A Graphene-Based Bioactive Product with a Non-Immunological Impact on Mononuclear Cell Populations from Healthy Volunteers
Abstract
1. Introduction
2. Methods
2.1. GMC Preparation and Characterization
2.2. Leucocyte Progenies’ Purification, Culture Conditions and Analysis
2.3. Cell Cycle Profiles
2.4. Apoptosis and Necrosis Analysis
2.5. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Statistical Analysis
3. Results
3.1. GMC Characterization and PBMC Viability After GMC Treatment
3.2. Immunological Activity of Lymphocytes and NK Progenies During GMC Treatment
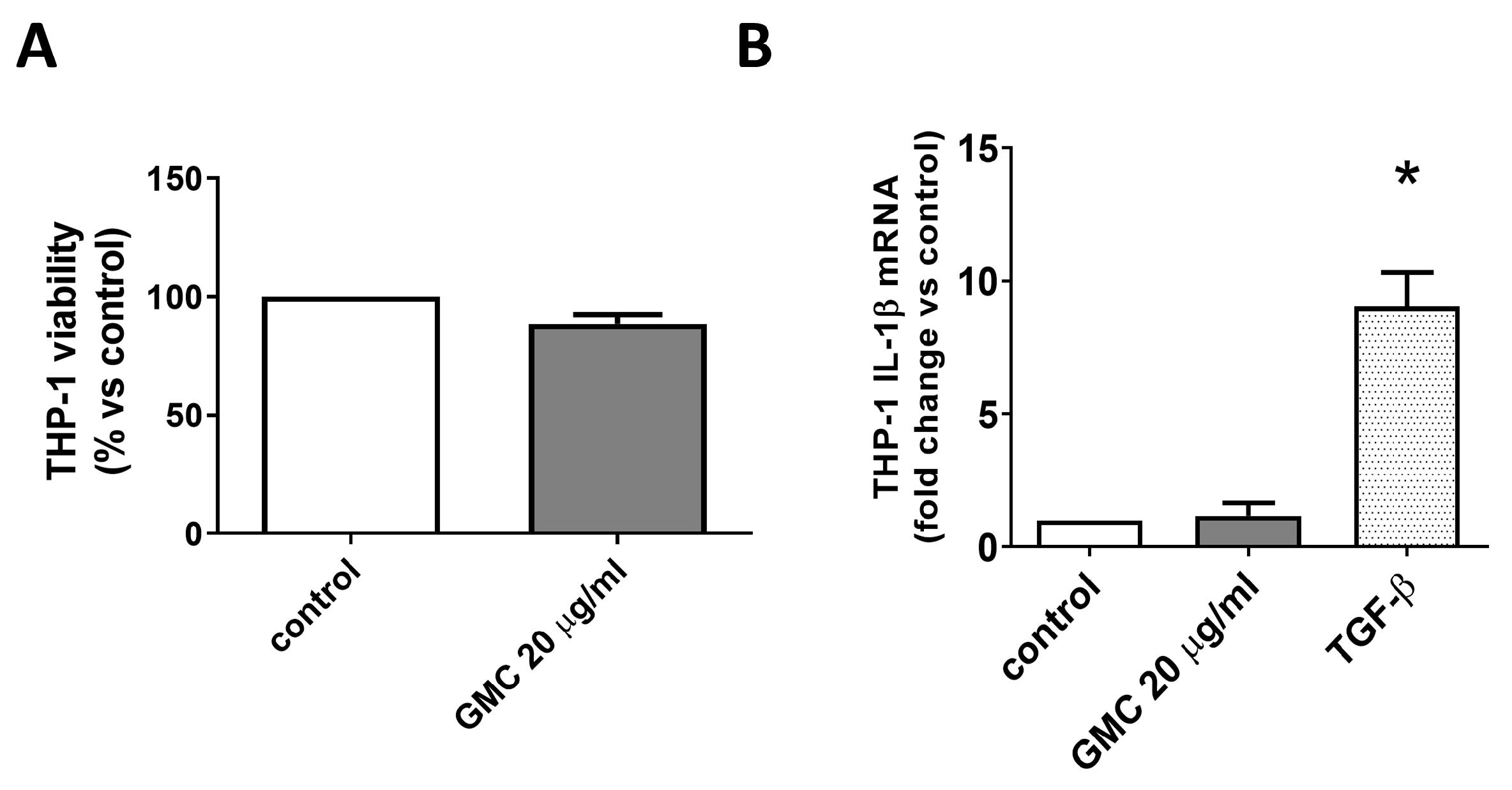
3.3. Viability and Immunological Activity of Cultured Human Monocytes THP-1 Cell Line
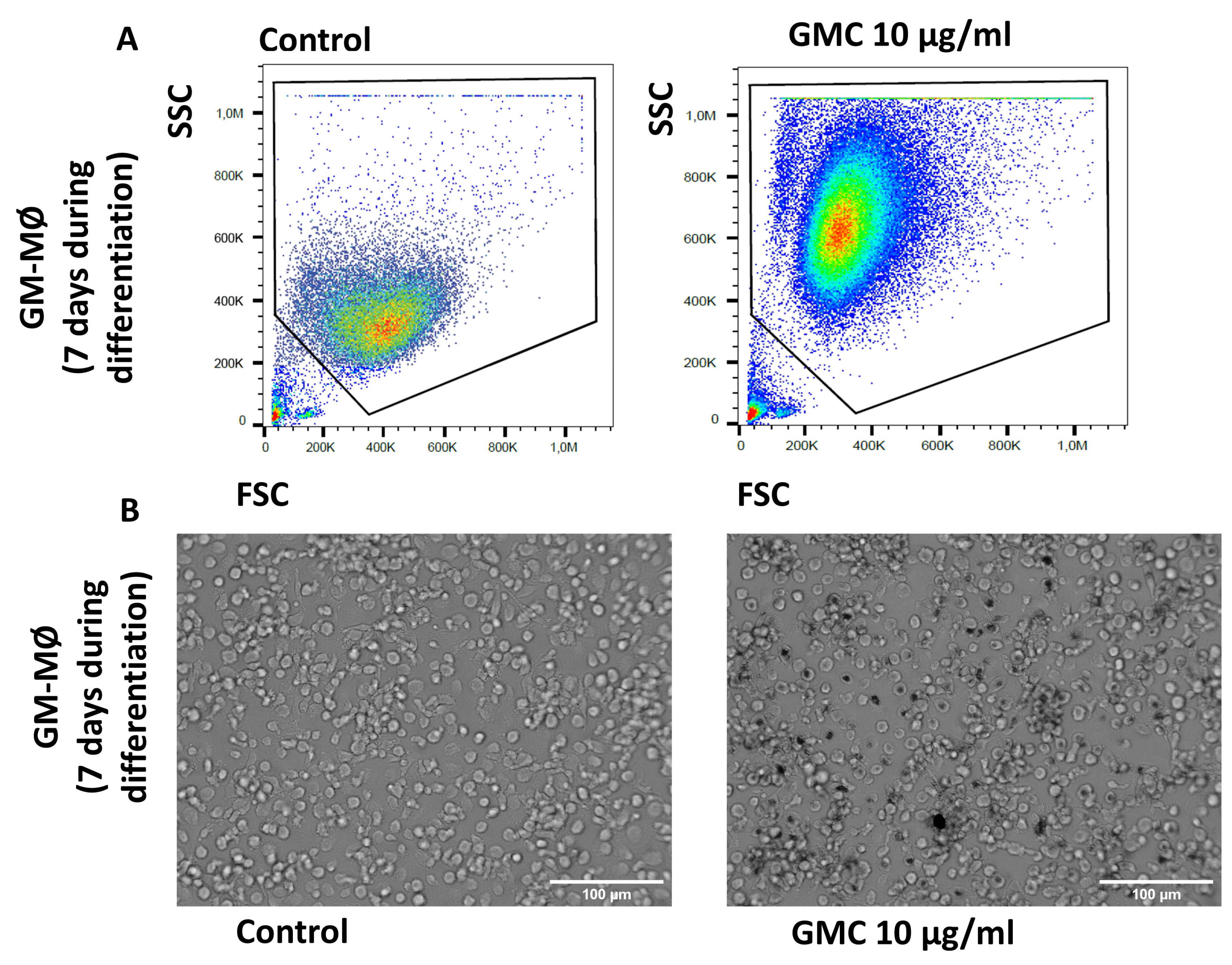
3.4. Viability, Immunological Activity and Phagocytosis During GMC Treatment of GM-MØ Macrophages
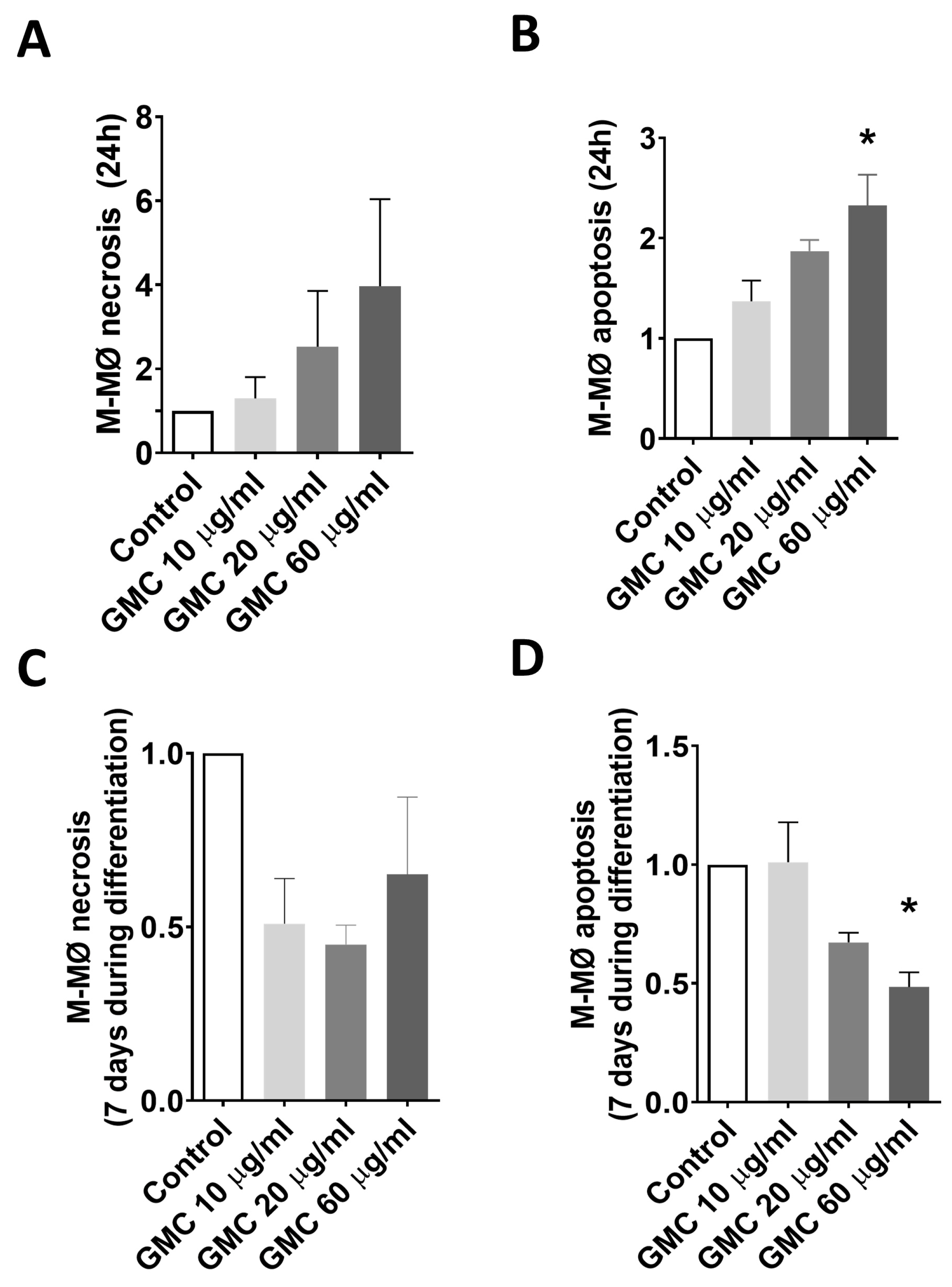
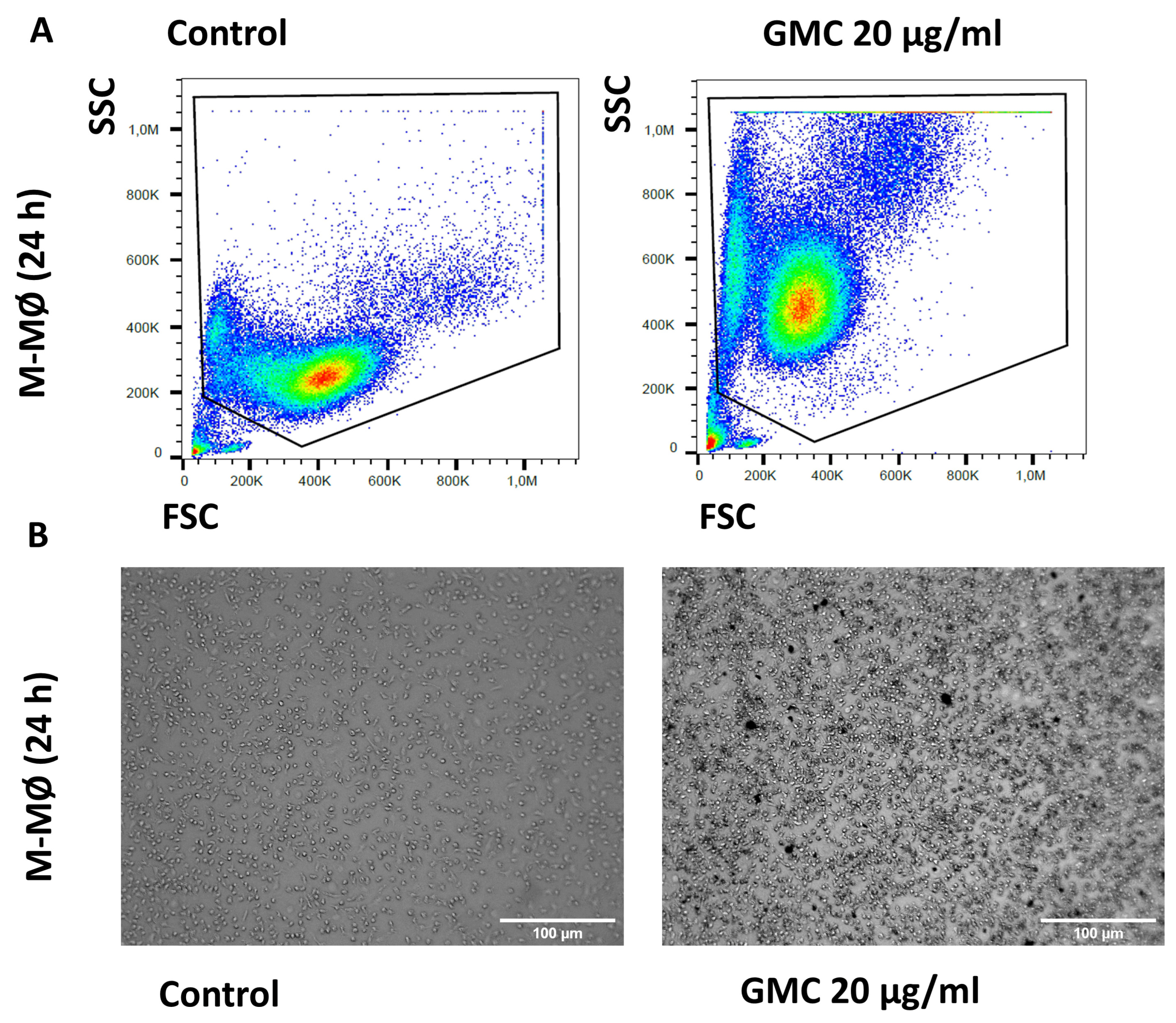
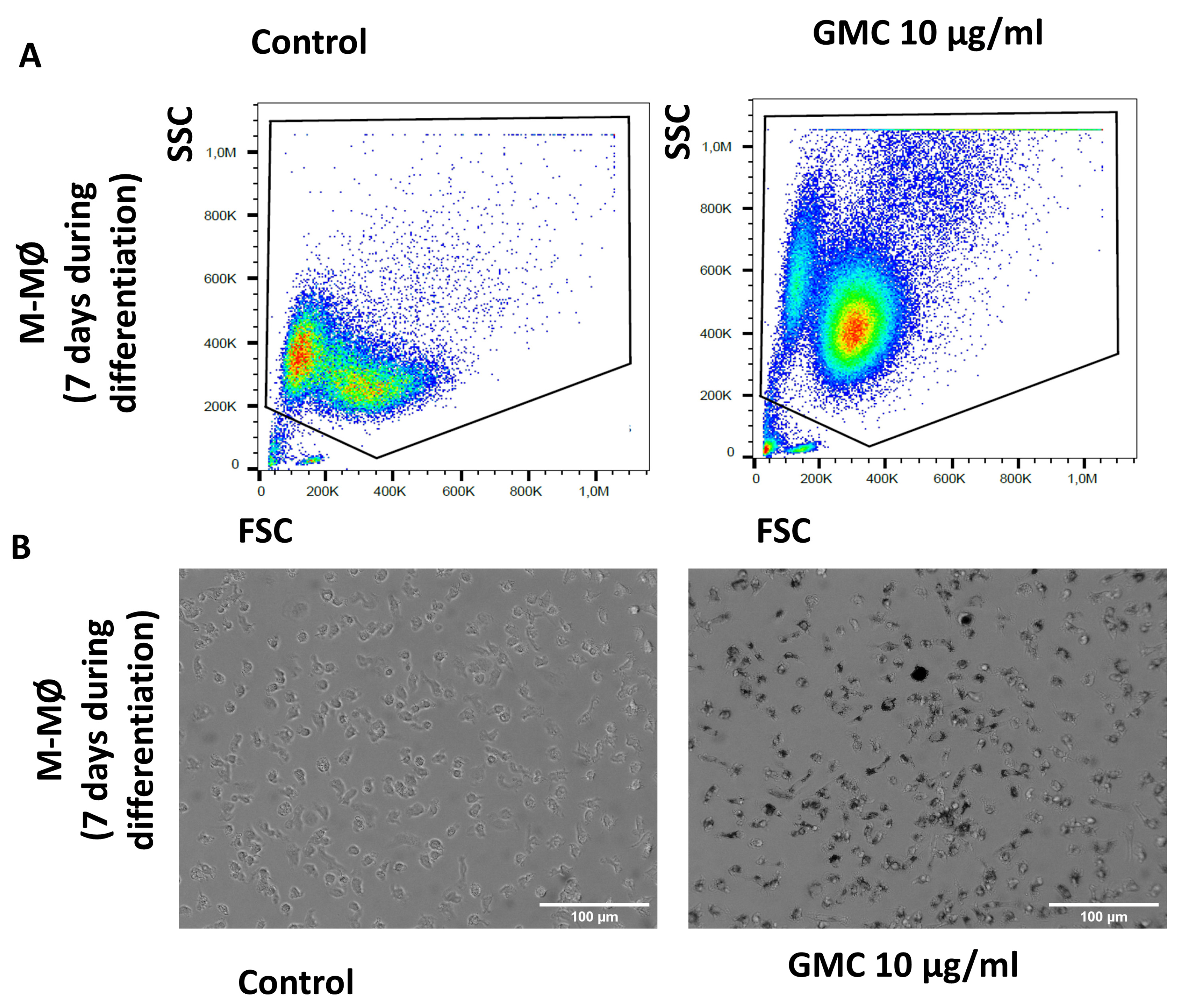
3.5. Viability, Immunological Activity and Phagocytosis During GMC Treatment of M-MØ
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahmati, M.; Mozafari, M. Biological Response to Carbon-Family Nanomaterials: Interactions at the Nano-Bio Interface. Front. Bioeng. Biotechnol. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30 years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.A. Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Curr. Pharm. Des. 2015, 21, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Shibata, K.; Kataoka, H.; Ogino, S.; Bunshi, F.; Yokoyama, A.; Tamura, K.; Akasaka, T.; Uo, M.; Motomiya, K.; et al. Strict preparation and evaluation of water-soluble hat-stacked carbon nanofibers for biomedical application and their high biocompatibility: Influence of nanofiber-surface functional groups on cytotoxicity. Mol. Biosyst. 2005, 1, 142–145. [Google Scholar] [CrossRef]
