Bimetallic Mesoporous MCM-41 Nanoparticles with Ta/(Ti, V, Co, Nb) with Catalytic and Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photocatalyst Preparation
2.3. Materials Characterization
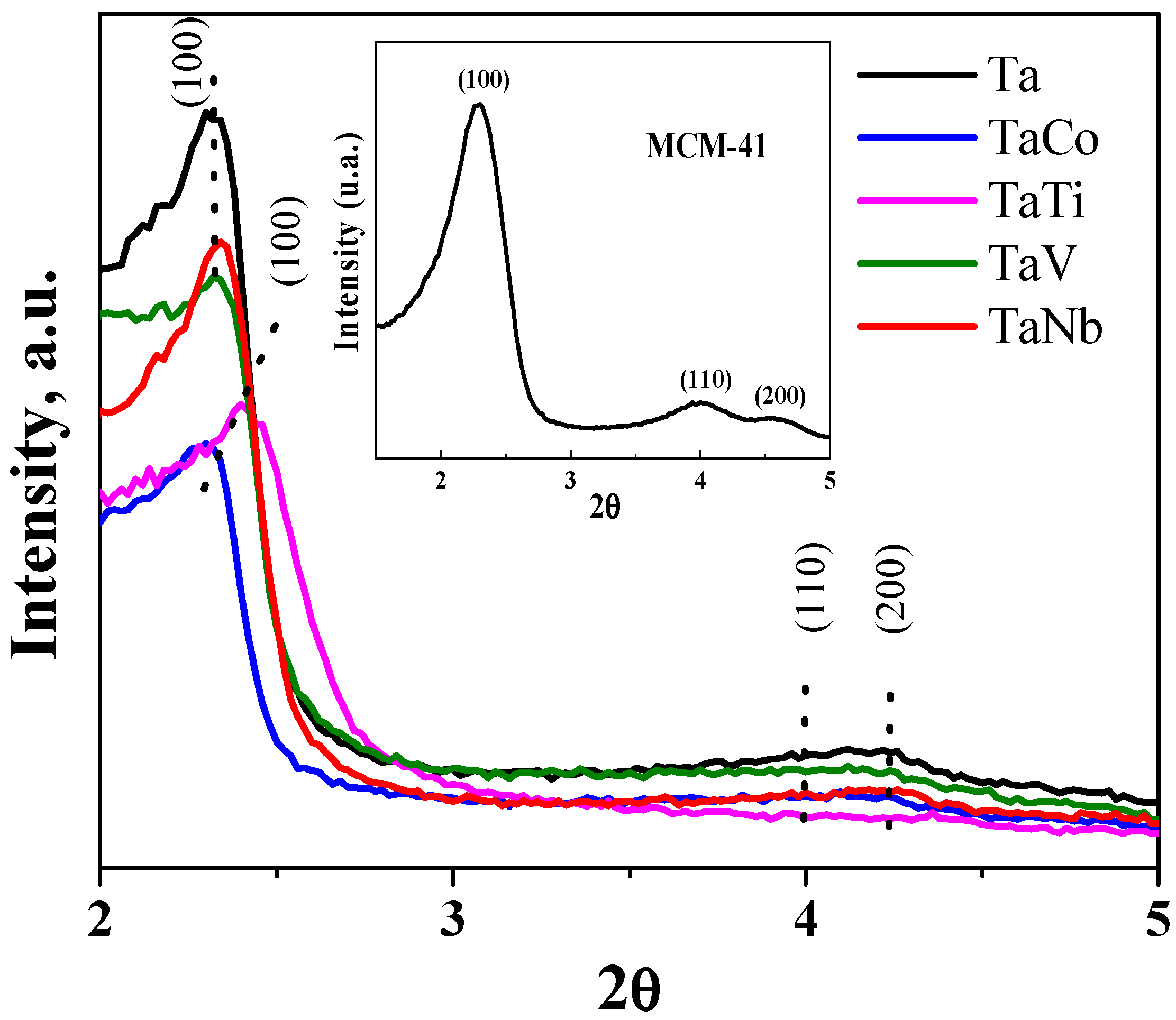
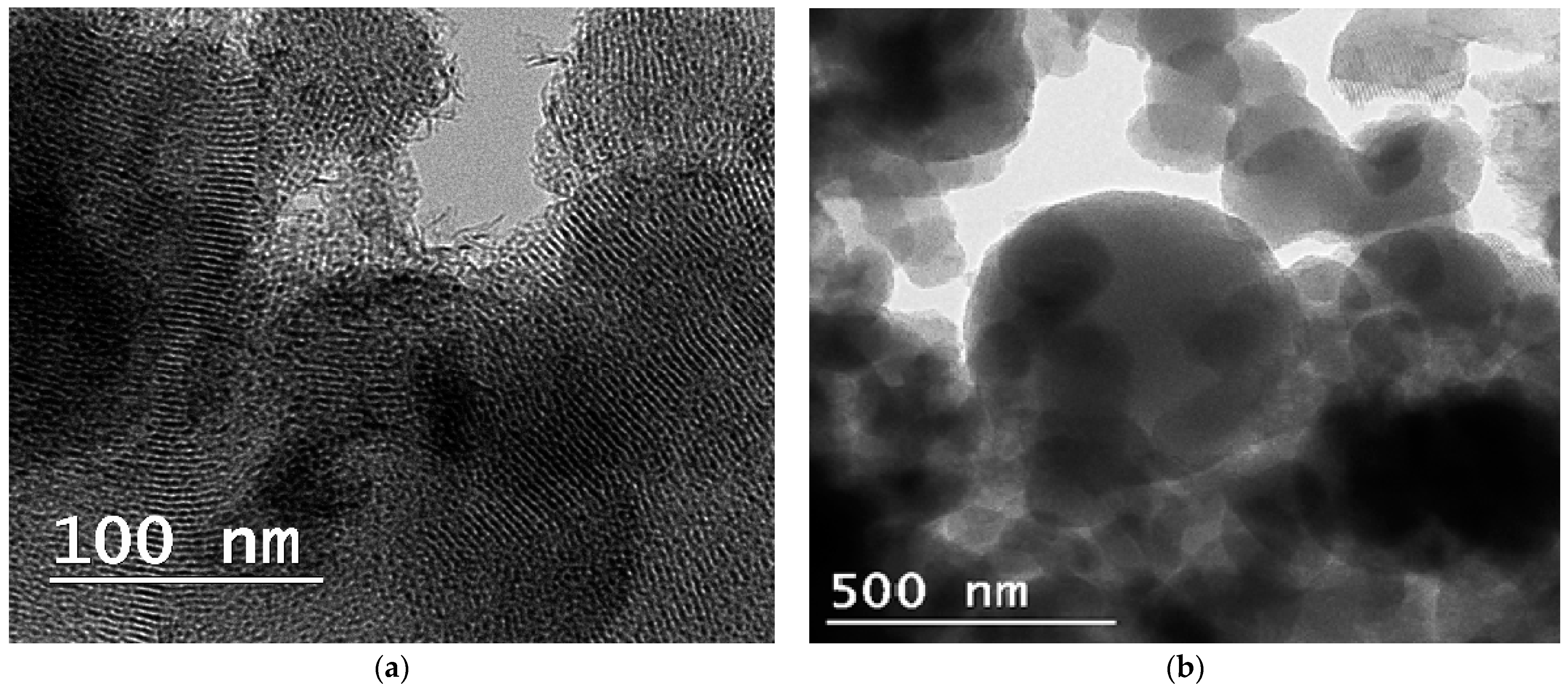
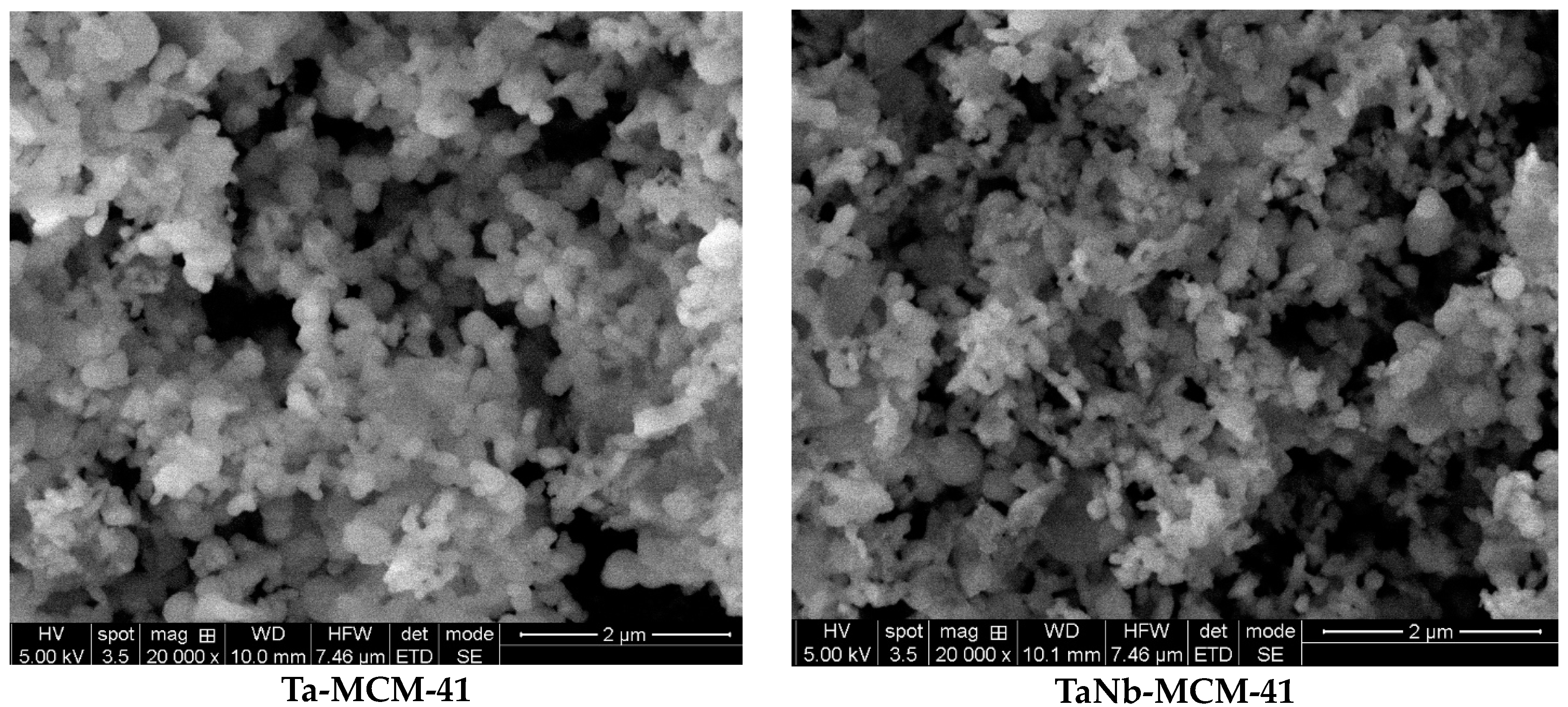
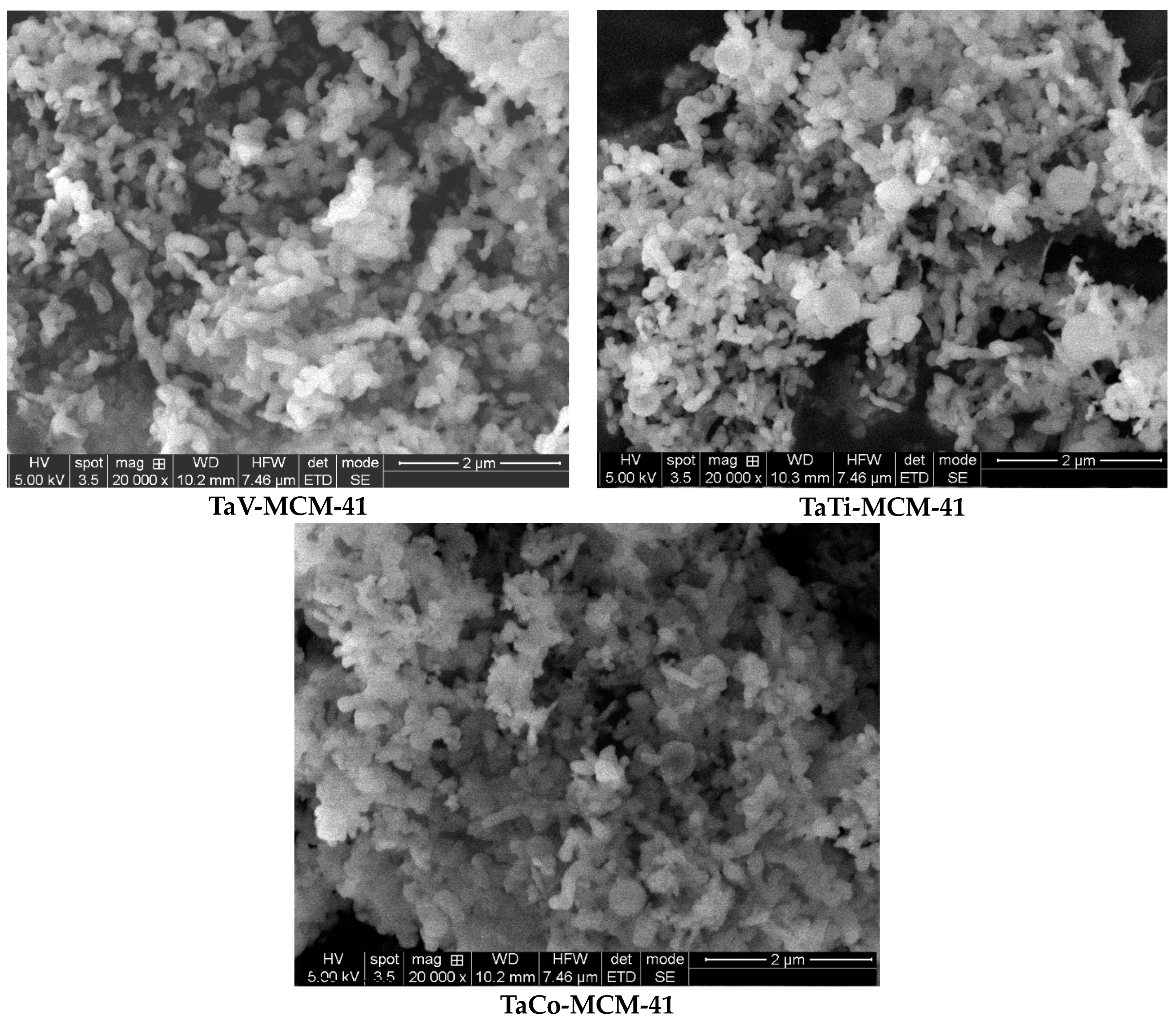
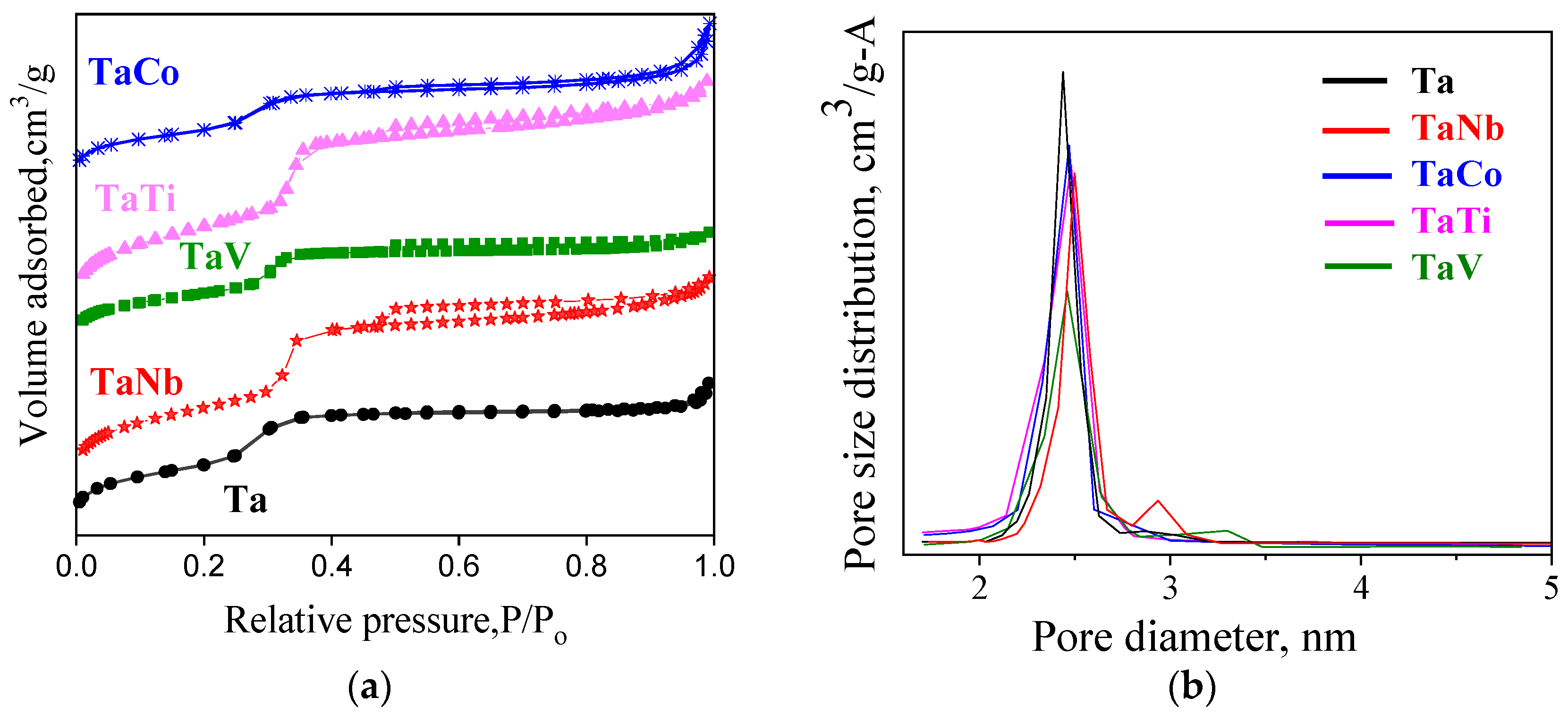
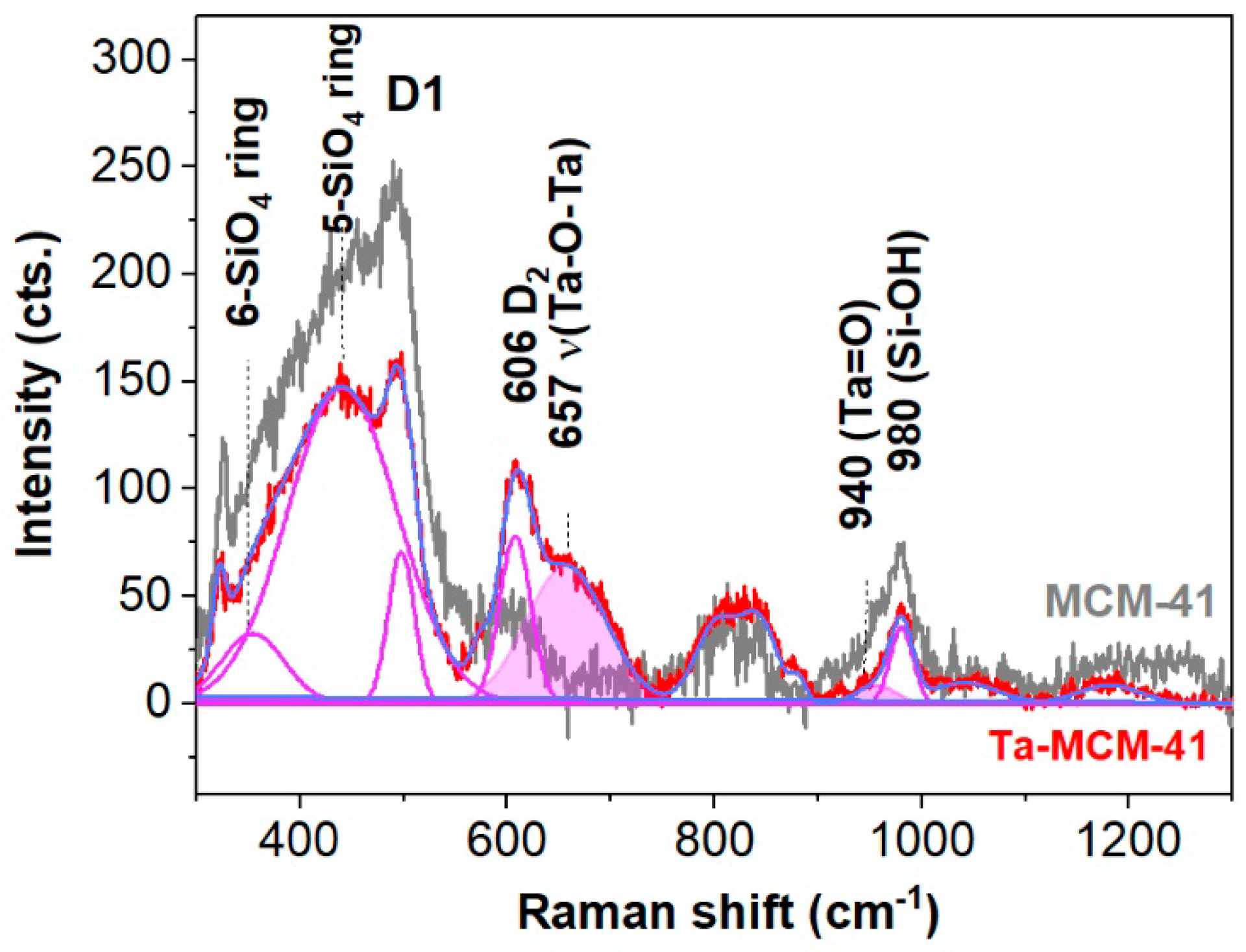
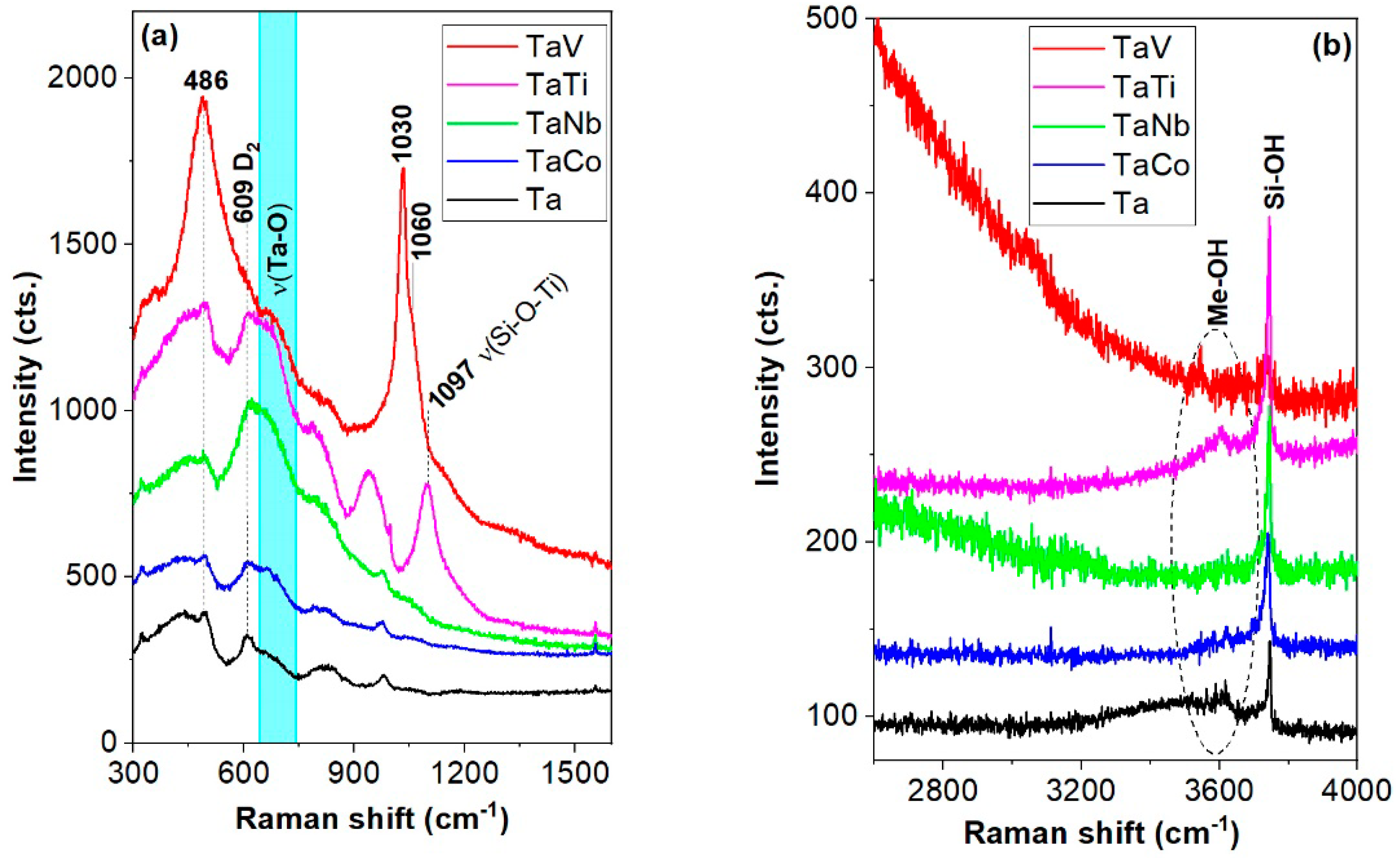
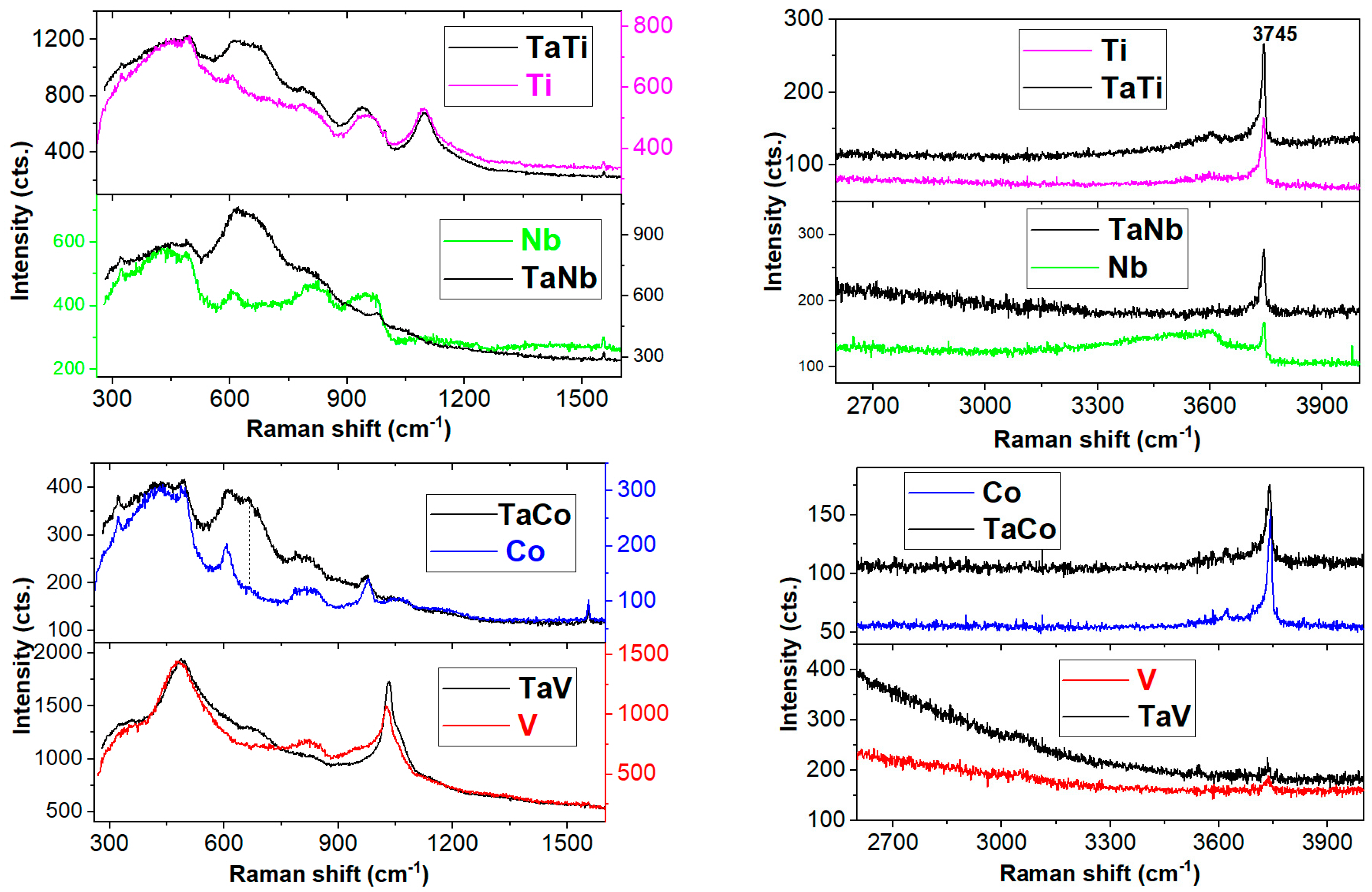
3. Results and Discussion
3.1. Characterization of Materials
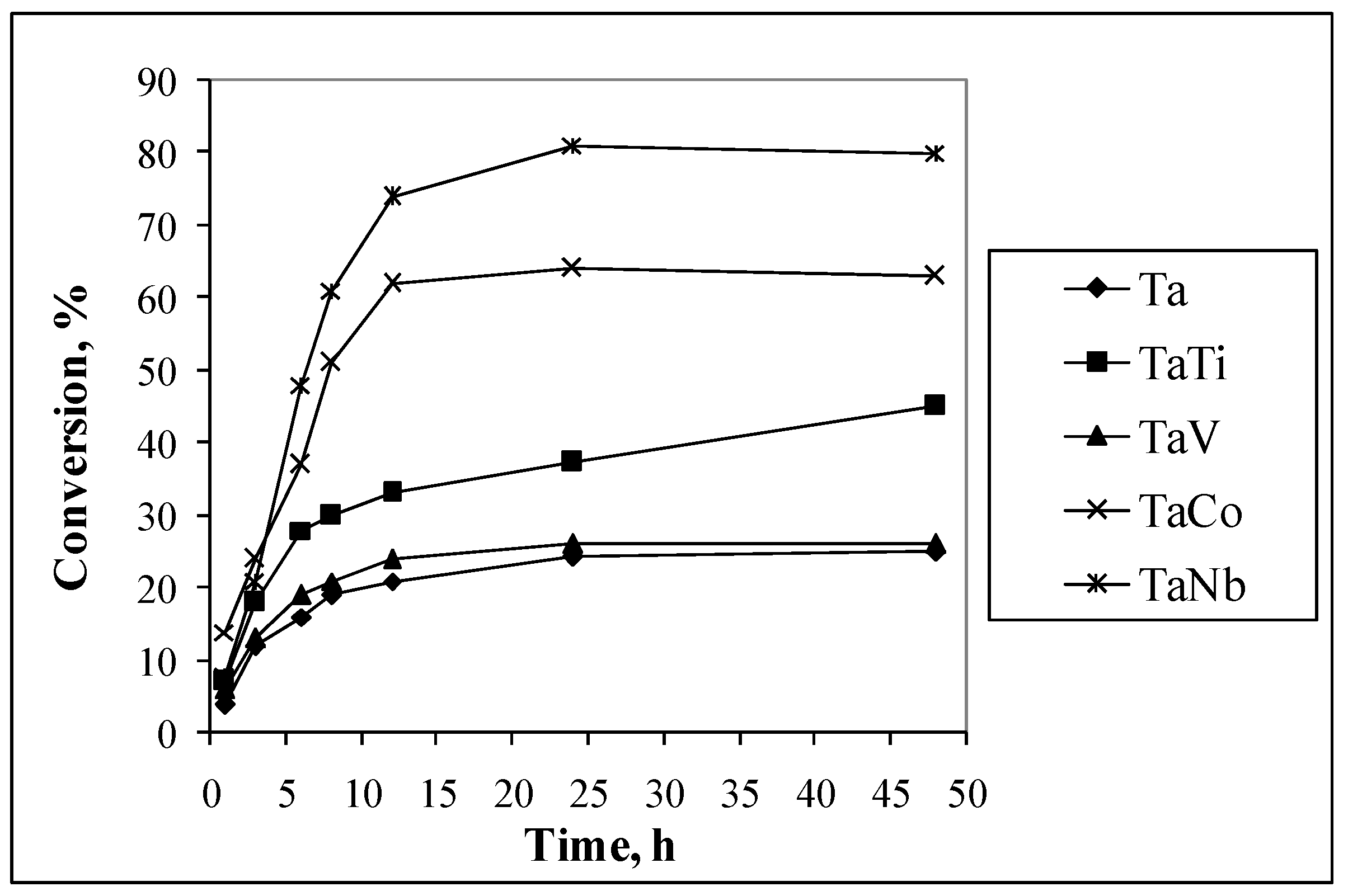
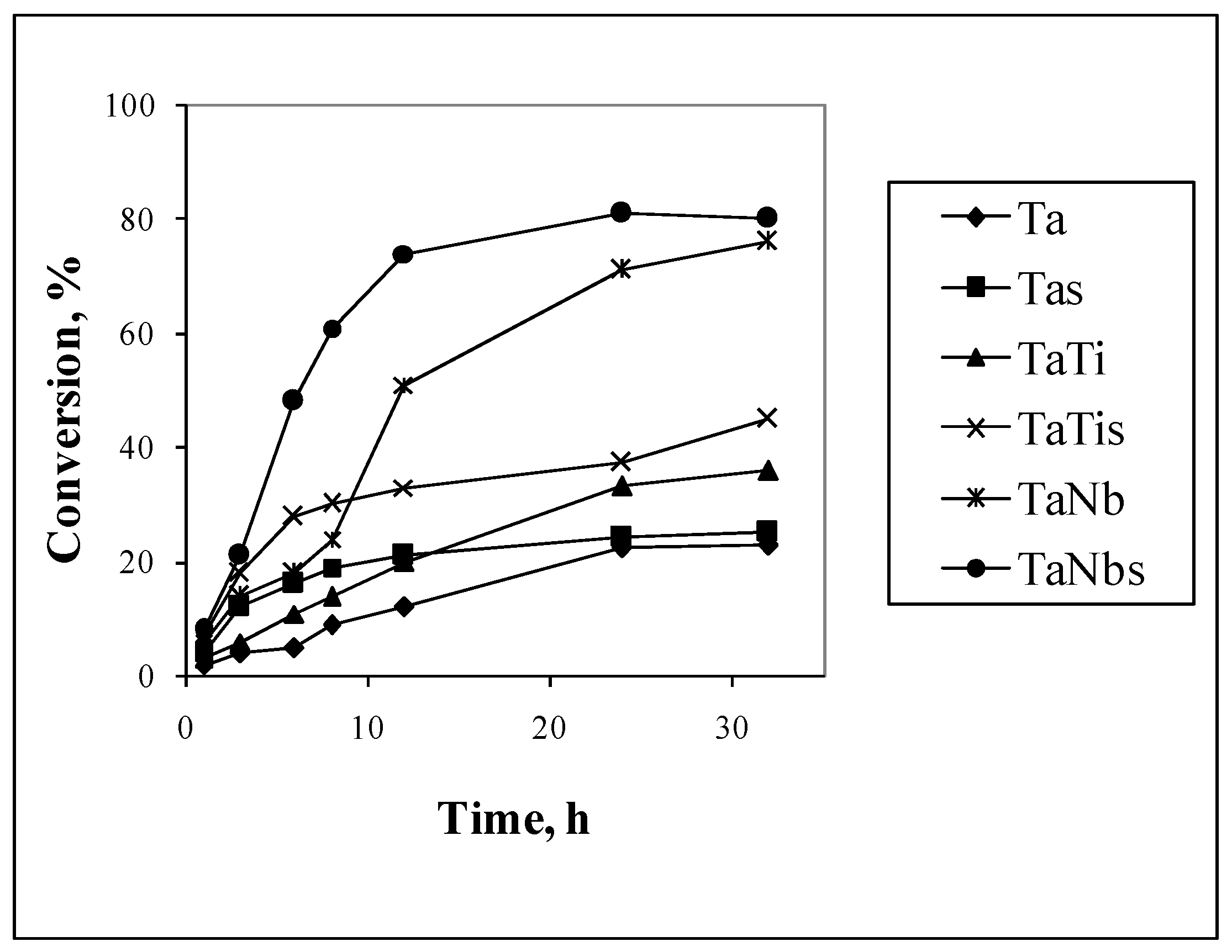
3.2. Catalytic Properties
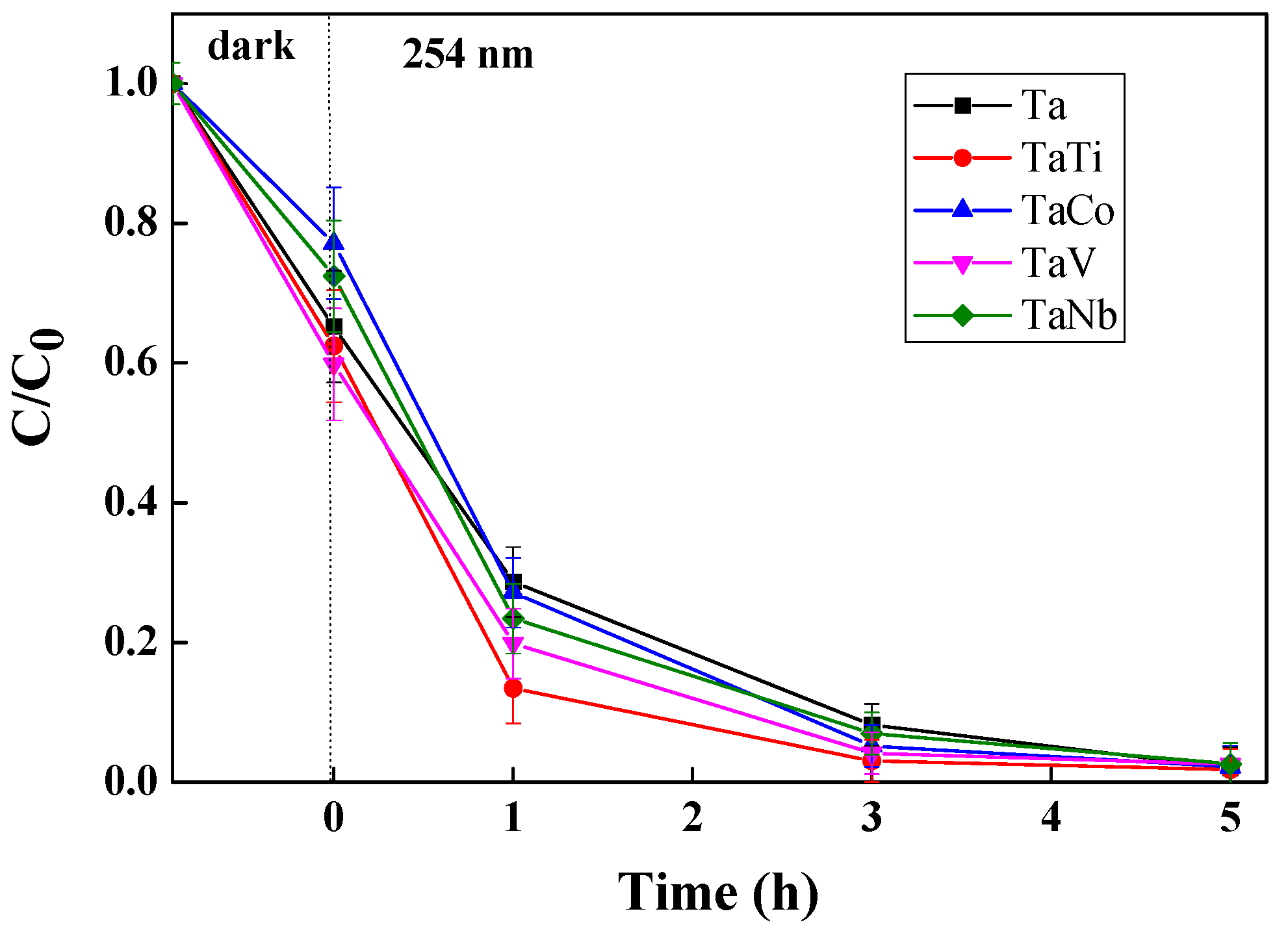
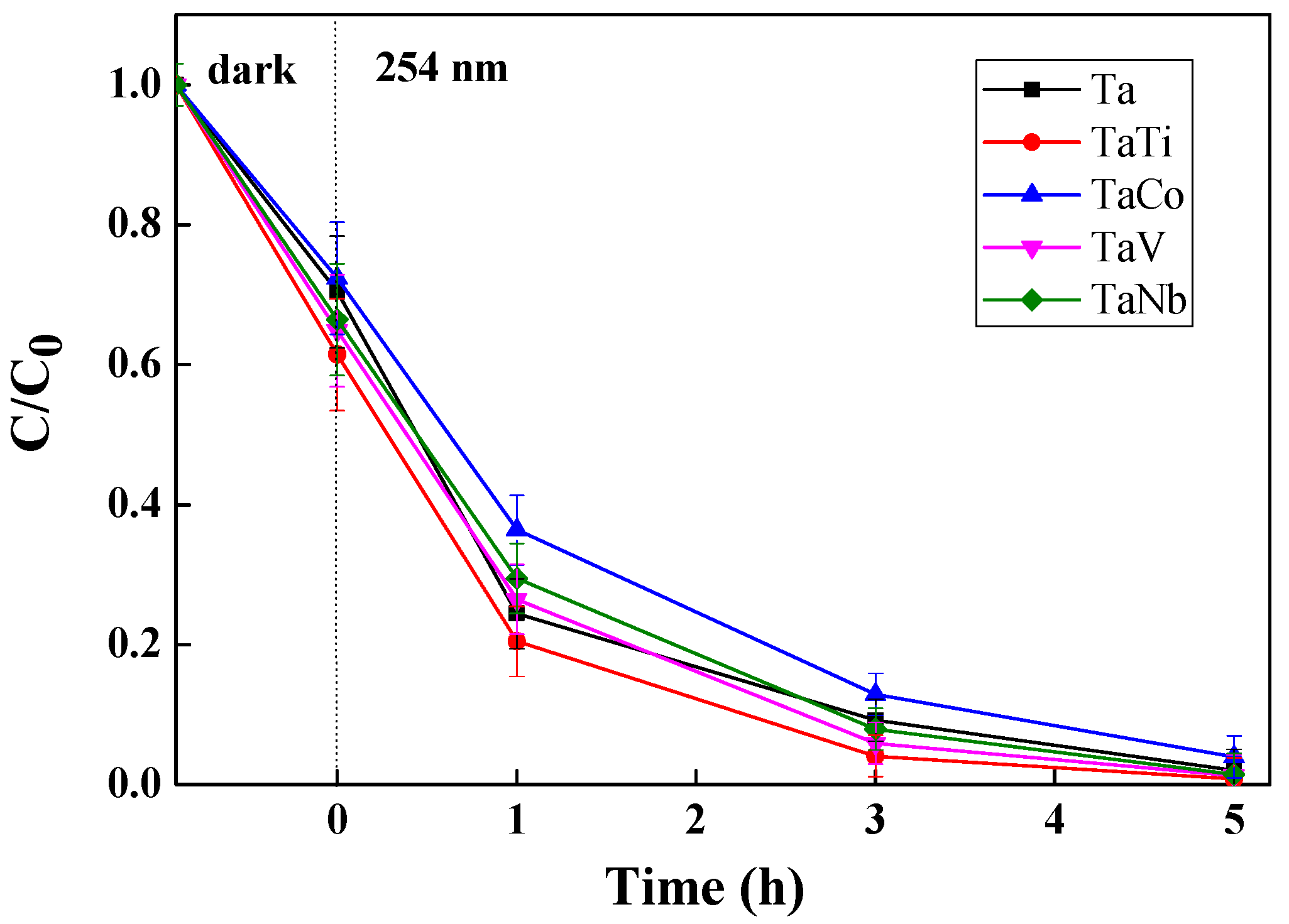
3.3. Photocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morais, L.A.; Castro, F.L.; Fernandes, G.J.T.; Araujo, M.D.S.; Farias, M.F.; Guedes, A.P.M.A.; Fernandes, V.J., Jr.; Araujo, A.S. Synthesis and Characterization of MCM-41 Nanomaterials Containing Titanium and Application for Catalytic Oxidation of BTEX. Catal. Res. 2023, 3, 17. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Molina, C.B.; Belver, C.; Bedia, J.; Mokhtar, A.; Hamacha, R.; Boukoussa, B. Metal-Loaded Mesoporous MCM-41 for the Catalytic Wet Peroxide Oxidation (CWPO) of Acetaminophen. Catalysts 2021, 11, 219. [Google Scholar] [CrossRef]
- Peng, W.; Cai, L.; Lu, Y.; Zhang, Y. Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B. Catalysts 2023, 13, 991. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Rath, D.; Nanda, B.; Parida, K.M. Transition metal/metal oxide modified MCM-41 for pollutant degradation and hydrogen energy production: A review. RSC Adv. 2015, 5, 83707–83724. [Google Scholar] [CrossRef]
- Schlichter, S.; Sapag, K.; Dennehy, M.; Alvarez, M. Metal-based mesoporous materials and their application as catalysts for the degradation of methyl orange azo dye. J. Environ. Chem. Eng. 2017, 5, 5207–5214. [Google Scholar] [CrossRef]
- Todorova, S.; Parvulescu, V.; Kadinov, G.; Tenchev, K.; Somacescu, S.; Su, B.-L. Metal states in cobalt- and cobalt-vanadium-modified MCM-41 mesoporous silica catalysts and their activity in selective hydrocarbons oxidation. Microporous Mesoporous Mater. 2008, 113, 22–30. [Google Scholar] [CrossRef]
- Kilos, B.; Aouine, M.; Nowak, I.; Ziolek, M.; Volta, J.C. The role of niobium in the gas- and liquid-phase oxidation on metallosilicate MCM-41-type materials. J. Catal. 2004, 224, 314–325. [Google Scholar] [CrossRef]
