic applications. Coord. Chem. Rev. 2024, 502, 215612. [Google Scholar] [CrossRef]
- Sharma, S.; Sonu; Sudhaik, A.; Khan, A.A.P.; Saini, A.K.; Mittal, D.; Nguyen, V.-H.; van Le, Q.; Ahamad, T.; Raizada, P. Potential of novel dual Z-scheme carbon quantum dots decorated MnIn2S4/CdS/Bi2S3 heterojunction for environmental applications. Environ. Sci. Pollut. Res. 2023, 30, 77622–77641. [Google Scholar] [CrossRef]
- Zhang, L.; Ai, Z.; Xu, X.; Shi, D.; Zhang, B.; Hu, H.; Yang, M.; Shao, Y.; Wu, Y.; Hao, X. Research progress on synthetic and modification strategies of CdS-based photocatalysts. Ionics 2023, 29, 2115–2139. [Google Scholar] [CrossRef]
- Yang, M.; Shi, Y.; Li, Y.; Li, H.; Luo, N.; Li, J.; Fan, J.; Zhou, A. Construction of 2D Bi2S3/CdS Nanosheet Arrays for Enhanced Photoelectrochemical Hydrogen Evolution. J. Electron. Mater. 2019, 48, 6397–6405. [Google Scholar] [CrossRef]
- Li, M.; Yao, H.; Yao, S.; Chen, G.; Sun, J. Vacancy-induced tensile strain of CdS/Bi2S3 as a highly performance and robust photocatalyst for hydrogen evolution. J. Colloid Interface Sci. 2023, 630, 224–234. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Wang, X.; Zhang, T.; Li, D.; Sun, M.; Deng, C. An intermediary route to CdS/Bi2S3 architectures with high-activity and stability in photocatalytic water splitting and dye degradation. Mater. Sci. Eng. B 2024, 305, 117431. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Li, H.; Li, J.; Xu, Y.; Liu, Y.; Zhou, J. Photoreduction of CO2 to methanol over Bi2S3/CdS photocatalyst under visible light irradiation. J. Nat. Gas Chem. 2011, 20, 413–417. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, Y.; Fan, Y.; Ni, Y.; Wei, X.; Tang, K.; Shen, J.; Chen, Y. Epitaxial growth of CdS nanoparticle on Bi2S3 nanowire and photocatalytic application of the heterostructure. J. Phys. Chem. C 2011, 115, 13968–13976. [Google Scholar] [CrossRef]
- Zhang, N.; Lü, J.; Cao, R. Photocatalytic CO2–to–Ethylene conversion over Bi2S3/CdS heterostructures constructed via facile cation exchange. Research 2022, 2022, 9805879. [Google Scholar]
- Yan, Y.; Zhou, Z.; Li, W.; Zhu, Y.; Cheng, Y.; Zhao, F.; Zhou, J. Facile ion-exchange synthesis of urchin-shaped CdS/Bi2S3 heterostructures with enhanced photostability and visible light photocatalytic activity. RSC Adv. 2014, 4, 38558–38567. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Tian, G.; Fu, H.; Pan, K.; Zhou, J.; Yan, H. One-pot controlled synthesis of sea-urchin shaped Bi2S3/CdS hierarchical heterostructures with excellent visible light photocatalytic activity. Dalton Trans. 2014, 43, 12396–12404. [Google Scholar] [CrossRef]
- Panmand, R.P.; Sethi, Y.A.; Deokar, R.S.; Late, D.J.; Gholap, H.M.; Baeg, J.-O.; Kale, B.B. In situ fabrication of highly crystalline CdS decorated Bi2S3 nanowires (nano-heterostructure) for visible light photocatalyst application. RSC Adv. 2016, 6, 23508–23517. [Google Scholar] [CrossRef]
- Uddin, M.R.; Khan, M.R.; Rahman, M.W.; Cheng, C.K.; Yousuf, A. Photocatalytic conversion of CO2 into methanol: Significant enhancement of the methanol yield over Bi2S3/CdS photocatalyst. Int. J. Eng. Technol. Sci. 2015, 3. [Google Scholar] [CrossRef]
- Desale, D.J.; Shaikh, S.; Ghosh, A.; Birajadar, R.; Siddiqui, F.; Ghule, A.; Sharma, R.B. Preparation and characterization of CdS–Bi2S3 nanocomposite thin film by successive ionic layer adsorption and reaction (SILAR) method. Compos. Part B Eng. 2012, 43, 1095–1100. [Google Scholar] [CrossRef]
- Mendhe, A.C.; Majumder, S.; Pawar, S.M.; Sankapal, B.R. Facile Bi2S3 nanoparticles on CdS nanowires surface: Core-shell nanostructured design towards solar cell application. Surf. Interfaces 2021, 27, 101457. [Google Scholar] [CrossRef]
