Surface-Functionalised Copper Oxide Nanoparticles: A Pathway to Multidrug-Resistant Pathogen Control in Medical Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of Copper Oxide Nanoparticles via Aqueous Precipitation
2.3. Glutamic Acid Coating of Copper Oxide Nanoparticles
2.4. Characterisation of Glutamic Acid–Copper Oxide Nanoparticles
2.4.1. Transmission Electron Microscopy
2.4.2. Dynamic Light Scattering and Zeta Potential Measurements
2.4.3. Thermogravimetric Analysis
2.5. Adhesion of Glutamic Acid–Copper Oxide Nanoparticles to Material Surfaces
2.6. Leaching Studies on Glutamic Acid-Coated Copper Oxide Nanoparticles from Silicone Tubing
2.7. Characterisation of Nanoparticle-Coated Materials
2.8. Bacterial Strains, Mammalian Cell Lines, Growth Conditions, and Testing
2.8.1. Minimum Inhibition and Bactericidal Concentration Assays
2.8.2. Toxicity Assays for Glutamic Acid–Copper Oxide Nanoparticles and 3-Mercaptopropyltrimethoxysilane
2.8.3. Modified Minimum Biofilm Eradication Concentration Assay
2.8.4. Centers for Disease Control and Prevention (CDC) Bioreactor Assay
3. Results and Discussion
3.1. Characterisation of Copper Oxide Nanoparticles and Glutamic Acid–Copper Oxide Nanoparticles
3.2. Antimicrobial Evaluation of the Glutamic Acid–Copper Oxide Nanoparticles
3.3. Adhesion of Glutamic Acid–Copper Oxide Nanoparticles to Medical-Grade Materials Results in an Evenly Distributed Antimicrobial Coating
3.4. Human Cell Line Toxicity Assessment of Antimicrobial-Coating Components Identifies Concentrations That Can Be Safely Applied to Medical Materials
3.5. Antimicrobial Activity of Glutamic Acid–Copper Oxide Nanoparticle Coatings
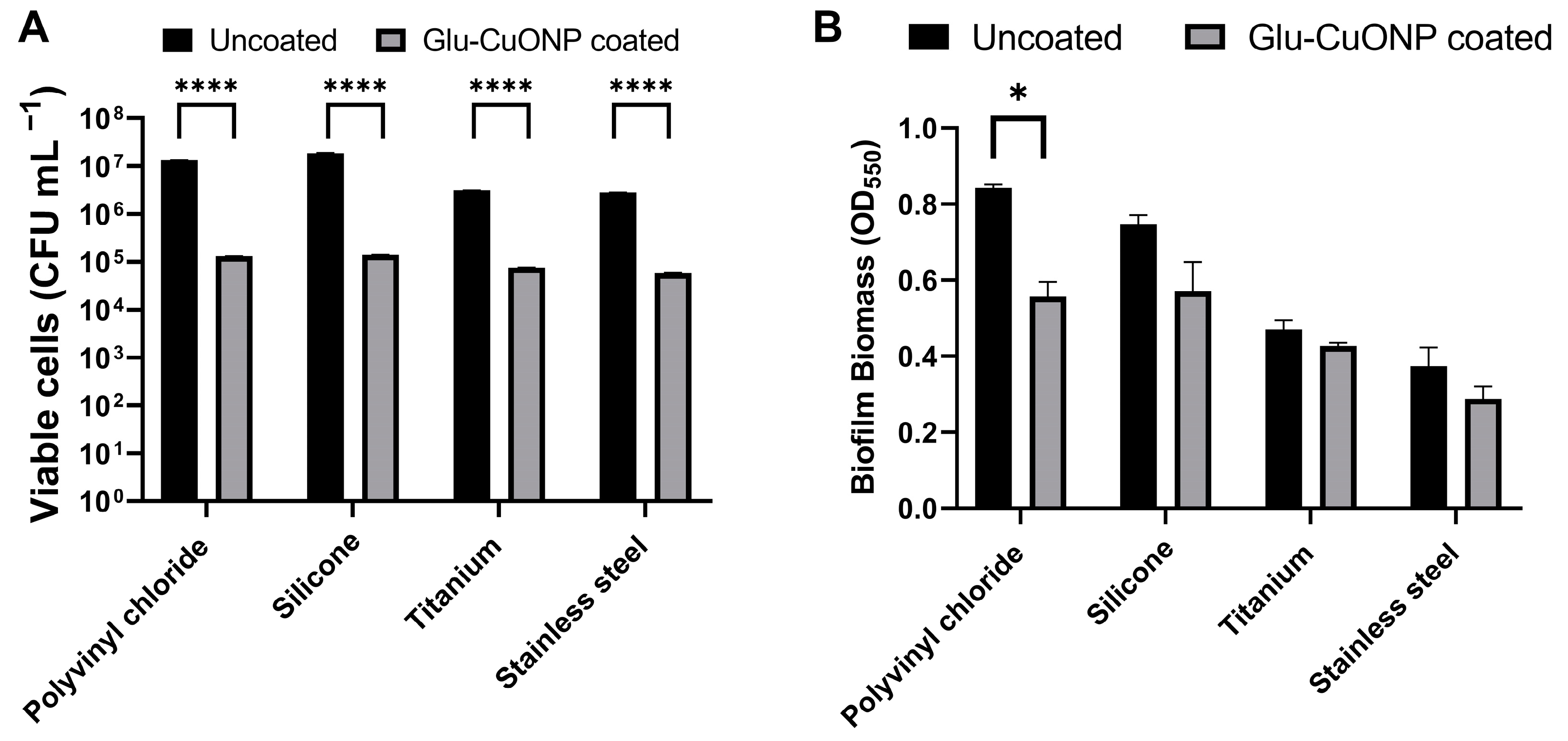
3.6. The Glutamic Acid–Copper Oxide Nanoparticle Coating Remains Active Across a Range of Medical Device Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleming, A. Sir Alexander Fleming Nobel Lecture. 1945. Available online: https://www.nobelprize.org/prizes/medicine/1945/fleming/lecture/ (accessed on 14 July 2024).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance Chaired by Jim O’Neill. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 14 July 2024).
