Synthesis and Characterization of Mesoporous Silica Nanoparticles Loaded with P-Cymene against Rice Bacterial Blight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MSNs
2.3. Synthesis of PC@MSNs
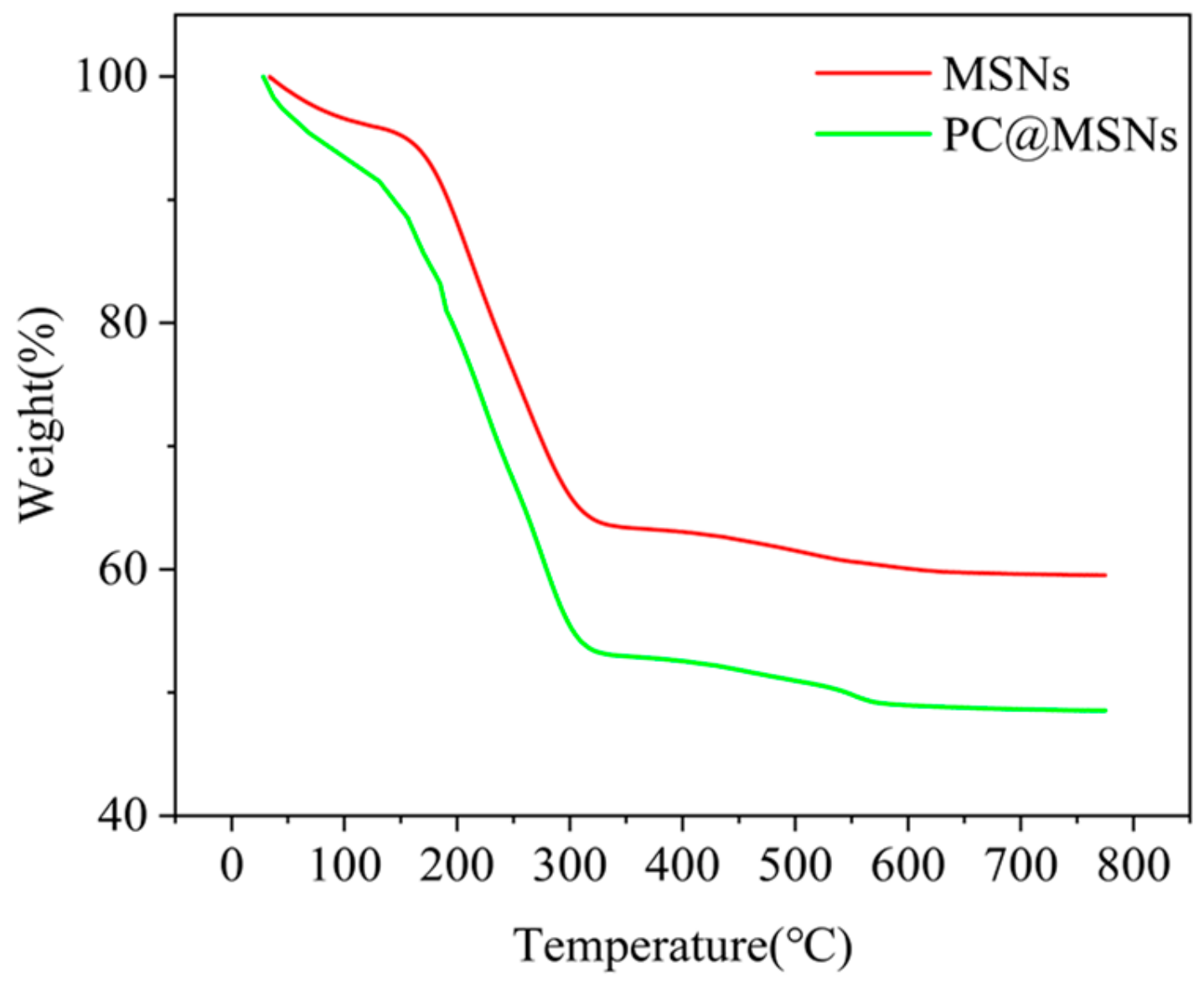
2.4. Characterization
2.5. Microscopic Morphology Observation
2.6. Drug Loading Content of PC@MSNs
2.7. In Vitro Release of PC@MSNs
2.8. In Vitro Bactericidal Activity
2.9. Acute Toxicity of PC and PC@MSNs to Zebrafish
2.10. Safety Evaluation of Rice
3. Results
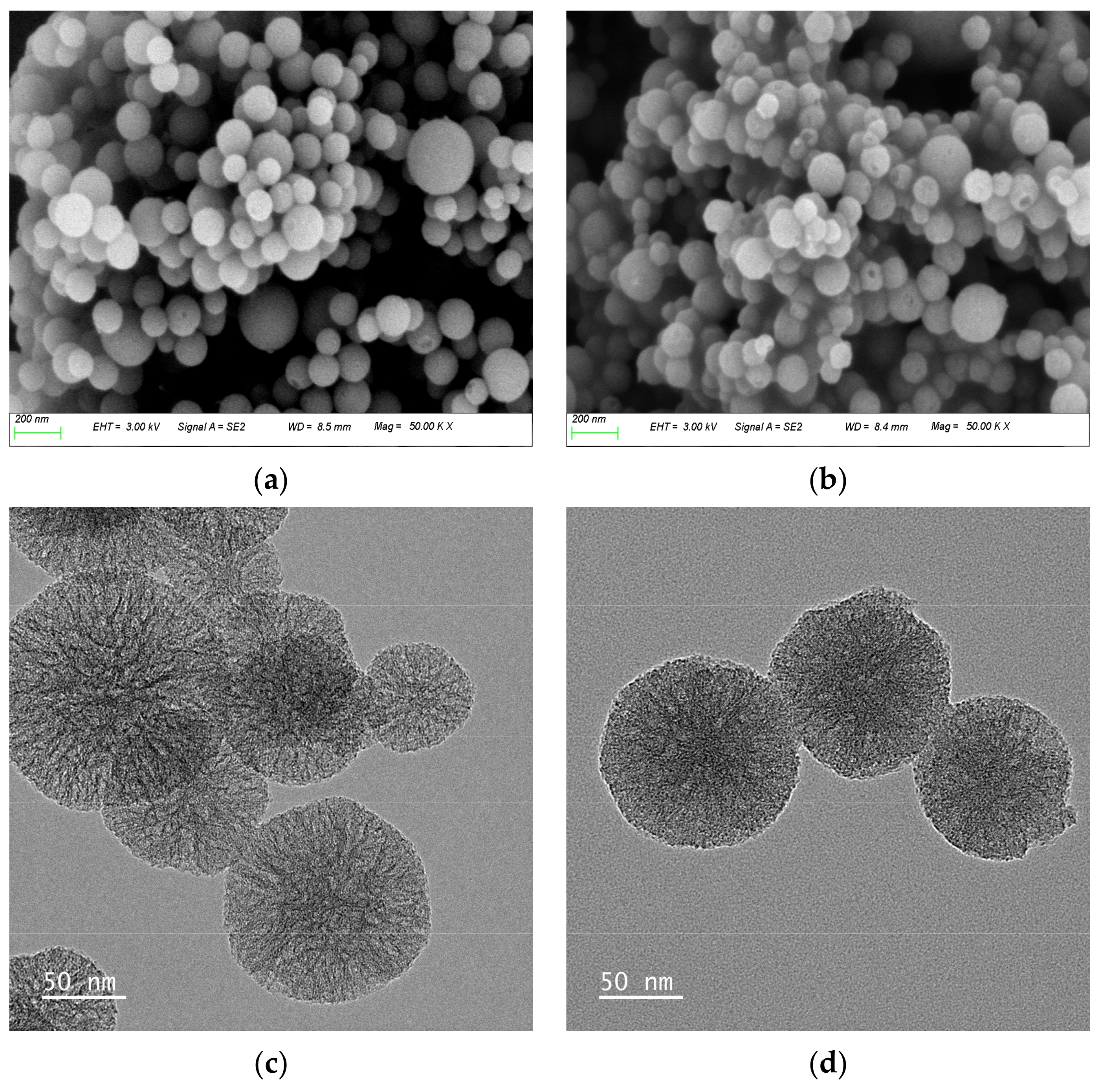
3.1. Morphological Analysis
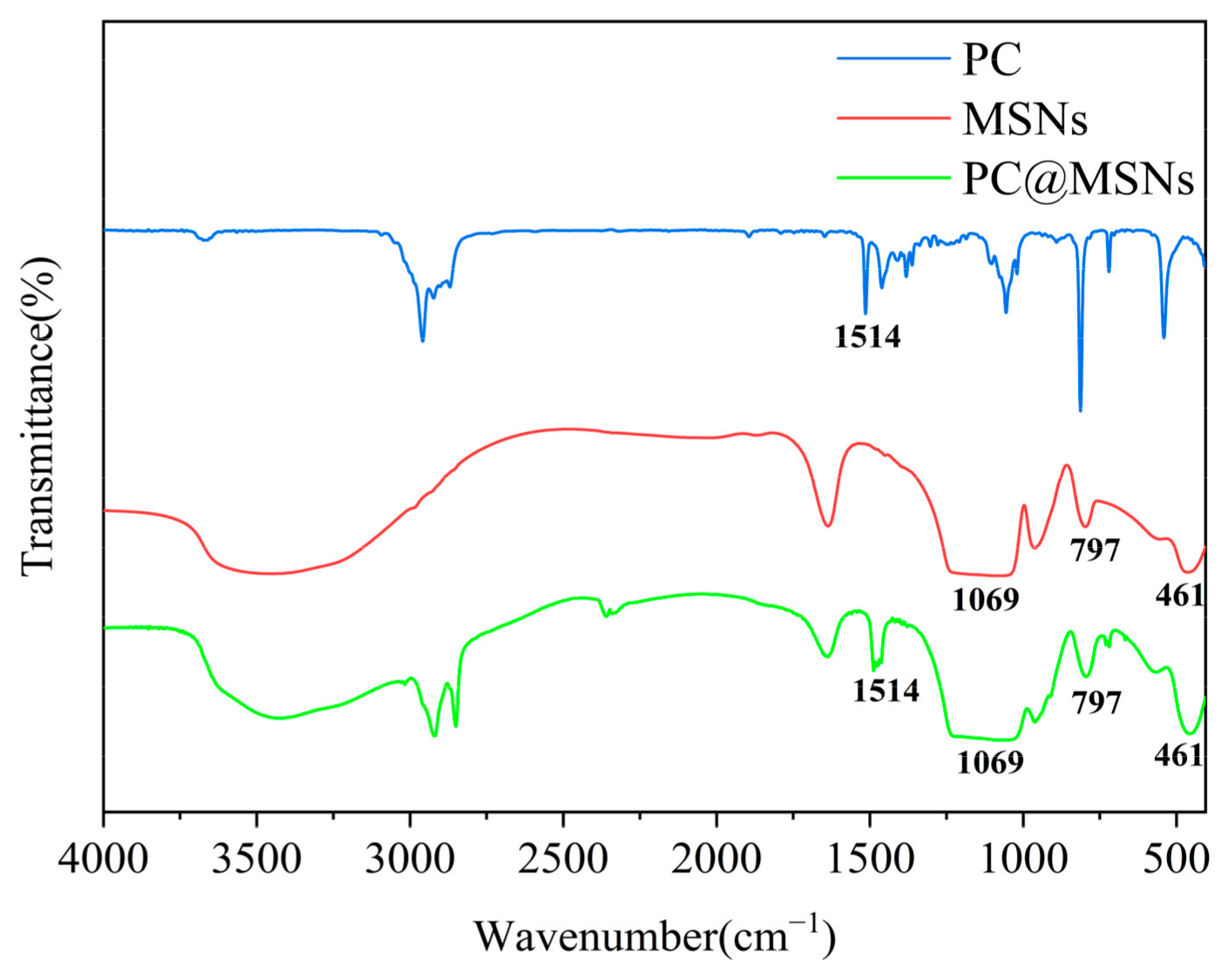
3.2. FTIR Analysis
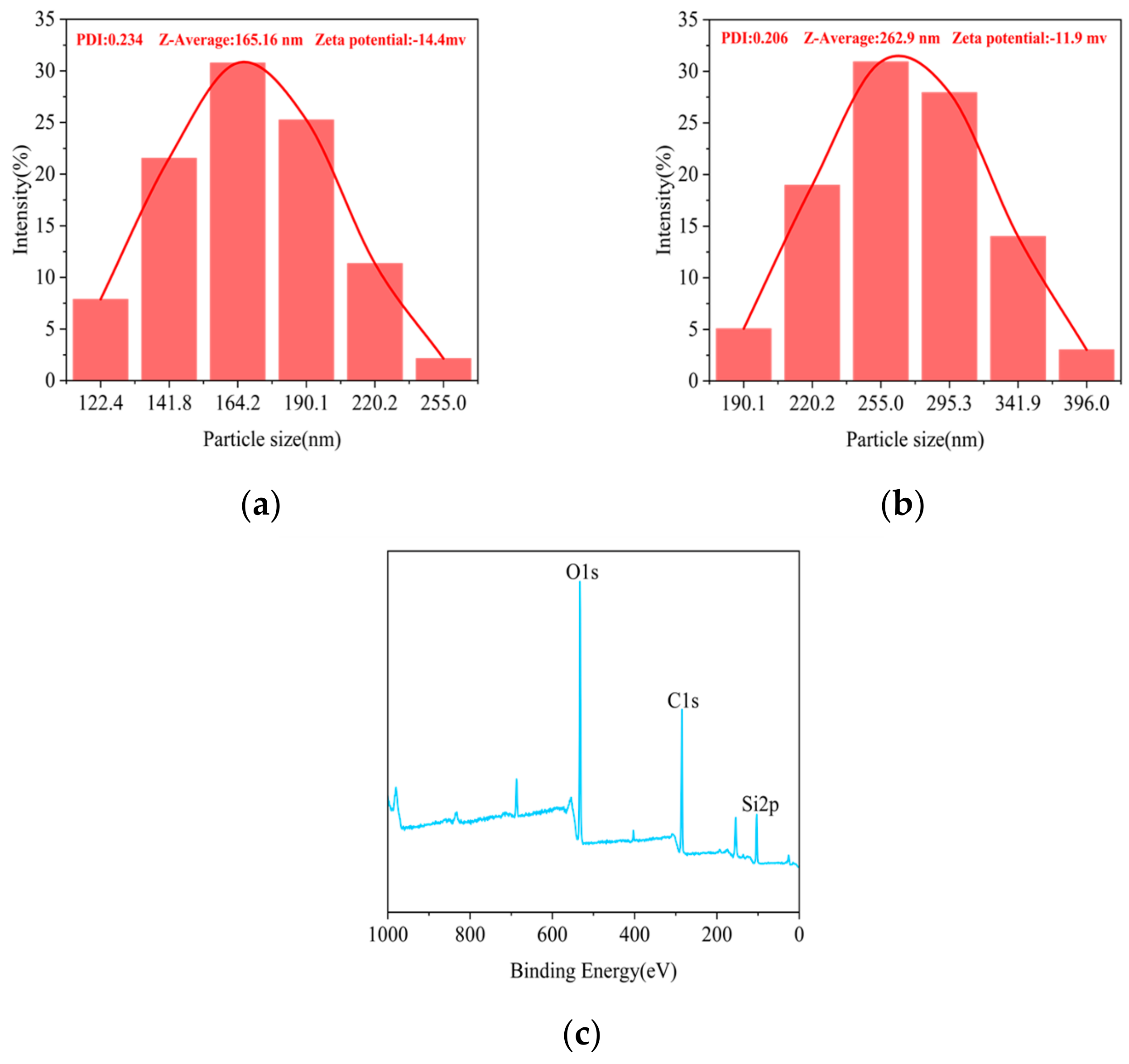
3.3. DLS and XPS Analysis
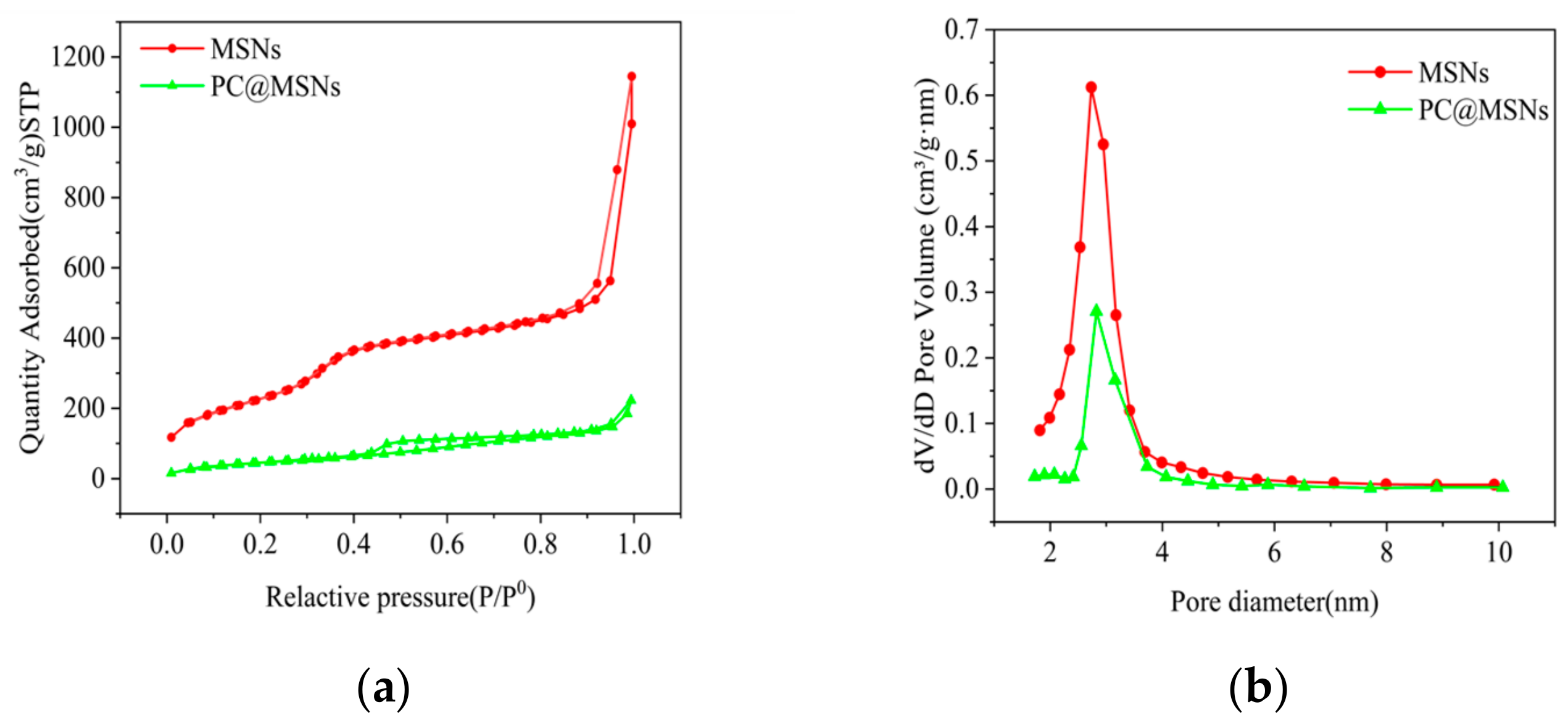
3.4. Specific Surface Area Analysis
3.5. Drug Loading Content of PC@MSNs
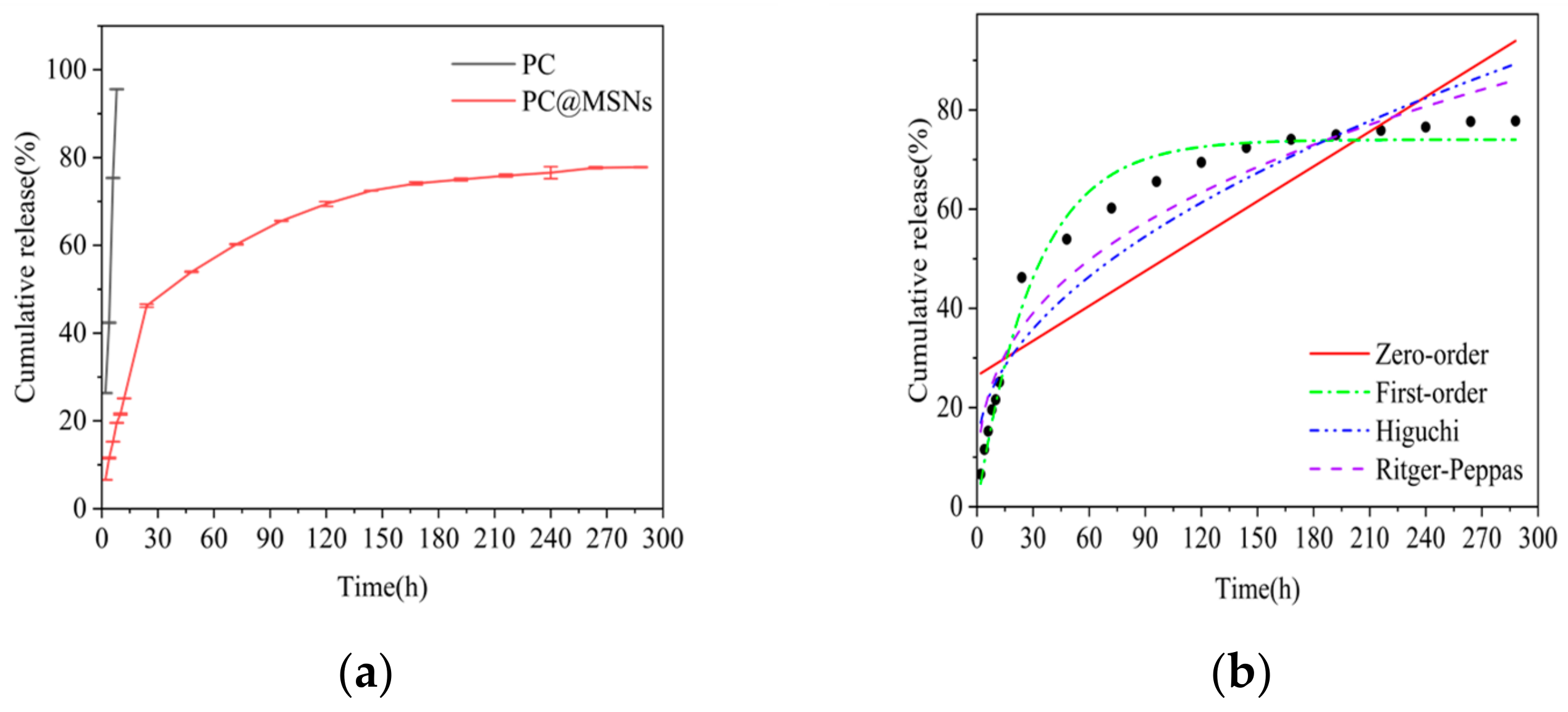
3.6. Release Behavior of PC@MSNs
3.7. Bactericidal Activity of PC@MSNs
3.8. Acute Toxicity to Zebrafish
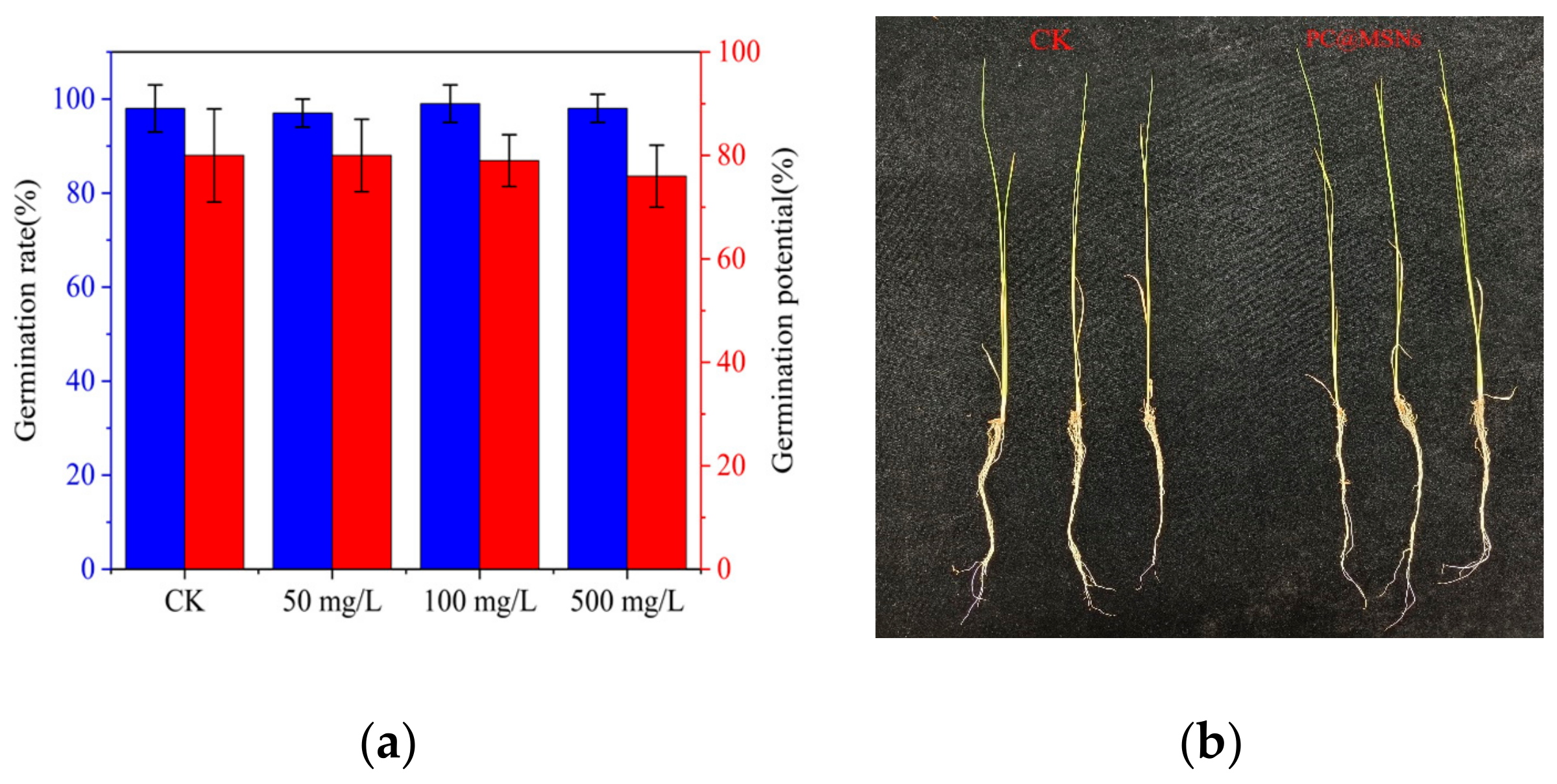
3.9. Plant Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Fraceto, L.F.; Amorim, M.J.B.; Scott-Fordsmand, J.J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J. Hazard. Mater. 2021, 404, 124148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, Y.; Tang, G.; Tian, Y.; Li, Y.; Wang, H.; Li, X.; Yu, X.; Zhang, Z.; Li, Y.; et al. Facile preparation of pH/pectinase responsive microcapsules based on CaCO3 using fungicidal ionic liquid as a nucleating agent for sustainable disease. Chem. Eng. J. 2022, 446, 137073. [Google Scholar] [CrossRef]