- Parvulescu, V.; Tablet, C.; Anastasescu, C.; Su, B.L. Activity and stability of bimetallic Co (V, Nb, La)-modified MCM-41 catalysts. Catal. Today 2004, 93–95, 307–313. [Google Scholar] [CrossRef]
- Parvulescu, V.; Anastasescu, C.; Su, B.L. Vanadium incorporated mesoporous silicates as catalysts for oxidation of alcohols and aromatics. J. Mol. Catal. A Chem. 2003, 198, 249–261. [Google Scholar] [CrossRef]
- Genel, S.; Durak, H.; Genel, Y. Catalytic effect of metal powder and MCM-41/metal catalysts on the pyrolysis of cellulose. Environ. Prog. Sustain. 2024, 43, 14225. [Google Scholar] [CrossRef]
- Parvulescu, V.; Anastasescu, C.; Su, B.L. Bimetallic Ru-(Cr, Ni, or Cu) and La-(Co or Mn) incorporated MCM-41 molecular sieves as catalysts for oxidation of aromatic hydrocarbons. J. Mol. Catal. A Chem. 2004, 211, 143–148. [Google Scholar] [CrossRef]
- Parvulecu, V. 2-Catalytic behavior of metal active sites from modified silicas in oxidation of organic compounds. In Redox; Khattak, R., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, T.; Pereira de Souza, C.; Lopes-Moriyama, A.L.; Pereira da Silva, M.L. In situ modification of MCM-41 using niobium and tantalum mixed oxide from columbite processing for methylene blue adsorption: Characterization, kinetic, isotherm, thermodynamic and mechanism study. Mater. Chem. Phys. 2023, 294, 127011. [Google Scholar] [CrossRef]
- Dubiel, W.; Kowalczyk, A.; Jankowska, A.; Michalik, M.; Mozgawa, W.; Kobielusz, M.; Macyk, W.; Chmielarz, L. Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2. Green Process. Synth. 2023, 12, 20230052. [Google Scholar] [CrossRef]
- Arellano, U.; Wang, J.A.; Chen, L.F.; Asomoza, M.; Guzmán, A.; Solís, S.; Estrella, A.; Cipagauta, S.; Noreña, L.E. Transition metal oxides dispersed on Ti-MCM-41 hybrid core-shell catalysts for the photocatalytic degradation of Congo red colorant. Catal. Today 2020, 349, 128–140. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lin, S.D.; Wu, J.C.-S. Synergetic photo-epoxidation of propylene over V-Ti/MCM-41 mesoporous photocatalysts. J. Catal. 2015, 331, 217–227. [Google Scholar] [CrossRef]
- Brutchey, R.L.; Lugmair, C.G.; Schebaum, L.O.; Tilley, T.D. Thermolytic conversion of a bis(alkoxy)tris(siloxy)tantalum(V) single-source molecular precursor to catalytic tantala–silica materials. J. Catal. 2005, 229, 72–81. [Google Scholar] [CrossRef]
- Jehng, J.M.; Tung, W.C.; Huang, C.H.; Wachs, I.E. Structural characteristics and reactivity properties of the tantalum modified mesoporous silicalite (MCM-41) catalysts. Microporous Mesoporous Mater. 2007, 99, 299–307. [Google Scholar] [CrossRef]
