Three-Dimensional Modeling with Osteoblast-like Cells under External Magnetic Field Conditions Using Magnetic Nano-Ferrite Particles for the Development of Cell-Derived Artificial Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Sterilization of NFPs
2.2. Preparation of Highly Dispersed NFPs
2.3. Cell Culture
2.4. Examination of Cell Proliferation
2.5. Evaluation of NFP Uptake
2.6. Subcellular Localization of NFP-MC
2.7. Assessment of the Effects of Calcification on NFP-MC
2.8. Evaluation of the Uptake of NFPs under External Magnetic Field Conditions on MC3T3-E1 Cells Movement
2.9. Evaluation of the 3D Modeling of NFP-MC under Magnetic Field Conditions
2.10. Statistical Analysis
3. Results
3.1. Properties of NFPs
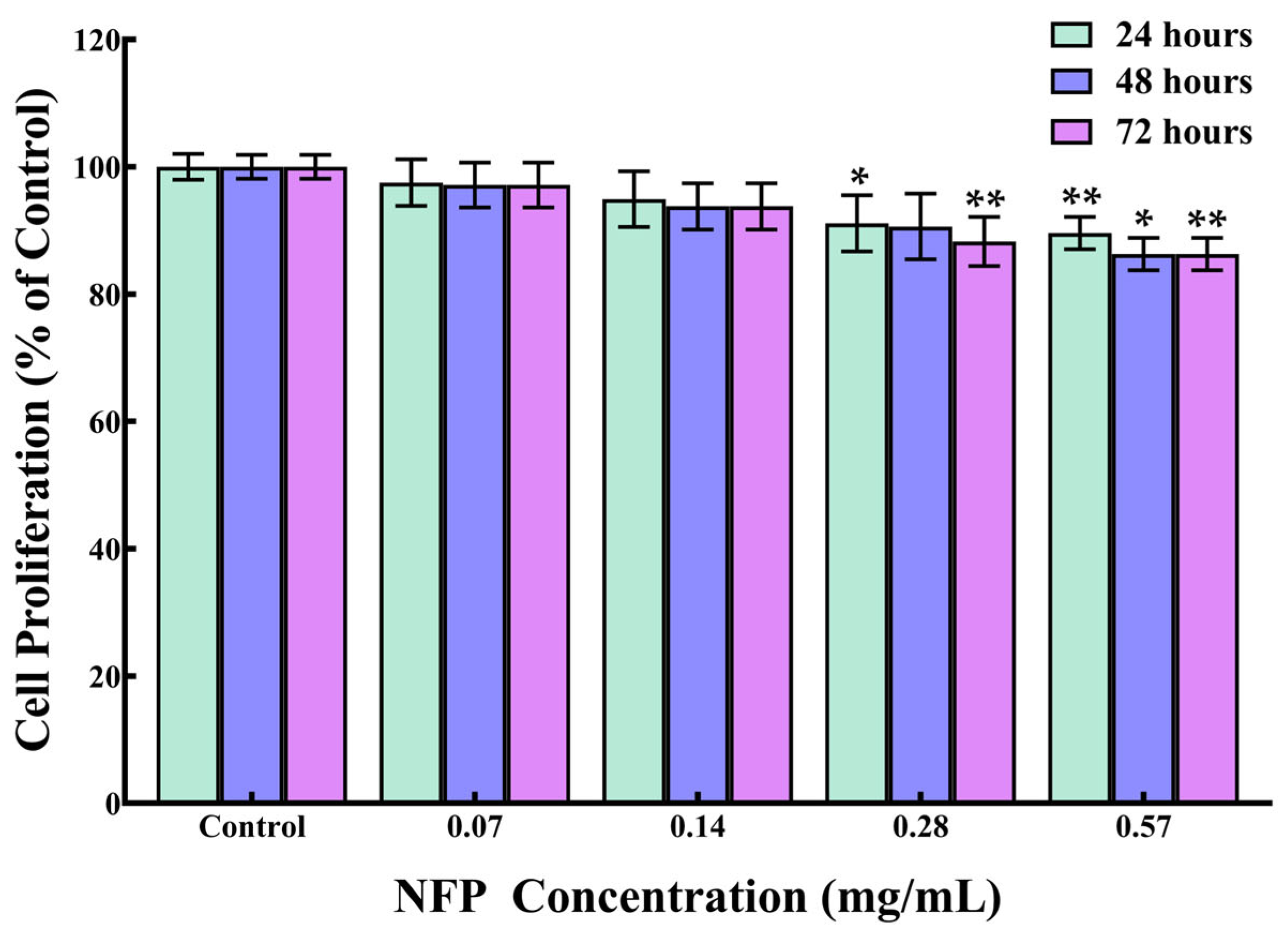
3.2. Effect of NFPs on the Cell Proliferation of MC3T3-E1 Cells
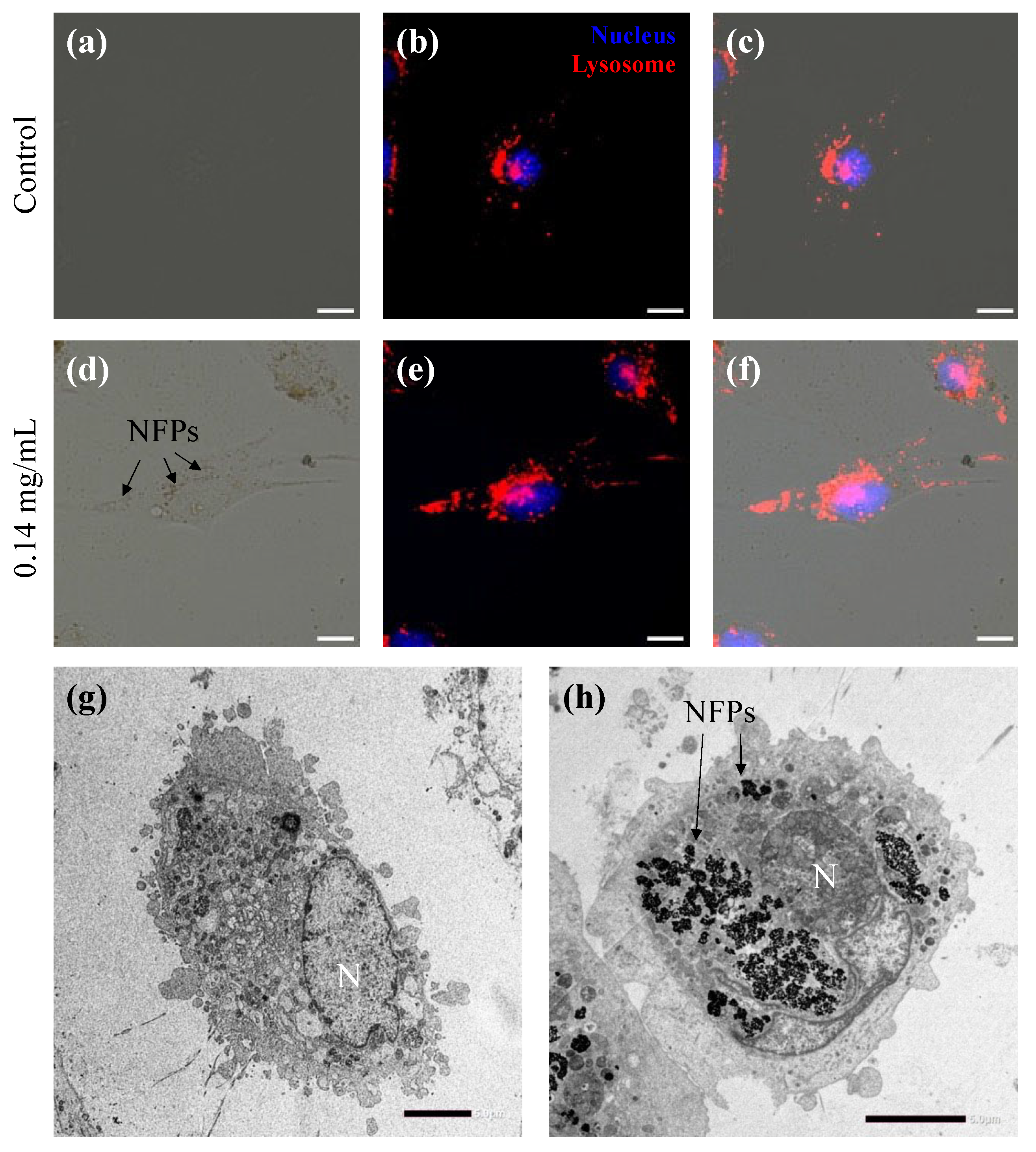
3.3. Intracellular Uptake of NFPs in MC3T3-E1 Cells
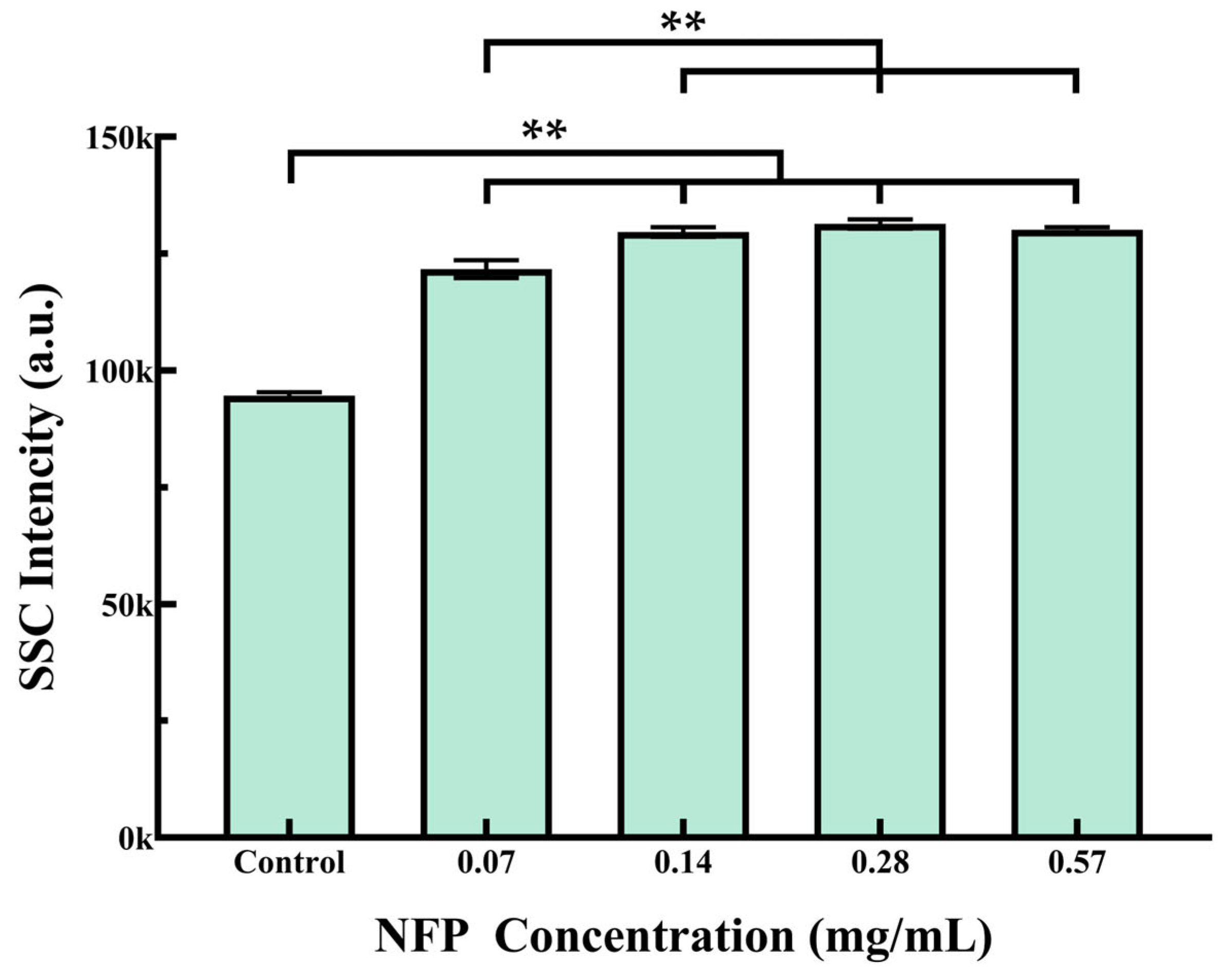
3.4. Flow Cytometry Analysis of NFP Uptake in MC3T3-E1 Cells
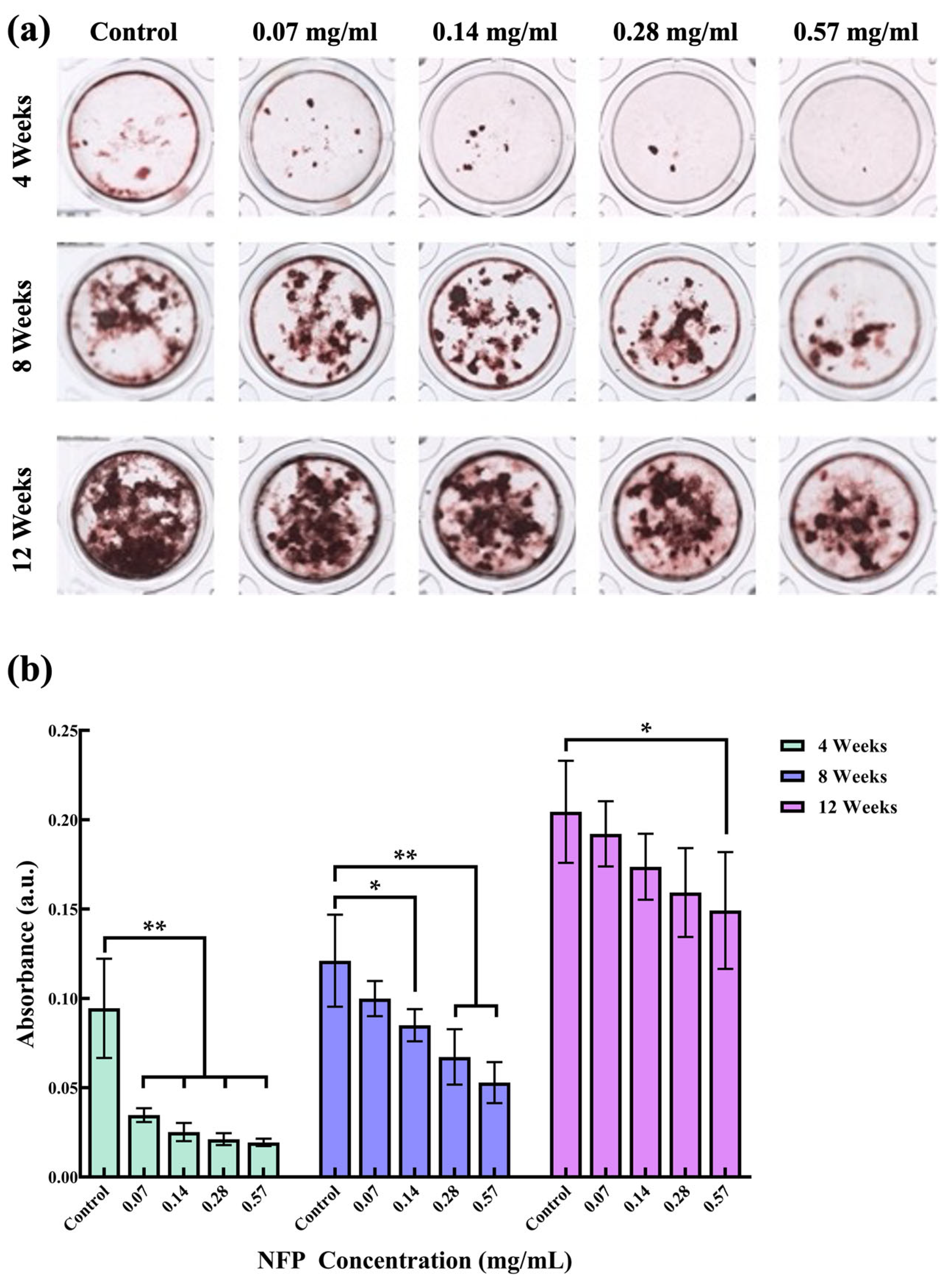
3.5. Effect of NFPs on MC3T3-E1 Cells under Calcified Conditions
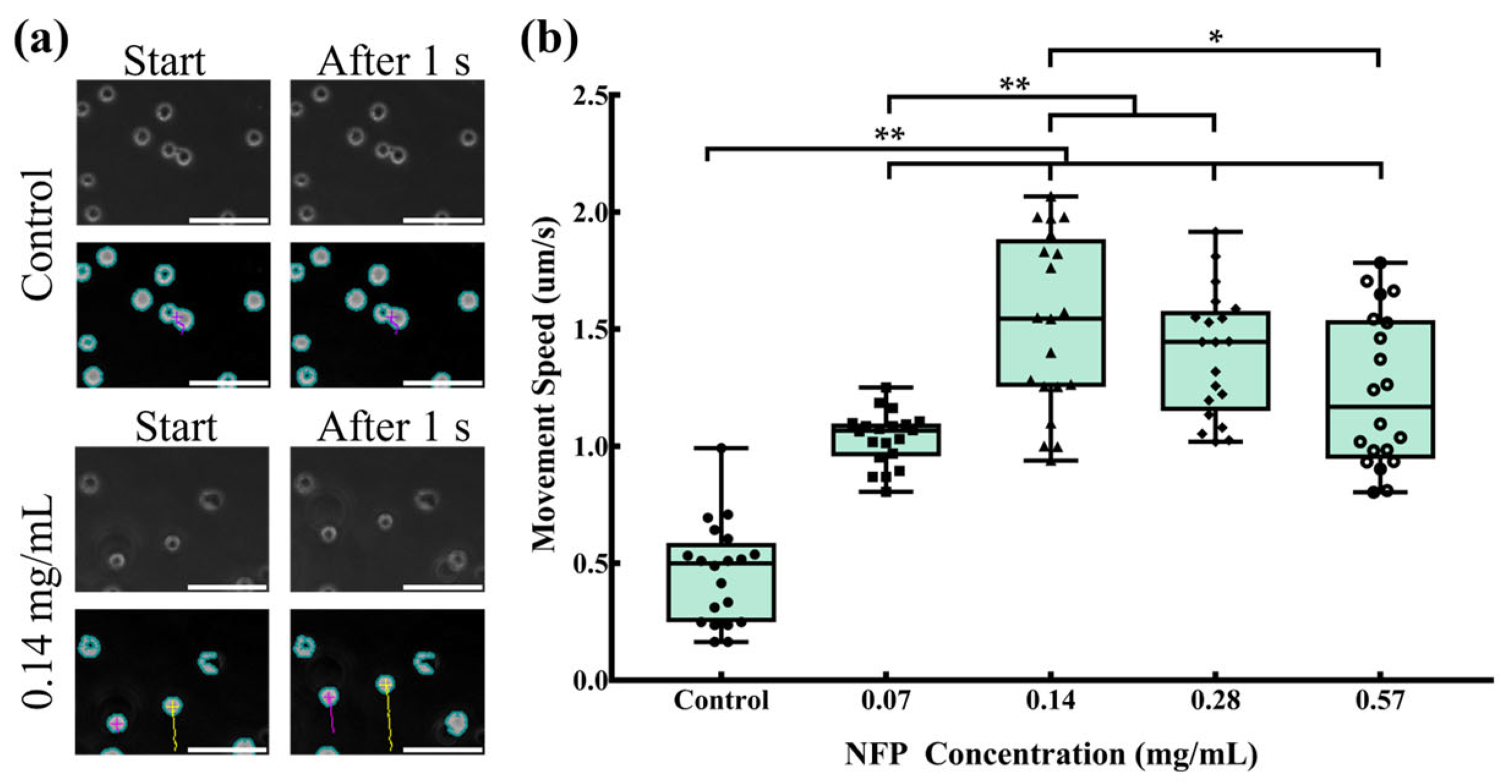
3.6. Evaluation of Cell Movement in NFP-MC under a Magnetic Field
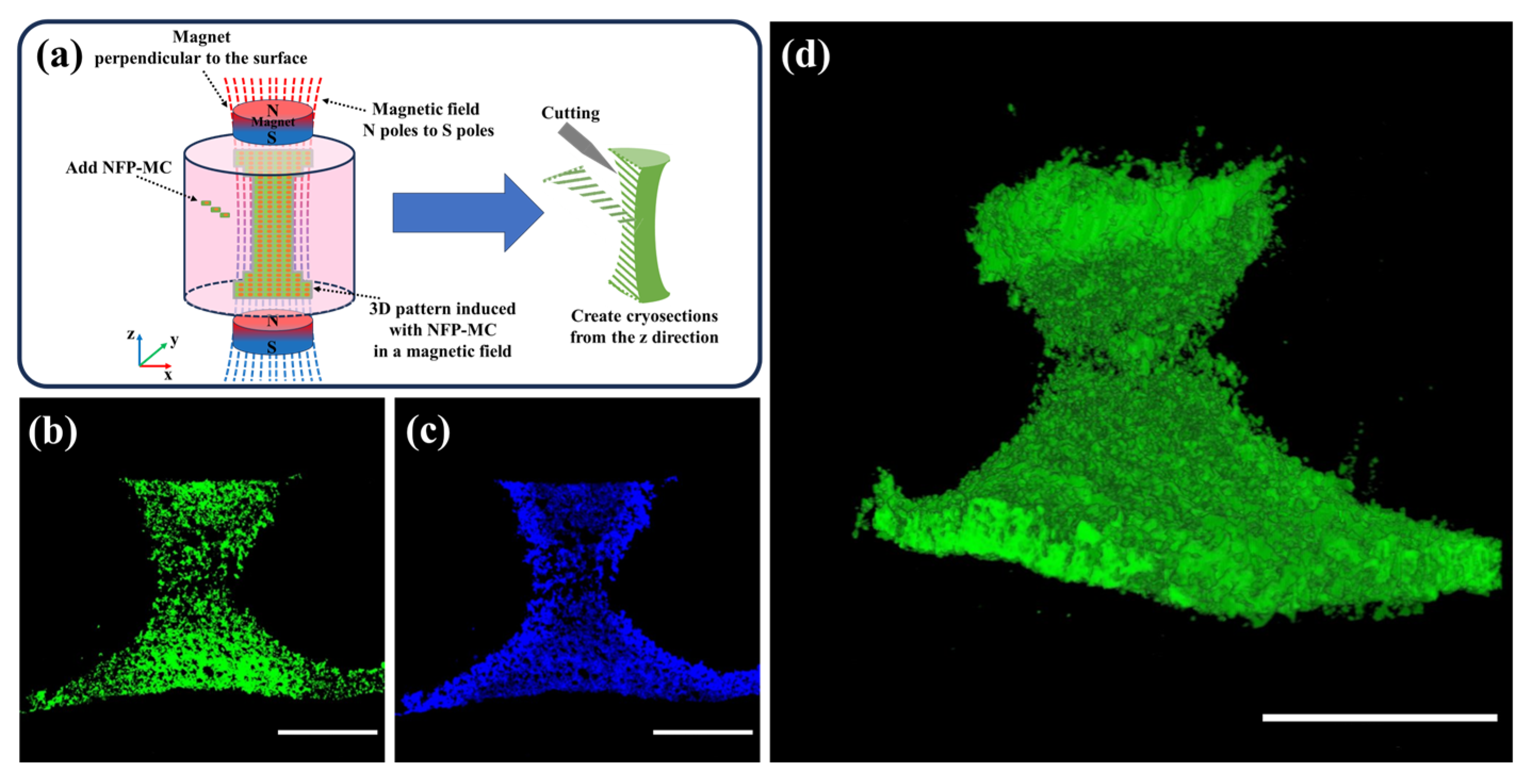
