Low-Temperature Hydrotreatment of C4/C5 Fractions Using a Dual-Metal-Loaded Composite Oxide Catalyst
Abstract
1. Introduction
2. Results and Discussion
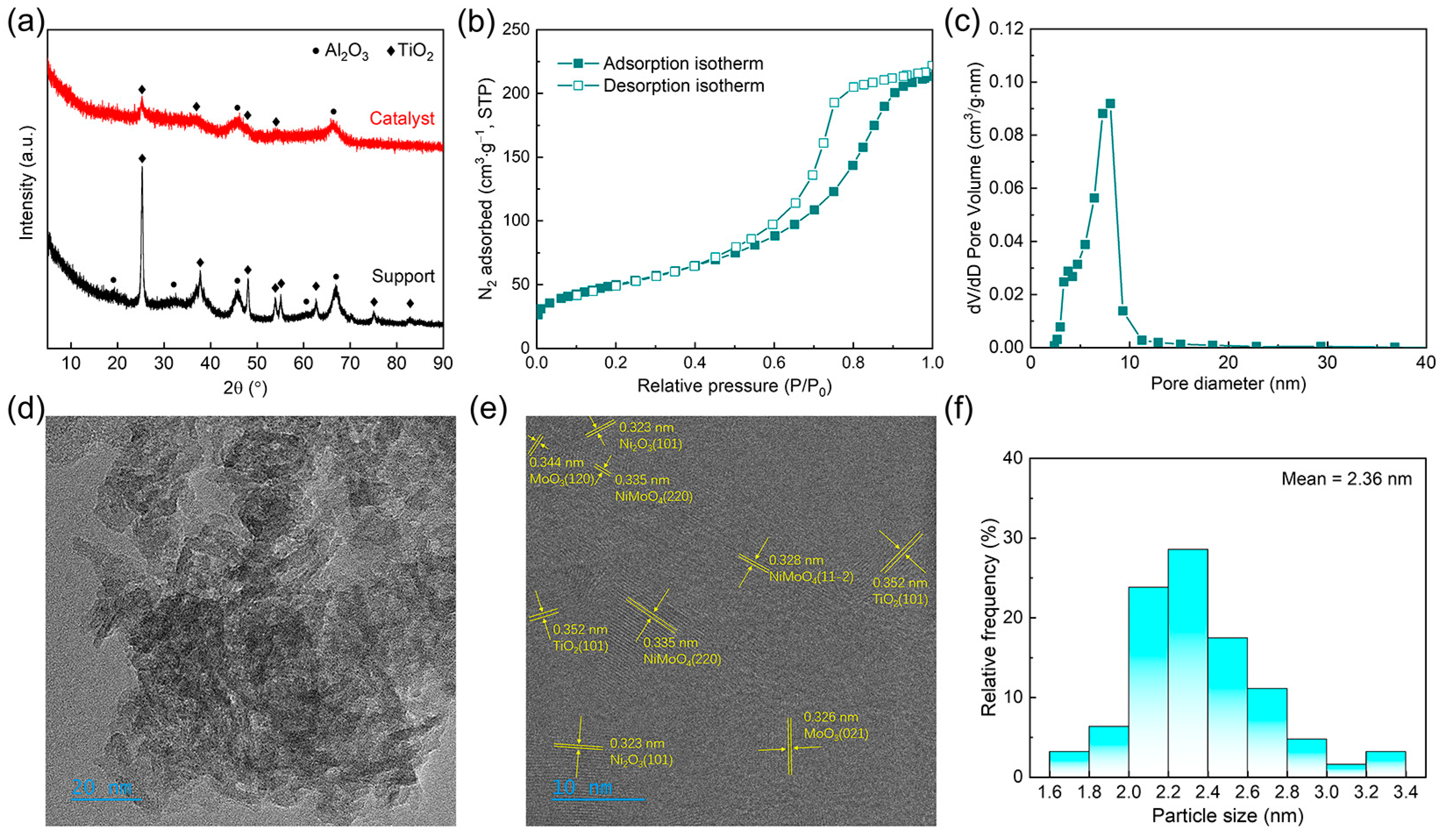
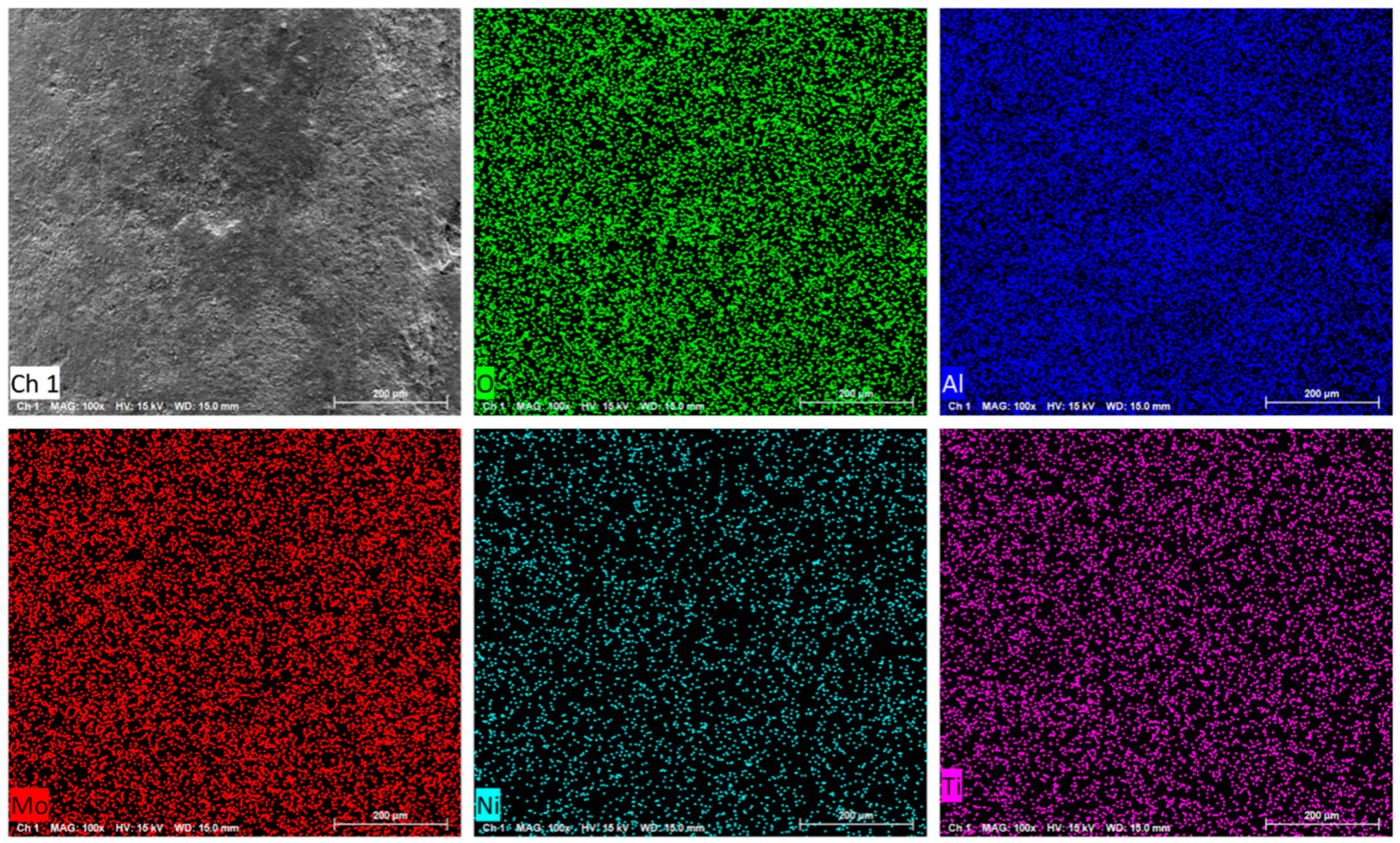
2.1. Catalyst Synthesis and Characterization
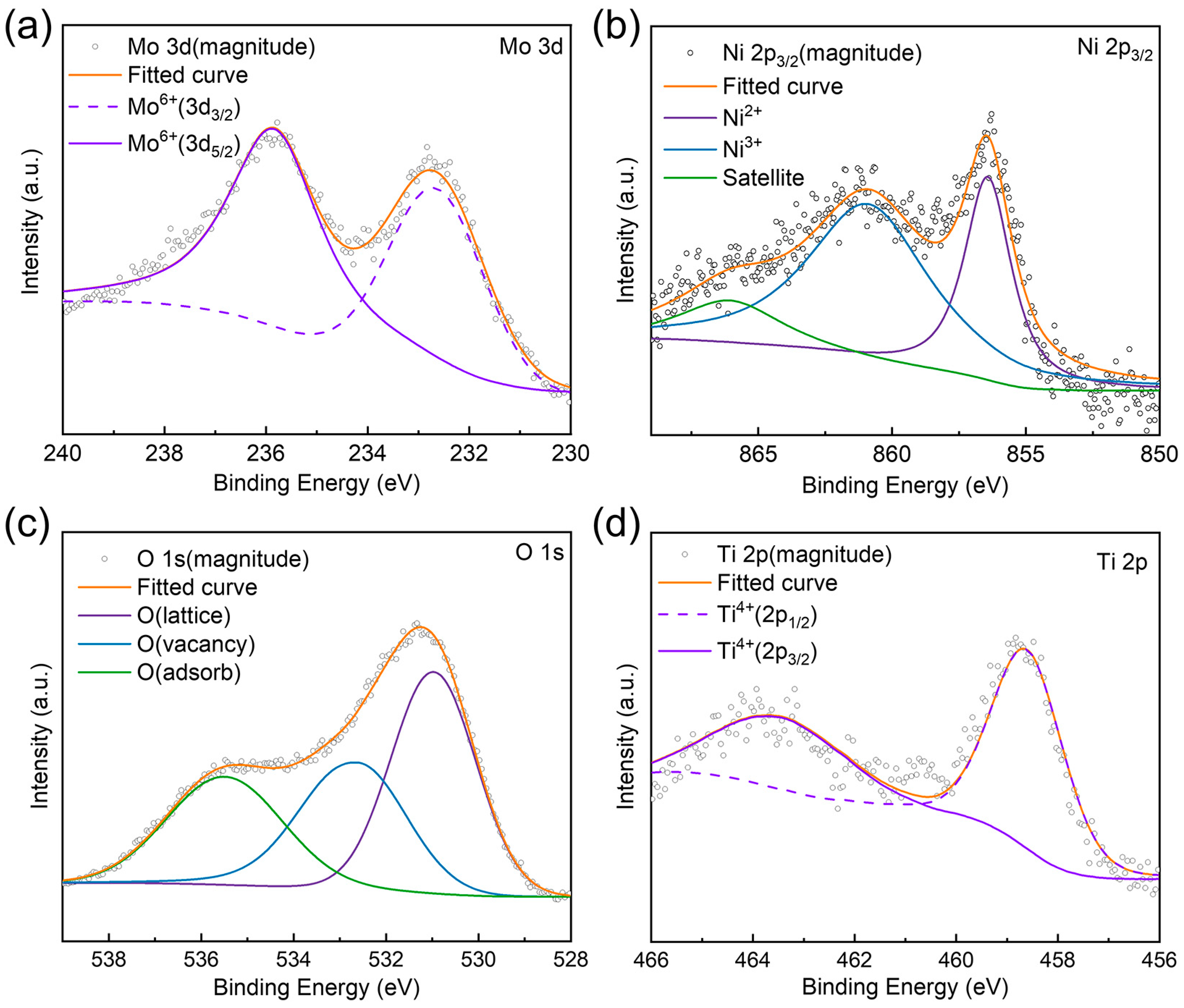
2.2. Characterization of the Surface Properties
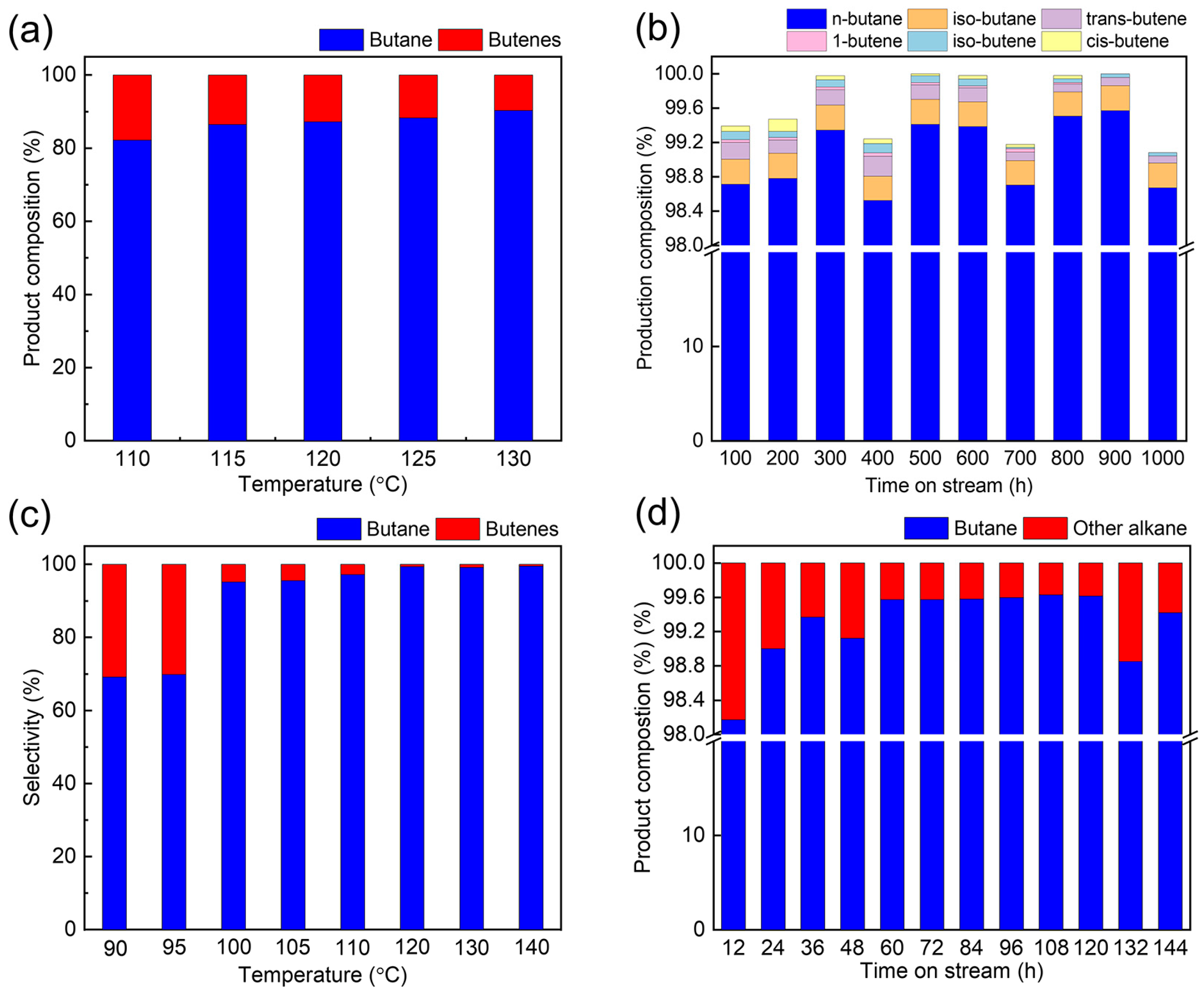
2.3. Catalytic Performance Evaluation
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longstaff, D.; Deo, M.D.; Hanson, F.; Oblad, A.; Tsai, C. Hydrotreating the bitumen-derived hydrocarbon liquid produced in a fluidized-bed pyrolysis reactor. Fuel 1992, 71, 1407–1419. [Google Scholar] [CrossRef]
- Li, Z.; Åhman, M.; Nilsson, L.J.; Bauer, F. Towards carbon neutrality: Transition pathways for the Chinese ethylene industry. Renew. Sustain. Energy Rev. 2024, 199, 114540. [Google Scholar] [CrossRef]
- Ju, F.; Li, L.; Wu, T.; Sun, Y.; Ling, H. Competition between reactive adsorption desulfurization and olefin hydrogenation over the NiO/ZnO-Al2O3-SiO2 adsorbent. New J. Chem. 2022, 46, 8144–8151. [Google Scholar] [CrossRef]
- Yao, S.; Zheng, Y.; Ding, L.; Ng, S.; Yang, H. Co-promotion of fluorine and boron on NiMo/Al2O3 for hydrotreating light cycle oil. Catal. Sci. Technol. 2012, 2, 1925–1932. [Google Scholar] [CrossRef]