- Oliveira, F.T.; Silva, P.M.L.; Lopes-Moriyama, A.L.; Souza, P.C. Facile preparation of ordered mesoporous Nb, Ta-MCM-41 by hydrothermal direct synthesis using columbite ore as metal source. Ceram. Int. 2021, 47, 29509–29514. [Google Scholar] [CrossRef]
- Lee, B.; Yamashita, T.; Lu, D.; Kondo, J.N.; Domen, K. Single-Crystal Particles of MesoporousNiobium-Tantalum Mixed Oxide. Chem. Mater. 2002, 14, 867–875. [Google Scholar] [CrossRef]
- Ziolek, M.; Nowak, I. Characterization techniques employed in the study of niobium and tantalum-containing materials. Catal. Today 2003, 78, 543–553. [Google Scholar] [CrossRef]
- Takahara, Y.; Kondo, J.N.; Lu, D.; Domen, K. Synthesis and application for overall water splitting of transition metal-mixed mesoporous Ta oxide. Solid State Ion. 2002, 151, 305–311. [Google Scholar] [CrossRef]
- Yang, X.; Roy, A.; Alhabradi, M.; Alruwaili, M.; Chang, H.; Tahir, A.A. Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution. Nanomaterials 2023, 13, 2464. [Google Scholar] [CrossRef] [PubMed]
- Fernandes de Oliveira, T.; de Souza, C.P.; Lopes-Moriyama, A.L. Acid leaching and thermal treatments in the obtaining of mixed oxides of Nb and Ta from ferrocolumbite. Miner. Eng. 2020, 147, 106157. [Google Scholar] [CrossRef]
- Talukdar, H.; Saikia, G.; Das, A.; Sultana, S.Y.; Islam, N.S. Organic-solvent-free oxidation of styrene, phenol and sulfides with H2O2 over eco-friendly niobium and tantalum based heterogeneous catalysts. J. Ind. Eng. Chem. 2023, 121, 249–263. [Google Scholar] [CrossRef]
- Paul, R.; Kavinarmatha, K.; Parthiban, S. Tantalum doped titanium dioxide nanoparticles for efficient photocatalytic degradation of dyes. J. Mol. Struct. 2023, 1277, 134869. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; José, M.; Fraile, J.M. Modified Ta/MCM-41 catalysts for enantioselective oxidation of thioanisole. J. Mol. Catal. A Chem. 2015, 410, 140–148. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Comparison of Ta-MCM-41 and Ti-MCM-41 as catalysts for the enantioselective epoxidation of styrene with TBHP. C. R. Chim. 2017, 20, 827–832. [Google Scholar] [CrossRef]
- Cimpeanu, V.; Pârvulescu, V.; Pârvulescu, V.I.; Capron, M.; Grange, P.; Thompson, J.M.; Hardacre, C. Selective oxidation of a pyrimidine thioether using supported tantalum catalysts. J. Catal. 2005, 235, 184–194. [Google Scholar] [CrossRef]
