Charge Transfer Mechanism in Type II WO3/Cu2O Heterostructure
Abstract
1. Introduction
- 2H2O + 4e− → H2 + 2OH−;
- 2H2O + 4h+ → O2 + 4H+;
- CO2 + 4e− + 4H+ → HCHO + H2O;
- CO2 + 6e− + 6H+ → CH3OH + H2O;
- CO2 + 8e− + 8H+ → CH4 + 2H2O.
2. Materials and Methods
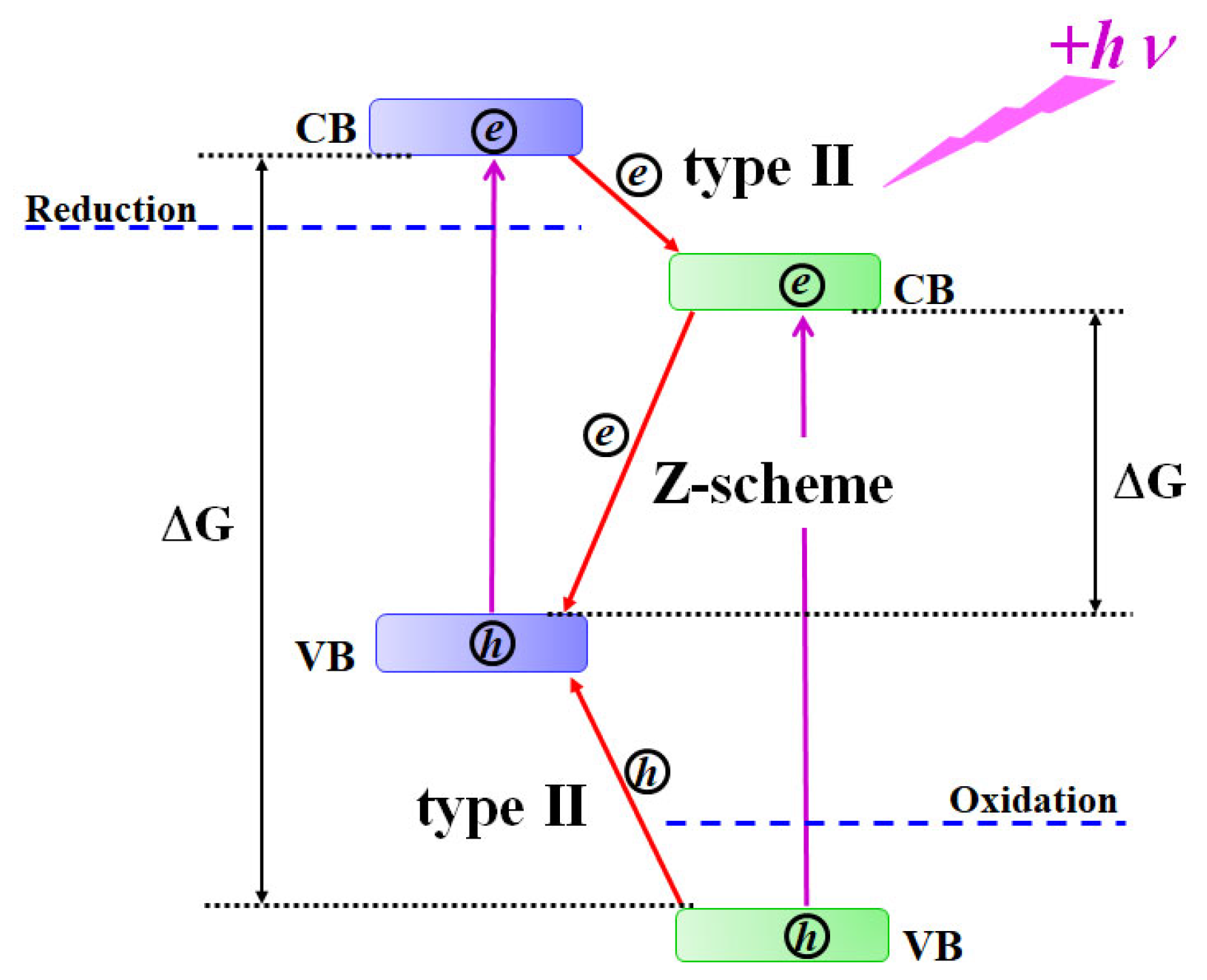
3. Results and Discussion
3.1. Physical–Chemical Characterization
3.2. Electrochemical Characterization
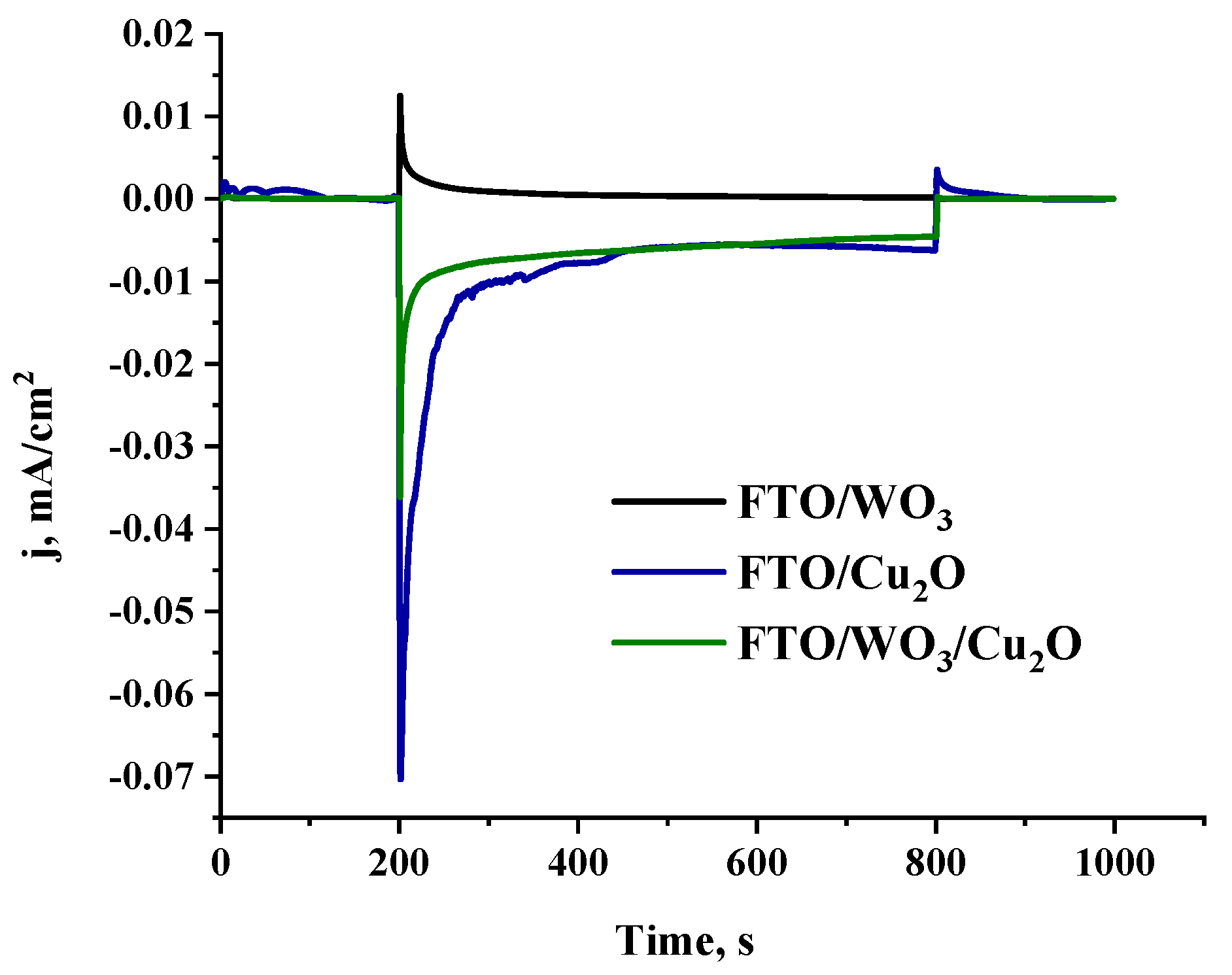
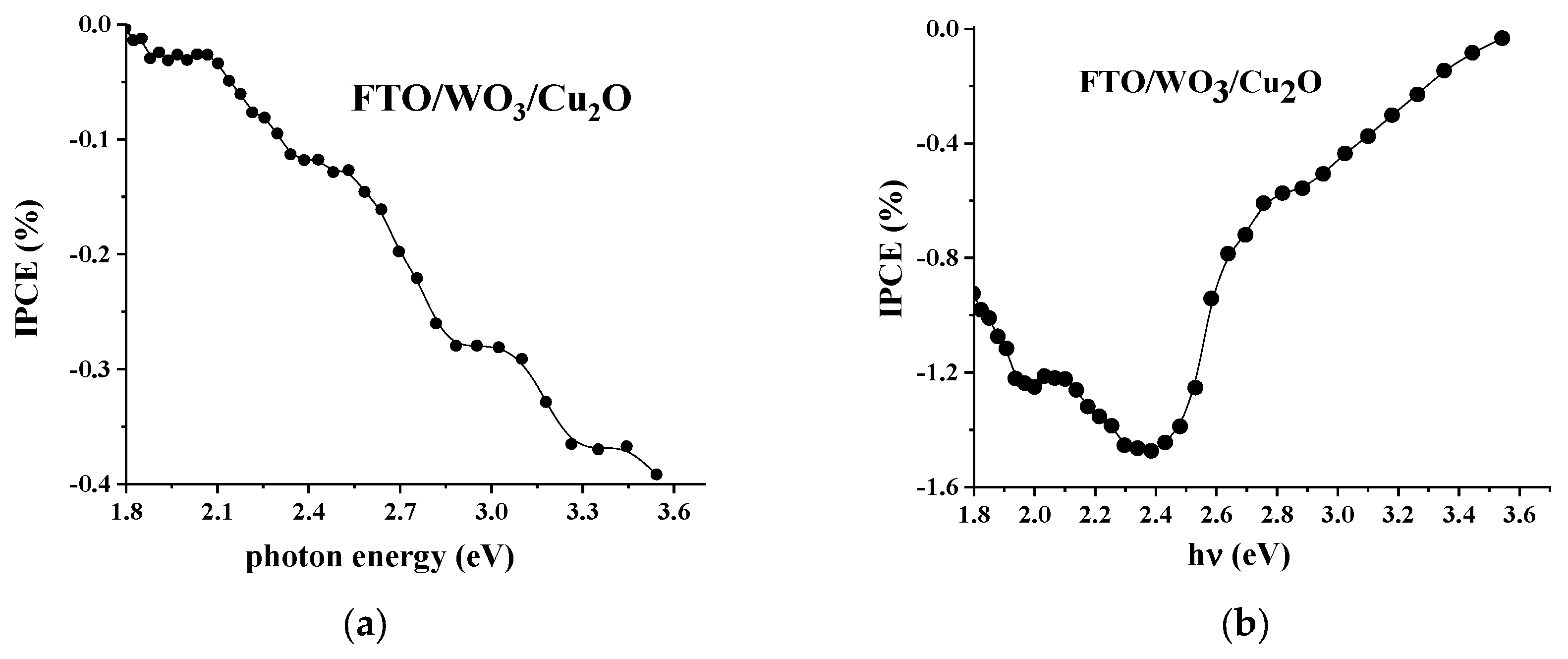
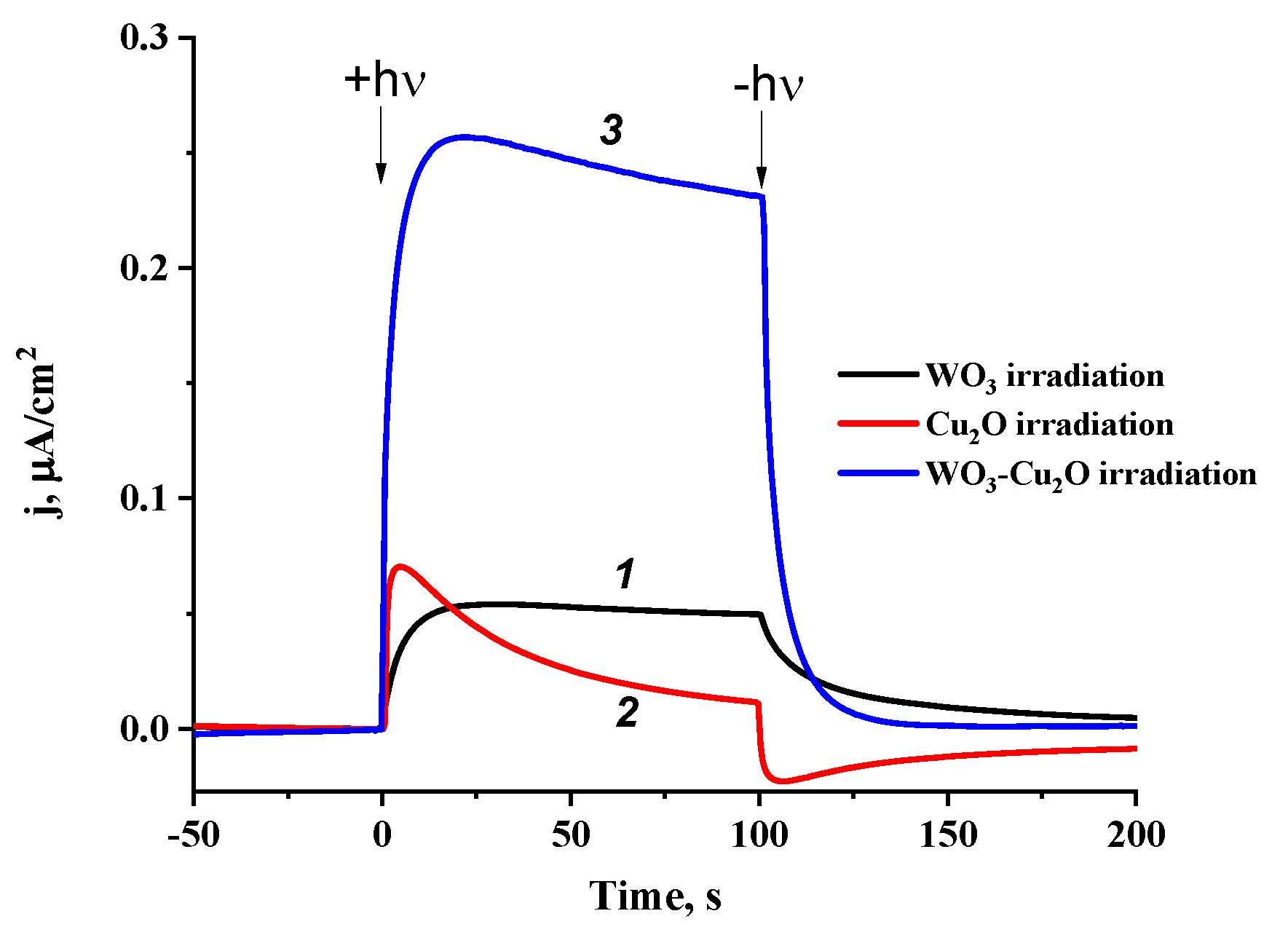
3.3. Photoelectrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Nishioka, S.; Osterloh, F.E.; Wang, X.; Mallouk, T.E.; Maeda, K. Photocatalytic water splitting. Nat. Rev. Methods Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Marschall, R. 50 years of materials research for photocatalytic water splitting. Eur. J. Inorg. Chem. 2021, 2021, 2435–2441. [Google Scholar] [CrossRef]
- Thompson, W.A.; Fernandez, E.S.; Maroto-Valer, M.M. Review and analysis of CO2 photoreduction kinetics. ACS Sustain. Chem. Eng. 2020, 8, 4677–4692. [Google Scholar] [CrossRef]
- Behera, A.; Kar, A.K.; Srivastava, R. Challenges and prospects in the selective photoreduction of CO2 to C1 and C2 products with nanostructured materials: A review. Mater. Horiz. 2022, 9, 607–639. [Google Scholar] [CrossRef]