- Kalman, J.; Merino, C.; Fernández-Cruz, M.L.; Navas, J.M. Usefulness of fish cell lines for the initial characterization of toxicity and cellular fate of graphene-related materials (carbon nanofibers and graphene oxide). Chemosphere 2019, 218, 347–358. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Jaurand, M.C.; Møller, P.; Morimoto, Y.; Kobayashi, N.; Pinkerton, K.E.; Sargent, L.M.; Vermeulen, R.C.; Fubini, B.; Kane, A.B. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit. Rev. Toxicol. 2017, 47, 1–58. [Google Scholar] [CrossRef]
- Salesa, B.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Biomedicines 2021, 9, 1155. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, Y.; Lian, C.; Prak, K.; Leo, H.L.; Tetley, T.D.; Braga, V.; Emerson, M.; Ahnström, J.; Yap, C.H. Safe and Efficacious Near Superhydrophobic Hemostat for Reduced Blood Loss and Easy Detachment in Traumatic Wounds. ACS Appl. Mater. Interfaces 2024, 16, 4307–4320. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019, 10, 5562. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, Á. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Lozano, N.; Kucki, M.; Del Rio-Castillo, A.E.; Newman, L.; Vazquez, E.; Kostarelos, K.; Wick, P.; Fadeel, B. Detection of Endotoxin Contamination of Graphene-Based Materials Using the TNF-A Expression Test and Guidelines for Endotoxin-Free Graphene Oxide Production. PLoS ONE 2016, 11, e0166816. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kinloch, I.A.; Bangert, U.; Windle, A.H.; Walter, D.M.; Walker, G.S.; Scotchford, C.A.; Donaldson, K.; Stone, V. An in vitro study of the potential of carbon nanotubes and nanofibers to induce inflammatory mediators and frustrated phagocytosis. Carbon 2007, 45, 1743–1756. [Google Scholar] [CrossRef]