- Wang, H.; Tang, G.; Zhou, Z.; Chen, X.; Liu, Y.; Yan, G.; Zhang, X.; Li, X.; Huang, Y.; Wang, J.; et al. Stable Fluorescent Nanoparticles Based on Co-assembly of Acifluorfen and Poly(salicylic acid) for Enhancing Herbicidal Activity and Reducing Environmental Risks. ACS Appl. Mater. Interfaces 2023, 15, 4303–4314. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Yang, J.; Gao, Y.; Zhou, Z.; Tang, J.; Tang, G.; Niu, J.; Chen, X.; Tian, Y.; Li, Y.; Cao, Y. A simple and green preparation process for PRO@PIL-PHS-PEC microcapsules by using phosphonium ionic liquid as a multifunctional additive. Chem. Eng. J. 2021, 424, 130371. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous materials for pesticide formulation and delivery in the agricultural sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Magalhaes, W.L.E.; Rojas, O.J. Controlled release for crop and wood protection: Recent progress toward sustainable and safe nanostructured biocidal systems. J. Control. Release 2017, 262, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yu, N.; Jiang, X.; Han, Z.; Wang, S.; Zhang, X.; Wei, S.; Giesy, J.P.; Yu, H. Influence of blooms of phytoplankton on concentrations of hydrophobic organic chemicals in sediments and snails in a hyper-eutrophic, freshwater lake. Water Res. 2017, 113, 22–31. [Google Scholar] [CrossRef]
