Lead Catalyzed GaAs Nanowires Grown by Molecular Beam Epitaxy
Abstract
:1. Introduction
2. Materials and Methods
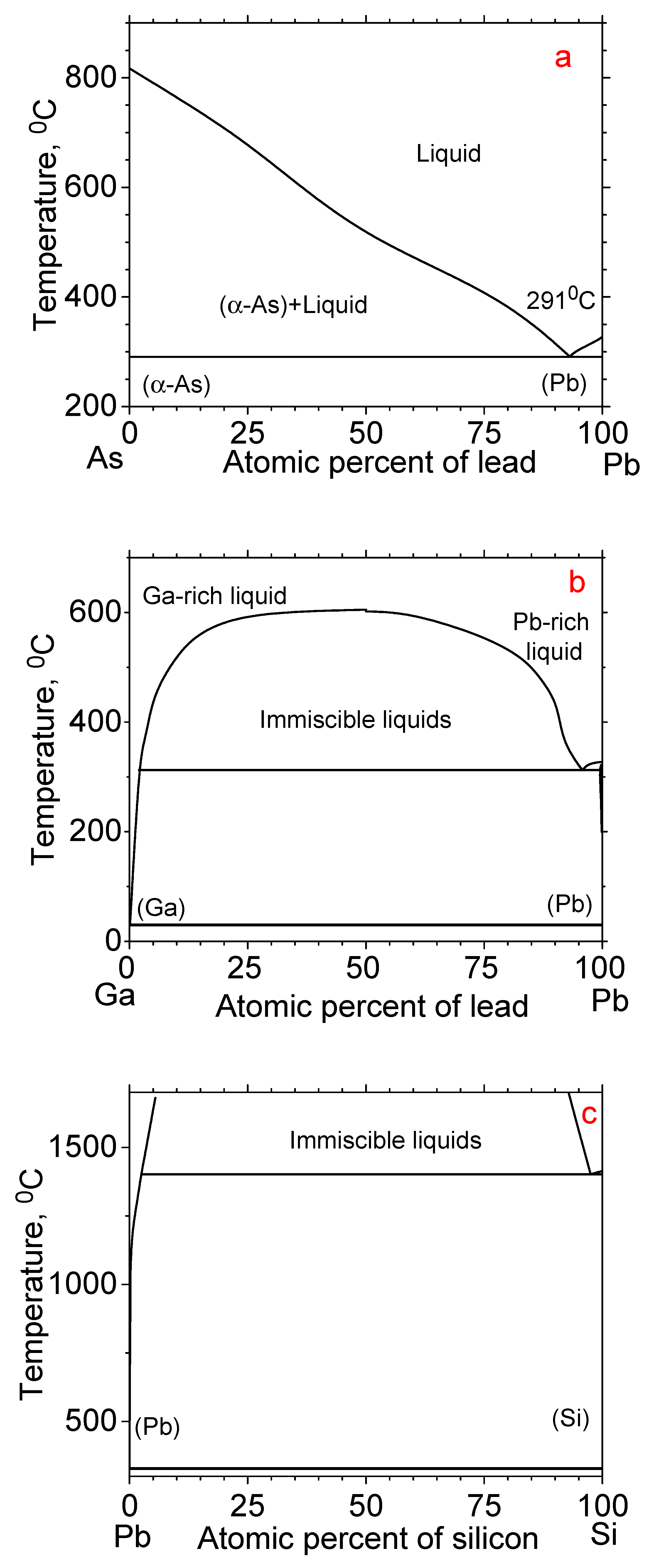
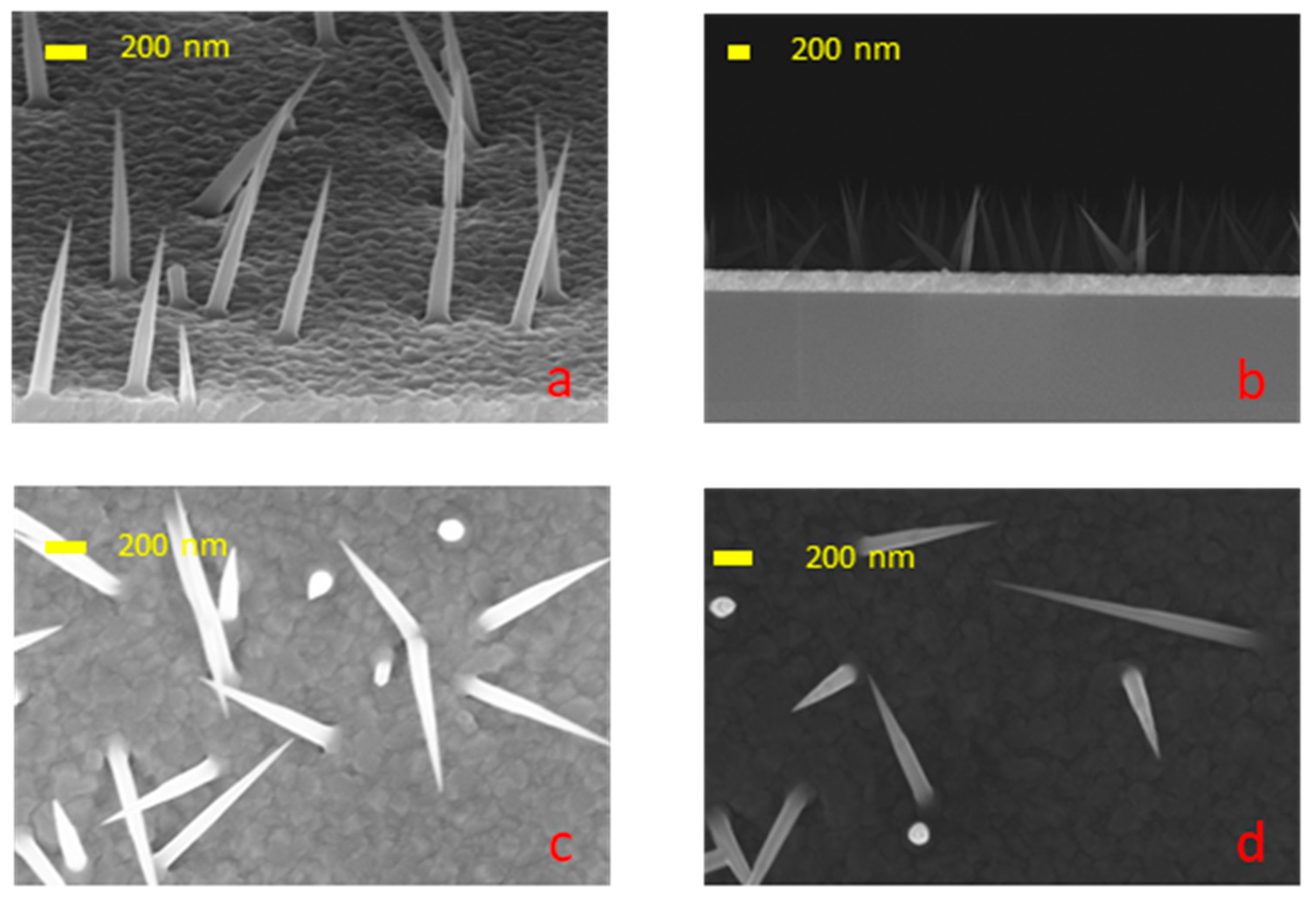
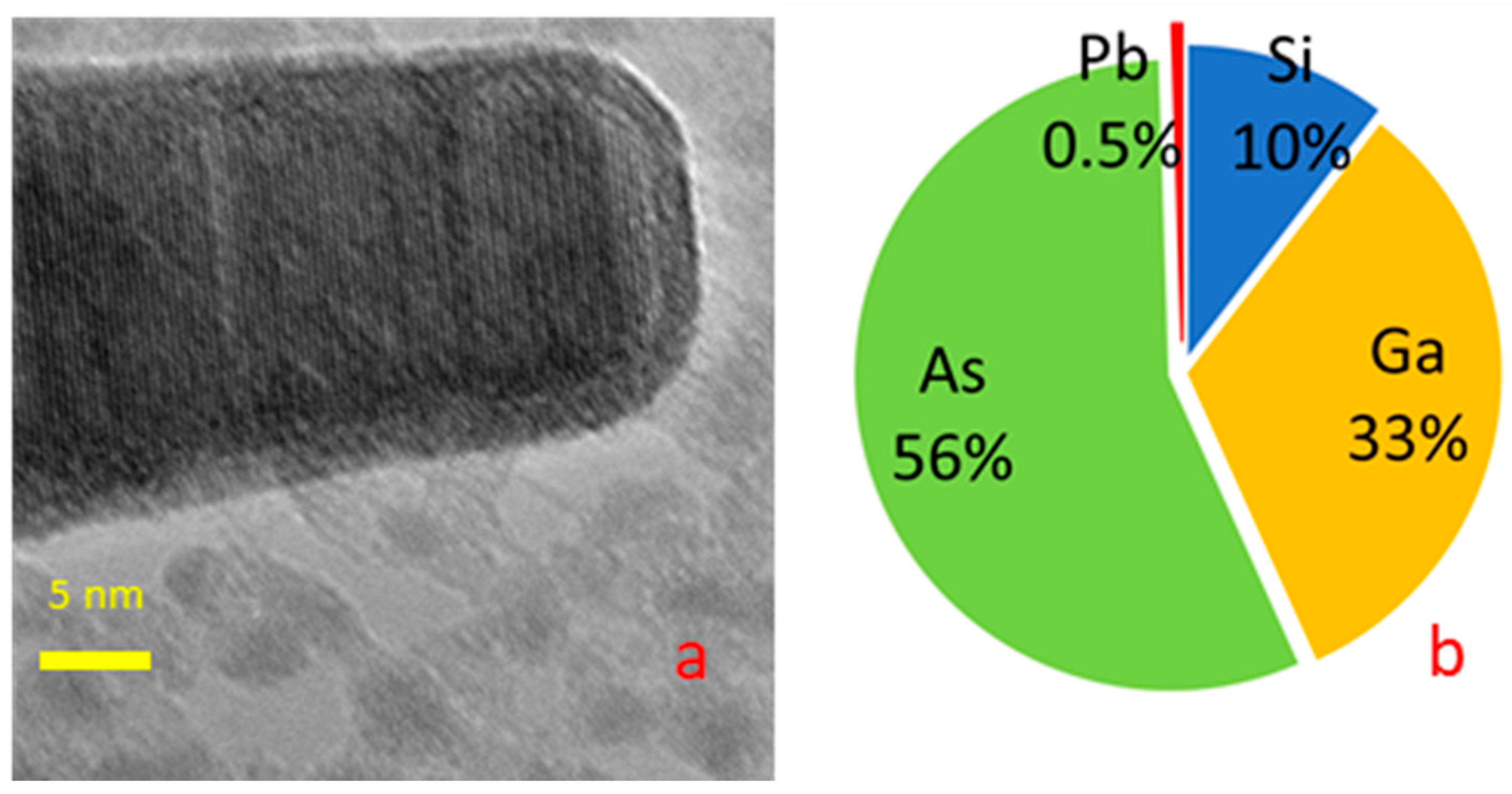
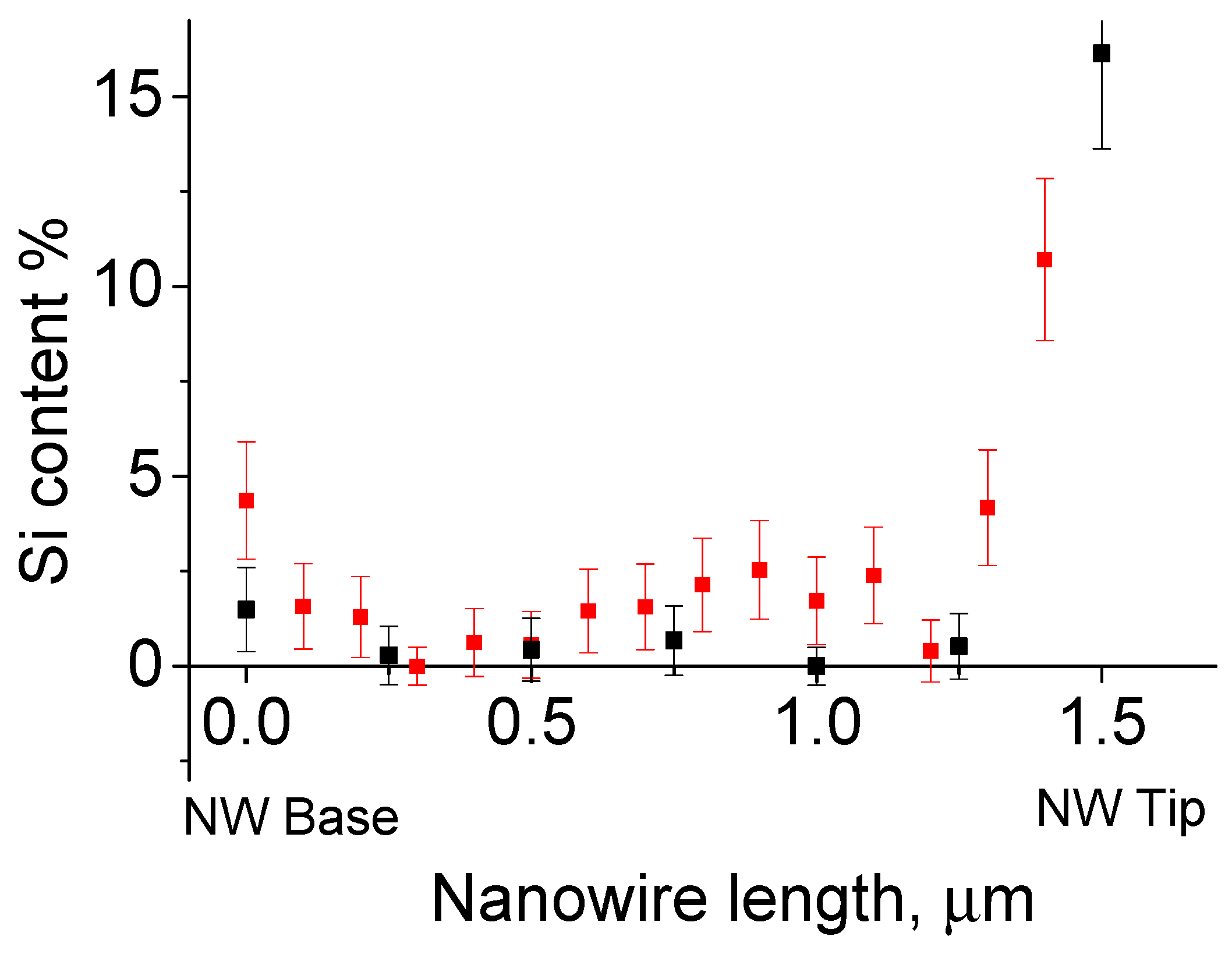
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boras, G.; Yu, X.; Liu, H. III–V Ternary Nanowires on Si Substrates: Growth, Characterization and Device Applications. J. Semicond. 2019, 40, 101301. [Google Scholar] [CrossRef]
- Hyun, J.K.; Zhang, S.; Lauhon, L.J. Nanowire Heterostructures. Annu. Rev. Mater. Res. 2013, 43, 451–479. [Google Scholar] [CrossRef]
- Claudon, J.; Bleuse, J.; Malik, N.S.; Bazin, M.; Jaffrennou, P.; Gregersen, N.; Sauvan, C.; Lalanne, P.; Gérard, J.-M. A Highly Efficient Single-Photon Source Based on a Quantum Dot in a Photonic Nanowire. Nat. Photonics 2010, 4, 174–177. [Google Scholar] [CrossRef]
- Reimer, M.E.; Bulgarini, G.; Akopian, N.; Hocevar, M.; Bavinck, M.B.; Verheijen, M.A.; Bakkers, E.P.A.M.; Kouwenhoven, L.P.; Zwiller, V. Bright Single-Photon Sources in Bottom-up Tailored Nanowires. Nat. Commun. 