- Ballesteros, S.; Domenech, J.; Velázquez, A.; Marcos, M.D.; García, J.R.; Alonso, M. Risk assessment of carbon nanotubes, graphene and their derivatives in health and environmental. J. Hazard. Mater. 2021, 414, 125471. [Google Scholar] [CrossRef]
- Kinaret, P.A.S.; Scala, G.; Federico, A.; Sund, J.; Greco, D. Carbon Nanomaterials Promote M1/M2 Macrophage Activation. Small 2020, 16, e1907609. [Google Scholar] [CrossRef]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of Carbon Nanotubes and Graphene on Immune Cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef]
- Feito, M.J.; Diez-Orejas, R.; Cicuéndez, M.; Casarrubios, L.; Rojo, J.M.; Portolés, M.T. Characterization of M1 and M2 Polarization Phenotypes in Peritoneal Macrophages after Treatment with Graphene Oxide Nanosheets. Colloids Surf. B Biointerfaces 2019, 176, 96–105. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Kyriakides, T.R. Nanomaterials, Inflammation, and Tissue Engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 355–370. [Google Scholar] [CrossRef]
- Hoyle, C.; Rivers-Auty, J.; Lemarchand, E.; Vranic, S.; Wang, E.; Buggio, M.; Rothwell, N.J.; Allan, S.M.; Kostarelos, K.; Brough, D. Small, Thin Graphene Oxide Is Anti-inflammatory Activating Nuclear Factor Erythroid 2-Related Factor 2 via Metabolic Reprogramming. ACS Nano 2018, 12, 11949–11962. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Samuelsen, M.; Marioara, C.D.; Løvik, M. Carbon Nanofibers Have IgE Adjuvant Capacity but Are Less Potent Than Nanotubes in Promoting Allergic Airway Responses. BioMed. Res. Int. 2013, 2013, 476010. [Google Scholar] [CrossRef]
- Piperno, A.; Scala, A.; Mazzaglia, A.; Neri, G.; Pennisi, R.; Sciortino, M.T.; Grassi, G. Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials. Int. J. Mol. Sci. 2018, 19, 3365. [Google Scholar] [CrossRef]