- Blaser, H.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective hydrogenation for fine chemicals: Recent trends and new developments. Adv. Synth. Catal. 2003, 345, 103–151. [Google Scholar] [CrossRef]
- Ardiaca, N.O.; Bressa, S.P.; Alves, J.A.; Martınez, O.M.; Barreto, G.F. Experimental procedure for kinetic studies on egg-shell catalysts: The case of liquid-phase hydrogenation of 1,3-butadiene and n-butenes on commercial Pd catalysts. Catal. Today 2001, 64, 205–215. [Google Scholar] [CrossRef]
- Masuda, T.; Kuwahara, H.; Mukai, S.; Hashimoto, K. Production of high quality gasoline from waste polyethylene derived heavy oil over Ni-REY catalyst in steam atmosphere. Chem. Eng. Sci. 1999, 54, 2773–2779. [Google Scholar] [CrossRef]
- Spanjers, C.S.; Sim, R.S.; Sturgis, N.P.; Kabius, B.; Rioux, R.M. In situ spectroscopic characterization of Ni1-xZnx/ZnO catalysts and their selectivity for acetylene semi-hydrogenation in excess ethylene. ACS Catal. 2015, 5, 3304–3315. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Zhan, W.; Cao, Z.; Chen, J.; Jiang, Q.; Jiang, Y.; Xie, Z.; Kuang, Q.; Zheng, L. Controlled encapsulation of flower-like Rh-Ni alloys with MOFs via tunable template sealloying for enhanced selective hydrogenation of alkyne. ACS Appl. Mater. Interfaces 2016, 8, 31059–31066. [Google Scholar] [CrossRef]