- Halioua, B.; Ziskind, B. Medicine in the Days of the Pharaohs; Harvard University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Zivic, F.; Grujovic, N.; Mitrovic, S.; Ahad, I.U.; Brabazon, D. Characteristics and Applications of Silver Nanoparticles. In Commercialization of Nanotechnologies–A Case Study Approach; Springer International Publishing: Cham, Switzerland, 2018; pp. 227–273. [Google Scholar]
- Franke, S.; Grass, G.; Nies, D.H. The Product of the ybdE Gene of the Escherichia coli Chromosome Is Involved in Detoxification of Silver Ions. Microbiology 2001, 147, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral Nanoparticles for Sanitizing Surfaces: A Roadmap to Self-Sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Coppers Fact Sheet. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1001BJR.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000003%5CP1001BJR.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 14 July 2024).
- Mekapothula, S.; Chrysanthou, E.; Hall, J.; Nekkalapudi, P.D.; McLean, S.; Cave, G.W.V. Antipathogenic Applications of Copper Nanoparticles in Air Filtration Systems. Materials 2024, 17, 2664. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The Use of Nanoparticles in Anti-Microbial Materials and Their Characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shaik, M.R.; Khan, S.T.; Adil, S.F.; Kuniyil, M.; Khan, M.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Nawaz Tahir, M. Enhanced Antimicrobial Activity of Biofunctionalized Zirconia Nanoparticles. ACS Omega 2020, 5, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e00221-20. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, N.; Vitacca, M. The Patient Needing Prolonged Mechanical Ventilation: A Narrative Review. Multidiscip. Respir. Med. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Cave, G.W.V.; Mundell, V.J. Coating Metal Oxide Particles. Patent WO2013136082A2, 19 September 2013. [Google Scholar]
- ISO 17294-2:2023; ISO/TC 147/SC 2 Part 2: Determination of Selected Elements Including Uranium Isotopes. Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). International Organization for Standardization: Geneva, Switzerland, 2023.