- Wang, W.-N.; Soulis, J.; Yang, Y.J.; Biswas, P. Comparison of CO2 photoreduction systems: A Review. Aerosol Air Qual. Res. 2014, 14, 533–549. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.-J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Fu, W.; Guan, X.; Wu, H.; Liu, M. Switching from two-electron to four-electron photocatalytic pure water splitting via band bending engineering with boosted activity. Appl. Catal. B 2022, 305, 121054. [Google Scholar] [CrossRef]
- Salazar-Marín, D.; Oza, G.; Díaz Real, J.A.; Cervantes-Uribe, A.; Pérez-Vidal, H.; Kesarla, M.K.; Torres, J.G.T.; Godavarthi, S. Distinguishing between type II and S-scheme heterojunction materials: A comprehensive review. Appl. Surf. Sci. Adv. 2024, 19, 100536. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Li, X.; Fang, B. Emerging polymeric carbon nitride Z-scheme systems for photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Song, W.; Chong, K.C.; Qi, G.; Xiao, Y.; Chen, G.; Li, B.; Tang, Y.; Zhang, X.; Yao, Y.; Lin, Z.; et al. Unraveling the transformation from type-II to Z-scheme in perovskite-based heterostructures for enhanced photocatalytic CO2 reduction. J. Am. Chem. Soc. 2024, 146, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Z-Scheme versus type-II junction in g-C3N4/TiO2 and g-C3N4/SrTiO3/TiO2 heterostructures. Catal. Sci. Technol. 2021, 11, 3589–3598. [Google Scholar] [CrossRef]
- Murashkina, A.A.; Bakiev, T.V.; Artemev, Y.M.; Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Photoelectrochemical behavior of the ternary heterostructured systems CdS/WO3/TiO2. Catalysts 2019, 9, 999. [Google Scholar] [CrossRef]