- Bregante, D.T.; Thornburg, N.E.; Notestein, J.M. Flaherty, Consequences of Confinement for Alkene Epoxidation with Hydrogen Peroxide on Highly Dispersed Group 4 and 5 Metal Oxide Catalysts. ACS Catal. 2018, 8, 2995–3010. [Google Scholar] [CrossRef]
- Suib, S.L.; Prěch, J.; Szaniawska, E.; Čejka, J. Recent Advances in Tetra- (Ti, Sn, Zr, Hf) and Pentavalent (Nb, V, Ta) Metal-Substituted Molecular Sieve Catalysis. Chem. Rev. 2023, 123, 877–917. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Thompson, A.B.; Notestein, J.M. Periodic Trends in Highly Dispersed Groups IV and V Supported Metal Oxide Catalysts for Alkene Epoxidation with H2O2. ACS Catal. 2015, 5, 5077–5088. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Teodorescu, C.M.; Esteva, J.M.; Karnatak, R.C.; El Afif, A. An approximation of the Voigt I profile for the fitting of experimental X-ray absorption data. Nucl. Instrum. Methods Phys. Res. Sect. A 1994, 345, 141–147. [Google Scholar] [CrossRef]
- Al Ebraheem, J.S.; Alkhoder, M.N.A.; Tulaimat, R.H. Synthesis and characterization of mesoporous V–Mo-MCM-41 nanocatalysts: Enhancing efficiency in oxalic acid synthesis. Heliyon 2024, 10, 24652. [Google Scholar] [CrossRef]
- Dubiel, W.; BaTran, L.; Jankowsk, A.; Kowalczyk, A.; Michalik, M.; Mozgawa, W.; Mazur, M.; Nguyen, N.H.; Chmielarz, L. Synergistic catalytic effect of titanium and iron incorporated to spherical MCM-41 in selective catalytic oxidation of diphenyl sulphide with H2O2. Polyhedron 2024, 262, 117158. [Google Scholar] [CrossRef]
- Jankowska, A.; Kowalczyk, A.; Rutkowska, M.; Michalik, M.; Chmielarz, L. Catalytic Performance of Bimetallic Systems (Cu-Fe, Cu-Mn, Fe-Mn) Based on Spherical MCM-41 Modified by Template Ion-Exchange in NH3-SCR Process. Catalysts 2022, 12, 885. [Google Scholar] [CrossRef]
- Sirinwaranon, P.; Sricharoenchaikul, V.; Atong, D. Catalytic performance of Co, Fe on MCM-41 synthesized from illite waste for gasification of torrefied cassava rhizome. Energy Rep. 2021, 7, 149–162. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Z.; Li, C. UV Raman Spectroscopic Studies on Active Sites and Synthesis Mechanisms of Transition Metal-Containing Microporous and Mesoporous Materials. Acc. Chem. Res. 2010, 43, 378–387. [Google Scholar] [CrossRef]
- Thu, J.; Dutta, P.K.; Kresge, C.T. Raman spectroscopic studies of the synthesis of faujasitic zeolites: Comparison of two silica sources. Zeolites 1991, 11, 672–679. [Google Scholar] [CrossRef]
