Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies
Abstract
1. Introduction
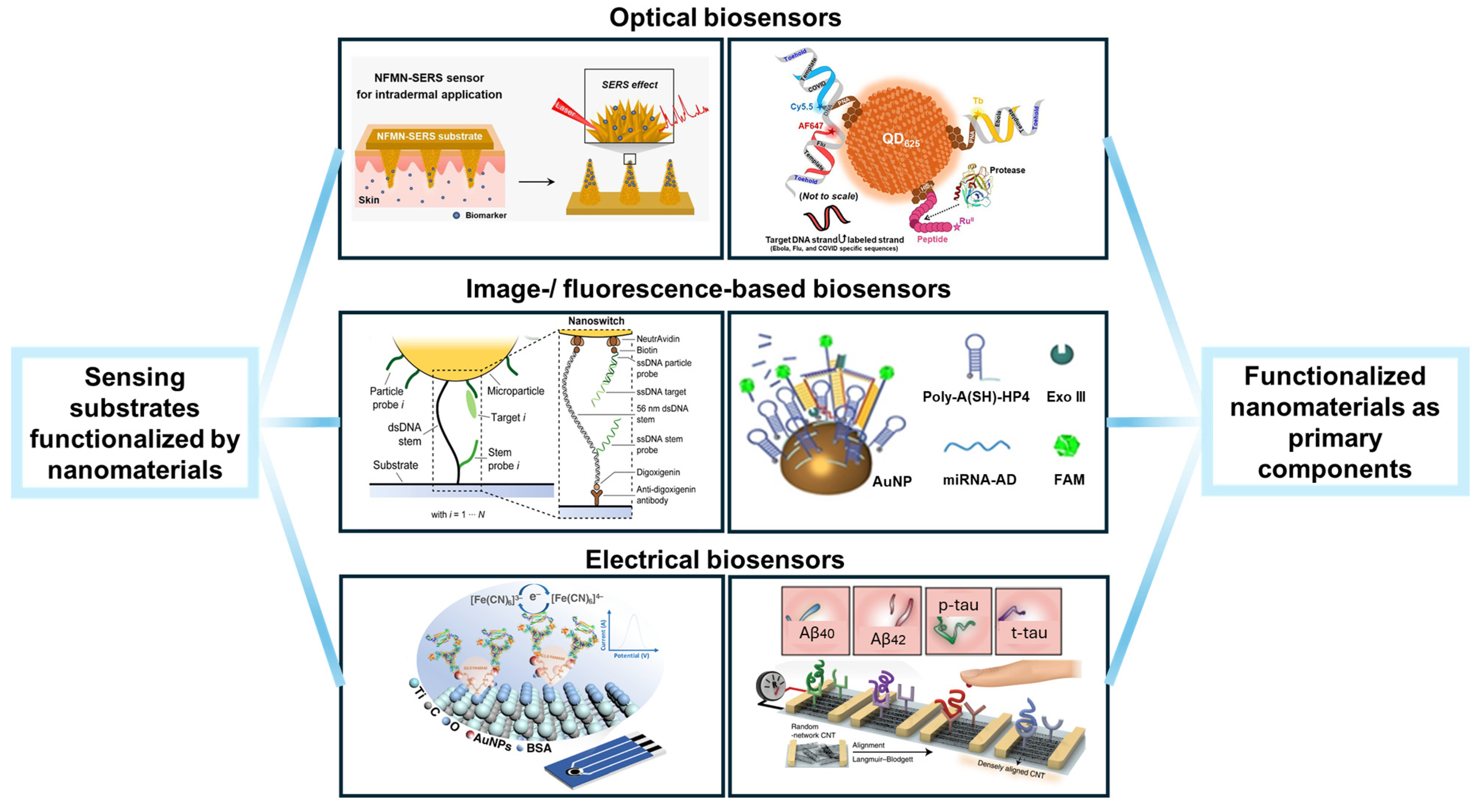
2. Surface Structures for Functionalization
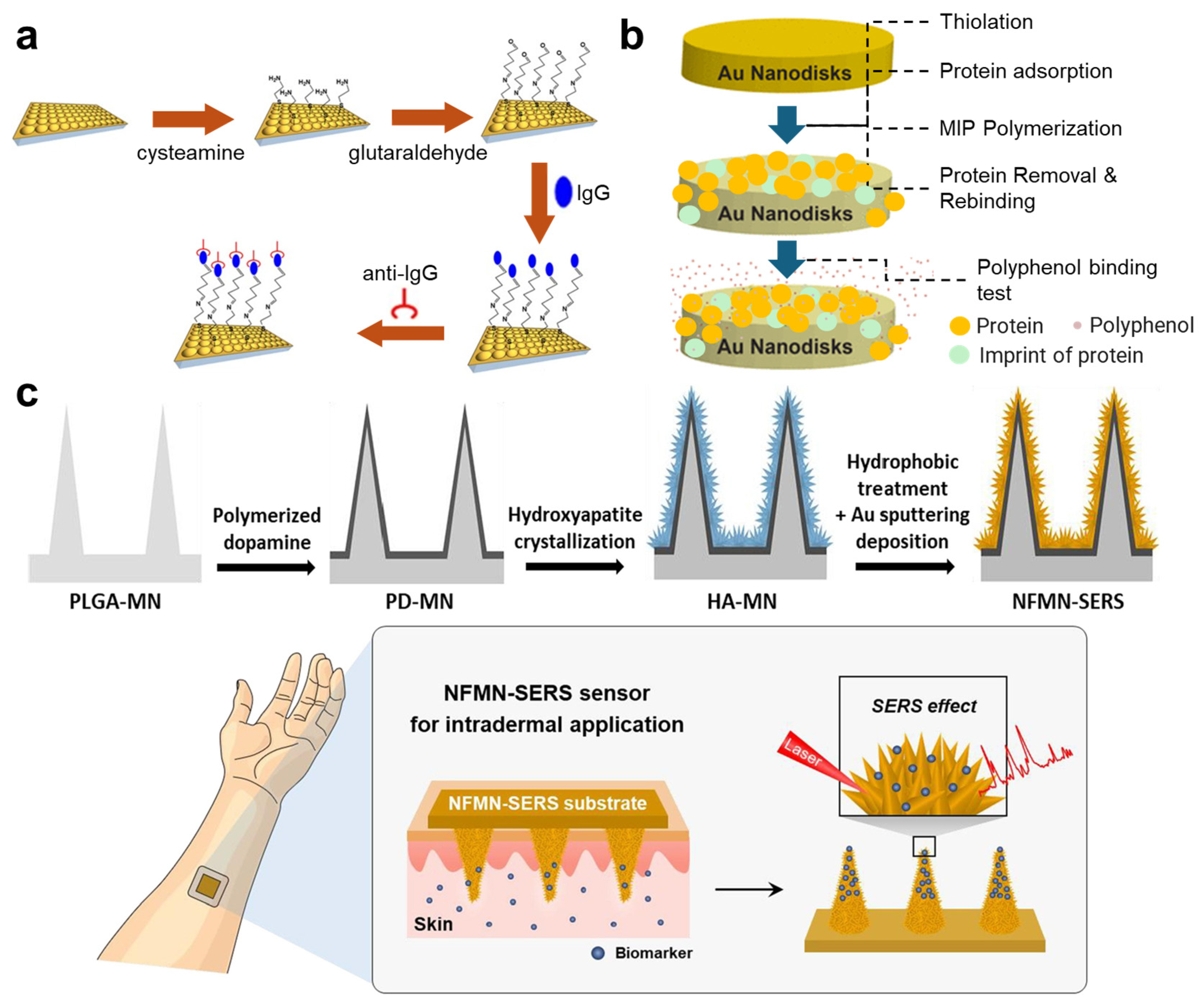
2.1. Plane Surfaces
2.2. 3D Structures
2.3. Nanoparticles
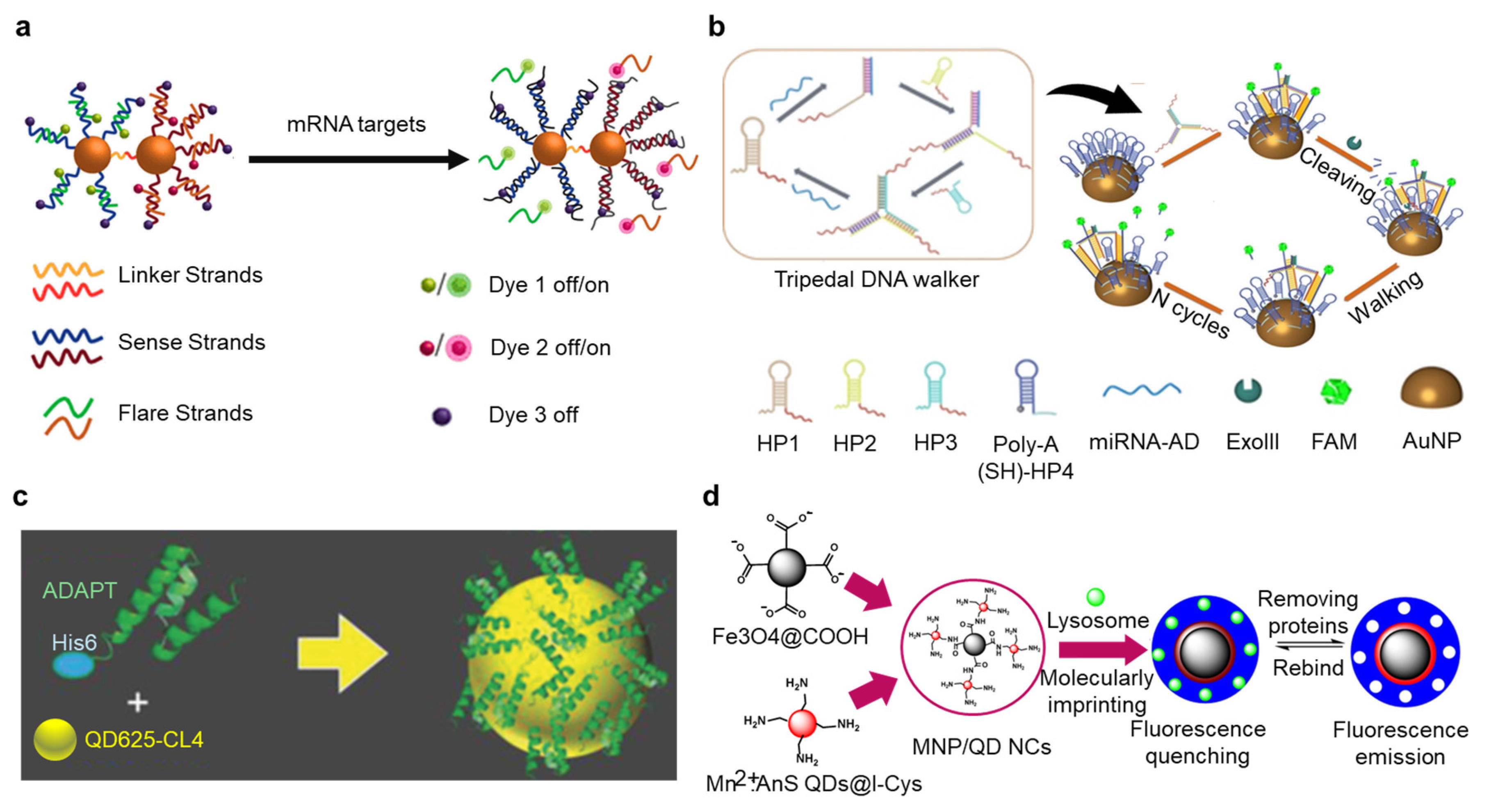
3. Nanomaterials for Improving Sensor Performance
3.1. Polymers
3.2. Composite Nanomaterials
4. Paradigms for Multiplexed Molecular Biosensing
4.1. Spatial Separation of Detecting Regions
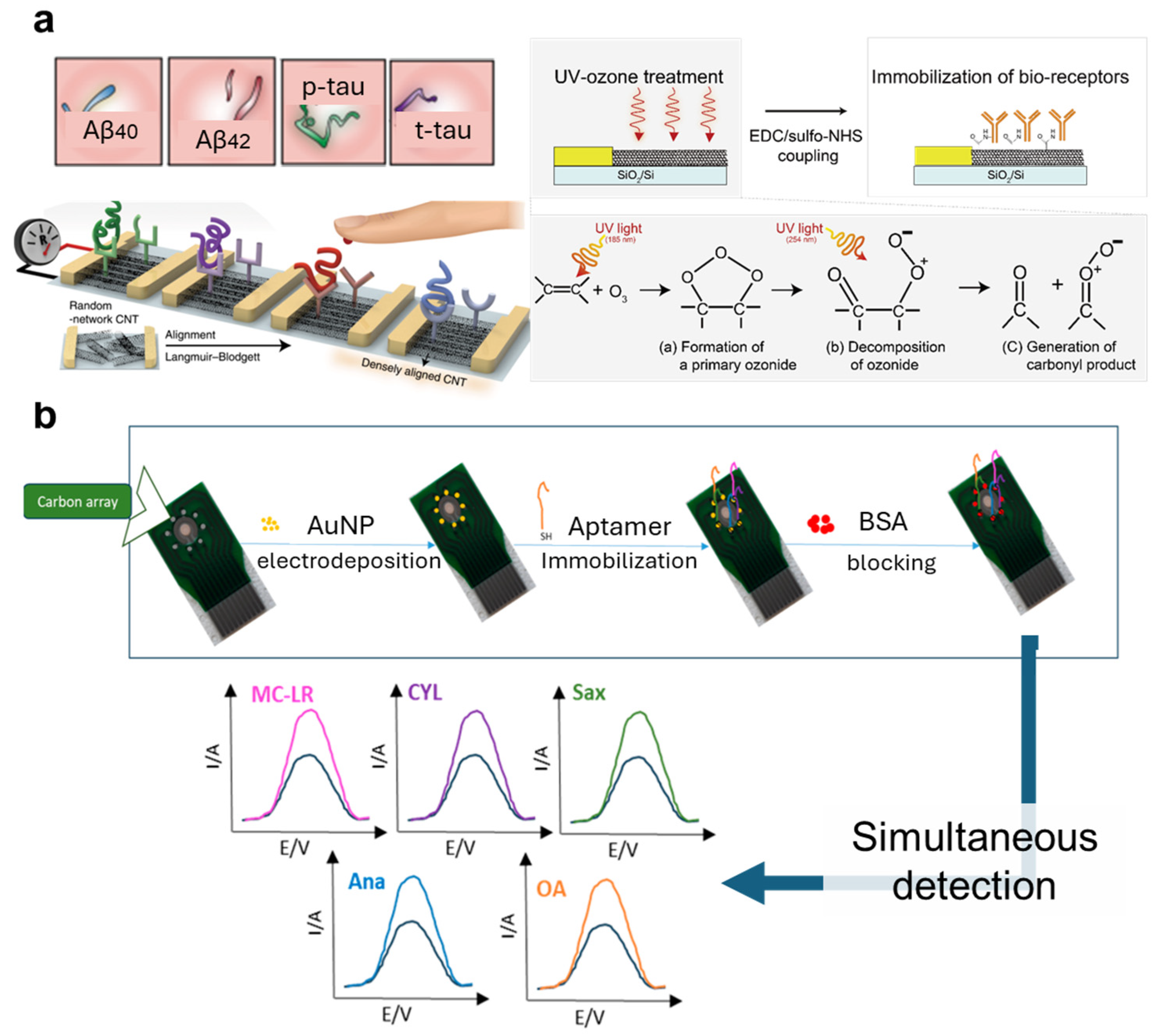
4.2. Unique Identifiers
4.3. Multimodal Detection
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Coskun, A.F.; Topkaya, S.N.; Yetisen, A.K.; Cetin, A.E. Portable Multiplex Optical Assays. Adv. Opt. Mater. 2019, 7, 1801109. [Google Scholar] [CrossRef]
- Terai, T.; Campbell, R.E. Barcodes, co-cultures, and deep learning take genetically encoded biosensor multiplexing to the nth degree. Mol. Cell 2022, 82, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Chi, W.-Y.; Liang, J.; Takayanagi, S.; Iglesias, P.A.; Huang, C.-H. Deciphering cell signaling networks with massively multiplexed biosensor barcoding. Cell 2021, 184, 6193–6206.e14. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, P.S.; Smith, S.J.; Chen, J.B.; Karlikow, M.; Tinafar, A.; Robinson, C.; Liu, W.; Ma, D.; Green, A.A.; Kelley, S.O.; et al. A multiplexed, electrochemical interface for gene-circuit-based sensors. Nat. Chem. 2020, 12, 48–55. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, Z.; Meng, Z.; Wang, P.; San, L.; Wang, W.; Aldalbahi, A.; Li, L.; Shen, J.; Mi, X. DNA Tetrahedral Nanostructure-Based Electrochemical miRNA Biosensor for Simultaneous Detection of Multiple miRNAs in Pancreatic Carcinoma. ACS Appl. Mater. Interfaces 2017, 9, 24118–24125. [Google Scholar] [CrossRef]
- Zupančič, U.; Jolly, P.; Estrela, P.; Moschou, D.; Ingber, D.E. Graphene Enabled Low-Noise Surface Chemistry for Multiplexed Sepsis Biomarker Detection in Whole Blood. Adv. Funct. Mater. 2021, 31, 2010638. [Google Scholar] [CrossRef]
- Lee, J.; Na Suh, H.; Park, H.-B.; Park, Y.M.; Kim, H.J.; Kim, S. Regenerative Strategy of Gold Electrodes for Long-Term Reuse of Electrochemical Biosensors. ACS Omega 2023, 8, 1389–1400. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Bauer, M.; Wunderlich, L.; Weinzierl, F.; Lei, Y.; Duerkop, A.; Alshareef, H.N.; Baeumner, A.J. Electrochemical multi-analyte point-of-care perspiration sensors using on-chip three-dimensional graphene electrodes. Anal. Bioanal. Chem. 2021, 413, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.T.; Pham, N.T.; Ngo, D.-H.; Kumar, S.; Cao, X.T. Covalently Functionalized Graphene with Molecularly Imprinted Polymers for Selective Adsorption and Electrochemical Detection of Chloramphenicol. ACS Omega 2023, 8, 25385–25391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dostalek, J.; Knoll, W. Magnetic Nanoparticle-Enhanced Biosensor Based on Grating-Coupled Surface Plasmon Resonance. Anal. Chem. 2011, 83, 6202–6207. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Z.; Li, W.; Yu, P.; Shi, Q.; Kong, F.; Zhang, Q.; Wang, P.; Wang, Y.; Shi, F.; et al. Digital Magnetic Detection of Biomolecular Interactions with Single Nanoparticles. Nano Lett. 2023, 23, 2636–2643. [Google Scholar] [CrossRef]
- Oropesa-Nuñez, R.; de la Torre, T.Z.G.; Stopfel, H.; Svedlindh, P.; Strömberg, M.; Gunnarsson, K. Insights into the Formation of DNA–Magnetic Nanoparticle Hybrid Structures: Correlations between Morphological Characterization and Output from Magnetic Biosensor Measurements. ACS Sens. 2020, 5, 3510–3519. [Google Scholar] [CrossRef]
- Downs, A.M.; Gerson, J.; Hossain, M.N.; Ploense, K.; Pham, M.; Kraatz, H.-B.; Kippin, T.; Plaxco, K.W. Nanoporous Gold for the Miniaturization of In Vivo Electrochemical Aptamer-Based Sensors. ACS Sens. 2021, 6, 2299–2306. [Google Scholar] [CrossRef]
- Kyriazi, M.-E.; Giust, D.; El-Sagheer, A.H.; Lackie, P.M.; Muskens, O.L.; Brown, T.; Kanaras, A.G. Multiplexed mRNA Sensing and Combinatorial-Targeted Drug Delivery Using DNA-Gold Nanoparticle Dimers. ACS Nano 2018, 12, 3333–3340. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Song, X.; Wang, H.; Wang, J.; Wang, Y.; Huang, J.; Yu, J. Robust and Universal SERS Sensing Platform for Multiplexed Detection of Alzheimer’s Disease Core Biomarkers Using PAapt-AuNPs Conjugates. ACS Sens. 2019, 4, 2140–2149. [Google Scholar] [CrossRef]