- Minggu, L.J.; Ng, K.H.; Kadir, H.A.; Kassim, M.B. Bilayer n-WO3/p-Cu2O photoelectrode with photocurrent enhancement in aqueous electrolyte photoelectrochemical reaction. Ceram. Int. 2014, 40, 16015–16021. [Google Scholar] [CrossRef]
- Khalifa, M.A.; Shen, L.; Zheng, J.; Xu, C. Boosting light harvesting and charge separation of WO3 via coupling with Cu2O/CuO towards highly efficient tandem photoanodes. RSC Adv. 2021, 11, 13513–13520. [Google Scholar] [CrossRef]
- Emeline, A.V.; Rudakova, A.V.; Ryabchuk, V.K.; Serpone, N. Recent advances in composite and heterostructured photoactive materials for the photochemical conversion of solar energy. Curr. Opin. Green Sustain. 2022, 34, 100588. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Kim, B.K.H.; Huang, J. The effect of morphology and crystal structure on the photocatalytic and photoelectrochemical performances of WO3. RSC Adv. 2024, 14, 2080–2087. [Google Scholar] [CrossRef]
- Shin, S.; Han, H.S.; Kim, J.S.; Park, I.J.; Lee, M.H.; Hong, K.S.; Cho, I.S. A tree-like nanoporous WO3 photoanode with enhanced charge transport efficiency for photoelectrochemical water oxidation. J. Mater. Chem. A 2015, 3, 12920–12926. [Google Scholar] [CrossRef]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A review on Cu2O-based composites in photocatalysis: Synthesis, modification, and applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef] [PubMed]