2012, 3, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.; Gunnarsson, C.P.; Reznik, R.; Jöns, K.D.; Shtrom, I.; Khrebtov, A.; Kasama, T.; Zwiller, V.; Cirlin, G.; Akopian, N. Nanowire Quantum Dots Tuned to Atomic Resonances. Nano Lett. 2018, 18, 7217–7221. [Google Scholar] [CrossRef]
- Tatebayashi, J.; Ota, Y.; Ishida, S.; Nishioka, M.; Iwamoto, S.; Arakawa, Y. Highly Uniform, Multi-Stacked InGaAs/GaAs Quantum Dots Embedded in a GaAs Nanowire. Appl. Phys. Lett. 2014, 105, 103104. [Google Scholar] [CrossRef]
- Loitsch, B.; Winnerl, J.; Grimaldi, G.; Wierzbowski, J.; Rudolph, D.; Morkötter, S.; Döblinger, M.; Abstreiter, G.; Koblmüller, G.; Finley, J.J. Crystal Phase Quantum Dots in the Ultrathin Core of GaAs–AlGaAs Core–Shell Nanowires. Nano Lett. 2015, 15, 7544–7551. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. (Ed.) Nucleation Theory and Growth of Nanostructures; NanoScience and Technology; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-39659-5. [Google Scholar]
- Krogstrup, P.; Jørgensen, H.I.; Johnson, E.; Madsen, M.H.; Sørensen, C.B.; Morral, A.F.I.; Aagesen, M.; Nygård, J.; Glas, F. Advances in the Theory of III–V Nanowire Growth Dynamics. J. Phys. D Appl. Phys. 2013, 46, 313001. [Google Scholar] [CrossRef]