- Li, W.; Dong, Y.; Wang, X.; Li, H.; Xu, D. PolyA-tailed and fluorophore-labeled aptamer-gold nanoparticle conjugate for fluorescence turn-on bioassay using iodide-induced ligand displacement. Biosens. Bioelectron. 2015, 66, 43–49. [Google Scholar] [CrossRef]
- Li, X.; Qian, Z.; Chang, R.; Peng, C.; Xie, Z.; Wang, Z. Non-thiolated nucleic acid functionalized gold nanoparticle–based aptamer lateral flow assay for rapid detection of kanamycin. Microchim. Acta 2022, 189, 244. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Yim, S.-G.; Mun, C.; Yang, J.-Y.; Lee, S.; Yoo, Y.W.; Sung, D.K.; Lee, Y.-I.; Kim, D.-H.; Park, S.-G.; et al. Bioinspired plasmonic nanoflower-decorated microneedle for label-free intradermal sensing. Appl. Surf. Sci. 2021, 551, 149411. [Google Scholar] [CrossRef]
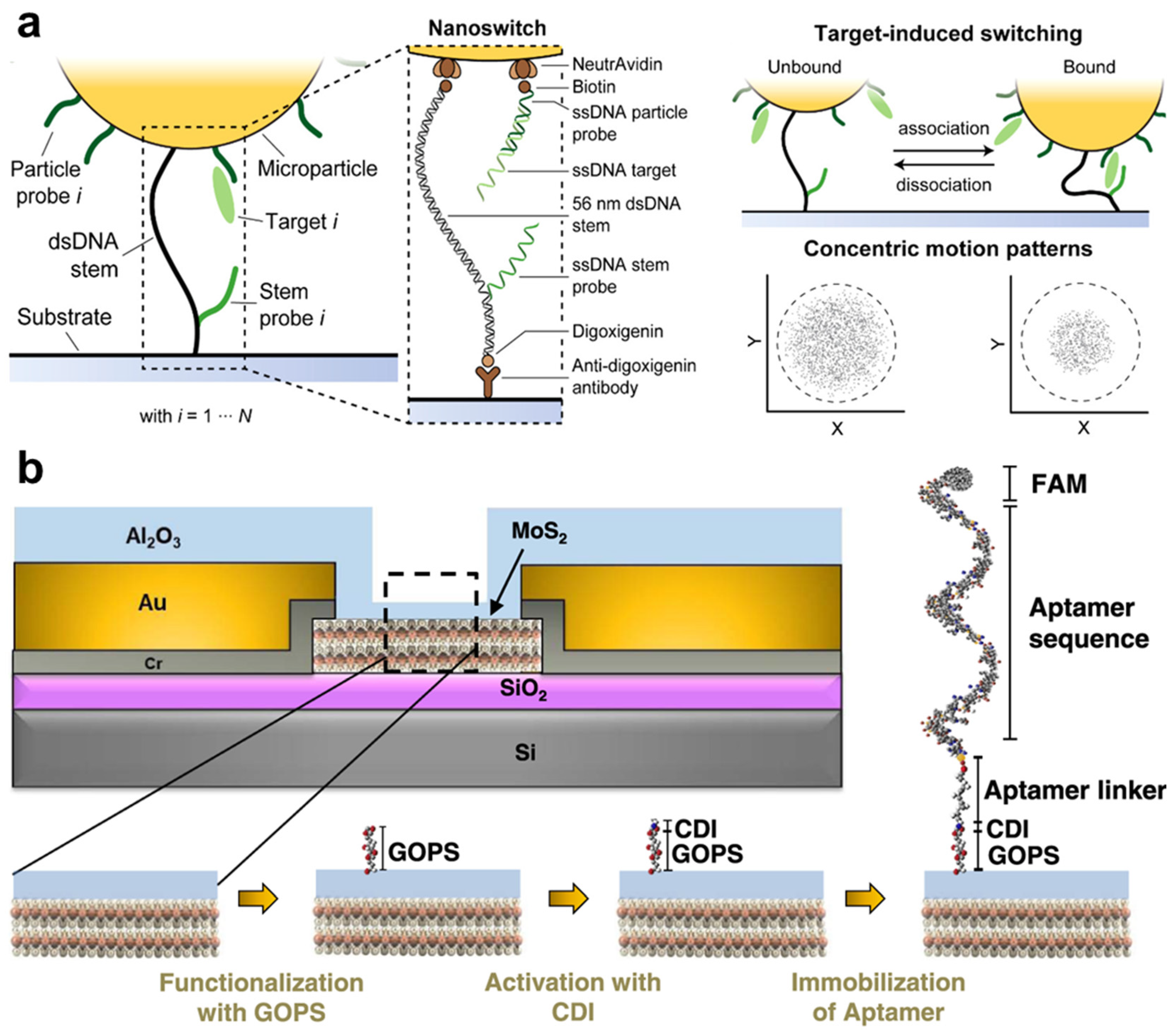
- Lubken, R.M.; de Jong, A.M.; Prins, M.W.J. Multiplexed Continuous Biosensing by Single-Molecule Encoded Nanoswitches. Nano Lett. 2020, 20, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, Y.; Jiang, D.; Li, F.; Gan, Y.; Zhu, Y.; Pan, Y.; Wan, H.; Wang, P. Covalently grafting first-generation PAMAM dendrimers onto MXenes with self-adsorbed AuNPs for use as a functional nanoplatform for highly sensitive electrochemical biosensing of cTnT. Microsyst. Nanoeng. 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
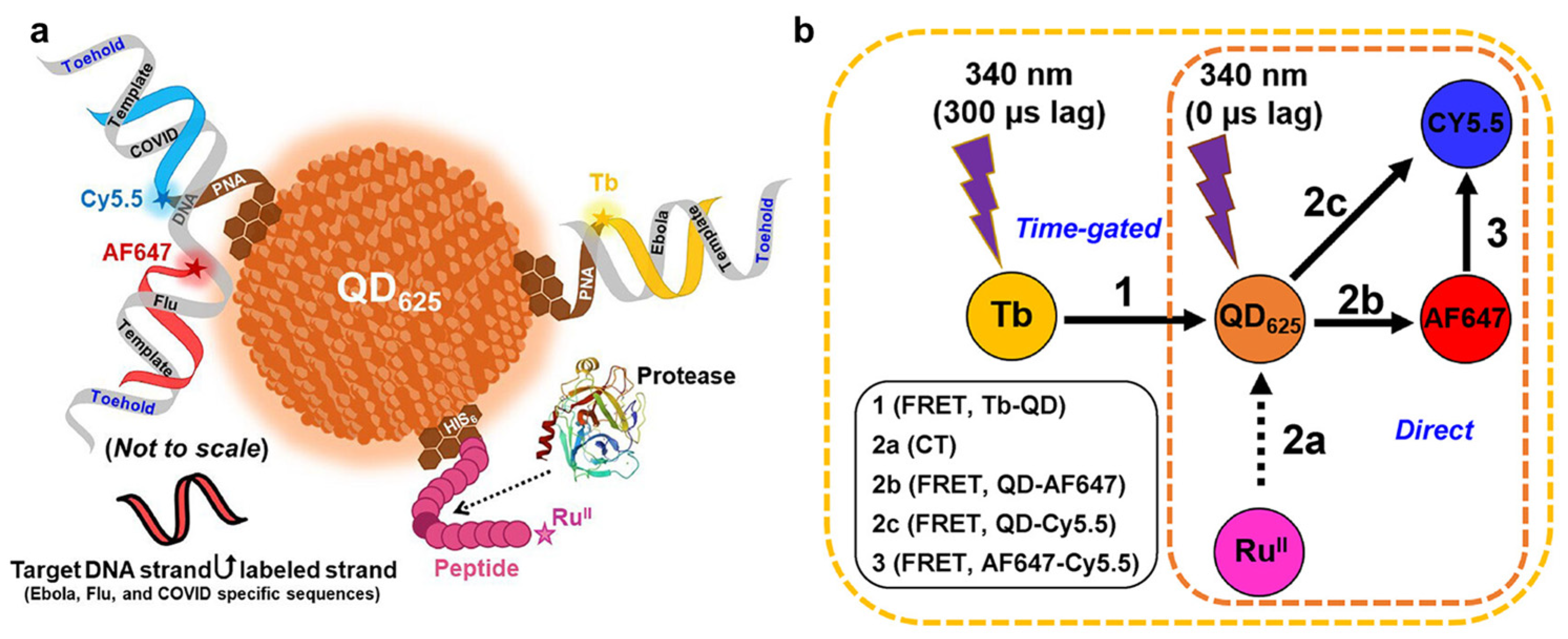
- Hastman, D.A.; Hooe, S.; Chiriboga, M.; Díaz, S.A.; Susumu, K.; Stewart, M.H.; Green, C.M.; Hildebrandt, N.; Medintz, I.L. Multiplexed DNA and Protease Detection with Orthogonal Energy Transfer on a Single Quantum Dot Scaffolded Biosensor. ACS Sens. 2024, 9, 157–170. [Google Scholar] [CrossRef]
- Li, J.; Li, H.-W. Ultrasensitive miRNA Detection by AuNP-based 3D DNA Walker and Catalytic Hairpin Assembly (CHA) Cascade Amplification for Early Cancer Diagnosis. Chem.—Asian J. 2023, 18, e202300367. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.-J.; Kim, D.W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 119. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Hu, C.; Hu, S. Ultrasensitive Photoelectrochemical Biosensing of Multiple Biomarkers on a Single Electrode by a Light Addressing Strategy. Anal. Chem. 2015, 87, 9368–9375. [Google Scholar] [CrossRef]
- Ji, X.; Lin, X.; Rivnay, J. Organic electrochemical transistors as on-site signal amplifiers for electrochemical aptamer-based sensing. Nat. Commun. 2023, 14, 1665. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Z.; Yang, S.; Rich, J.; Zhao, S.; Klingeborn, M.; Huang, P.-H.; Li, Z.; Stout, A.; Murphy, Q.; et al. Electrochemical micro-aptasensors for exosome detection based on hybridization chain reaction amplification. Microsyst. Nanoeng. 2021, 7, 63. [Google Scholar] [CrossRef]
- Kesler, V.; Fu, K.; Chen, Y.; Park, C.H.; Eisenstein, M.; Murmann, B.; Soh, H.T. Tailoring Electrode Surface Charge to Achieve Discrimination and Quantification of Chemically Similar Small Molecules with Electrochemical Aptamers. Adv. Funct. Mater. 2023, 33, 2208534. [Google Scholar] [CrossRef]
- Keyvani, F.; GhavamiNejad, P.; Saleh, M.A.; Soltani, M.; Zhao, Y.; Sadeghzadeh, S.; Shakeri, A.; Chelle, P.; Zheng, H.; Rahman, F.A.; et al. Integrated Electrochemical Aptamer Biosensing and Colorimetric pH Monitoring via Hydrogel Microneedle Assays for Assessing Antibiotic Treatment. Adv. Sci. 2024, 11, e2309027. [Google Scholar] [CrossRef] [PubMed]
- Wirde, M.; Gelius, U.; Nyholm, L. Self-Assembled Monolayers of Cystamine and Cysteamine on Gold Studied by XPS and Voltammetry. Langmuir 1999, 15, 6370–6378. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Burgos-Flórez, F.; Posada, J.D.; Cervera, E.; Zucolotto, V.; Sanjuán, H.; Sanjuán, M.; Villalba, P.J. Electrochemical Immunosensor for the Quantification of S100B at Clinically Relevant Levels Using a Cysteamine Modified Surface. Sensors 2021, 21, 1929. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Wang, H.; Jiang, J.; Shen, G.; Yu, R. An amperometric horseradish peroxidase inhibition biosensor based on a cysteamine self-assembled monolayer for the determination of sulfides. Sens. Actuators B Chem. 2004, 102, 162–168. [Google Scholar] [CrossRef]