- Toe, C.Y.; Zheng, Z.; Wu, H.; Scott, J.; Amal, R.; Ng, W.H. Photocorrosion of cuprous oxide in hydrogen production: Rationalising self-oxidation or self-reduction. Angew. Chem. 2018, 57, 13613–13617. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.-W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef]
- Knöppel, J.; Kormányos, A.; Mayerhöfer, B.; Hofer, A.; Bierling, M.; Bachmann, J.; Thiele, S.; Cherevko, S. Photocorrosion of WO3 photoanodes in different electrolytes. ACS Phys. Chem Au 2021, 1, 6–13. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Li, L.; Gu, Y.; BoK-Hee, K.; Huang, J. Construction of Z-scheme WO3-Cu2O nanorods array heterojunction for efficient photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2022, 138, 109248. [Google Scholar] [CrossRef]
- Mandyal, P.; Sharma, R.; Sambyal, S.; Islam, N.; Priye, A.; Kumar, M.; Chauhan, V.; Shandilya, P. Cu2O/WO3: A promising S-scheme heterojunction for photocatalyzed degradation of carbamazepine and reduction of nitrobenzene. J. Water Process Eng. 2024, 59, 105008. [Google Scholar] [CrossRef]
- Ali, H.; Guler, A.; Masar, M.; Urbanek, P.; Urbanek, M.; Skoda, D.; Suly, P.; Machovsky, M.; Galusek, D.; Kuritka, I. Solid-state synthesis of direct Z-scheme Cu2O/WO3 nanocomposites with enhanced visible-light photocatalytic performance. Catalysts 2021, 11, 293. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, J.; Wang, J.; Cao, L. Effects of Cu2O thickness on the photoelectrochemical properties of Cu2O/WO3 heterostructure. Vacuum 2023, 207, 111680. [Google Scholar] [CrossRef]