- Shalabayev, Z.; Baláž, M.; Khan, N.; Nurlan, Y.; Augustyniak, A.; Daneu, N.; Tatykayev, B.; Dutková, E.; Burashev, G.; Casas-Luna, M.; et al. Sustainable synthesis of cadmium sulfide, with applicability in photocatalysis, hydrogen production, and as an antibacterial agent, using two mechanochemical protocols. Nanomaterials 2022, 12, 1250. [Google Scholar] [CrossRef]
- Burashev, G.; Tatykayev, B.; Baláž, M.; Khan, N.; Jumagazieva, A.; Iskakbayeva, Z.; Seysembekova, A.; Tugelbay, S.; Turgynbay, N.; Niyazbayeva, A.; et al. The superiority of the photocatalytic and antibacterial performance of mechanochemically synthesized CdS nanoparticles over solvothermal-prepared ones. Semicond. Sci. Technol. 2024, 39, 045006. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Dai, K.; Zhang, J. Review on inorganic–organic S-scheme photocatalysts. J. Mater. Sci. Technol. 2023, 165, 187–218. [Google Scholar] [CrossRef]
- Colacino, E.; Dayaker, G.; Morère, A.; Friščić, T. Introducing students to mechanochemistry via environmentally friendly organic synthesis using a solvent-free mechanochemical preparation of the antidiabetic drug tolbutamide. J. Chem. Educ. 2019, 96, 766–771. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal–organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Malca, M.Y.; Bao, H.; Bastaille, T.; Saadé, N.K.; Kinsella, J.M.; Friščić, T.; Moores, A. Mechanically activated solvent-free assembly of ultrasmall Bi2S3 nanoparticles: A novel, simple, and sustainable means to access chalcogenide nanoparticles. Chem. Mater. 2017, 29, 7766–7773. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, E.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 photocatalysts prepared by thermolysis of g-CN/MIL-125(Ti) composites for efficient pollutant degradation and hydrogen production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Evans, J.S. Advanced input files & parametric quantitative analysis using topas. In Material Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2010; Volume 651, pp. 1–9. [Google Scholar]
- Motaung, M.P.; Onwudiwe, D.C.; Lei, W. Microwave-assisted synthesis of Bi2S3 and Sb2S3 nanoparticles and their photoelectrochemical properties. ACS Omega 2021, 6, 18975–18987. [Google Scholar] [CrossRef]
- Kumari, P.; Awasthi, K.; Agarwal, S.; Ichikawa, T.; Kumar, M.; Jain, A. Flower-like Bi2S3 nanostructures as highly efficient anodes for all-solid-state lithium-ion batteries. RSC Adv. 2019, 9, 29549–29555. [Google Scholar] [CrossRef]
- Kumar, P.; Saxena, N.; Chandra, R.; Gupta, V.; Agarwal, A.; Kanjilal, D. Nanotwinning and structural phase transition in CdS quantum dots. Nanoscale Res. Lett. 2012, 7, 584. [Google Scholar] [CrossRef]
- Briggs, R.; Ramdas, A. Piezospectroscopic study of the Raman spectrum of cadmium sulfide. Phys. Rev. B 1976, 13, 5518. [Google Scholar] [CrossRef]
- Tell, B.; Damen, T.; Porto, S. Raman effect in cadmium sulfide. Phys. Rev. 1966, 144, 771. [Google Scholar] [CrossRef]
- Chuu, D.; Dai, C.; Hsieh, W.; Tsai, C. Raman investigations of the surface modes of the crystallites in CdS thin films grown by pulsed laser and thermal evaporation. J. Appl. Phys. 1991, 69, 8402–8404. [Google Scholar] [CrossRef]
- Richter, H.; Wang, Z.; Ley, L. The one phonon Raman spectrum in microcrystalline silicon. Solid State Commun. 1981, 39, 625–629. [Google Scholar] [CrossRef]
- Sambathkumar, C.; Manirathinam, V.; Manikandan, A.; Krishna Kumar, M.; Sudhahar, S.; Devendran, P. Solvothermal synthesis of Bi2S3 nanoparticles for active photocatalytic and energy storage device applications. J. Mater. Sci. Mater. Electron. 2021, 32, 20827–20843. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Police, A.K.R.; Shim, J.; Byon, C. Sacrificial-template-free synthesis of core-shell C@Bi2S3 heterostructures for efficient supercapacitor and H2 production applications. Sci. Rep. 2018, 8, 4194. [Google Scholar] [CrossRef]