- Presentato, A.; Armetta, F.; Spinella, A.; Chillura Martino, D.F.; Alduina, R.; Saladino, M.L. Formulation of Mesoporous Silica Nanoparticles for Controlled Release of Antimicrobials for Stone Preventive Conservation. Front. Chem. 2020, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Gao, Z.D.; Feng, H.J.; Wang, S.Y.; Wang, Q.Y. Cellulose based materials for controlled release formulations of agrochemicals: A review of modifications and applications. J. Control. Release 2019, 316, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development Strategies and Prospects of Nano-based Smart Pesticide Formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. [Google Scholar] [CrossRef] [PubMed]
- Nong, W.; Wu, J.; Ghiladi, R.A.; Guan, Y. The structural appeal of metal-organic frameworks in antimicrobial applications. Coord. Chem. Rev. 2021, 442, 214007. [Google Scholar] [CrossRef]
- Hou, R.; Zhou, J.; Song, Z.; Zhang, N.; Huang, S.; Kaziem, A.E.; Zhao, C.; Zhang, Z. pH-responsive λ-cyhalothrin nanopesticides for effective pest control and reduced toxicity to Harmonia axyridis. Carbohydr. Polym. 2023, 302, 120373. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Chu, X.; Feng, W.; Li, J.; Huang, X.; Zhou, N.; Shen, J. A new temperature-responsive controlled-release pesticide formulation—Poly(N-isopropylacrylamide) modified graphene oxide as the nanocarrier for lambda-cyhalothrin delivery and their application in pesticide transportation. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 612, 125987. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Qin, X.; Guo, Z.; Li, D.; Li, C.; Wan, H.; Zhu, F.; Li, J.; Zhang, Z.; et al. Dual stimuli-responsive fungicide carrier based on hollow mesoporous silica/hydroxypropyl cellulose hybrid nanoparticles. J. Hazard. Mater. 2021, 414, 125513. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patriaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sahu, B.K.; Cao, L.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.; Shanmugam, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog. Mater. Sci. 2021, 121, 100812. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Yan, S.; Gu, N.; Peng, M.; Jiang, Q.; Liu, E.; Li, Z.; Yin, M.; Shen, J.; Du, X.; Dong, M. A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials 2022, 12, 2419. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, X.; Shangguan, S.; Zhao, L.; Fang, X.; Huang, Y.; Hermanowicz, S.W. Public Perceptions and Willingness-to-Pay for Nanopesticides. Nanomaterials 2022, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wen, H.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. Coordination bonding-based polydopamine-modified mesoporous silica for sustained avermectin release. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110073. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chem. Eng. J. 2020, 395, 125093. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Yang, L.P.; Lin, Y.G.; Song, Z.X.; Xu, H.H.; Zhang, Z.X. Efficiency of mesoporous silica/carboxymethyl β-glucan as a fungicide nano-delivery system for improving chlorothalonil bioactivity and reduce biotoxicity. Chemosphere 2022, 287, 131902. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized Chitosan-Capped Mesoporous Silica Nanoparticles as Nanocarriers for Controlled Pesticide Release. Nanomaterials 2016, 6, 126. [Google Scholar] [CrossRef]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery—Current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, X.; Cao, L.; Cao, C.; Li, F.; Chen, C.; Xu, C.; Huang, Q.; Du, F. Uptake and Distribution of Fenoxanil-Loaded Mesoporous Silica Nanoparticles in Rice Plants. Int. J. Mol. Sci. 2018, 19, 2854. [Google Scholar] [CrossRef]
- Wang, Y.; Dimkpa, C.; Deng, C.; Elmer, W.H.; Gardea-Torresdey, J.; White, J.C. Impact of engineered nanomaterials on rice (Oryza sativa L.): A critical review of current knowledge. Environ. Pollut. 2022, 297, 118738. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ming, H.; Shanshan, C.; Anqun, H.; Shimao, L.; Haiya, H.; Guangtao, L.; Lisha, Z.; Jianuan, Z. Enterobacter asburiae and Pantoea ananatis causing rice bacterial blight in China. Plant Dis. 2021, 105, 2078–2088. [Google Scholar] [CrossRef]
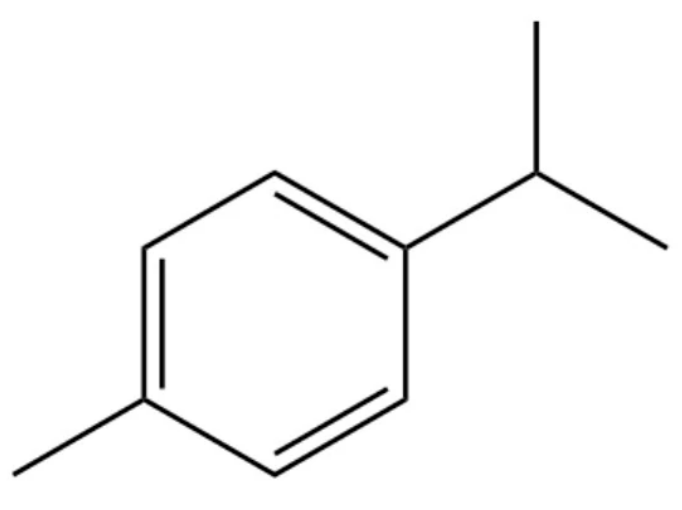