- Hao, J.; Qi, B.; Wei, J.; Li, D.; Zeng, F. A Z-scheme Cu2O/WO3 heterojunction for production of renewable hydrocarbon fuel from carbon dioxide. Fuel 2021, 287, 119439. [Google Scholar] [CrossRef]
- Shi, W.; Guo, X.; Cui, C.; Jiang, K.; Li, Z.; Qu, L.; Wang, J.-C. Controllable synthesis of Cu2O decorated WO3 nanosheets with dominant (0 0 1) facets for photocatalytic CO2 reduction under visible-light irradiation. Appl. Catal. B 2019, 243, 236–242. [Google Scholar] [CrossRef]
- Hu, C.-C.; Nian, J.-N.; Teng, H. Electrodeposited p-type Cu2O as photocatalyst for H2 evolution from water reduction in the presence of WO3. Sol. Energy Mater. Sol. Cells 2008, 92, 1071–1076. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Li, H.; Li, Y.; Hu, P. WO3/Cu2O heterojunction for the efficient photoelectrochemical property without external bias. Appl. Catal. B 2024, 265, 118561. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Liu, Z. Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Appl. Catal. B 2017, 201, 84–91. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lai, Y.H.; Mersch, D.; Reisner, E. Cu2O|NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting. Chem. Sci. 2012, 3, 3482–3487. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.C.; Chen, A.; Xu, X.; Wang, S.; Li, R.; Zhang, W.; Hou, Y. Cu nanoparticles modified step-scheme Cu2O/WO3 heterojunction nanoflakes for visible-light-driven conversion of CO2 to CH4. Nanomaterials 2022, 12, 2284. [Google Scholar] [CrossRef]