- Watkins, Z.; Karajic, A.; Young, T.; White, R.; Heikenfeld, J. Week-Long Operation of Electrochemical Aptamer Sensors: New Insights into Self-Assembled Monolayer Degradation Mechanisms and Solutions for Stability in Serum at Body Temperature. ACS Sens. 2023, 8, 1119–1131. [Google Scholar] [CrossRef]
- Radi, A.-E.; Abd-Ellatief, M.R. Electrochemical Aptasensors: Current Status and Future Perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef]
- Kanyong, P.; Davis, J.J. Homogeneous functional self-assembled monolayers: Faradaic impedance baseline signal drift suppression for high-sensitivity immunosensing of C-reactive protein. J. Electroanal. Chem. 2020, 856, 113675. [Google Scholar] [CrossRef]
- Xia, N.; Sun, Z.; Ding, F.; Wang, Y.; Sun, W.; Liu, L. Protease Biosensor by Conversion of a Homogeneous Assay into a Surface-Tethered Electrochemical Analysis Based on Streptavidin–Biotin Interactions. ACS Sens. 2021, 6, 1166–1173. [Google Scholar] [CrossRef]
- Liu, G.; Wan, Y.; Gau, V.; Zhang, J.; Wang, L.; Song, S.; Fan, C. An Enzyme-Based E-DNA Sensor for Sequence-Specific Detection of Femtomolar DNA Targets. J. Am. Chem. Soc. 2008, 130, 6820–6825. [Google Scholar] [CrossRef]
- Bai, H.; Wang, R.; Hargis, B.; Lu, H.; Li, Y. A SPR Aptasensor for Detection of Avian Influenza Virus H5N1. Sensors 2012, 12, 12506–12518. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Thakur, M.; Díaz, S.A.; Susumu, K.; Stewart, M.H.; Oh, E.; Walper, S.A.; Medintz, I.L. Hybrid Nucleic Acid-Quantum Dot Assemblies as Multiplexed Reporter Platforms for Cell-Free Transcription Translation-Based Biosensors. ACS Synth. Biol. 2022, 11, 4089–4102. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Dou, Y.; Liu, X.; Song, S.; Jiang, H.; Fan, C. Electrochemical Biosensor for Point-of-Care Testing of Low-Abundance Biomarkers of Neurological Diseases. Anal. Chem. 2024, 96, 10332–10340. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lan, Q.; Li, J.; Wu, J.; Tang, Y.; Hu, X. Efficient streptavidin-functionalized nitrogen-doped graphene for the development of highly sensitive electrochemical immunosensor. Biosens. Bioelectron. 2017, 89, 312–318. [Google Scholar] [CrossRef]
- Moreira, G.; Qian, H.; Datta, S.P.A.; Bliznyuk, N.; Carpenter, J.; Dean, D.; McLamore, E.; Vanegas, D. A capacitive laser-induced graphene based aptasensor for SARS-CoV-2 detection in human saliva. PLoS ONE 2023, 18, e0290256. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef]
- Osbeck, S.; Bradley, R.; Liu, C.; Idriss, H.; Ward, S. Effect of an ultraviolet/ozone treatment on the surface texture and functional groups on polyacrylonitrile carbon fibres. Carbon 2011, 49, 4322–4330. [Google Scholar] [CrossRef]
- Boonkaew, S.; Jang, I.; Noviana, E.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Electrochemical paper-based analytical device for multiplexed, point-of-care detection of cardiovascular disease biomarkers. Sens. Actuators B Chem. 2021, 330, 129336. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
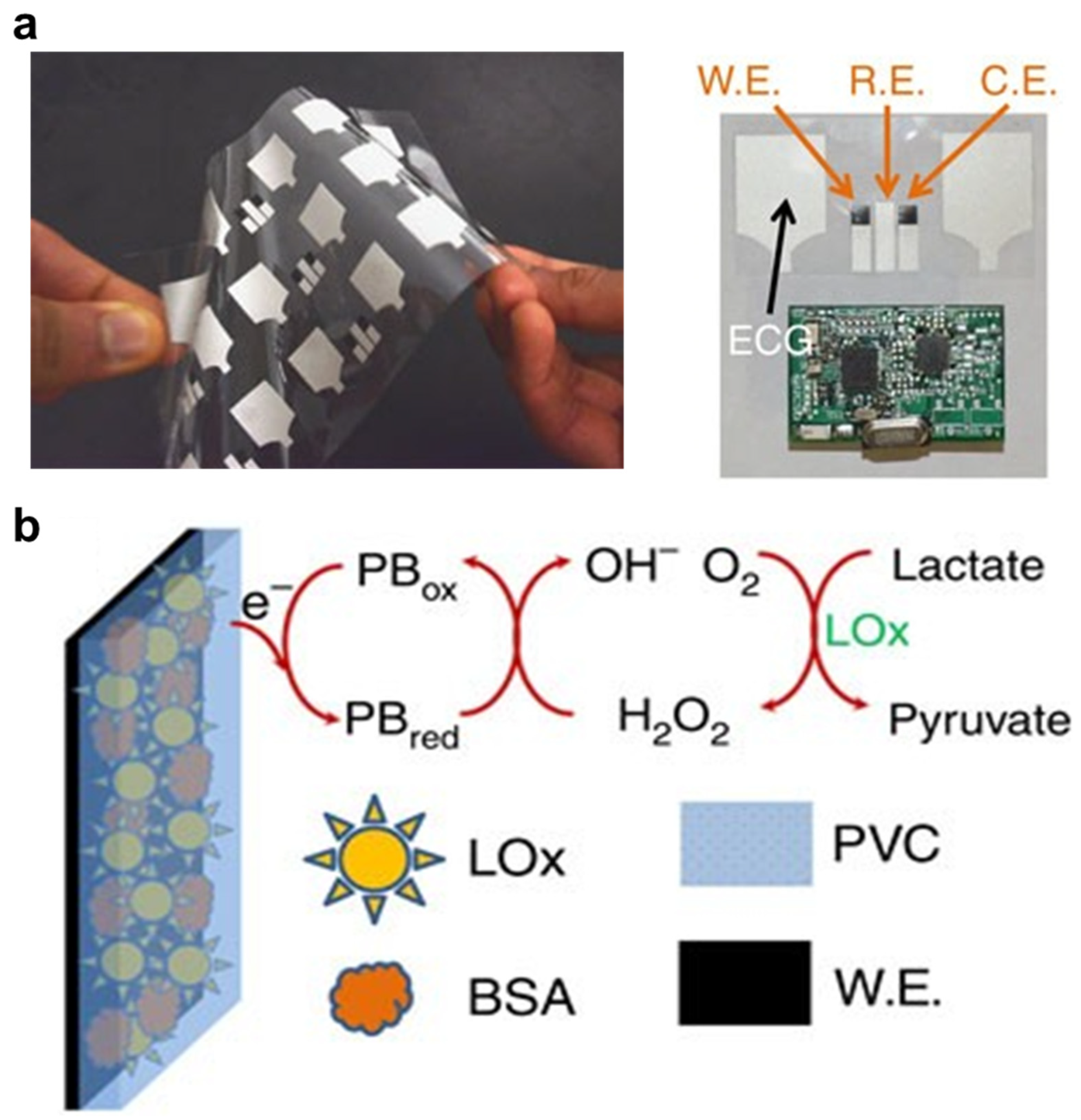
- Zahed, A.; Kim, D.K.; Jeong, S.H.; Reza, S.; Sharifuzzaman, M.; Pradhan, G.B.; Song, H.; Asaduzzaman, M.; Park, J.Y. Microfluidic-Integrated Multimodal Wearable Hybrid Patch for Wireless and Continuous Physiological Monitoring. ACS Sens. 2023, 8, 2960–2974. [Google Scholar] [CrossRef]
- Song, W.; Li, H.; Liang, H.; Qiang, W.; Xu, D. Disposable Electrochemical Aptasensor Array by Using in Situ DNA Hybridization Inducing Silver Nanoparticles Aggregate for Signal Amplification. Anal. Chem. 2014, 86, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Wang, M.; Min, J.; Tay, R.Y.; Lukas, H.; Sempionatto, J.R.; Li, J.; Xu, C.; Gao, W. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. 2024, 19, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-S.; Xu, J.-K.; Lu, L.-M.; Wu, L.-P.; Zhang, K.-X.; Nie, T.; Zhu, X.-F.; Wu, Y. Overoxidized polypyrrole/graphene nanocomposite with good electrochemical performance as novel electrode material for the detection of adenine and guanine. Biosens. Bioelectron. 2014, 62, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Sipuka, D.S.; Olorundare, F.O.G.; Makaluza, S.; Midzi, N.; Sebokolodi, T.I.; Arotiba, O.A.; Nkosi, D. Dendrimer─Gold Nanocomposite-Based Electrochemical Aptasensor for the Detection of Dopamine. ACS Omega 2023, 8, 33403–33411. [Google Scholar] [CrossRef]