- Magne, T.M.; de Oliveira Vieira, T.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.U.D.; Freire, R.M.; Golokhvast, K.; Metrangolo, P.; et al. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostruct. Chem. 2021, 11, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Lin, S.; Song, B.; Liu, J.; Lai, R.; Shao, L. The mechanisms of graphene-based materials-induced programmed cell death: A review of apoptosis, autophagy, and programmed necrosis. Int. J. Nanomed. 2017, 12, 6633–6646. [Google Scholar] [CrossRef]
- Xiaoli, F.; Qiyue, C.; Weihong, G.; Yaqing, Z.; Chen, H.; Junrong, W.; Longquan, S. Toxicology data of graphene-family nanomaterials: An update. Arch. Toxicol. 2020, 94, 1915–1939. [Google Scholar] [CrossRef] [PubMed]
- de Frutos, S.; Griera, M.; Lavín-López, M.D.P.; Martínez-Rovira, M.; Martínez-Rovira, J.A.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D. A New Graphene-Based Nanomaterial Increases Lipolysis and Reduces Body Weight Gain through Integrin Linked Kinase (ILK). Biomater. Sci. 2023, 11, 4916–4929. [Google Scholar] [CrossRef] [PubMed]
- Soler Palacios, B.; Nieto, C.; Fajardo, P.; González de la Aleja, A.; Andrés, N.; Dominguez-Soto, Á.; Lucas, P.; Cuenda, A.; Rodríguez-Frade, J.M.; Martínez-A, C.; et al. Growth Hormone Reprograms Macrophages toward an Anti-Inflammatory and Reparative Profile in an MAFB-Dependent Manner. J. Immunol. 2020, 205, 776–788. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Puig-Kröger, A.; Blanco, F.J.; Nieto, C.; Bragado, R.; Palomero, M.I.; Bernabéu, C.; Vega, M.A.; Corbí, A.L. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011, 117, 5092–5101. [Google Scholar] [CrossRef]
- Souza, I.L.M.; Suzukawa, A.A.; Josino, R.; Marcon, B.H.; Robert, A.W.; Shigunov, P.; Correa, A.; Stimamiglio, M.A. Cellular in vitro responses induced by human mesenchymal stem/stromal cell-derived extracellular vesicles obtained from suspension culture. Int. J. Mol. Sci. 2024, 25, 7605. [Google Scholar] [CrossRef]