- Wu, L.; Tsui, L.; Swami, N.; Zangari, G. Photoelectrochemical stability of electrodeposited Cu2O films. J. Phys. Chem. C 2010, 114, 11551–11556. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J. Photoelectrochemistry of electrodeposited Cu2O. J. Electrochem. Soc. 2000, 147, 486. [Google Scholar] [CrossRef]
- Li, X.; Kong, W.; Qin, X.; Qu, F.; Lu, L. Self-powered cathodic photoelectrochemical aptasensor based on in situ–synthesized CuO-Cu2O nanowire array for detecting prostate-specific antigen. Microchim. Acta 2020, 187, 325. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Y.; Sha, H. Cuprous oxide-modified diatomite waste from the brewery used as an effective adsorbent for removal of organic dye: Adsorption performance, kinetics and mechanism studies. Water Air Soil Pollut. 2018, 229, 322. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, Q.; Song, P.; Zhang, X.; Xie, J.; Dong, Q.; Qi, J.; Xing, X.S.; Du, J. Integration of a Cu2O/ZnO heterojunction and Ag@SiO2 into a photoanode for enhanced solar water oxidation. RSC Adv. 2024, 14, 4568–4574. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, Q.; Yang, Y.; Liu, Y.; Wang, K.; Li, Y.; Li, D.; Qiu, X.; Li, W.; Li, J. An efficient tandem photoelectrochemical cell composed of FeOOH/TiO2/BiVO4 and Cu2O for self-driven solar water splitting. Int. J. Hydrogen Energy 2019, 44, 594–604. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 19 December 2024).