- Shen, Z.; Ke, M.; Yu, P.; Liu, S.; Song, Z.; Jiang, Q. Catalytic activities of Mo-modified Ni/Al2O3 catalysts for thioetherification of mercaptans and di-olefins in fluid catalytic cracking naphtha. Transit. Met. Chem. 2012, 37, 587–593. [Google Scholar] [CrossRef]
- Niu, M.; Ji, P.; Fan, Z.; Yan, Y.; Li, D.; Li, W. Hydrofining process of coal tar based on four kinds of catalyst grading. Energy Fuels 2020, 34, 6510–6517. [Google Scholar] [CrossRef]
- Mo, L.; Saw, E.-T.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Preparation of highly dispersed Cu/SiO2 doped with CeO2 and its application for high temperature water gas shift reaction. Int. J. Hydrogen Energy 2018, 43, 15891–15897. [Google Scholar] [CrossRef]
- Gao, X.; Hou, X.; Ma, Z.; Xiao, C.; Jia, L. Highly dispersed Ag nanocrystals functionalized ZIF-8 derived ZnO hollow structures for superior sensitive and selective detection of nitric oxide. Sens. Actuators B Chem. 2025, 422, 136646. [Google Scholar] [CrossRef]
- Gong, S.; Liu, L.; He, H.; Cui, Q. Dibenzothiophene hydrodesulfurization over MoP/SiO2 catalyst prepared with sol-gel method. Korean J. Chem. Eng. 2010, 27, 1419–1422. [Google Scholar] [CrossRef]
- Ma, L.; Huang, C.; Li, X.; An, X.; Li, J.; Zou, L.; Zhu, X.; Yuan, C.; Dai, X.; Zhou, Y.; et al. In situ synthesis of mesoporous TiO2 doped with bismuth single atoms for tunable photocatalytic degradation of formaldehyde. Sep. Purif. Technol. 2025, 354, 128635. [Google Scholar] [CrossRef]