3.7. Evaluation of the 3D Modeling of NFP-MC under a Magnetic Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.; Mazzoni, E.; Bononi, I.; Rotondo, J.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Haniu, H.; Takeuchi, A.; Ueda, K.; Sano, M.; Tanaka, M.; Takizawa, T.; Sobajima, A.; Kamanaka, T.; Saito, N. In Vitro and In Vivo Evaluation of Starfish Bone-Derived β-Tricalcium Phosphate as a Bone Substitute Material. Materials 2019, 12, 1881. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Han, Y.; Sun, H.; Shi, J. Click chemistry-based biopolymeric hydrogels for regenerative medicine. Biomed. Mater. 2021, 16, 022003. [Google Scholar] [CrossRef] [PubMed]
- Gavinho, S.R.; Pádua, A.S.; Holz LI, V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Oh, J. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Kheradmandi, R.; Salehi, M.; Alizadeh, M.; Hagen, T.; Falahati, M. Criteria, Challenges, and Opportunities for Acellularized Allogeneic/Xenogeneic Bone Grafts in Bone Repairing. ACS Biomater. Sci. Eng. 2022, 8, 3199–3219. [Google Scholar] [CrossRef]
- Zhang, Y.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.; Lee, J.; Lutolf, M.; McAlpine, M.; Yeong, W.; Zenobi-Wong, M.; Malda, J. 3D extrusion bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, S.; Schröder, H.; Al-Nawas, B.; Wang, X.; Mueller, W. 