- Xie, D.; Li, C.; Shangguan, L.; Qi, H.; Xue, D.; Gao, Q.; Zhang, C. Click chemistry-assisted self-assembly of DNA aptamer on gold nanoparticles-modified screen-printed carbon electrodes for label-free electrochemical aptasensor. Sens. Actuators B Chem. 2014, 192, 558–564. [Google Scholar] [CrossRef]
- Nie, Y.; Yuan, X.; Zhang, P.; Chai, Y.-Q.; Yuan, R. Versatile and Ultrasensitive Electrochemiluminescence Biosensor for Biomarker Detection Based on Nonenzymatic Amplification and Aptamer-Triggered Emitter Release. Anal. Chem. 2019, 91, 3452–3458. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Hu, Z.; Chen, Y.; Tao, Y.; Wang, L.; Li, L.; Wang, P.; Li, H.-Y.; Zhang, J.; et al. All-solid-state SARS-CoV-2 protein biosensor employing colloidal quantum dots-modified electrode. Biosens. Bioelectron. 2022, 202, 113974. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Enzyme-Free Electrochemical Nano-Immunosensor Based on Graphene Quantum Dots and Gold Nanoparticles for Cardiac Biomarker Determination. Nanomaterials 2021, 11, 578. [Google Scholar] [CrossRef]
- Liu, G.; Ling, J.; Li, J. Extremely Sensitive Molecularly Imprinted ECL Sensor with Multiple Probes Released from Liposomes Immobilized by a Light-Triggered Click Reaction. ACS Sens. 2021, 6, 4185–4192. [Google Scholar] [CrossRef]
- Pan, Y.; Shan, D.; Ding, L.; Yang, X.; Wang, J.; Wu, B.; Ren, H. Ultra-fast Redox Pulse for Stable Electrochemiluminescence on AuNP-Based Biosensors and Mechanism Investigation. Anal. Chem. 2023, 95, 2975–2982. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Barton, S.J.; Wren, S.P.; Barker, J. Review on molecularly imprinted polymers with a focus on their application to the analysis of protein biomarkers. TrAC Trends Anal. Chem. 2021, 144, 116431. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Han, R.; Chen, Q.; Jiang, L.; Luo, X. Click reaction-assisted construction of antifouling immunosensors for electrochemical detection of cancer biomarkers in human serum. Sens. Actuators B Chem. 2022, 363, 131810. [Google Scholar] [CrossRef]
- Han, L.; Shen, H.; Zhu, J.-X.; Li, Y.-T. Mini review: Electrochemical electrode based on graphene and its derivatives for heavy metal ions detection. Talanta Open 2022, 6, 100153. [Google Scholar] [CrossRef]
- Demir, E.; Kırboga, K.K.; Işık, M. Chapter 8—An overview of stability and lifetime of electrochemical biosensors. In Novel Nanostructured Materials for Electrochemical Bio-Sensing Applications; Manjunatha, J.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 129–158. [Google Scholar]
- Wan, Y.; Wang, H.; Zhang, L.; Chen, Y.; Li, S.; Zhou, J.; Zhang, Q.; Xia, L. Highly stable acetylcholinesterase electrochemical biosensor based on polymerized ionic liquids microgel for pesticides detection. Microchim. Acta 2022, 189, 300. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M.; et al. Human ACE2-Functionalized Gold “Virus-Trap” Nanostructures for Accurate Capture of SARS-CoV-2 and Single-Virus SERS Detection. Nanomicro Lett. 2021, 13, 109. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, J.U.; Song, S.; Kim, S.; Choi, Y.J.; Sim, S.J. A label-free, ultra-highly sensitive and multiplexed SERS nanoplasmonic biosensor for miRNA detection using a head-flocked gold nanopillar. Analyst 2019, 144, 1768–1776. [Google Scholar] [CrossRef]
- Najiminaini, M.; Ertorer, E.; Kaminska, B.; Mittler, S.; Carson, J.J.L. Surface plasmon resonance sensing properties of a 3D nanostructure consisting of aligned nanohole and nanocone arrays. Analyst 2014, 139, 1876–1882. [Google Scholar] [CrossRef]
