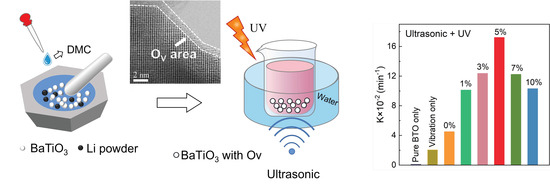
Boosting Piezocatalytic Performance of BaTiO3 by Tuning Defects at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization
2.3. Piezocatalytic Activity Experiments
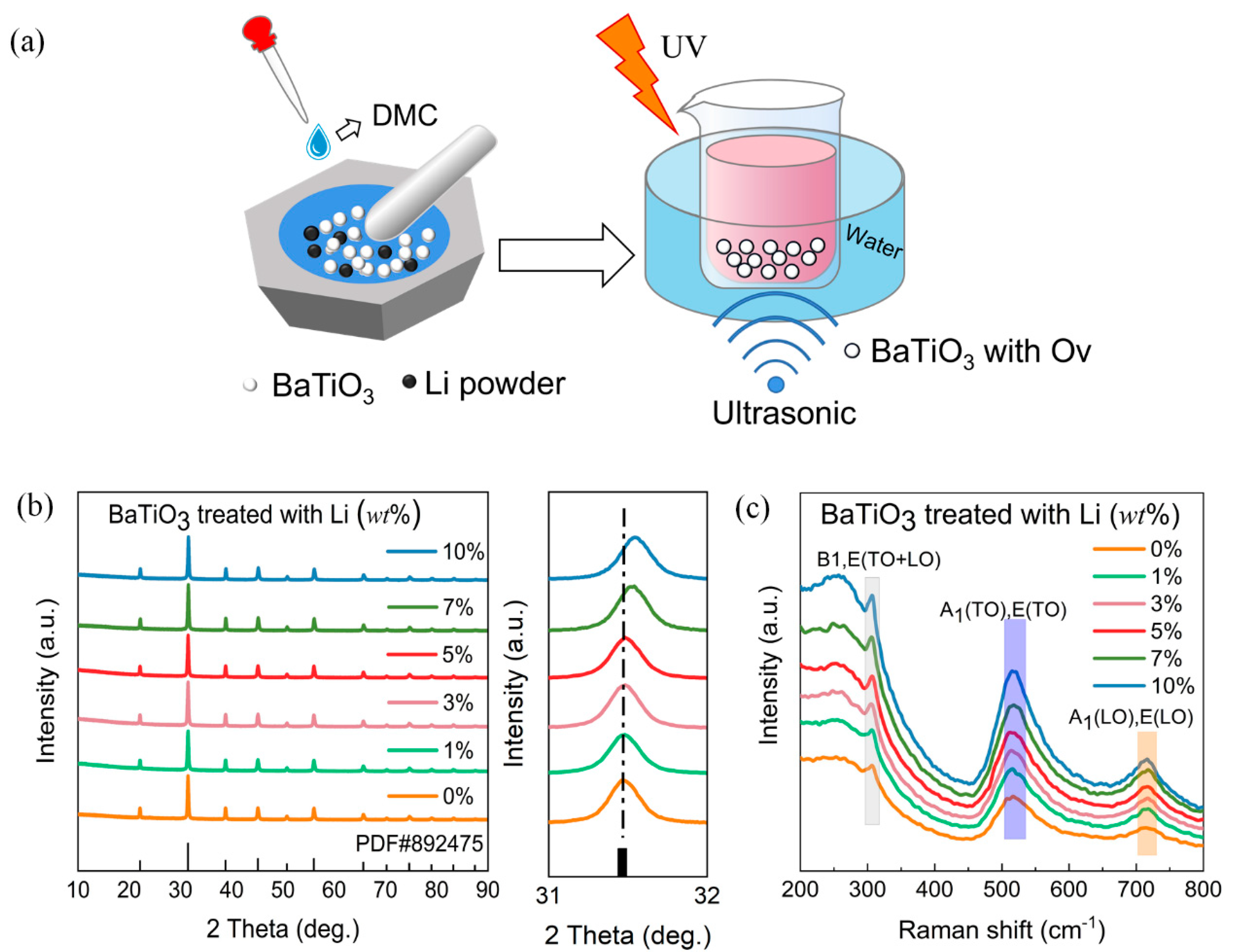
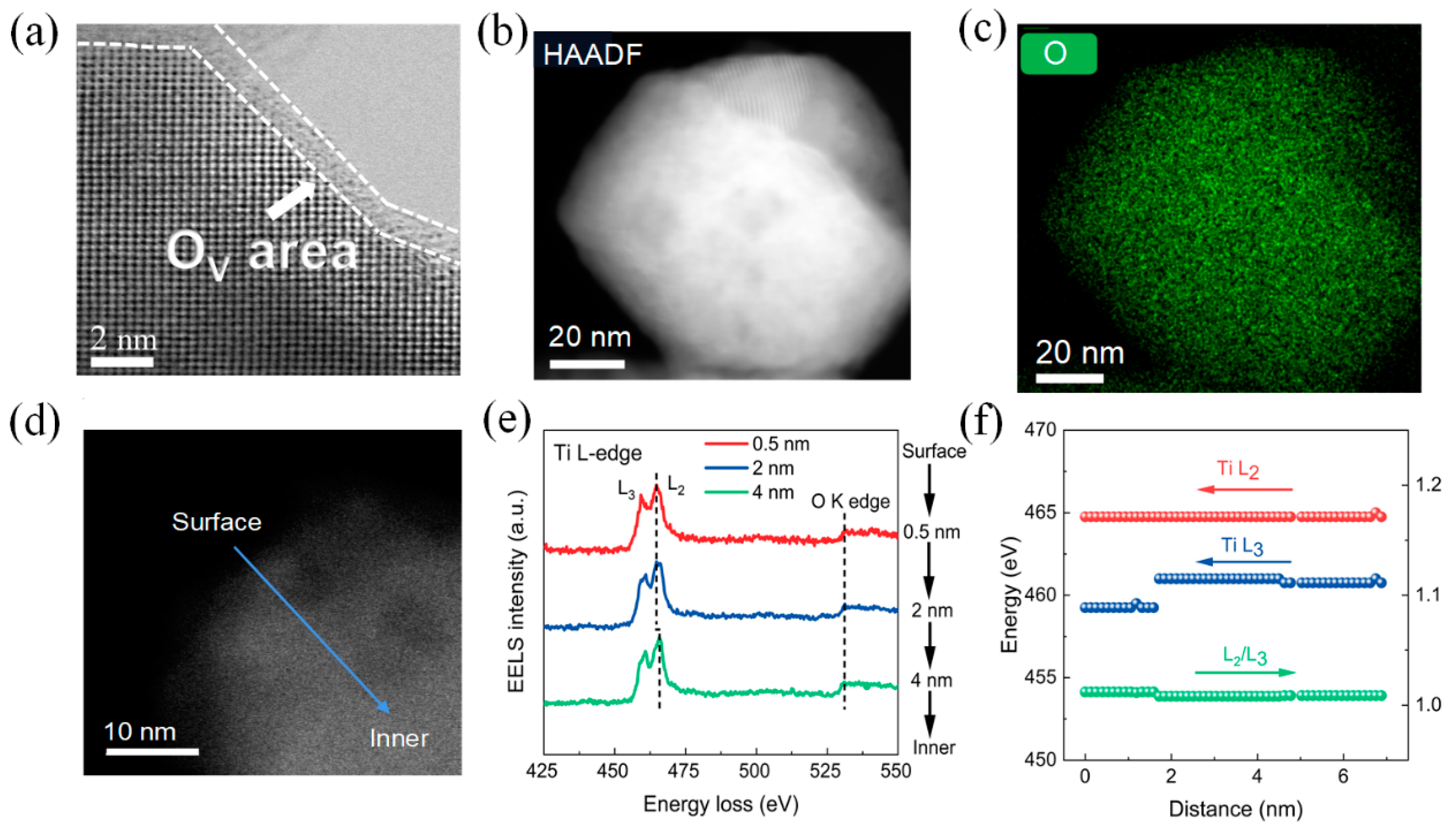