- Tsarenko, A.; Gorshenkov, M.; Yatsenko, A.; Zhigunov, D.; Butova, V.; Kaichev, V.; Ulyankina, A. Electrochemical synthesis-dependent photoelectrochemical properties of tungsten oxide powders. ChemEngineering 2022, 6, 31. [Google Scholar] [CrossRef]

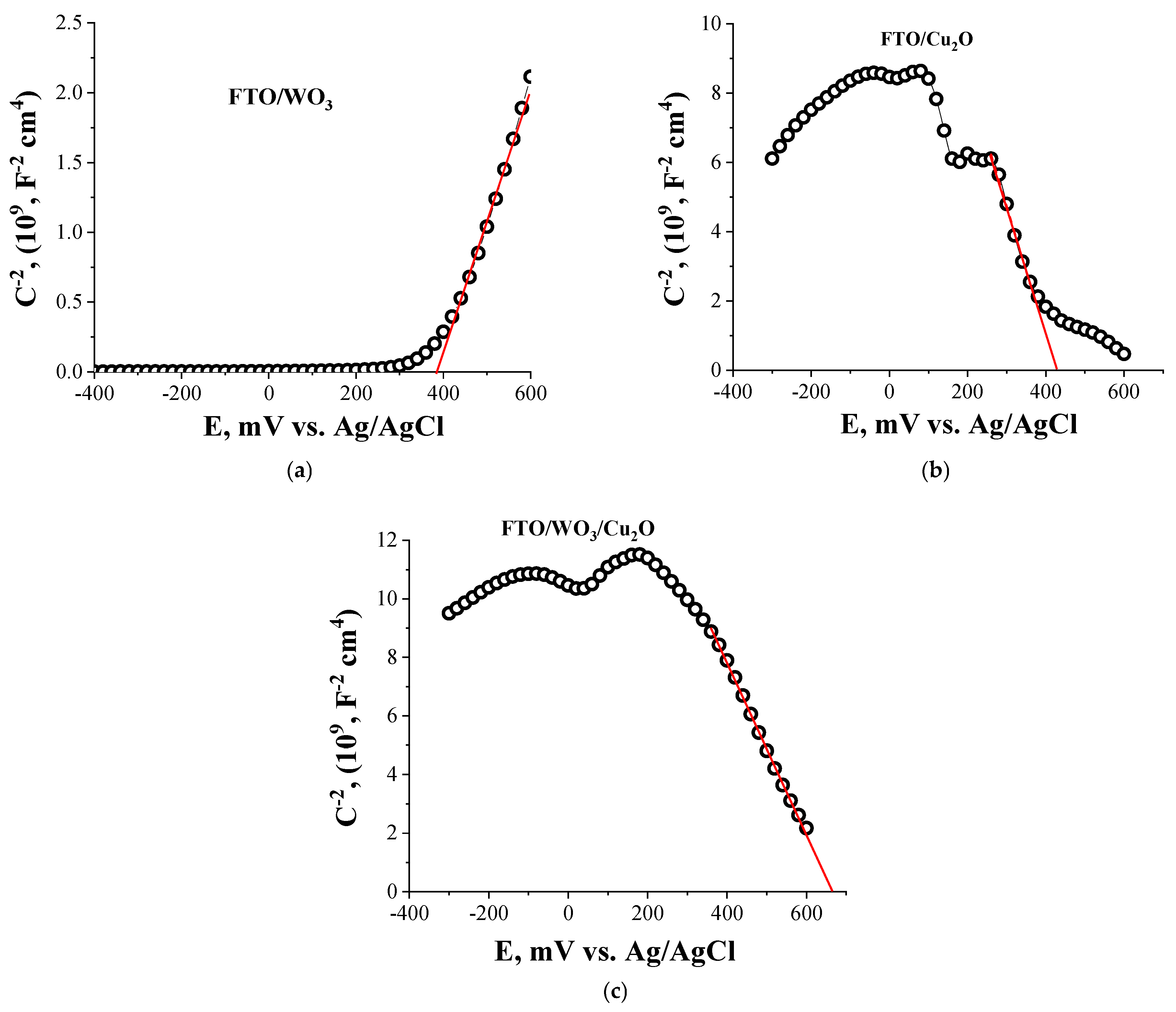
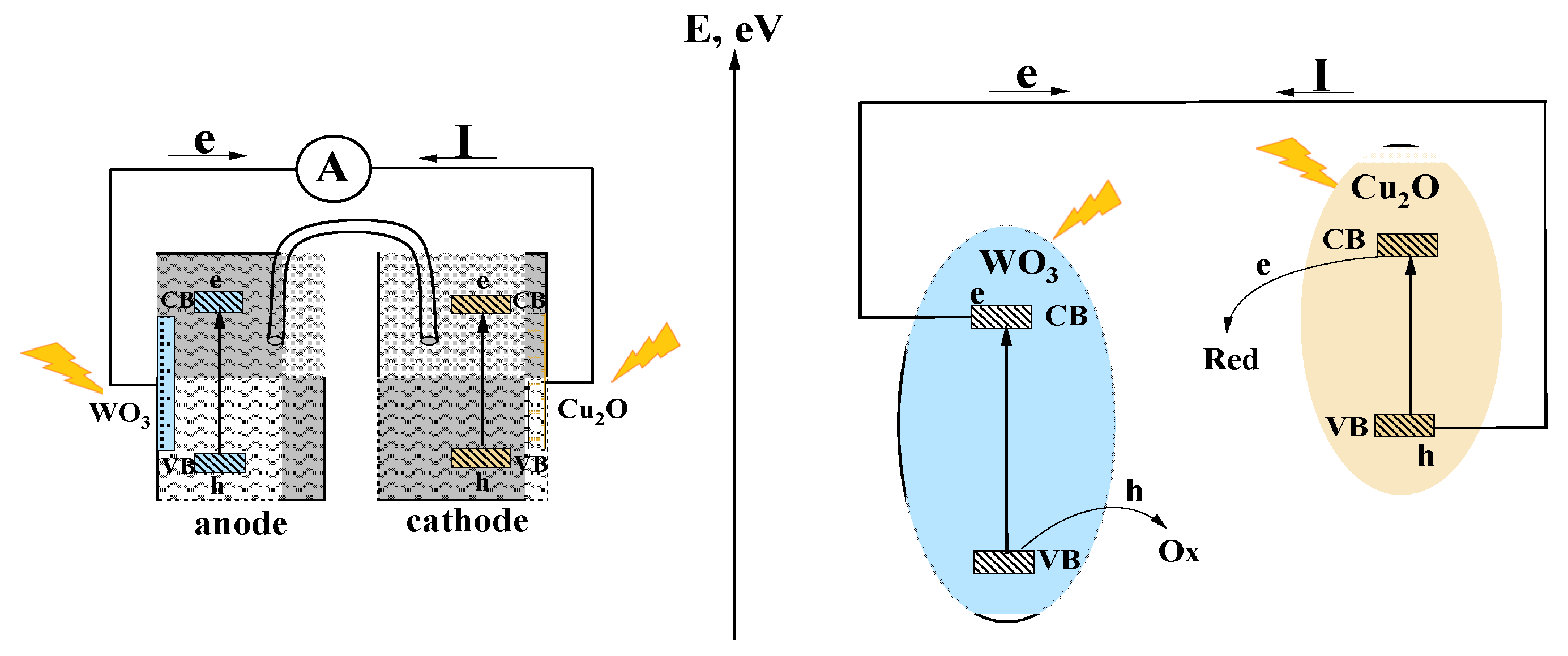
| Electrode | Eg, eV | EVB, eV | ECB, eV | EF, eV | WF, eV |
|---|---|---|---|---|---|
| FTO/WO3 | 2.8 | −7.3 | −4.5 | −4.89 | 5.0 ± 0.1 |
| FTO/Cu2O | 2.4 | −5.9 | −3.5 | −4.83 | 4.8 ± 0.1 |
| FTO/WO3/Cu2O | 2.5 (Cu2O) 2.8 (WO3) | −6.1 | −3.6 | −4.85 | 4.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murashkina, A.A.; Rudakova, A.V.; Bakiev, T.V.; Emeline, A.V.; Bahnemann, D.W. Charge Transfer Mechanism in Type II WO3/Cu2O Heterostructure. Nanomaterials 2024, 14, 2057. https://doi.org/10.3390/nano14242057
Murashkina AA, Rudakova AV, Bakiev TV, Emeline AV, Bahnemann DW. Charge Transfer Mechanism in Type II WO3/Cu2O Heterostructure. Nanomaterials. 2024; 14(24):2057. https://doi.org/10.3390/nano14242057
Chicago/Turabian StyleMurashkina, Anna A., Aida V. Rudakova, Tair V. Bakiev, Alexei V. Emeline, and Detlef W. Bahnemann. 2024. "Charge Transfer Mechanism in Type II WO3/Cu2O Heterostructure" Nanomaterials 14, no. 24: 2057. https://doi.org/10.3390/nano14242057
APA StyleMurashkina, A. A., Rudakova, A. V., Bakiev, T. V., Emeline, A. V., & Bahnemann, D. W. (2024). Charge Transfer Mechanism in Type II WO3/Cu2O Heterostructure. Nanomaterials, 14(24), 2057. https://doi.org/10.3390/nano14242057