3D bioprinting of tissue units with mesenchymal stem cells, retaining their proliferative and differentiating potential, in polyphosphate-containing bio-ink. Biofabrication 2022, 14, 015016. [Google Scholar] [CrossRef]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Labusca, L.; Herea, D.; Danceanu, C.; Minuti, A.; Stavila, C.; Grigoras, M.; Gherca, D.; Stoian, G.; Ababei, G.; Chiriac, H.; et al. The effect of magnetic field exposure on differentiation of magnetite nanoparticle-loaded adipose-derived stem cells. Mater. Sci. Eng. C 2020, 109, 110652. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.; Phull, A.; Ali, J.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Gudoshnikov, S.; Liubimov, B.; Matveets, L.; Ranchinski, M.; Usov, N.; Gubin, S.; Yurkov, G.; Snigirev, O.; Volkov, I. Magnetic properties of Fe-based nanoparticle assembly. J. Magn. Magn. Mater. 2003, 258-259, 54–56. [Google Scholar] [CrossRef]
- Angeloni, L.; Passeri, D.; Reggente, M.; Mantovani, D.; Rossi, M. Removal of electrostatic artifacts in magnetic force microscopy by controlled magnetization of the tip: Application to superparamagnetic nanoparticles. Sci. Rep. 2016, 6, 26293. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Guo, Z.; Zhang, G.; Zhang, H. Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling. Cells 2022, 11, 3298. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, J.; Long, Y.; Xie, Y.; Nie, J.; Chen, L. Magnetic Materials in Promoting Bone Regeneration. Front. Mater. 2019, 6, 268. [Google Scholar] [CrossRef]
- Na, H.; Song, I.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Huang, J.; Song, L.; Chen, Z.; Liu, H.; Li, Y.; Zhang, Y.; Gu, N. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci. Rep. 2014, 4, 5125. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Leesungbok, R.; Lee, S.; Lee, H.; Park, S.; Mah, S.; Ahn, S. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics 2015, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, M.; Haniu, M.; Ueda, K.; Ishida, H.; Ma, C.; Ideta, H.; Sobajima, A.; Ueshiba, K.; Uemura, T.; Saito, N.; et al. Evaluation of MC3T3-E1 Cell Osteogenesis in Different Cell Culture Media. Int. J. Mol. Sci. 2021, 22, 7752. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Izumiya, M.; Haniu, H.; Ueda, K.; Ma, C.; Ueshiba, K.; Ideta, H.; Sobajima, A.; Uchiyama, S.; Takahashi, J.; et al. Current Methods in the Study of Nanomaterials for Bone Regeneration. Nanomaterials 2022, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Ma, C.; Izumiya, M.; Kuroda, C.; Ishida, H.; Uemura, T.; Saito, N.; Aoki, K.; Haniu, H. Biocompatibility