- Zuo, X.-Q.; Yang, X.; Zhou, L.; Yang, B.; Li, G.; Tang, H.-B.; Zhang, H.-J.; Wu, M.-Z.; Ma, Y.-Q.; Jin, S.-W. Facile synthesis of Bi₂S3–C composite microspheres as low-cost counter electrodes for dye-sensitized solar cells. RSC Adv. 2014, 4, 57412–57418. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Boughdachi, S.; Azizian-Kalandaragh, Y.; Badali, Y.; Altındal, Ş. Facile ultrasound-assisted and microwave-assisted methods for preparation of Bi2S3-PVA nanostructures: Exploring their pertinent structural and optical properties and comparative studies on the electrical, properties of Au/(Bi2S3-PVA)/n-Si Schottky structure. J. Mater. Sci. Mater. Electron. 2017, 28, 17948–17960. [Google Scholar]
- López-Cabaña, Z.; Torres, C.M.S.; González, G. Semiconducting properties of layered cadmium sulphide-based hybrid nanocomposites. Nanoscale Res. Lett. 2011, 6, 523. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, P.; Sharma, V. Redshift in Absorption Edge of Cd1−xCoxS Nanofilms. IEEE Trans. Nanotechnol. 2014, 13, 343–348. [Google Scholar] [CrossRef]
- Tauc, J.; Scott, T.A. The optical properties of solids. Phys. Today 1967, 20, 105–107. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hou, C.; Chen, Y.; He, T. Highly efficient visible-light driven solar-fuel production over tetra(4-carboxyphenyl)porphyrin iron(III) chloride using CdS/Bi2S3 heterostructure as photosensitizer. Appl. Catal. B Environ. 2018, 238, 656–663. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Wei, Z.; Liu, H.; Peng, J.; Zhou, L.; Li, H.; Fan, H. Cell imaging using two-photon excited CdS fluorescent quantum dots working within the biological window. Nanomaterials 2019, 9, 369. [Google Scholar] [CrossRef]
- Wei, Z.; Mao, W.; Liu, J.; Xiao, Y.; Zhu, M.; Tian, Y. CdS nanorods decorated with ultrathin MoS2 nanosheets for efficient visible-light photocatalytic H2 production. J. Mater. Sci. Mater. Electron. 2020, 31, 4574–4581. [Google Scholar] [CrossRef]
- Khan, M.S.; Gul, B.; Khan, G.; Benaadad, M.; Ghlamallah, B.; Khattak, S.A.; Khan, T.; Zulfiqar, S.; Shah, S.K.; Khan, M.A. Ab-initio study about the electronic, optical and thermoelectric nature of α-, β-, and γ-phases of CdS semiconductor: Using the accurate m-BJ approach. Phys. Scr. 2021, 96, 055803. [Google Scholar]
- Luo, X.; Wang, G.; Huang, Y.; Wang, B.; Yuan, H.; Chen, H. A two-dimensional layered CdS/C2 N heterostructure for visible-light-driven photocatalysis. Phys. Chem. Chem. Phys. 2017, 19, 28216–28224. [Google Scholar] [CrossRef]
- Mahendra, K.; Anupriya, J.; Gajendra, N.; Madhusudhan, C.; Nagaraja, B.; Pattar, J.; Udayashankar, N. Visible-light-driven photocatalytic degradation of organic dyes over cubic-phase and hexagonal-phase CdS: A comparative study. J. Mater. Sci. Mater. Electron. 2023, 34, 730. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Mu, Y.; Zheng, M.; Wang, Y. Photocatalytic performance of cubic and hexagonal phase CdS synthesized via different Cd sources. J. Electron. Mater. 2019, 48, 2895–2901. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Sahu, M.; Park, C. A comprehensive review on bismuth-sulfide-based compounds. Mater. Today Sustain. 2023, 23, 100441. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal sulfide photocatalysts for hydrogen generation: A review of recent advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Lin, L.; Yang, X.; Luo, X.; Guo, S.; Xu, X. Single-atomic activation on ZnIn2S4 basal planes boosts photocatalytic hydrogen evolution. Nano Res. 2024, 17, 5949–5955. [Google Scholar] [CrossRef]