- Hao, X.; Wu, S.; Lv, X.; Chen, J.; Jia, H. In situ reaction-induced strong metal-support interaction to enhance catalytic performance and stability of toluene oxidation. Sep. Purif. Technol. 2025, 358, 130266. [Google Scholar] [CrossRef]
- Huang, W.; Liu, H.; Huang, M.; Jia, Y.; Tao, J.; Liu, C.; Deng, K.; Zhao, L.; Liu, X.; Wei, Q.; et al. Effect of TiO2-Al2O3 support surface properties on active phase structure and hydrodenitrogenation performances of the corresponding NiWS supported catalysts. Fuel 2023, 343, 127922. [Google Scholar] [CrossRef]
- Ding, L.; Xiong, T.; Zhao, Z.; Liao, J.; Zhang, Y. In-situ synthesis of Al2O3-TiO2 nanocomposite with enhanced adsorption performance to uranium(VI) from aqueous solution. J. Mol. Liq. 2022, 362, 119731. [Google Scholar] [CrossRef]
- Phan, D.-P.; Vo, T.K.; Le, V.N.; Kim, J.; Lee, E.Y. Spray pyrolysis synthesis of bimetallic NiMo/Al2O3-TiO2 catalyst for hydrodeoxygenation of guaiacol: Effects of bimetallic composition and reduction temperature. J. Ind. Eng. Chem. 2020, 83, 351–358. [Google Scholar] [CrossRef]
- Wang, J.; Ren, D.; Zhang, N.; Lang, J.; Du, Y.; He, W.; Norinaga, K.; Huo, Z. Boosting in-situ hydrodeoxygenation of fatty acids over a fine and oxygen-vacancy-rich NiAl catalyst. Renew. Energy 2023, 202, 952–960. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.R.; Li, J.Y.; Qu, J.J.; Li, S.S. Sensitive detection of Hg(II) on MoS2/NiS2 based on interfacial engineering to accelerate the Ni2+/Ni3+ cycle: Identification the role of atomic-level heterojunction-induced electron transfer in electroanalysis. Anal. Chim. Acta 2024, 1331, 343339. [Google Scholar] [CrossRef]
- Genel, S.; Durak, H. The utilization of novel NaF/Al2O3 catalysts in the process of hydrothermal liquefaction of Crambe orientalis: Synthesis and characterization. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 3748–3759. [Google Scholar]
- Ferguson, J.D.; Weimer, A.W.; George, S.M. Atomic layer deposition of Al2O3 films on polyethylene particles. Chem. Mater. 2004, 16, 5602–5609. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, C.; Cui, Y.; Dai, S.; Zhao, X.; Luo, R.; An, P.; Li, H.; Wang, H.; Hou, Z. Direct transformation of glycerol to propanal using zirconium phosphate-supported bimetallic catalysts. ChemSusChem 2020, 13, 4954–4966. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Weng, W.; Wan, H.; Toghiani, H.; Toghiani, R.; Pittman, C. Activation of methane to syngas over a Ni/TiO2 catalyst. Acta Phys. Chim. Sin. 2001, 17, 733–738. [Google Scholar] [CrossRef]
- Cao, J.; Xia, J.; Zhang, Y.; Liu, X.; Bai, L.; Xu, J.; Yang, C.-A.; Zheng, S.; Yang, T.; Tang, K.; et al. Influence of the alumina crystal phase on the performance of CoMo/Al2O3 catalysts for the selective hydrodesulfurization of fluid catalytic cracking naphtha. Fuel 2021, 289, 119843. [Google Scholar] [CrossRef]