Evaluation of Carbon Nanohorns in Bone Tissues. Nanomaterials 2023, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Nelissen, I.; Haase, A.; Åberg, C.; Moya, S.; Jacobs, A.; Alnasser, F.; Bewersdorff, T.; Deville, S.; Luch, A.; et al. Quantitative measurement of nanoparticle uptake by flow cytometry illustrated by an interlaboratory comparison of the uptake of labelled polystyrene nanoparticles. NanoImpact 2018, 9, 42–50. [Google Scholar] [CrossRef]
- Bartucci, R.; Åberg, C.; Melgert, B.; Boersma, Y.; Olinga, P.; Salvati, A. Time-Resolved Quantification of Nanoparticle Uptake, Distribution, and Impact in Precision-Cut Liver Slices. Small 2020, 16, 1906523. [Google Scholar] [CrossRef]
- Shin, H.; Kwak, M.; Joo, S.; Lee, J. Quantifying fluorescent nanoparticle uptake in mammalian cells using a plate reader. Sci. Rep. 2022, 12, 20146. [Google Scholar] [CrossRef]
- Entezari, A.; Swain, M.; Gooding, J.; Roohani, I.; Li, Q. A modular design strategy to integrate mechanotransduction concepts in scaffold-based bone tissue engineering. Acta Biomater. 2020, 118, 100–112. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.; Rowe, D.; Wei, M. Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef] [PubMed]