- Perna, N.T.; Plunkett, G.; Burland, V.; Mau, B.; Glasner, J.D.; Rose, D.J.; Mayhew, G.F.; Evans, P.S.; Gregor, J.; Kirkpatrick, H.A.; et al. Genome Sequence of Enterohaemorrhagic Escherichia coli O157:H7. Nature 2001, 409, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D.; Endres, J.L.; Yajjala, V.K.; Widhelm, T.J.; Boissy, R.J.; Bose, J.L.; Bayles, K.W. A Genetic Resource for Rapid and Comprehensive Phenotype Screening of Nonessential Staphylococcus aureus Genes. mBio 2013, 4, e00537-12. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Sissions, C.H. A Comparison of Human Dental Plaque Microcosm Biofilms Grown in an Undefined Medium and a Chemically Defined Artificial Saliva. Arch. Oral. Biol. 2001, 46, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, T.; Tardito, S. Cell Culture Medium Formulation and Its Implications in Cancer Metabolism. Trends Cancer 2019, 5, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Voorde, J.V.; Ackermann, T.; Pfetzer, N.; Sumpton, D.; Mackay, G.; Kalna, G.; Nixon, C.; Blyth, K.; Gottlieb, E.; Tardito, S. Improving the Metabolic Fidelity of Cancer Models with a Physiological Cell Culture Medium. Sci. Adv. 2019, 5, eaau7314. [Google Scholar] [CrossRef] [PubMed]
- Varney, A.M.; Smitten, K.L.; Thomas, J.A.; Mclean, S. Transcriptomic Analysis of the Activity and Mechanism of Action of a Ruthenium(II)-Based Antimicrobial That Induces Minimal Evolution of Pathogen Resistance. ACS Pharmacol. Transl. Sci. 2021, 4, 168–178. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Technical Committee ISO/TC 194 Part 5: Tests for in Vitro Cytotoxicity. Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2009.
- Cave, G. Reactor. Patent WO2017033005A1, 10 March 2015. [Google Scholar]
- Smith, N.; Raston, C.L.; Saunders, M.; Woodward, R. Synthesis of Magnetic Nanoparticles Using Spinning Disc Processing. In Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show, Boston, MA, USA, 7–11 May 2006. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Varney, A.M.; Mannix-Fisher, E.; Thomas, J.C.; McLean, S. Evaluation of Phenotypic and Genotypic Methods for the Identification and Characterization of Bacterial Isolates Recovered from Catheter-Associated Urinary Tract Infections. J. Appl. Microbiol. 2024, 135, lxae155. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Keevil, C.W. Lack of Involvement of Fenton Chemistry in Death of Methicillin-Resistant and Methicillin-Sensitive Strains of Staphylococcus aureus and Destruction of Their Genomes on Wet or Dry Copper Alloy Surfaces. Appl. Environ. Microbiol. 2016, 82, 2132–2136. [Google Scholar] [CrossRef]