- Poirier, S.J.; Boudreau, L.H.; Flamand, N.; Surette, M.E. LPS induces ALOX5 promoter activation and 5-lipoxygenase expression in human monocytic cells. Prostaglandins Leukot. Essent. Fatty Acids 2020, 154, 102078. [Google Scholar] [CrossRef]
- Brubaker, Z.E.; Langford, J.J.; Kapsimalis, R.J.; Niedziela, J.L. Quantitative analysis of Raman spectral parameters for carbon fibers: Practical considerations and connection to mechanical properties. J. Mater. Sci. 2021, 56, 15087–15121. [Google Scholar] [CrossRef]
- Romero, A.; Lavin-Lopez, M.P.; Sánchez-Silva, M.L.; Valverde, J.L.; Paton-Carrero, A. Effects of oxidizing procedures on carbon nanofibers surface and dispersability in an epoxy resin. Mater. Chem. Phys. 2020, 243, 122571. [Google Scholar] [CrossRef]
- Watari, F.; Takashi, N.; Yokoyama, A.; Uo, M.; Akasaka, T.; Sato, Y.; Abe, S.; Totsuka, Y.; Tohji, K. Material Nanosizing Effect on Living Organisms: Non-Specific, Biointeractive, Physical Size Effects. J. R. Soc. Interface 2009, 6 (Suppl. S3), S371–S388. [Google Scholar] [CrossRef] [PubMed]
- Ikram, R.; Shamsuddin, S.A.A.; Mohamed Jan, B.; Abdul Qadir, M.; Kenanakis, G.; Stylianakis, M.M.; Anastasiadis, S.H. Impact of Graphene Derivatives as Artificial Extracellular Matrices on Mesenchymal Stem Cells. Molecules 2022, 27, 379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Meng, X.; Sun, G.; Gao, C. Influences of Acid-Treated Multiwalled Carbon Nanotubes on Fibroblasts: Proliferation, Adhesion, Migration, and Wound Healing. Ann. Biomed. Eng. 2011, 39, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.T.; Yang, L.; Lee, K.B. Guiding Stem Cell Differentiation into Oligodendrocytes Using Graphene-Nanofiber Hybrid Scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; Anderson, D.E.; Biris, A.S.; Bourdo, S.E.; Dhar, M. Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar] [CrossRef]