- Agulló, L.; Romero-Silva, M.J.; Domenech, M.; Seeger, M. p-Cymene Promotes Its Catabolism through the p-Cymene and the p-Cumate Pathways, Activates a Stress Response and Reduces the Biofilm Formation in Burkholderia xenovorans LB400. PLoS ONE 2017, 12, e0169544. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Sparling, M.; Dabeka, R. p-Cymene, a natural antioxidant, in Canadian total diet foods: Occurrence and dietary exposures. J. Sci. Food Agric. 2019, 99, 5606–5609. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, H.; Zhou, Z.; Xu, C.; Shan, Y.; Lin, Y.; Huang, Q. Fluorophore-free luminescent double-shelled hollow mesoporous silica nanoparticles as pesticide delivery vehicles. Nanoscale 2018, 10, 20354–20365. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.-T.; Chang, X.-L. Bioaccumulation and Toxicity of 13C-Skeleton Labeled Graphene Oxide in Wheat. Environ. Sci. Technol. 2017, 51, 10146–10153. [Google Scholar] [CrossRef]
- Wang, A.; Cui, J.; Wang, Y.; Zhu, H.; Li, N.; Wang, C.; Shen, Y.; Liu, P.; Cui, B.; Sun, C.; et al. Preparation and characterization of a novel controlled-release nano-delivery system loaded with pyraclostrobin via high-pressure homogenization. Pest Manag. Sci. 2020, 76, 2829–2837. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.; Mao, K.; Qin, X.; Zhang, Y.; Li, D.; Zhang, Y.; Li, J.; Wan, H.; He, S. Thermoresponsive polymer-encapsulated hollow mesoporous silica nanoparticles and their application in insecticide delivery. Chem. Eng. J. 2020, 383, 123169. [Google Scholar] [CrossRef]