- Focsan, M.; Craciun, A.M.; Potara, M.; Leordean, C.; Vulpoi, A.; Maniu, D.; Astilean, S. Flexible and Tunable 3D Gold Nanocups Platform as Plasmonic Biosensor for Specific Dual LSPR-SERS Immuno-Detection. Sci. Rep. 2017, 7, 14240. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Bochenkov, V.E.; Runager, K.; Aslan, H.; Dong, M.; Enghild, J.J.; De Freitas, V.; Ferreira Sales, M.G.; Sutherland, D.S. Molecular Imprinting of Complex Matrices at Localized Surface Plasmon Resonance Biosensors for Screening of Global Interactions of Polyphenols and Proteins. ACS Sens. 2016, 1, 258–264. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.R.; Kim, M.; Lim, H.; Jung, Y.S.; Park, C.B. Metallic Woodpile Nanostructures for Femtomolar Sensing of Alzheimer’s Neurofilament Lights. ACS Nano 2020, 14, 10376–10384. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Zhong, G.; Xu, T.; Zhang, X. Wearable Vertical Graphene-Based Microneedle Biosensor for Real-Time Ketogenic Diet Management. Anal. Chem. 2024, 96, 8713–8720. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chino, Y.; Kasama, T.; Miyake, R.; Mitsuzawa, S.; Luan, Y.; Ahmad, N.B.; Hibino, H.; Takai, M.; Zhou, S.; et al. Biocompatible Core–Shell Microneedle Sensor Filled with Zwitterionic Polymer Hydrogel for Rapid Continuous Transdermal Monitoring. ACS Nano 2024, 18, 26541–26559. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tehrani, F.; Teymourian, H.; Mack, J.; Shaver, A.; Reynoso, M.; Kavner, J.; Huang, N.; Furmidge, A.; Duvvuri, A.; et al. Microneedle Aptamer-Based Sensors for Continuous, Real-Time Therapeutic Drug Monitoring. Anal. Chem. 2022, 94, 8335–8345. [Google Scholar] [CrossRef]
- Downs, A.M.; Bolotsky, A.; Weaver, B.M.; Bennett, H.; Wolff, N.; Polsky, R.; Miller, P.R. Microneedle electrochemical aptamer-based sensing: Real-time small molecule measurements using sensor-embedded, commercially-available stainless steel microneedles. Biosens. Bioelectron. 2023, 236, 115408. [Google Scholar] [CrossRef]
- Reynoso, M.; Chang, A.-Y.; Wu, Y.; Murray, R.; Suresh, S.; Dugas, Y.; Wang, J.; Arroyo-Currás, N. 3D-printed, aptamer-based microneedle sensor arrays using magnetic placement on live rats for pharmacokinetic measurements in interstitial fluid. Biosens. Bioelectron. 2024, 244, 115802. [Google Scholar] [CrossRef]
- Mirsky, V.M.; Riepl, M.; Wolfbeis, O.S. Capacitive monitoring of protein immobilization and antigen–antibody reactions on monomolecular alkylthiol films on gold electrodes. Biosens. Bioelectron. 1997, 12, 977–989. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Scida, K.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. High Surface Area Electrodes Generated via Electrochemical Roughening Improve the Signaling of Electrochemical Aptamer-Based Biosensors. Anal. Chem. 2017, 89, 12185–12191. [Google Scholar] [CrossRef]
- Srivastava, M.; Nirala, N.R.; Srivastava, S.K.; Prakash, R. A comparative Study of Aptasensor Vs Immunosensor for Label-Free PSA Cancer Detection on GQDs-AuNRs Modified Screen-Printed Electrodes. Sci. Rep. 2018, 8, 1923. [Google Scholar] [CrossRef]
- Martín, M.; Salazar, P.; Álvarez, R.; Palmero, A.; López-Santos, C.; González-Mora, J.; González-Elipe, A.R. Cholesterol biosensing with a polydopamine-modified nanostructured platinum electrode prepared by oblique angle physical vacuum deposition. Sens. Actuators B Chem. 2017, 240, 37–45. [Google Scholar] [CrossRef]
- Dutta, G.; Fernandes, F.C.; Estrela, P.; Moschou, D.; Bueno, P.R. Impact of surface roughness on the self-assembling of molecular films onto gold electrodes for label-free biosensing applications. Electrochim. Acta 2021, 378, 138137. [Google Scholar] [CrossRef]
- Butterworth, A.; Blues, E.; Williamson, P.; Cardona, M.; Gray, L.; Corrigan, D.K. SAM Composition and Electrode Roughness Affect Performance of a DNA Biosensor for Antibiotic Resistance. Biosensors 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.; Zhang, N.; Gilchrist, M.D. Electropolishing and Shaping of Micro-Scale Metallic Features. Micromachines 2022, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Pardo, W.A.; Mir, M.; Samitier, J. Signal enhancement in ultraflat electrochemical DNA biosensors. Electrophoresis 2015, 36, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, B.; Liang, P.; Lee, J.-H.; Yang, Y.; Yu, H.; Canoura, J.; He, J.; Li, W.; Weizmann, Y.; Xiao, Y. Ambient Filtration Method to Rapidly Prepare Highly Conductive, Paper-Based Porous Gold Films for Electrochemical Biosensing. ACS Appl. Mater. Interfaces 2015, 7, 27049–27058. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Xu, Z.; Wang, Y.; Zhang, W.; Shi, P. Interrogation of Cellular Innate Immunity by Diamond-Nanoneedle-Assisted Intracellular Molecular Fishing. Nano Lett. 2015, 15, 7058–7063. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, L.; Yang, Y.; Lu, M.; Xie, K.; Zhao, X.; Cheung, E.H.C.; Wang, Y.; Jiang, X.; Zhang, W.; et al. High-throughput intracellular biopsy of microRNAs for dissecting the temporal dynamics of cellular heterogeneity. Sci. Adv. 2020, 6, eaba4971. [Google Scholar] [CrossRef]