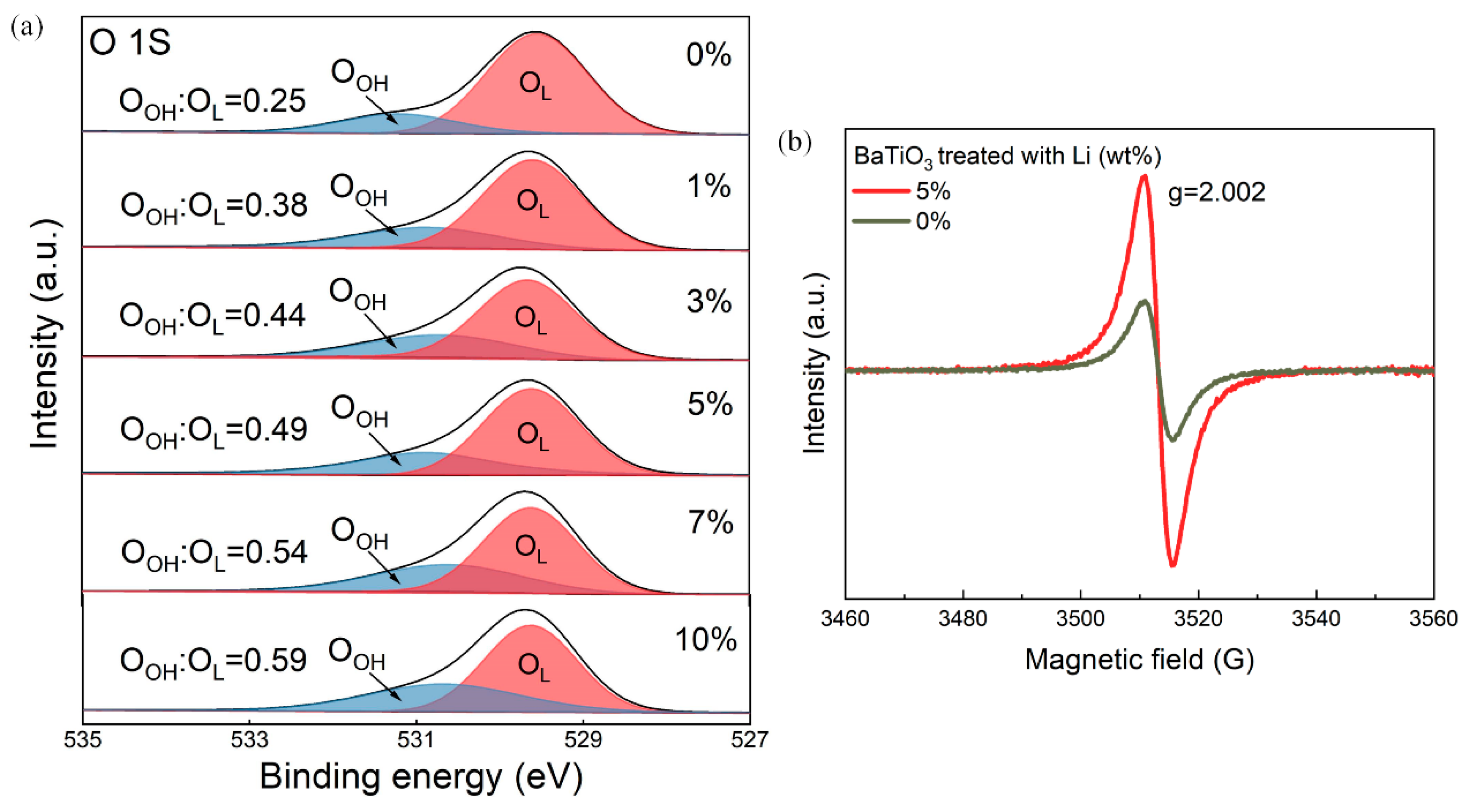
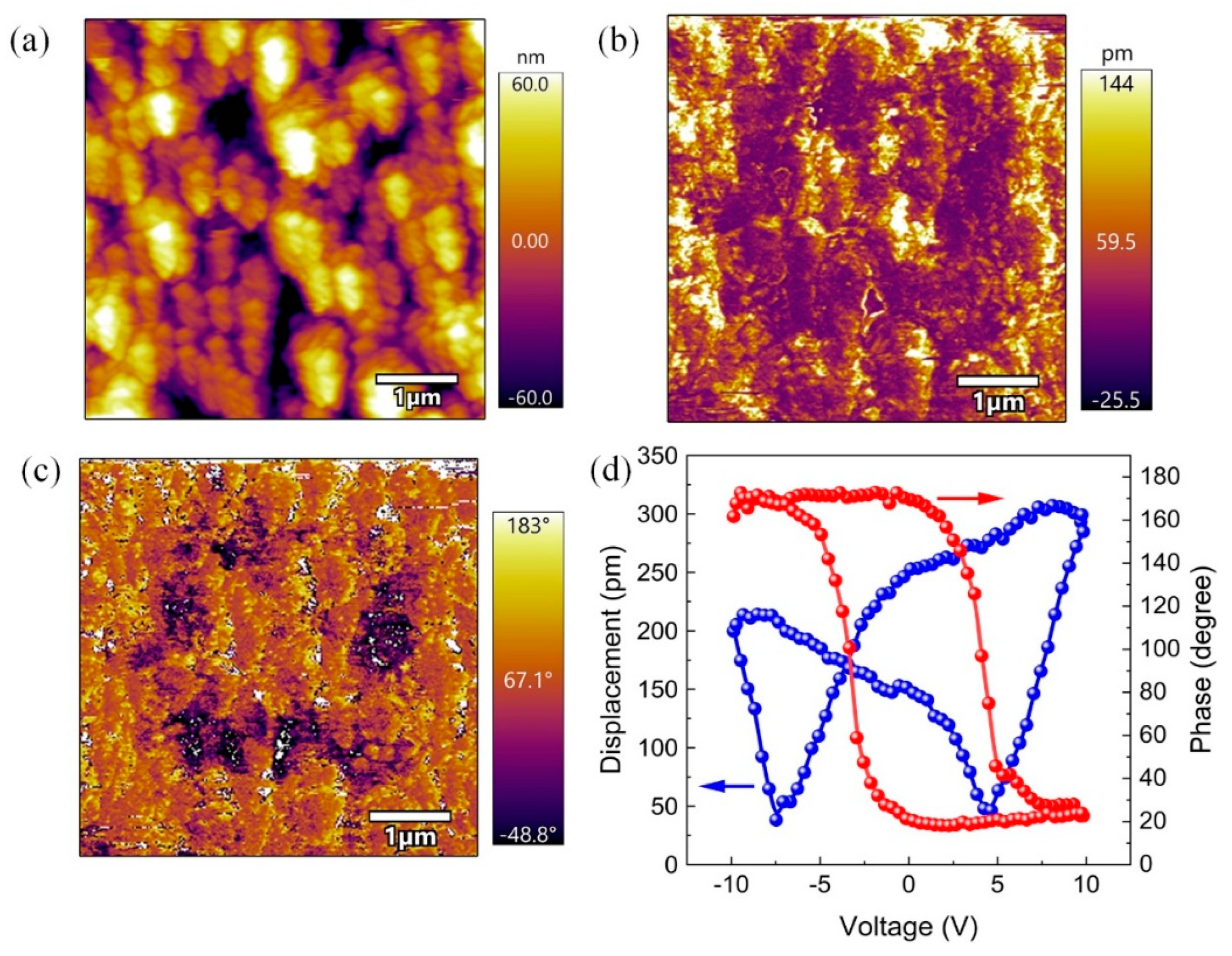