- Jin, S.; Feng, Z.; Fan, F.; Li, C. UV Raman Spectroscopic Characterization of Catalysts and Catalytic Active Sites. Catal. Lett. 2015, 145, 468–481. [Google Scholar] [CrossRef]
- Lewandowska, A.E.; Banares, M.A.; Tielens, F.; Che, M.; Dzwigaj, S. Different Kinds of Tetrahedral V Species in Vanadium-Containing Zeolites Evidenced by Diffuse Reflectance UV-vis, Raman, and Periodic Density Functional Theory. J. Phys. Chem. C 2010, 114, 19771–19776. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Ohishi, Y.; Shishido, T.; Takehira, K. Vanadium-Containing MCM-41 for Partial Oxidation of Lower Alkanes. J. Catal. 2002, 202, 308–318. [Google Scholar] [CrossRef]
- Wetherall, K.M.; Doughty, P.; Mountjoy, G.; Bettinelli, M.; Speghini, A.; Casula, M.F.; Cesare-Marincola, F.; Locci, E.; Newport, R.J. The atomic structure of niobium and tantalum containing borophosphate glasses. J. Phys. Condens. Matter 2009, 21, 375106. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Sastre, Á.; Boix, A.V.; Cocero, M.J.; Alonso, E. Cobalt oxide nanoparticles on mesoporous MCM-41 and Al-MCM-41 by supercritical CO2 deposition. Microporous Mesoporous Mater. 2012, 148, 53–61. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Z.; Xu, L.; Li, M.; Xin, Q.; Liu, Z.; Li, C. Ti-MCM-41 Synthesized from Colloidal Silica and Titanium Trichloride: Synthesis, Characterization, and Catalysis. Chem. Mater. 2001, 13, 994–998. [Google Scholar] [CrossRef]
- Nitsche, D.; Hess, C. Structure of Isolated Vanadia and Titania: A Deep UV Raman, UV−Vis, and IR Spectroscopic Study. J. Phys. Chem. C 2016, 120, 1025–1037. [Google Scholar] [CrossRef]
- Malfait, B.; Moréac, A.; Jani, A.; Lefort, R.; Huber, P.; Fröba, M.; Morineau, D. Structure of Water at Hydrophilic and Hydrophobic Interfaces: Raman Spectroscopy of Water Confined in Periodic Mesoporous (Organo)Silicas. J. Phys. Chem. C 2022, 126, 3520–3531. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Cherian, M.; Baerns, M.; Su, D.; Schlögl, R.; Wang, X.; Wachs, I.E. Oxidative dehydrogenation of propane over V/MCM-41 catalysts: Comparison of O2 and N2O as oxidants. J. Catal. 2005, 234, 131–142. [Google Scholar] [CrossRef]
- Petrescu, S.; Constantinescu, M.; Anghel, E.M.; Atkinson, I.; Olteanu, M.; Zaharescu, M. Structural and physico-chemical characterization of some soda lime zinc alumino-silicate glasses. J. Non-Crystall Solids 2022, 358, 3280–3288. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E.; Wong, M.S.; Ying, J.Y. Structural and Reactivity Properties of Nb–MCM-41: Comparison with That of Highly Dispersed Nb2O5/SiO2 Catalysts. J. Catal. 2001, 203, 18–24. [Google Scholar] [CrossRef]