- Landázuri, N.; Tong, S.; Suo, J.; Joseph, G.; Weiss, D.; Sutcliffe, D.; Giddens, D.; Bao, G.; Taylor, W. Magnetic Targeting of Human Mesenchymal Stem Cells with Internalized Superparamagnetic Iron Oxide Nanoparticles. Small 2013, 9, 4017–4026. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5. Tests for In Vitro Cytotoxicity. ISO (International Organization for Standardization): Geneva, Switzerland, 2009.
- Rennick, J.; Johnston, A.; Parton, R. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, Q.; Li, X.; Zhou, X.; Zang, J.; Xue, W.; Xiang, J.; Guo, C. Biocompatibility of magnetic Fe3O4 nanoparticles and their cytotoxic effect on MCF-7 cells. Int. J. Nanomed. 2012, 7, 4973–4982. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Wilhelm, C.; Clément, O.; Gazeau, F. Cell labeling with magnetic nanoparticles: Opportunity for magnetic cell imaging and cell manipulation. J. Nanobiotechnol. 2013, 11, S7. [Google Scholar] [CrossRef] [PubMed]
- Haniu, H.; Saito, N.; Matsuda, Y.; Kim, Y.; Park, K.; Tsukahara, T.; Usui, Y.; Aoki, K.; Shimizu, M.; Ogihara, N.; et al. Effect of dispersants of multi-walled carbon nanotubes on cellular uptake and biological responses. Int. J. Nanomed. 2011, 6, 3295–3307. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Su, X.; Liang, J.; Yang, L.; Hu, Q.; Shan, X.; Wan, J.; Hu, Z. Synthesis of polymer-functionalized nanoscale graphene oxide with different surface charge and its cellular uptake, biosafety and immune responses in Raw264.7 macrophages. Mater. Sci. Eng. C 2018, 90, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef]
- Pauluhn, J. Subchronic inhalation toxicity of iron oxide (magnetite, Fe3O4) in rats: Pulmonary toxicity is determined by the particle kinetics typical of poorly soluble particles. J. Appl. Toxicol. 2012, 32, 488–504. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Lee, W.; Nelson, K.; Yoon, J.; Sedlak, D. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef]
- Preger, C.; Josefsson, M.; Westerström, R.; Messing, M.E. Bottom-up field-directed self-assembly of magnetic nanoparticles into ordered nano- and macrostructures. Nanotechnology 2021, 32, 195603. [Google Scholar] [CrossRef] [PubMed]
- Han, K. Electric and Magnetic Field-Driven Dynamic Structuring for Smart Functional Devices. Micromachines 2023, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Giżynski, K.; Lee, T.; Grzybowski, B.A. Dynamic Self-Assembly of Magnetic/Polymer Composites in Rotating Frames of Reference. Adv. Mater. 2017, 29, 1700614. [Google Scholar] [CrossRef] [PubMed]
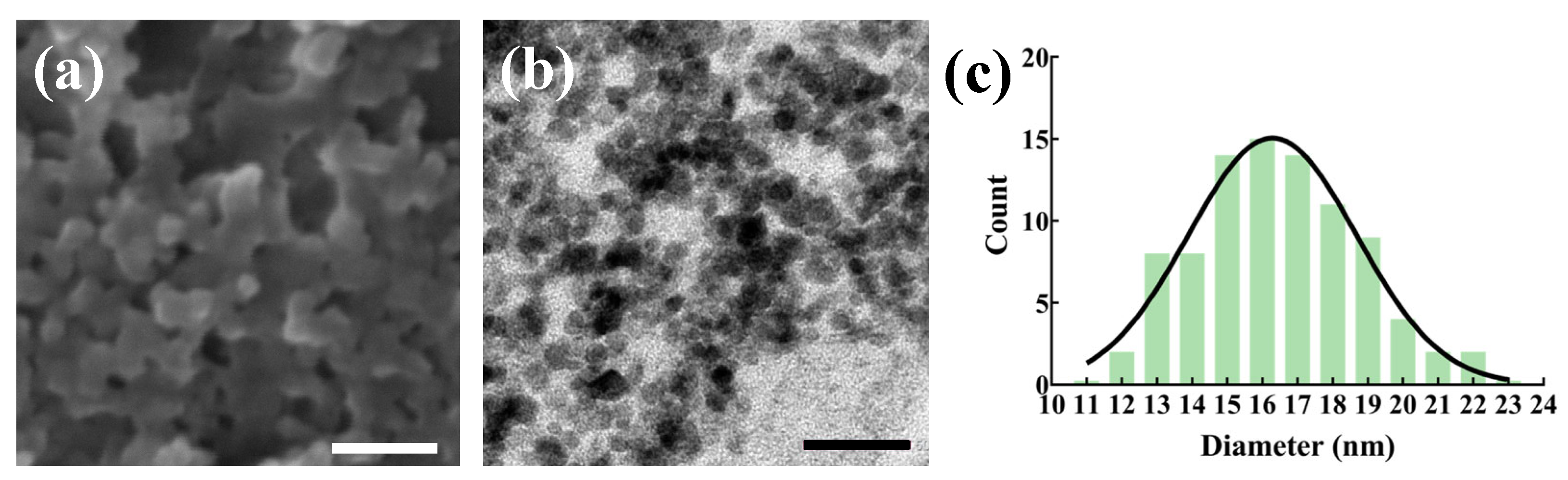