- Wang, S.; Liao, W.; Su, H.; Pang, S.; Yang, C.; Fu, Y.; Zhang, Y. Review on the application of semiconductor heterostructures in photocatalytic hydrogen evolution: State-of-the-art and outlook. Energy Fuels 2023, 37, 1633–1656. [Google Scholar] [CrossRef]
- Li, C.; Yuan, J.; Han, B.; Shangguan, W. Synthesis and photochemical performance of morphology-controlled CdS photocatalysts for hydrogen evolution under visible light. Int. J. Hydrogen Energy 2011, 36, 4271–4279. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Peng, S.; Li, Z.; Lu, G. Phosphate-assisted hydrothermal synthesis of hexagonal CdS for efficient photocatalytic hydrogen evolution. CrystEngComm 2012, 14, 6974–6982. [Google Scholar] [CrossRef]
- Li, X.; Jun, L.; Xiao, J.; Xu, Y.; Yang, C.; Tang, J.; Zhou, K.; Gong, X.; Zhou, X.; Zou, H. Study on the relationship between Bi2S3 with different morphologies and its photocatalytic hydrogen production performance. J. Anal. Sci. Technol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Nagajyothi, P.; Shim, J. Fabrication of CdS quantum dot/Bi2S3 nanocomposite photocatalysts for enhanced H2 production under simulated solar light. J. Mater. Sci. Mater. Electron. 2019, 30, 5681–5690. [Google Scholar] [CrossRef]
- Nethercot Jr, A.H. Prediction of Fermi energies and photoelectric thresholds based on electronegativity concepts. Phys. Rev. Lett. 1974, 33, 1088. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Li, H. Comparative study on the mechanism in photocatalytic degradation of different-type organic dyes on SnS2 and CdS. Appl. Catal. B Environ. 2012, 123, 174–181. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wu, C.; Gao, J.; Li, M.; Xing, Z.; Li, Z.; Zhou, W. Hollow nanoboxes Cu2−xS@ZnIn2S4 core-shell S-scheme heterojunction with broad-spectrum response and enhanced photothermal-photocatalytic performance. Small 2022, 18, 2202544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Hasija, V.; Malhotra, M.; Verma, P.K.; Khan, A.A.P.; Thakur, S.; van Le, Q.; Quang, H.H.P.; Nguyen, V.-H.; Singh, P.; et al. A review of CdS-based S-scheme for photocatalytic water splitting: Synthetic strategy and identification techniques. Int. J. Hydrogen Energy 2023, 52, 804–818. [Google Scholar] [CrossRef]
- Hasija, V.; Kumar, A.; Sudhaik, A.; Raizada, P.; Singh, P.; van Le, Q.; Le, T.T.; Nguyen, V.-H. Step-scheme heterojunction photocatalysts for solar energy, water splitting, CO2 conversion, and bacterial inactivation: A review. Environ. Chem. Lett. 2021, 19, 2941–2966. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalabayev, Z.; Abilkhan, A.; Khan, N.; Tugelbay, S.; Seisembekova, A.; Tatykayev, B.; Balaz, M. Sustainable Scalable Mechanochemical Synthesis of CdS/Bi2S3 Nanocomposites for Efficient Hydrogen Evolution. Nanomaterials 2024, 14, 1785. https://doi.org/10.3390/nano14221785
Shalabayev Z, Abilkhan A, Khan N, Tugelbay S, Seisembekova A, Tatykayev B, Balaz M. Sustainable Scalable Mechanochemical Synthesis of CdS/Bi2S3 Nanocomposites for Efficient Hydrogen Evolution. Nanomaterials. 2024; 14(22):1785. https://doi.org/10.3390/nano14221785
Chicago/Turabian StyleShalabayev, Zhandos, Abylay Abilkhan, Natalya Khan, Saparbek Tugelbay, Anar Seisembekova, Batukhan Tatykayev, and Matej Balaz. 2024. "Sustainable Scalable Mechanochemical Synthesis of CdS/Bi2S3 Nanocomposites for Efficient Hydrogen Evolution" Nanomaterials 14, no. 22: 1785. https://doi.org/10.3390/nano14221785
APA StyleShalabayev, Z., Abilkhan, A., Khan, N., Tugelbay, S., Seisembekova, A., Tatykayev, B., & Balaz, M. (2024). Sustainable Scalable Mechanochemical Synthesis of CdS/Bi2S3 Nanocomposites for Efficient Hydrogen Evolution. Nanomaterials, 14(22), 1785. https://doi.org/10.3390/nano14221785