- Ding, G.; Li, D.; Liu, Y.; Guo, M.; Duan, Y.; Li, J.; Cao, Y. Preparation and characterization of kasuga-silica-conjugated nanospheres for sustained antimicrobial activity. J. Nanoparticle Res. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Qian, K.; Shi, T.; He, S.; Luo, L.; Liu, X.; Cao, Y. Release kinetics of tebuconazole from porous hollow silica nanospheres prepared by miniemulsion method. Microporous Mesoporous Mater. 2013, 169, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, L.; Li, W.; Yang, C. Dual Functional N-Doped TiO2-Carbon Composite Fibers for Efficient Removal of Water Pollutants. ACS Sustain. Chem. Eng. 2018, 6, 12893–12905. [Google Scholar] [CrossRef]
- Zhang, W.; He, S.; Liu, Y.; Geng, Q.; Ding, G.; Guo, M.; Deng, Y.; Zhu, J.; Li, J.; Cao, Y. Preparation and Characterization of Novel Functionalized Prochloraz Microcapsules Using Silica-Alginate-Elements as Controlled Release Carrier Materials. ACS Appl. Mater. Interfaces 2014, 6, 11783–11790. [Google Scholar] [CrossRef]

| MSNs | PC@MSNs | |
|---|---|---|
| Specific Surface Area (m2/g) | 926.0 | 176.6 |
| Pore Volume (cm3/g) | 1.7 | 0.3 |
| Average Pore Diameter (nm) | 8.4 | 7.8 |
| Fitting Method | Equation | R2 |
|---|---|---|
| Zero-Order Fitting | Q = 25.45066 + 0.2344t | 0.75217 |
| First-Order Fitting | Q = 74.04867(1 − e0.03261t) | 0.9824 |
| Higuchi Fitting | Q = 10.40539 + 4.64955t1/2 | 0.90414 |
| Ritger–Peppas Fitting | Q = 11.91204t0.34917 | 0.93925 |
| Treatment | Equation | R2 | EC50 (mg/L) | 95% Confidence Interval |
|---|---|---|---|---|