- Christesen, J.D.; Pinion, C.W.; Zhang, X.; Mcbride, J.R.; Cahoon, J.F. Encoding Abrupt and Uniform Dopant Pro Fi Les in Vapor À Liquid À Solid Nanowires by Suppressing the Reservoir Effect of the Liquid Catalyst. ACS Nano 2014, 8, 11790–11798. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Hijazi, H.; Goktas, N.I.; LaPierre, R.R. Be, Te, and Si Doping of GaAs Nanowires: Theory and Experiment. J. Phys. Chem. C 2020, 124, 17299–17307. [Google Scholar] [CrossRef]
- Hijazi, H.; Monier, G.; Gil, E.; Trassoudaine, A.; Bougerol, C.; Leroux, C.; Castellucci, D.; Robert-Goumet, C.; Hoggan, P.E.; André, Y.; et al. Si Doping of Vapor-Liquid-Solid Gaas Nanowires: N-Type or p-Type? Nano Lett. 2019, 19, 4498–4504. [Google Scholar] [CrossRef] [PubMed]
- Metaferia, W.; Sivakumar, S.; Persson, A.R.; Geijselaers, I.; Wallenberg, L.R.; Deppert, K.; Samuelson, L.; Magnusson, M.H. N-Type Doping and Morphology of GaAs Nanowires in Aerotaxy. Nanotechnology 2018, 29, 285601. [Google Scholar] [CrossRef] [PubMed]
- Dick, K.A.; Bolinsson, J.; Borg, B.M.; Johansson, J. Nanowires: Beyond the Reservoir Effect. Nano Lett. 2012, 12, 3200–3206. [Google Scholar] [CrossRef] [PubMed]