- Zheng, H.; GhavamiNejad, A.; GhavamiNejad, P.; Samarikhalaj, M.; Giacca, A.; Poudineh, M. Hydrogel Microneedle-Assisted Assay Integrating Aptamer Probes and Fluorescence Detection for Reagentless Biomarker Quantification. ACS Sens. 2022, 7, 2387–2399. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Chang, J.Y.H.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-Coated Microneedle Arrays for Minimally Invasive Sampling and Sensing of Specific Circulating Nucleic Acids from Skin Interstitial Fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum dots: An overview of synthesis, properties, and applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar] [CrossRef]
- Kalkal, A.; Pradhan, R.; Kadian, S.; Manik, G.; Packirisamy, G. Biofunctionalized Graphene Quantum Dots Based Fluorescent Biosensor toward Efficient Detection of Small Cell Lung Cancer. ACS Appl. Bio Mater. 2020, 3, 4922–4932. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Pang, G.; Zhang, Y.; Guo, S. Tuning the Aggregation/Disaggregation Behavior of Graphene Quantum Dots by Structure-Switching Aptamer for High-Sensitivity Fluorescent Ochratoxin A Sensor. Anal. Chem. 2017, 89, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gong, X.; Song, T.; Yang, J.; Zhu, S.; Li, Y.; Cui, Y.; Li, Y.; Zhang, B.; Chang, J. Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosens. Bioelectron. 2011, 30, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhou, S.; Chen, T.; Li, J.; Shen, H.; Chai, Y.; Li, L.S. Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip. Anal. Chim. Acta 2018, 1008, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Meng, H.-M.; Chen, J.; Liu, J.; Zhang, L.; Qu, L.; Li, Z.; Lin, Y. Quantum Dot-Based Lateral Flow Test Strips for Highly Sensitive Detection of the Tetanus Antibody. ACS Omega 2019, 4, 6789–6795. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, X.; Lindbo, S.; Susumu, K.; Medintz, I.L.; Hober, S.; Hildebrandt, N. Quantum Dot–Based FRET Immunoassay for HER2 Using Ultrasmall Affinity Proteins. Small 2018, 14, 1802266. [Google Scholar] [CrossRef]
- Green, C.M.; Spangler, J.; Susumu, K.; Stenger, D.A.; Medintz, I.L.; Díaz, S.A. Quantum Dot-Based Molecular Beacons for Quantitative Detection of Nucleic Acids with CRISPR/Cas(N) Nucleases. ACS Nano 2022, 16, 20693–20704. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Li, Y.; Liu, C.; Jiao, P.; Wei, Y. Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme. Nanomaterials 2021, 11, 1575. [Google Scholar] [CrossRef]
- He, Y.; Zeng, Z.; Cao, Y.; Zhang, X.; Wu, C.; Luo, X. Ultrasenstive SERS biosensor based on Zn2+ from ZnO nanoparticle assisted DNA enzyme amplification for detection of miRNA. Anal. Chim. Acta 2022, 1228, 340340. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Poly(amidoamine) (PAMAM): An emerging material for electrochemical bio(sensing) applications. Talanta 2016, 148, 427–438. [Google Scholar] [CrossRef]
- Hoffman, L.W.; Andersson, G.G.; Sharma, A.; Clarke, S.R.; Voelcker, N.H. New Insights into the Structure of PAMAM Dendrimer/Gold Nanoparticle Nanocomposites. Langmuir 2011, 27, 6759–6767. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.; Dasgupta, C.; Maiti, P.K. Engineering Gold Nanoparticle Interaction by PAMAM Dendrimer. J. Phys. Chem. C 2013, 117, 13627–13636. [Google Scholar] [CrossRef]
- Zhou, Y.; Lü, H.; Zhang, D.; Xu, K.; Hui, N.; Wang, J. Electrochemical biosensors based on conducting polymer composite and PAMAM dendrimer for the ultrasensitive detection of acetamiprid in vegetables. Microchem. J. 2023, 185, 108284. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhang, Y.; Chen, Z.; Liu, Y.; Li, Z.; Li, J. Sensitive Electrochemical Aptamer Biosensor for Dynamic Cell Surface N-Glycan Evaluation Featuring Multivalent Recognition and Signal Amplification on a Dendrimer–Graphene Electrode Interface. Anal. Chem. 2014, 86, 4278–4286. [Google Scholar] [CrossRef]
- Li, F.; Peng, J.; Zheng, Q.; Guo, X.; Tang, H.; Yao, S. Carbon Nanotube-Polyamidoamine Dendrimer Hybrid-Modified Electrodes for Highly Sensitive Electrochemical Detection of MicroRNA24. Anal. Chem. 2015, 87, 4806–4813. [Google Scholar] [CrossRef]