- Altimira, F.; Yáñez, C.; Bravo, G.; González, M.; Rojas, L.A.; Seeger, M. Characterization of Copper-Resistant Bacteria and Bacterial Communities from Copper-Polluted Agricultural Soils of Central Chile. BMC Microbiol. 2012, 12, 193. [Google Scholar] [CrossRef] [PubMed]

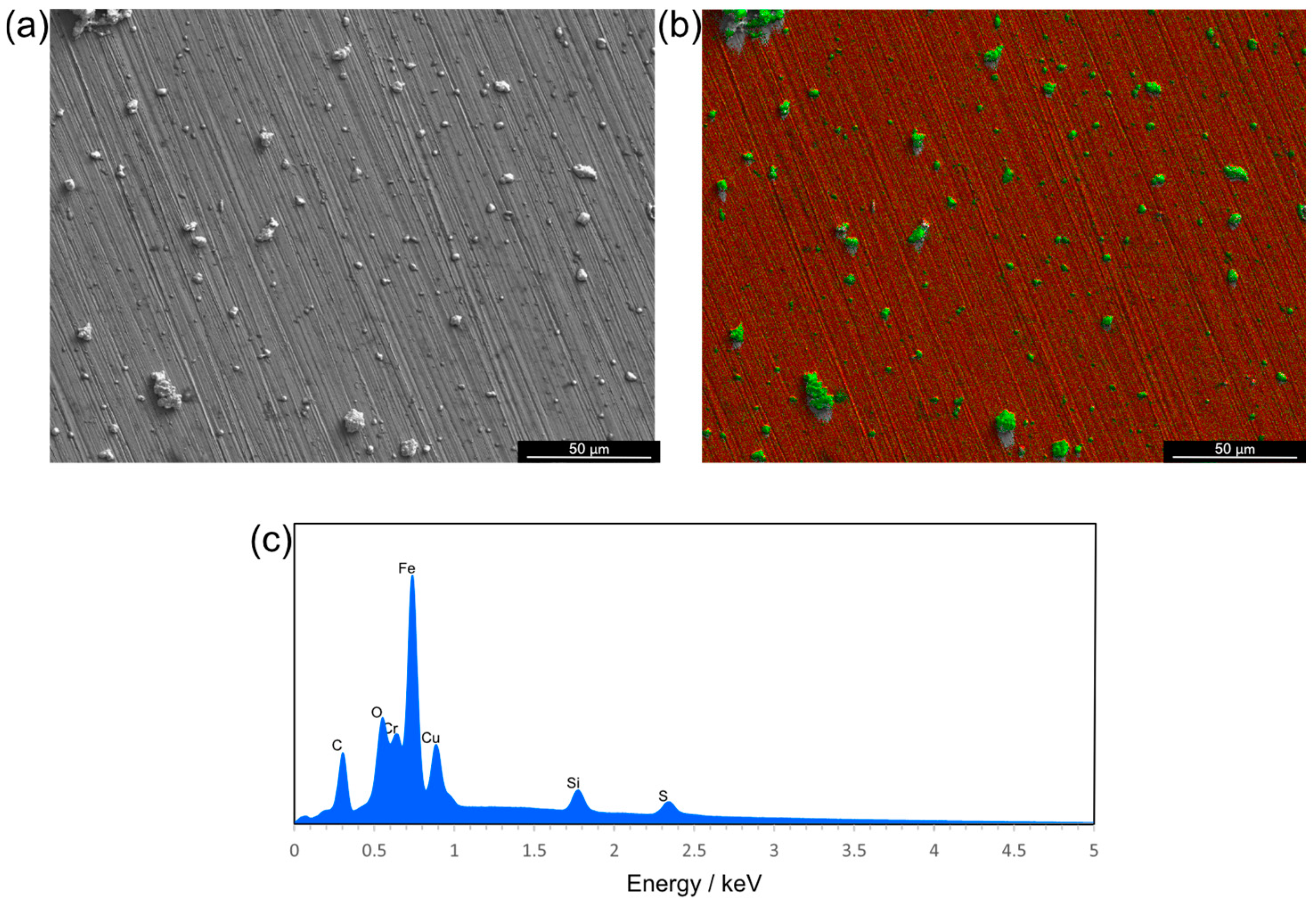


| Bacterial Pathogen | Details/Origin | MIC (mg L−1) | MBC (mg L−1) |
|---|---|---|---|
| Escherichia coli | 0157:H7 Poole RK, University of Sheffield | 258 | 325 |
| Staphylococcus aureus | USA 300 LAC JE2 Poole RK, University of Sheffield | 258 | 325 |
| Pseudomonas aeruginosa | Clinical isolate, neonatal sepsis Forsythe SJ, Nottingham Trent University | 325 | 325 |
| Klebsiella pneumoniae | Clinical isolate, neonatal enterocolitis Forsythe SJ, Nottingham Trent University | 325 | 325 |
| Staphylococcus epidermidis | ATCC 12228 | 258 | 325 |
| Acinetobacter pittii | PS_Acine7: clinical isolate, bronchial lavage Hoyles L, Nottingham Trent University | 258 | 325 |
| Acinetobacter baumannii | PS_Acine9: clinical isolate, wound Hoyles L, Nottingham Trent University | 258 | 325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, J.; Mekapothula, S.; Coxhill, R.; Craske, D.; Varney, A.M.; Cave, G.W.V.; McLean, S. Surface-Functionalised Copper Oxide Nanoparticles: A Pathway to Multidrug-Resistant Pathogen Control in Medical Devices. Nanomaterials 2024, 14, 1899. https://doi.org/10.3390/nano14231899
Hall J, Mekapothula S, Coxhill R, Craske D, Varney AM, Cave GWV, McLean S. Surface-Functionalised Copper Oxide Nanoparticles: A Pathway to Multidrug-Resistant Pathogen Control in Medical Devices. Nanomaterials. 2024; 14(23):1899. https://doi.org/10.3390/nano14231899
Chicago/Turabian StyleHall, James, Subbareddy Mekapothula, Rebecca Coxhill, Dominic Craske, Adam M. Varney, Gareth W. V. Cave, and Samantha McLean. 2024. "Surface-Functionalised Copper Oxide Nanoparticles: A Pathway to Multidrug-Resistant Pathogen Control in Medical Devices" Nanomaterials 14, no. 23: 1899. https://doi.org/10.3390/nano14231899
APA StyleHall, J., Mekapothula, S., Coxhill, R., Craske, D., Varney, A. M., Cave, G. W. V., & McLean, S. (2024). Surface-Functionalised Copper Oxide Nanoparticles: A Pathway to Multidrug-Resistant Pathogen Control in Medical Devices. Nanomaterials, 14(23), 1899. https://doi.org/10.3390/nano14231899