- Priante, G.; Glas, F.; Patriarche, G.; Pantzas, K.; Oehler, F.; Harmand, J.C. Sharpening the Interfaces of Axial Heterostructures in Self-Catalyzed AlGaAs Nanowires: Experiment and Theory. Nano Lett. 2016, 16, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Ansara, I.; Ajersch, F. The Ga-Pb (Gallium-Lead) System. J. Phase Equilibria 1991, 12, 73–77. [Google Scholar] [CrossRef]
- Anderson, T.J.; Ansara, I. The Ga-Sn (Gallium-Tin) System. J. Phase Equilibria 1992, 13, 181–189. [Google Scholar] [CrossRef]
- Gokcen, N.A. The As-Sn (Arsenic-Tin) System. Bull. Alloy Phase Diagr. 1990, 11, 271–278. [Google Scholar] [CrossRef]
- Gokcen, N.A. The As-Pb (Arsenic-Lead) System. Bull. Alloy Phase Diagr. 1990, 11, 120–124. [Google Scholar] [CrossRef]
- Sun, R.; Vainorius, N.; Jacobsson, D.; Pistol, M.E.; Lehmann, S.; Dick, K.A. Sn-Seeded GaAs Nanowires Grown by MOVPE. Nanotechnology 2016, 27, 215603. [Google Scholar] [CrossRef]
- Karlina, L.B.; Vlasov, A.S.; Smirnova, I.P.; Soshnikov, I.P. Sn-Assisted Growth of Ga(In)AsP Nanowires from Vapor Phase in a Quasi-Closed Volume. J. Phys. Conf. Ser. 2020, 1697, 012109. [Google Scholar] [CrossRef]