- Cao, J.; Zheng, Z.; Sun, D.; Chen, X.; Cheng, R.; Lv, T.; An, Y.; Zheng, J.; Song, J.; Wu, L.; et al. Decoder-seq enhances mRNA capture efficiency in spatial RNA sequencing. Nat. Biotechnol. 2024, 42, 1735–1746. [Google Scholar] [CrossRef]
- Santos, C.S.; Mossanha, R.; Pessôa, C.A. Biosensor for carbaryl based on gold modified with PAMAM-G4 dendrimer. J. Appl. Electrochem. 2015, 45, 325–334. [Google Scholar] [CrossRef]
- Tang, D.; Hou, L.; Niessner, R.; Xu, M.; Gao, Z.; Knopp, D. Multiplexed electrochemical immunoassay of biomarkers using metal sulfide quantum dot nanolabels and trifunctionalized magnetic beads. Biosens. Bioelectron. 2013, 46, 37–43. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Wang, C.; Chu, Y.; Wang, S.; Sun, M.Y.; Ji, H.; Gao, Y.; Wang, Y.; Han, Y.; et al. Poly-l-Lysine-Modified Graphene Field-Effect Transistor Biosensors for Ultrasensitive Breast Cancer miRNAs and SARS-CoV-2 RNA Detection. Anal. Chem. 2022, 94, 1626–1636. [Google Scholar] [CrossRef]
- Kaneshiro, T.L.; Wang, X.; Lu, Z.-R. Synthesis, Characterization, and Gene Delivery of Poly-l-lysine Octa(3-aminopropyl)silsesquioxane Dendrimers: Nanoglobular Drug Carriers with Precisely Defined Molecular Architectures. Mol. Pharm. 2007, 4, 759–768. [Google Scholar] [CrossRef]
- Avaritt, B.R.; Swaan, P.W. Internalization and Subcellular Trafficking of Poly-l-lysine Dendrimers Are Impacted by the Site of Fluorophore Conjugation. Mol. Pharm. 2015, 12, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Asong, J.; Wolfert, M.A.; Boons, G.-J. Multifunctional Surface Modification of Gold-Stabilized Nanoparticles by Bioorthogonal Reactions. J. Am. Chem. Soc. 2011, 133, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Shen, M.; Shi, X. Polyethylenimine-Assisted Generation of Optical Nanoprobes for Biosensing Applications. ACS Appl. Bio Mater. 2020, 3, 3935–3955. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef] [PubMed]
- Jamei, H.R.; Rezaei, B.; Ensafi, A.A. Ultra-sensitive and selective electrochemical biosensor with aptamer recognition surface based on polymer quantum dots and C60/MWCNTs-polyethylenimine nanocomposites for analysis of thrombin protein. Bioelectrochemistry 2021, 138, 107701. [Google Scholar] [CrossRef]
- Ma, L.; Sun, N.; Zhang, J.; Tu, C.; Cao, X.; Duan, D.; Diao, A.; Man, S. Polyethylenimine-coated Fe3O4 nanoparticles effectively quench fluorescent DNA, which can be developed as a novel platform for protein detection. Nanoscale 2017, 9, 17699–17703. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, A.; Pandey, G.; Kumar, S.; Awasthi, K.; Awasthi, A. Carbon Nano-Onion-Decorated ZnO Composite-Based Enzyme-Less Electrochemical Biosensing Approach for Glucose. ACS Omega 2022, 7, 37748–37756. [Google Scholar] [CrossRef]
- Khoshhesab, Z.M. Simultaneous electrochemical determination of acetaminophen, caffeine and ascorbic acid using a new electrochemical sensor based on CuO–graphene nanocomposite. RSC Adv. 2015, 5, 95140–95148. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Chang, Y.-J.; Chen, S.-M. Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2011, 80, 114–120. [Google Scholar] [CrossRef]
- Viswanathan, S.; Rani, C.; Anand, A.V.; Ho, J.-A.A. Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode. Biosens. Bioelectron. 2009, 24, 1984–1989. [Google Scholar] [CrossRef]
- De Silva, T.; Fawzy, M.; Hasani, A.; Ghanbari, H.; Abnavi, A.; Askar, A.; Ling, Y.; Mohammadzadeh, M.R.; Kabir, F.; Ahmadi, R.; et al. Ultrasensitive rapid cytokine sensors based on asymmetric geometry two-dimensional MoS2 diodes. Nat. Commun. 2022, 13, 7593. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, J.H.; Kim, H.J.; Lee, D.; Lee, D.S.; Yoon, D.S.; Hwang, K.S. Multiplexed femtomolar detection of Alzheimer’s disease biomarkers in biofluids using a reduced graphene oxide field-effect transistor. Biosens. Bioelectron. 2020, 167, 112505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Wu, Y.; Xia, X.; Liao, Y.; Li, Q. Fluorescent Probe-Based Lateral Flow Assay for Multiplex Nucleic Acid Detection. Anal. Chem. 2014, 86, 5611–5614. [Google Scholar] [CrossRef]