- Klimova, T.E.; Valencia, D.; Mendoza-Nieto, J.A.; Hernández-Hipólito, P. Behavior of NiMo/SBA-15 catalysts prepared with citric acid in simultaneous hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. J. Catal. 2013, 304, 29–46. [Google Scholar] [CrossRef]
- Peña, L.; Valencia, D.; Klimova, T. CoMo/SBA-15 catalysts prepared with EDTA and citric acid and their performance in hydrodesulfurization of dibenzothiophene. Appl. Catal. B Environ. 2014, 147, 879–887. [Google Scholar] [CrossRef]
- Kinnunen, N.M.; Hirvi, J.T.; Venäläinen, T.; Suvanto, M.; Pakkanen, T.A. Procedure to tailor activity of methane combustion catalyst: Relation between Pd/PdO2 active sites and methane oxidation activity. Appl. Catal. A Gen. 2011, 397, 54–61. [Google Scholar] [CrossRef]
- Chen, L.; Janssens, T.V.W.; Skoglundh, M.; Grönbeck, H. Interpretation of NH3-TPD profiles from Cu-CHA using first-principles calculations. Top. Catal. 2019, 62, 93–99. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, S.; Wang, H.; Ye, D.; Xie, S.; Pei, Y.; Hu, H.; Hua, W.; Li, Z.H.; Qiao, M.; et al. Nanoparticulate Pt on mesoporous SBA-15 doped with extremely low amount of Was a highly selective catalyst for glycerol hydrogenolysis to 1,3-propanediol. Green Chem. 2017, 19, 2174–2183. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, Z.; Pan, H.; Guo, A.; Jiao, S.; Wang, F.; Chen, K.; Wang, Z. Molybdenum carbide and sulfide nanoparticles as selective hydrotreating catalysts for FCC slurry oil to remove olefins and sulfur. Nanomaterials 2021, 11, 2721. [Google Scholar] [CrossRef] [PubMed]
- Badawi, M.; Vivier, L.; Duprez, D. Kinetic study of olefin hydrogenation on hydrotreating catalysts. J. Mol. Catal. A Chem. 2010, 320, 34–39. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Li, R.; Shen, Z.; Hai, X.; Zou, R. Low-Temperature Hydrotreatment of C4/C5 Fractions Using a Dual-Metal-Loaded Composite Oxide Catalyst. Nanomaterials 2024, 14, 1934. https://doi.org/10.3390/nano14231934
Du Z, Li R, Shen Z, Hai X, Zou R. Low-Temperature Hydrotreatment of C4/C5 Fractions Using a Dual-Metal-Loaded Composite Oxide Catalyst. Nanomaterials. 2024; 14(23):1934. https://doi.org/10.3390/nano14231934
Chicago/Turabian StyleDu, Zhou, Renyi Li, Zhenghui Shen, Xiao Hai, and Ruqiang Zou. 2024. "Low-Temperature Hydrotreatment of C4/C5 Fractions Using a Dual-Metal-Loaded Composite Oxide Catalyst" Nanomaterials 14, no. 23: 1934. https://doi.org/10.3390/nano14231934
APA StyleDu, Z., Li, R., Shen, Z., Hai, X., & Zou, R. (2024). Low-Temperature Hydrotreatment of C4/C5 Fractions Using a Dual-Metal-Loaded Composite Oxide Catalyst. Nanomaterials, 14(23), 1934. https://doi.org/10.3390/nano14231934