- Sun, R.; Jacobsson, D.; Chen, I.-J.; Nilsson, M.; Thelander, C.; Lehmann, S.; Dick, K.A. Sn-Seeded GaAs Nanowires as Self-Assembled Radial p–n Junctions. Nano Lett. 2015, 15, 3757–3762. [Google Scholar] [CrossRef] [PubMed]
- Olesinski, R.W.; Abbaschian, G.J. The Pb-Si (Lead-Silicon) System. Bull. Alloy Phase Diagr. 1984, 5, 271–273. [Google Scholar] [CrossRef]
- Bao, X.-Y.; Soci, C.; Susac, D.; Bratvold, J.; Aplin, D.P.R.; Wei, W.; Chen, C.-Y.; Dayeh, S.A.; Kavanagh, K.L.; Wang, D. Heteroepitaxial Growth of Vertical GaAs Nanowires on Si (111) Substrates by Metal-Organic Chemical Vapor Deposition. Nano Lett. 2008, 8, 3755–3760. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, I.; Prete, P.; Marzo, F.; Cannoletta, D.; Lovergine, N. Synthesis of Vertically-Aligned GaAs Nanowires on GaAs/(111)Si Hetero-Substrates by Metalorganic Vapour Phase Epitaxy. Cryst. Res. Technol. 2001, 46, 795–800. [Google Scholar] [CrossRef]
- Shandyba, N.; Kirichenko, D.; Sharov, V.; Chernenko, N.; Balakirev, S.; Solodovnik, M. Modulation of GaAs Nanowire Growth by Pre-Treatment of Si Substrate Using a Ga Focused Ion Beam. Nanotechnology 2023, 34, 465603. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Sun, J.; Liu, C.; Brittman, S.; Andrews, S.C.; Lim, J.; Gao, H.; Yan, R.; Yang, P. 25th Anniversary Article: Semiconductor Nanowires—Synthesis, Characterization, and Applications. Adv. Mater. 