- Lee, E.L.; Wachs, I.E. In Situ Spectroscopic Investigation of the Molecular and Electronic Structures of SiO2 Supported Surface Metal Oxides. J. Phys. Chem. C 2007, 111, 14410–14425. [Google Scholar] [CrossRef]
- Liu, W.-S.; Liao, M.-W.; Huang, S.-H.; Reyes, Y.L.A.; Chen, H.-Y.T.; Perng, T.-P. Formation and characterization of gray Ta2O5 and its enhanced photocatalytic hydrogen generation activity. Int. J. Hydrogen Energy 2020, 45, 16560–16568. [Google Scholar] [CrossRef]
- Xiong, G.; Li, C.; Li, H.; Xin, Q.; Feng, Z. Direct spectroscopic evidence for vanadium species in V-MCM-41 molecular sieve characterized by UV resonance Raman spectroscopy. Chem. Commun. 2000, 8, 677–678. [Google Scholar] [CrossRef]
- Samek, I.A.; Bobbitt, N.S.; Snurr, R.Q.; Stair, P.C. Interactions of VOx Species with Amorphous TiO2 Domains on ALD Derived Alumina-Supported Materials. J. Phys. Chem. C 2019, 123, 7988–7999. [Google Scholar] [CrossRef]
- Fan, M.; Xu, S.; An, B.; Sheveleva, A.M.; Betts, A.; Hurd, J.; Zhu, Z.; He, M.; Iuga, D.; Lin, L.; et al. Bimetallic Aluminum- and Niobium-Doped MCM-41 for Efficient Conversion of Biomass-Derived 2-Methyltetrahydrofuran to Pentadienes. Angew. Chem. Int. Ed. 2022, 61, e202212164. [Google Scholar] [CrossRef]
- Wachs, I.E.; Chen, Y.; Jehng, J.-M.; Briand, L.E.; Tanaka, T. Molecular structure and reactivity of the Group V metal oxides. Catal. Today 2003, 78, 13–24. [Google Scholar] [CrossRef]
- Pedersen, C.S.; Chang, J.H.; Li, Y.; Pryds, N.; Garcia Lastra, J.M. Phase separation in amorphous tantalum oxide from first principles. APL Mater. 2020, 8, 071108. [Google Scholar] [CrossRef]
- Lu, Q. How to Correctly Analyze 2p X-ray Photoelectron Spectra of 3d Transition-Metal Oxides: Pitfalls and Principles. ACS Nano 2024, 18, 13973–13982. [Google Scholar] [CrossRef]
- Prokopenko, V.B.; Gurin, V.S.; Alexeenko, A.A.; Kulikauskas, V.S.; Kovalenko, D.I. Surface segregation of transition metals in sol–gel silica films. J. Phys. D Appl. Phys. 2000, 33, 3152–3155. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, J.; Pu, X.; Wang, J.; Huang, Z.; Yin, G. Effects of incorporated vanadium and its chemical states on morphology and mesostructure of mesoporous bioactive glass particles. Microporous Mesoporous Mater. 2021, 319, 111061. [Google Scholar] [CrossRef]
- Available online: https://www.thermofisher.com/ro/en/home/materials-science/learning-center/periodic-table/transition-metal/cobalt.html (accessed on 22 November 2024).