- Han, S.; Zhou, T.; Yin, B.; He, P. A sensitive and semi-quantitative method for determination of multi-drug residues in animal body fluids using multiplex dipstick immunoassay. Anal. Chim. Acta 2016, 927, 64–71. [Google Scholar] [CrossRef]
- Han, M.; Gong, L.; Wang, J.; Zhang, X.; Jin, Y.; Zhao, R.; Yang, C.; He, L.; Feng, X.; Chen, Y. An octuplex lateral flow immunoassay for rapid detection of antibiotic residues, aflatoxin M1 and melamine in milk. Sens. Actuators B Chem. 2019, 292, 94–104. [Google Scholar] [CrossRef]
- Hendriks, J.; Stojanovic, I.; Schasfoort, R.B.M.; Saris, D.B.F.; Karperien, M. Nanoparticle Enhancement Cascade for Sensitive Multiplex Measurements of Biomarkers in Complex Fluids with Surface Plasmon Resonance Imaging. Anal. Chem. 2018, 90, 6563–6571. [Google Scholar] [CrossRef]
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [Google Scholar] [CrossRef]
- Bruch, R.; Johnston, M.; Kling, A.; Mattmüller, T.; Baaske, J.; Partel, S.; Madlener, S.; Weber, W.; Urban, G.A.; Dincer, C. CRISPR-powered electrochemical microfluidic multiplexed biosensor for target amplification-free miRNA diagnostics. Biosens. Bioelectron. 2021, 177, 112887. [Google Scholar] [CrossRef]
- Liu, T.; Sin, M.L.; Pyne, J.D.; Gau, V.; Liao, J.C.; Wong, P.K. Electrokinetic stringency control in self-assembled monolayer-based biosensors for multiplex urinary tract infection diagnosis. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 159–166. [Google Scholar] [CrossRef]
- Rhouati, A.; Zourob, M. Development of a Multiplexed Electrochemical Aptasensor for the Detection of Cyanotoxins. Biosensors 2024, 14, 268. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Li, H.; Yin, H.; Hao, P.; Dai, X.; Jiang, K.; Liu, C.; Zhang, T.; Yin, J.; et al. Ultrasensitive SERS Analysis of Liquid and Gaseous Putrescine and Cadaverine by a 3D-Rosettelike Nanostructure-Decorated Flexible Porous Substrate. Anal. Chem. 2022, 94, 5273–5283. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Dang, H.; Das, A.; Sim, M.S.; Chung, I.Y.; Choo, J. SERS biosensors for ultrasensitive detection of multiple biomarkers expressed in cancer cells. Biosens. Bioelectron. 2020, 164, 112326. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Wang, J.; Fu, W.; Yao, C. A SPR biosensor based on signal amplification using antibody-QD conjugates for quantitative determination of multiple tumor markers. Sci. Rep. 2016, 6, 33140. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with Luminescent Semiconductor Quantum Dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Zhang, S.; Song, P.; Guo, B.; Zhao, Y.; Wu, H. Simultaneous Quantification of Multiple Cancer Biomarkers in Blood Samples through DNA-Assisted Nanopore Sensing. Angew. Chem. Int. Ed. 2018, 57, 11882–11887. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, M.I.; et al. Supramolecular chemistry in solution and solid–gas interfaces: Synthesis and photophysical properties of monocolor and bicolor fluorescent sensors for barium tagging in neutrinoless double beta decay. RSC Appl. Interfaces 2025. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Sun, J.; Wu, X.; Xiao, J.; Zhang, Y.; Ding, J.; Jiang, J.; Chen, Z.; Liu, X.; Wei, D.; Zhou, L.; et al. Hydrogel-Integrated Multimodal Response as a Wearable and Implantable Bidirectional Interface for Biosensor and Therapeutic Electrostimulation. ACS Appl. Mater. Interfaces 2023, 15, 5897–5909. [Google Scholar] [CrossRef]
- Tort, N.; Salvador, J.-P.; Marco, M.-P. Multimodal plasmonic biosensing nanostructures prepared by DNA-directed immobilization of multifunctional DNA-gold nanoparticles. Biosens. Bioelectron. 2017, 90, 13–22. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Peng, G.; Ma, Z. Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies. Nanomaterials 2024, 14, 2014. https://doi.org/10.3390/nano14242014
Zou S, Peng G, Ma Z. Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies. Nanomaterials. 2024; 14(24):2014. https://doi.org/10.3390/nano14242014
Chicago/Turabian StyleZou, Shangjie, Guangdun Peng, and Zhiqiang Ma. 2024. "Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies" Nanomaterials 14, no. 24: 2014. https://doi.org/10.3390/nano14242014
APA StyleZou, S., Peng, G., & Ma, Z. (2024). Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies. Nanomaterials, 14(24), 2014. https://doi.org/10.3390/nano14242014