- Aventaggiato, M.; Valentini, F.; Caissutti, D.; Relucenti, M.; Tafani, M.; Misasi, R.; Zicari, A.; Di Martino, S.; Virtuoso, S.; Neri, A.; et al. Biological Effects of Small Sized Graphene Oxide Nanosheets on Human Leukocytes. Biomedicines 2024, 12, 256. [Google Scholar] [CrossRef]
- Turabekova, M.; Rasulev, B.; Theodore, M.; Jackman, J.; Leszczynska, D.; Leszczynski, J. Immunotoxicity of Nanoparticles: A Computational Study Suggests That CNTs and C60 Fullerenes Might Be Recognized as Pathogens by Toll-Like Receptors. Nanoscale 2014, 6, 3488–3495. [Google Scholar] [CrossRef]
- Chen, G.Y.; Yang, H.J.; Lu, C.H.; Chao, Y.C.; Hwang, S.M.; Chen, C.L.; Lo, K.W.; Sung, L.Y.; Luo, W.Y.; Tuan, H.Y.; et al. Simultaneous Induction of Autophagy and Toll-Like Receptor Signaling Pathways by Graphene Oxide. Biomaterials 2012, 33, 6559–6569. [Google Scholar] [CrossRef]
- Qu, G.; Liu, S.; Zhang, S.; Wang, L.; Wang, X.; Sun, B.; Yin, N.; Gao, X.; Xia, T.; Chen, J.J.; et al. Graphene oxide induces toll-like receptor 4 (TLR4)-dependent necrosis in macrophages. ACS Nano 2013, 7, 5732–5745. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Kanno, S.; Furuyama, A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol. Appl. Pharmacol. 2008, 232, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lohray, R.; Chow, E.; Gangupantula, P.; Smith, L.; Draper, R. Selective Uptake of Carboxylated Multi-Walled Carbon Nanotubes by Class A Type 1 Scavenger Receptors and Impaired Phagocytosis in Alveolar Macrophages. Nanomaterials 2020, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.T.; Mikoryak, C.; Pantano, P.; Draper, R. Scavenger Receptor A1 Mediates the Uptake of Carboxylated and Pristine Multi-Walled Carbon Nanotubes Coated with Bovine Serum Albumin. Nanomaterials 2021, 11, 539. [Google Scholar] [CrossRef]