2014, 26, 2137–2184. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtrom, I.V.; Sibirev, N.V.; Soshnikov, I.P.; Ilkiv, I.V.; Ubyivovk, E.V.; Reznik, R.R.; Cirlin, G.E. Lead Catalyzed GaAs Nanowires Grown by Molecular Beam Epitaxy. Nanomaterials 2024, 14, 1860. https://doi.org/10.3390/nano14231860
Shtrom IV, Sibirev NV, Soshnikov IP, Ilkiv IV, Ubyivovk EV, Reznik RR, Cirlin GE. Lead Catalyzed GaAs Nanowires Grown by Molecular Beam Epitaxy. Nanomaterials. 2024; 14(23):1860. https://doi.org/10.3390/nano14231860
Chicago/Turabian StyleShtrom, Igor V., Nickolai V. Sibirev, Ilya P. Soshnikov, Igor V. Ilkiv, Evgenii V. Ubyivovk, Rodion R. Reznik, and George E. Cirlin. 2024. "Lead Catalyzed GaAs Nanowires Grown by Molecular Beam Epitaxy" Nanomaterials 14, no. 23: 1860. https://doi.org/10.3390/nano14231860
APA StyleShtrom, I. V., Sibirev, N. V., Soshnikov, I. P., Ilkiv, I. V., Ubyivovk, E. V., Reznik, R. R., & Cirlin, G. E. (2024). Lead Catalyzed GaAs Nanowires Grown by Molecular Beam Epitaxy. Nanomaterials, 14(23), 1860. https://doi.org/10.3390/nano14231860