- Chen, J.Y.; Leng, Y.X.; Zhang, X.; Yang, P.; Sun, H.; Wang, J.; Wan, G.J.; Zhao, A.S.; Huang, N.; Chu, P.K. Effect of tantalum content of titanium oxide film fabricated by magnetron sputtering on the behavior of cultured human umbilical vein endothelial cells (HUVEC). Nucl. Instrum. Methods Phys. Res. B 2006, 242, 26–29. [Google Scholar] [CrossRef]
- Lozada, A.B.; Sango, A.; Sangurima-Cedillo, S.-C.; Debut, A.; Endara, D.; De la Torre, E.; Gaigneaux, E.M.; Manangon-Perugachi, L.E. Mesoporous titanosilicate-silica-coated cobalt ferrite core-shell catalysts for the oxidation of styrene. Catal. Today 2024, 430, 114513. [Google Scholar] [CrossRef]
- Galacho, C.; Ribeiro Carrott, M.; Carrott, P.; Cansado, I. Hydrothermal Stability of Ordered Mesoporous Titanosilicate Materials Prepared at Room Temperature. Adv. Mater. Res. 2010, 107, 63–70. [Google Scholar] [CrossRef]
- Garcia, L.M.P.; Tavares, M.T.S.; Andrade Neto, N.F.; Nascimento, R.M.; Paskocimas, C.A.; ·Longo, E.; Bomio, M.R.D.; Motta, F.V. Photocatalytic activity and photoluminescence properties of TiO2, In2O3,TiO2/In2O3 thin films multilayer. J. Mater. Sci. Mater. Electron. 2018, 29, 6530–6542. [Google Scholar] [CrossRef]
- Negoescu, D.; Atkinson, I.; Gherendi, M.; Culita, D.C.; Baran, A.; Petrescu, S.; Trica, B.; Pelinescu, D.; Ionescu, R.; Bratan, V.; et al. Brij58–activated carbon assisted synthesis of Ag/Ag2O/TiO2-ACphotocatalysts for efficient organic pollutants degradation. J. Alloys Compd. 2023, 931, 167528. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Zha, X.; He, X.; Gu, X. Crystallite structure, surface morphology and optical properties of In2O3–TiO2 composite thin films by sol–gel method. Mater. Sci. Eng. B. 2008, 151, 179–186. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Xin, Y.; Liu, F.; Xuan, J.; Guo, M.; Duan, T. IrO2-Ta2O5 Anode for Oxygen Evolution with TaOx Interlayer Prepared by Thermal Decomposition in Inert Atmosphere. J. Electrochem. Soc. 2022, 169, 046516. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Visinescu, C.; Parvulescu, V.; Marcu, V.; Levy, F. Comparative photocatalytic behavior of Ta catalysts prepared by d.c.-sputtering, sol–gel and grafting in acetone degradation. Catal. Today 2006, 118, 433–439. [Google Scholar] [CrossRef]
- Ramanathan, A.; Maheswari, R.; Subramaniam, B. Facile Styrene Epoxidation with H2O2 over Novel Niobium Containing Cage Type Mesoporous Silicate, Nb-KIT5. Top. Catal. 2015, 58, 314–324. [Google Scholar] [CrossRef]
- Ziolek, M.; Lewandowska, A.; Renn, M.; Nowak, I. The use of niobium containing mesoporous molecular sieves in the liquid phase oxidation. Surf. Sci. Catal. 2004, 154, 2610–2617. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Pastore, H.O.; Schuchardt, U. Silylation of [Nb]-MCM-41 as an efficient tool to improve epoxidation activity and selectivity. J. Catal. 2006, 243, 57–63. [Google Scholar] [CrossRef]
- Nandi, M.; Sarkar, K.; Bhaumik, A. Liquid phase partial oxidation of olefins over mesoporous titanium silicate molecular sieve synthesized by non-ionic templating route. Mater. Chem. Phys. 2008, 107, 499–504. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Modified Ti/MCM-41 catalysts for enantioselective epoxidation of styrene. J. Mol. Catal. A Chem. 2016, 420, 282–289. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Hitam, C.N.C.; Sawal, M.H.; Rahim, M.N.S.; Hussain, I.; Jusoh, N.W.C.; Saravanan, R.; Prasetyoko, D. Enhanced photooxidative desulphurization of dibenzothiophene over fibrous silica tantalum: Influence of metal-disturbance electronic band structure. Int. J. Hydrogen Energy 2023, 48, 6575–6585. [Google Scholar] [CrossRef]
- Emeline, A.V.; Zhang, X.; Murakami, T.; Fujishima, A. Activity and selectivity of photocatalysts in photodegradation of phenols. J. Hazard. Mater. 2012, 211–212, 154–160. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laıné, J.-M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl. Catal. B Environ. 2004, 50, 259–269. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, T.; Xiao, Z.; Zhu, H.; Yu, M. Photocatalytic Treatment of Methyl Orange Dye Wastewater by Porous Floating Ceramsite Loaded with Cuprous Oxide. Coatings 2022, 12, 286. [Google Scholar] [CrossRef]
- Din, S.T.U.; Xie, W.-F.; Yang, W. Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA. Int. J. Mol. Sci. 2022, 23, 15028. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Highly Visible Light Responsive, Narrow Band gap TiO2 Nanoparticles Modified by Elemental Red Phosphorus for Photocatalysis and Photoelectrochemical Applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef] [PubMed]
- Petcu, G.; Anghel, E.M.; Atkinson, I.; Culita, D.C.; Apostol, N.G.; Kuncser, A.; Papa, F.; Baran, A.; Blin, J.-L.; Parvulescu, V. Composite Photocatalysts with Fe, Co, and Ni Oxides on Supports with Tetracoordinated Ti Embedded into Aluminosilicate Gel during Zeolite Y Synthesis. Gels 2024, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Makota, O.; Dutková, E.; Briancin, J.; Bednarcik, J.; Lisnichuk, M.; Yevchuk, I.; Melnyk, I. Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment. Molecules 2024, 29, 1190. [Google Scholar] [CrossRef] [PubMed]