- Keshavan, S.; Andón, F.T.; Gallud, A.; Chen, W.; Reinert, K.; Tran, L.; Fadeel, B. Profiling of Sub-Lethal in Vitro Effects of Multi-Walled Carbon Nanotubes Reveals Changes in Chemokines and Chemokine Receptors. Nanomaterials 2021, 11, 883. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis. Nanotoxicology 2018, 12, 153–168. [Google Scholar] [CrossRef]
- Otsuka, K.; Yamada, K.; Taquahashi, Y.; Arakaki, R.; Ushio, A.; Saito, M.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Kanno, J.; et al. Long-term polarization of alveolar macrophages to a profibrotic phenotype after inhalation exposure to multi-wall carbon nanotubes. PLoS ONE 2018, 13, e0205702. [Google Scholar] [CrossRef]
- Korejwo, D.; Chortarea, S.; Louka, C.; Buljan, M.; Rothen-Rutishauser, B.; Wick, P.; Buerki-Thurnherr, T. Gene expression profiling of human macrophages after graphene oxide and graphene nanoplatelets treatment reveals particle-specific regulation of pathways. NanoImpact 2023, 29, 100452. [Google Scholar] [CrossRef]
- Ding, X.; Pang, Y.; Liu, Q.; Zhang, H.; Wu, J.; Lei, J.; Zhang, T. GO-PEG Represses the Progression of Liver Inflammation via Regulating the M1/M2 Polarization of Kupffer Cells. Small 2024, e2306483. [Google Scholar] [CrossRef]
- Huaux, F.; d’Ursel de Bousies, V.; Parent, M.A.; Orsi, M.; Uwambayinema, F.; Devosse, R.; Ibouraadaten, S.; Yakoub, Y.; Panin, N.; Palmai-Pallag, M.; et al. Mesothelioma response to carbon nanotubes is associated with an early and selective accumulation of immunosuppressive monocytic cells. Part. Fibre Toxicol. 2016, 13, 46. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, M.; Zhang, J.; Yao, Z.; Zhu, J.; Yang, S.; Zhu, Q.; Shen, T. Carbon nanotubes promote alveolar macrophages toward M2 polarization mediated epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Nanotoxicology 2021, 15, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef] [PubMed]




| % of Total PBMCs | Control | 20 µg/mL | 60 µg/mL |
|---|---|---|---|