| TC@SC | Y = −8.96 + 3.38x | 0.983 | 451.482 | 415.59–497.913 |
| PC | Y = −1.01 + 2.01x | 0.996 | 3.178 | 2.809–3.599 |
| PC@MSNs | Y = 1.89 + 3.01x | 0.989 | 4.223 | 3.863–4.667 |
| Treatment Time (h) | LC50 (95% Confidence Interval) (mg/L) | |
|---|---|---|
| PC | PC@MSNs | |
| 24 | 16.443(16.179–16.675) | 259.511(256.984–264.874) |
| 48 | 15.992(15.769–16.254) | 258.412(256.367–262.952) |
| 72 | 15.624(15.422–15.922) | 258.023(255.066–261.876) |
| 96 | 15.165(14.986–15.533) | 257.867(256.986–258.453) |
| Treatment | Fresh Weight (mg) | Stem Length (cm) | Root Length (cm) |
|---|---|---|---|
| CK | 330 ± 0.4 | 30 ± 1.0 | 17.8 ± 0.6 |
| PC@MSNs | 321 ± 0.3 | 29.3 ± 0.7 | 17.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Mo, Y.; Jiao, L.; Liu, Y.; Li, X. Synthesis and Characterization of Mesoporous Silica Nanoparticles Loaded with P-Cymene against Rice Bacterial Blight. Nanomaterials 2024, 14, 250. https://doi.org/10.3390/nano14030250
Li C, Mo Y, Jiao L, Liu Y, Li X. Synthesis and Characterization of Mesoporous Silica Nanoparticles Loaded with P-Cymene against Rice Bacterial Blight. Nanomaterials. 2024; 14(3):250. https://doi.org/10.3390/nano14030250
Chicago/Turabian StyleLi, Chaonan, Yalan Mo, Luying Jiao, Yiping Liu, and Xiaogang Li. 2024. "Synthesis and Characterization of Mesoporous Silica Nanoparticles Loaded with P-Cymene against Rice Bacterial Blight" Nanomaterials 14, no. 3: 250. https://doi.org/10.3390/nano14030250