| Materials | TEM-Observed Particle Size (nm) | Rheological Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| NFPs | 16.5 ± 2.4 | 164.7 ± 54.0 | −9.9 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Izumiya, M.; Nobuoka, H.; Ueno, R.; Mimura, M.; Ueda, K.; Ishida, H.; Tomotsune, D.; Johkura, K.; Yue, F.; et al. Three-Dimensional Modeling with Osteoblast-like Cells under External Magnetic Field Conditions Using Magnetic Nano-Ferrite Particles for the Development of Cell-Derived Artificial Bone. Nanomaterials 2024, 14, 251. https://doi.org/10.3390/nano14030251
Ma C, Izumiya M, Nobuoka H, Ueno R, Mimura M, Ueda K, Ishida H, Tomotsune D, Johkura K, Yue F, et al. Three-Dimensional Modeling with Osteoblast-like Cells under External Magnetic Field Conditions Using Magnetic Nano-Ferrite Particles for the Development of Cell-Derived Artificial Bone. Nanomaterials. 2024; 14(3):251. https://doi.org/10.3390/nano14030251
Chicago/Turabian StyleMa, Chuang, Makoto Izumiya, Hidehiko Nobuoka, Rintaro Ueno, Masaki Mimura, Katsuya Ueda, Haruka Ishida, Daihachiro Tomotsune, Kohei Johkura, Fengming Yue, and et al. 2024. "Three-Dimensional Modeling with Osteoblast-like Cells under External Magnetic Field Conditions Using Magnetic Nano-Ferrite Particles for the Development of Cell-Derived Artificial Bone" Nanomaterials 14, no. 3: 251. https://doi.org/10.3390/nano14030251