| G0/G1 phase | 90.3 ± 2.0 | 92.5 ± 1.3 | 93.5 ± 0.7 |
| G2/M phase | 3.4 ± 0.2 | 3.3 ± 0.8 | 2.3 ± 0.5 |
| S phase | 0.9 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.3 |
| Apoptosis | 3.1 ± 1.0 | 1.9 ± 0.4 | 2.1 ± 0.5 |
| Necrosis | 4.9 ± 1.8 | 2.7 ± 0.6 | 2.8 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavín-López, M.d.P.; Torres-Torresano, M.; García-Cuesta, E.M.; Soler-Palacios, B.; Griera, M.; Martínez-Rovira, M.; Martínez-Rovira, J.A.; Rodríguez-Puyol, D.; de Frutos, S. A Graphene-Based Bioactive Product with a Non-Immunological Impact on Mononuclear Cell Populations from Healthy Volunteers. Nanomaterials 2024, 14, 1945. https://doi.org/10.3390/nano14231945
Lavín-López MdP, Torres-Torresano M, García-Cuesta EM, Soler-Palacios B, Griera M, Martínez-Rovira M, Martínez-Rovira JA, Rodríguez-Puyol D, de Frutos S. A Graphene-Based Bioactive Product with a Non-Immunological Impact on Mononuclear Cell Populations from Healthy Volunteers. Nanomaterials. 2024; 14(23):1945. https://doi.org/10.3390/nano14231945
Chicago/Turabian StyleLavín-López, María del Prado, Mónica Torres-Torresano, Eva María García-Cuesta, Blanca Soler-Palacios, Mercedes Griera, Martín Martínez-Rovira, José Antonio Martínez-Rovira, Diego Rodríguez-Puyol, and Sergio de Frutos. 2024. "A Graphene-Based Bioactive Product with a Non-Immunological Impact on Mononuclear Cell Populations from Healthy Volunteers" Nanomaterials 14, no. 23: 1945. https://doi.org/10.3390/nano14231945
APA StyleLavín-López, M. d. P., Torres-Torresano, M., García-Cuesta, E. M., Soler-Palacios, B., Griera, M., Martínez-Rovira, M., Martínez-Rovira, J. A., Rodríguez-Puyol, D., & de Frutos, S. (2024). A Graphene-Based Bioactive Product with a Non-Immunological Impact on Mononuclear Cell Populations from Healthy Volunteers. Nanomaterials, 14(23), 1945. https://doi.org/10.3390/nano14231945