- Parvulescu, V.; Constantin, C.; Su, B.L. Liquid phase oxidation of aromatic hydrocarbons using highly ordered Nb and NbCo-MCM-41 nanoreactors. J. Mol. Catal. A Chem. 2003, 202, 171–178. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef]

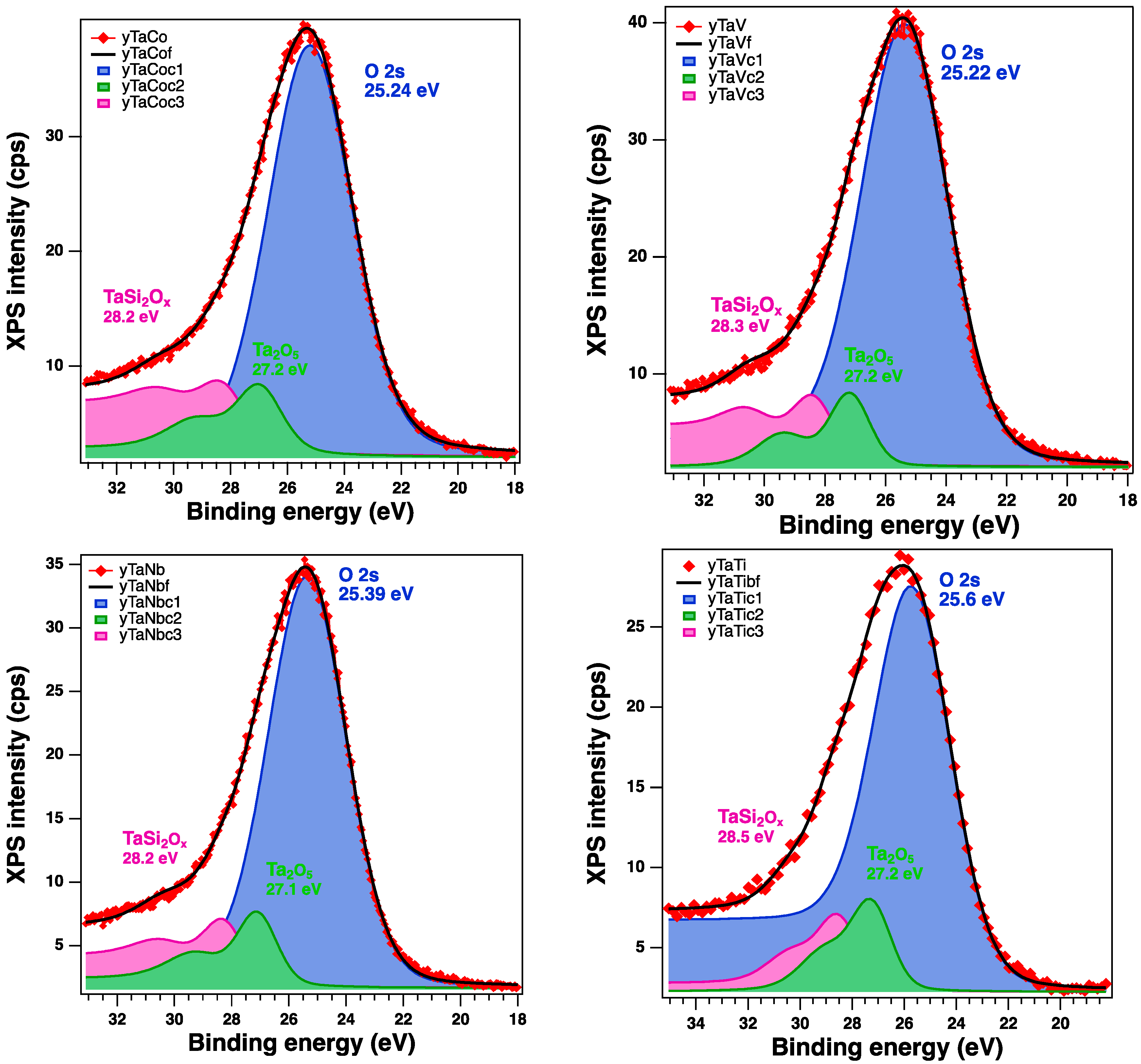

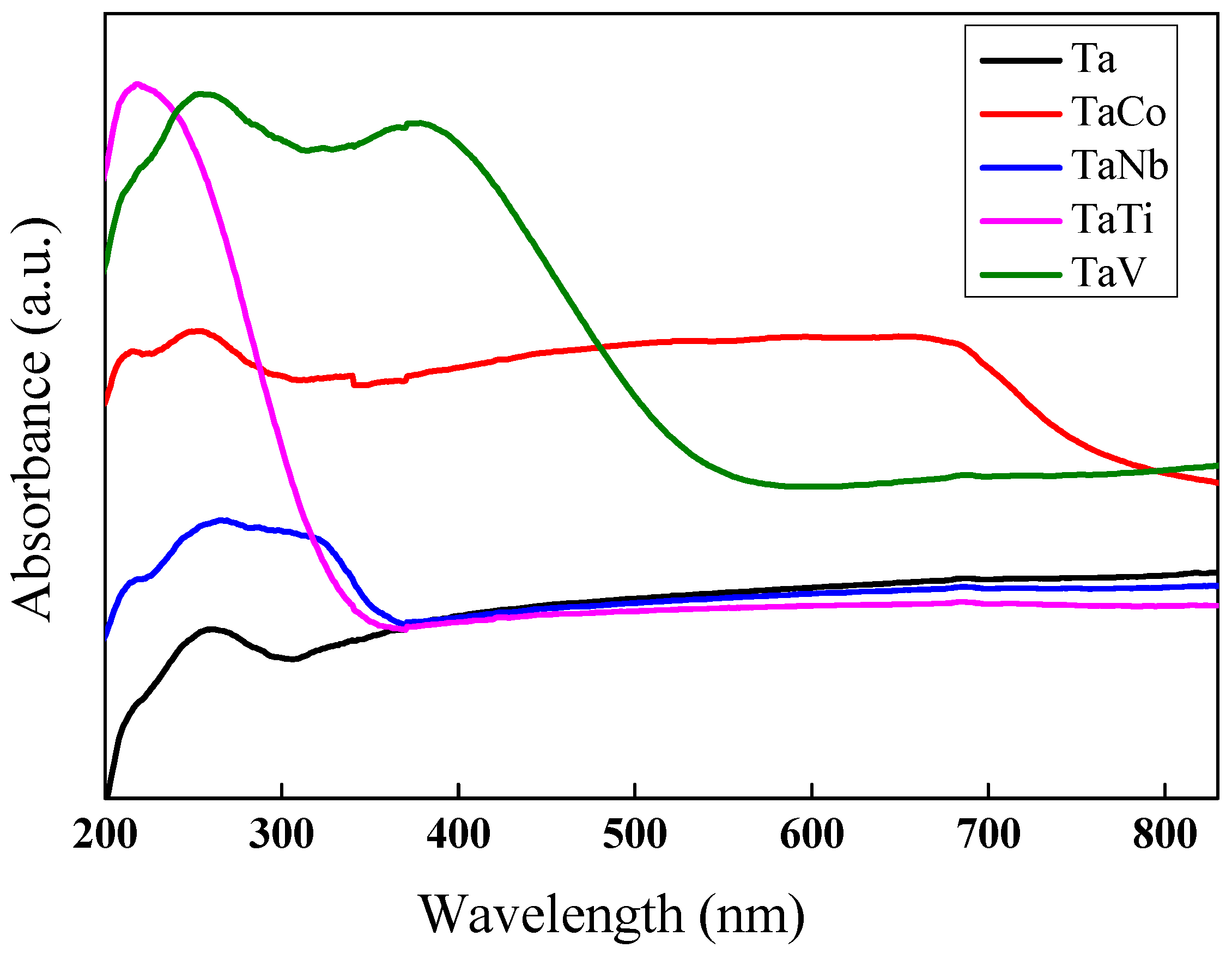

| Sample | Sample Code | Ta, % | Nb, % | V, % | Ti, % | Co, % | Ta/Me b |
|---|---|---|---|---|---|---|---|
| Ta-MCM-41 | Ta | 5.36 | - | - | - | - | - |
| TaNb-MCM-41 | TaNb | 5.36 | 3.28 | - | - | - | 0.829 |
| TaV-MCM-41 | TaV | 5.55 | - | 1.96 | - | - | 0.789 |
| TaTi-MCM-41 | TaTi | 5.34 | - | - | 4.23 | - | 0.329 |
| TaCo-MCM-41 | TaCo | 3.33 | - | - | - | 2.44 | 0.445 |
| Sample | 1,4-Cyclohexadiene | Cyclohexene | Styrene | |||
|---|---|---|---|---|---|---|
| C (%) | SHOL (%) | C (%) | SHO (%) | C (%) | SSO (%) | |
| Ta | 25.2 | 91.2 | 28.1 | 25.2 | 48.1 | 21.2 |
| Ta Nb | 81.1 | 85.2 | 65.7 | 71.2 | 76.4 | 65.0 |
| TaV | 36.1 | 84.5 | 32.4 | 78.9 | 89.0 | 66.8 |
| TaTi | 37.4 | 98.6 | 56,3 | 89.4 | 95.0 | 62.6 |
| TaCo | 64.0 | 89.0 | 64.2 | 84.4 | 78.2 | 71.2 |
| Catalyst | Substrate | Reaction Conditions | C, % | SEPO, % | SHOL, % | SDOL/SCO, % | Ref. |
|---|---|---|---|---|---|---|---|
| Ta-SiO2 | Styrene | 104 mg, 6 h, 65 °C, H2O2, acetonitrile | 37 | 36 | - | - | [32] |
| Nb-KIT-6 | Styrene | 20 mg, 6 h, 50 °C, H2O2, methanol | 51 | 13 | - | - | [73] |
| NbV-MCM-41 | Phenol | 200 mg, 5 h, 80 °C, H2O2, H2O | 2.55 | - | 100 | - | [74] |
| Nb-MCM-41 | Cys-cyclooctene | 100 mg, 24 h, 80 °C, ethyl acetate | 46 | 77 | - | - | [75] |
| Ti-MCM-41 | Styrene | 20 mg, 36 h, 600 °C | 48.8 | 72.3 | - | 27.7 | [76] |
| Ti(OPr)4-MCM-41 | Styrene | 40 mg, 24 h, 70 °C, tert-butyl hydroperoxide, decane (acetonitrile) | 68 (79) | 44 (36) | - | - | [77] |
| Ta(OEt)5-MCM-41 | Styrene | 40 mg, 24 h, 70 °C, tert-butyl hydroperoxide, decane (acetonitrile) | 59 (69) | 24 (23) | - | - | [28] |
| Ta2O5·6SiO2 | Cyclohexene | 35 mg, 65 °C, 2 h, H2O2 acetonitrile | 2.61 | 9.6 | 58 | 31.8 | [17] |
| Ta-SBA-15 | Cyclohexene | 35 mg, 65 °C, 2 h, H2O2 acetonitrile | 13.6 | 36 | 32.7 | 31.3 | [17] |
| Sample | MO | Ph | ||||||
|---|---|---|---|---|---|---|---|---|
| PFO | PSO | PFO | PSO | |||||
| k1 (min−1) | R2adj | k2 (Lmol−1min−1) | R2adj | k1 (min−1) | R2adj | k2 (Lmol−1min−1) | R2adj | |
| Ta | 0.67009 ± 0.01747 | 0.99729 | 40.13985 ± 11.16201 | 0.74893 | 0.66294 ± 0.03919 | 0.98617 | 5.51095 ± 1.60835 | 0.72864 |
| TaTi | 0.7217 ± 0.10731 | 0.91707 | 50.09469 ± 5.7599 | 0.94914 | 0.83548 ± 0.02653 | 0.99598 | 14.07729 ± 4.28598 | 0.70989 |
| TaCo | 0.694 ± 0.05896 | 0.97177 | 39.02368 ± 7.10775 | 0.87931 | 0.55547 ± 0.01693 | 0.99629 | 2.82933 ± 0.69338 | 0.79644 |
| TaV | 0.65621 ± 0.08449 | 0.93684 | 34.7876 ± 4.09714 | 0.94673 | 0.7582 ± 0.02175 | 0.99672 | 9.04504 ± 2.68858 | 0.72063 |
| TaNb | 0.64324 ± 0.04877 | 0.9774 | 32.06994 ± 6.38889 | 0.85814 | 0.72291 ± 0.03025 | 0.99303 | 7.68671 ± 2.43553 | 0.69138 |
| Equation [87] | ln(C0/C) = k1t * | 1/C-1/C0 = k2t * | ln(C0/C) = k1t * | 1/C-1/C0 = k2t * | ||||
| Sample | Ta | TaNb | TaV | TaTi | TaCo |
|---|---|---|---|---|---|
| SBET (m2/g) | 867 | 856 | 862 | 826 | 817 |
| VBJH (cm3/g) | 0.877 | 0.942 | 0.894 | 1.012 | 1.025 |
| DBJH (nm) | 2.9 | 3.1 | 2.8 | 3.0 | 3.9 |
| Ta | TaTi | TaNb | TaCo | TaV | ||
|---|---|---|---|---|---|---|
| Eg (eV) | 5.64 | 3.62 | 3.32 | 2.25 | 2.18 | |
| MO Degradation Efficiency (%) | 1 h | 71.34 | 86.55 | 76.56 | 72.86 | 80.13 |
| 3 h | 91.79 | 96.92 | 93.01 | 94.78 | 95.85 | |
| 5 h | 97.99 | 98.19 | 97.40 | 97.80 | 97.39 | |
| Ph Degradation Efficiency (%) | 1 h | 75.57 | 79.55 | 70.56 | 63.61 | 73.56 |
| 3 h | 90.79 | 95.92 | 92.10 | 87.12 | 94.10 | |
| 5 h | 97.98 | 99.19 | 98.55 | 96.05 | 98.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvulescu, V.; Petcu, G.; Apostol, N.G.; Atkinson, I.; Petrescu, S.; Baran, A.; Culita, D.C.; Ene, R.; Trica, B.; Anghel, E.M. Bimetallic Mesoporous MCM-41 Nanoparticles with Ta/(Ti, V, Co, Nb) with Catalytic and Photocatalytic Properties. Nanomaterials 2024, 14, 2025. https://doi.org/10.3390/nano14242025
Parvulescu V, Petcu G, Apostol NG, Atkinson I, Petrescu S, Baran A, Culita DC, Ene R, Trica B, Anghel EM. Bimetallic Mesoporous MCM-41 Nanoparticles with Ta/(Ti, V, Co, Nb) with Catalytic and Photocatalytic Properties. Nanomaterials. 2024; 14(24):2025. https://doi.org/10.3390/nano14242025
Chicago/Turabian StyleParvulescu, Viorica, Gabriela Petcu, Nicoleta G. Apostol, Irina Atkinson, Simona Petrescu, Adriana Baran, Daniela C. Culita, Ramona Ene, Bogdan Trica, and Elena M. Anghel. 2024. "Bimetallic Mesoporous MCM-41 Nanoparticles with Ta/(Ti, V, Co, Nb) with Catalytic and Photocatalytic Properties" Nanomaterials 14, no. 24: 2025. https://doi.org/10.3390/nano14242025
APA StyleParvulescu, V., Petcu, G., Apostol, N. G., Atkinson, I., Petrescu, S., Baran, A., Culita, D. C., Ene, R., Trica, B., & Anghel, E. M. (2024). Bimetallic Mesoporous MCM-41 Nanoparticles with Ta/(Ti, V, Co, Nb) with Catalytic and Photocatalytic Properties. Nanomaterials, 14(24), 2025. https://doi.org/10.3390/nano14242025