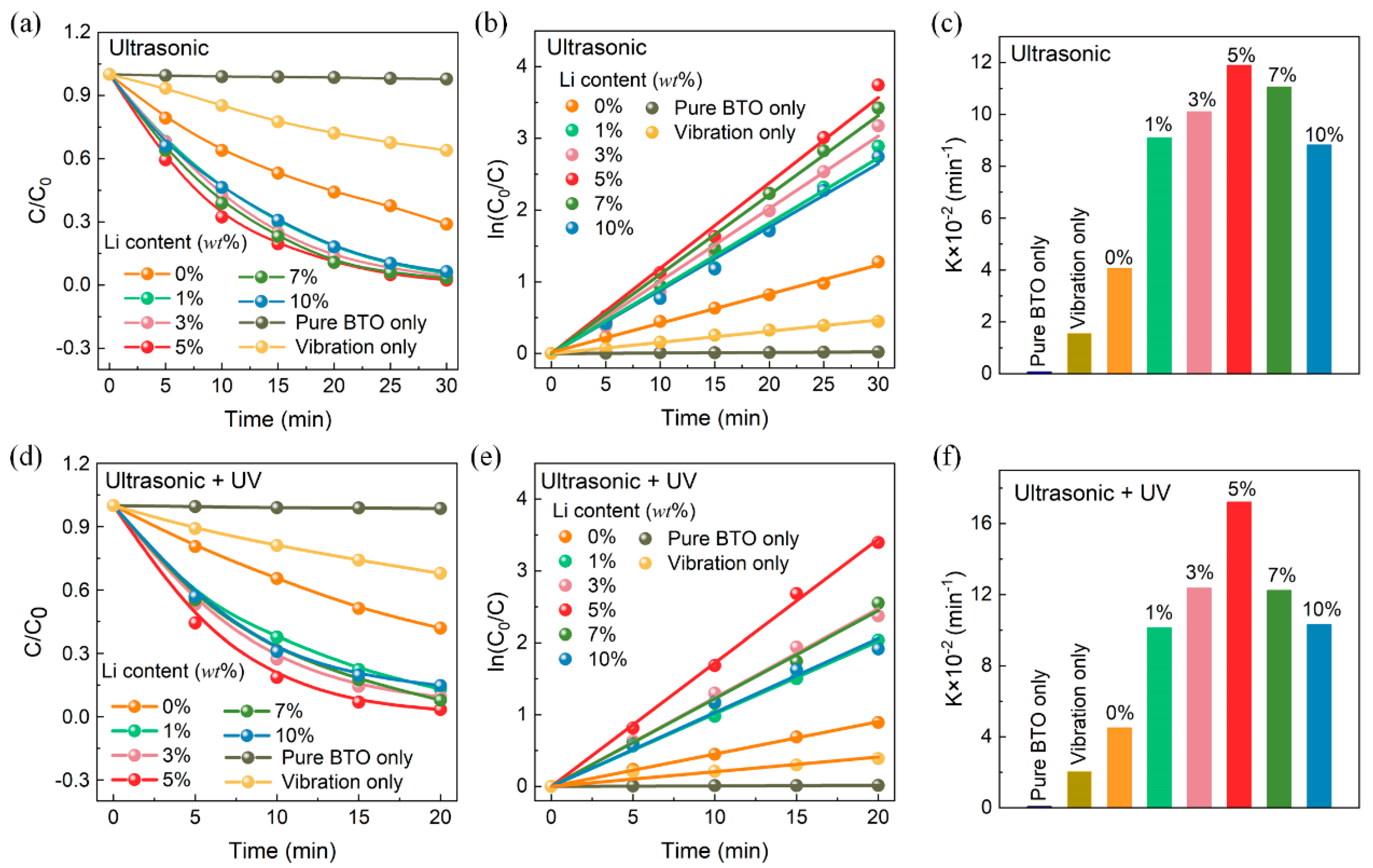
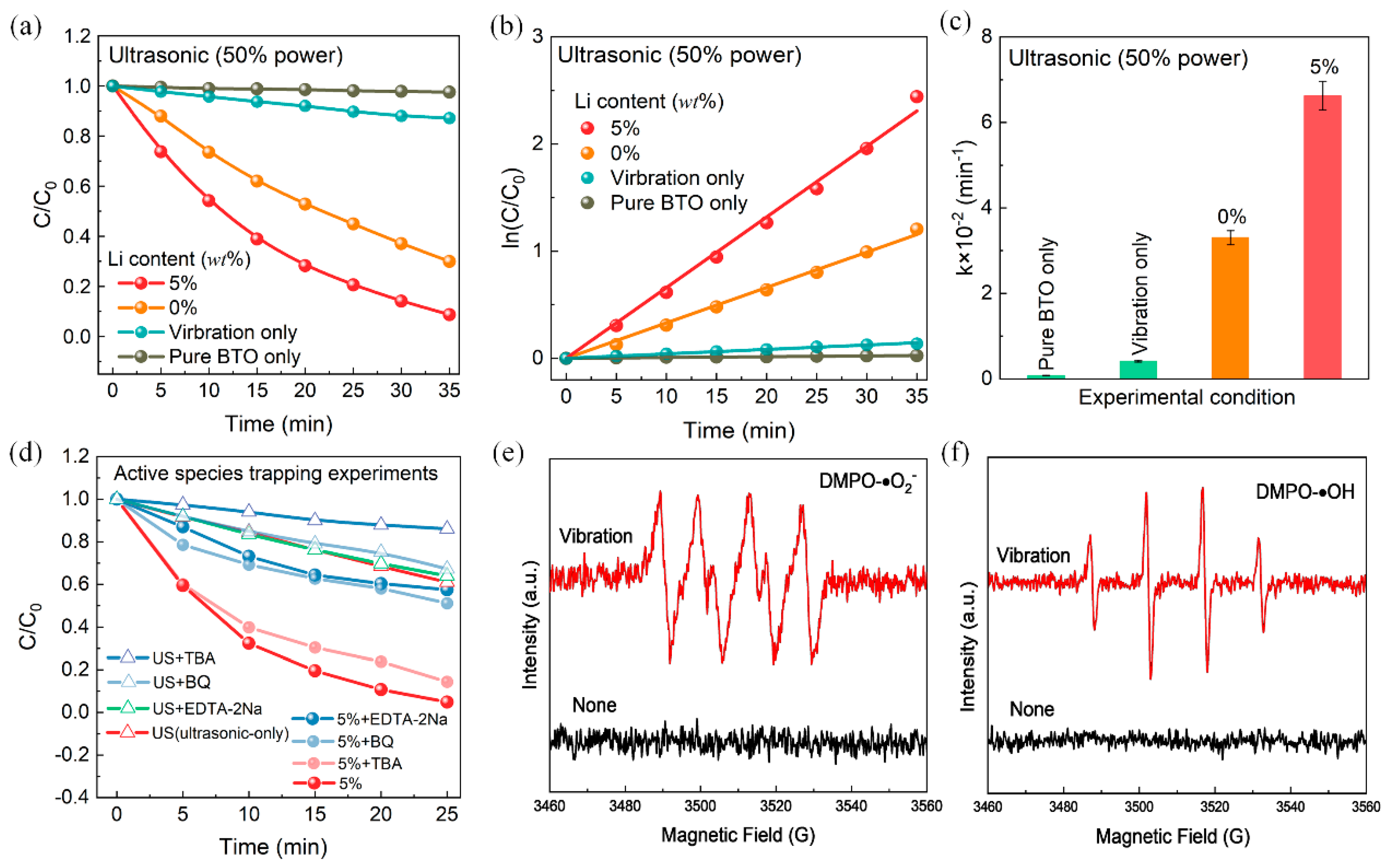
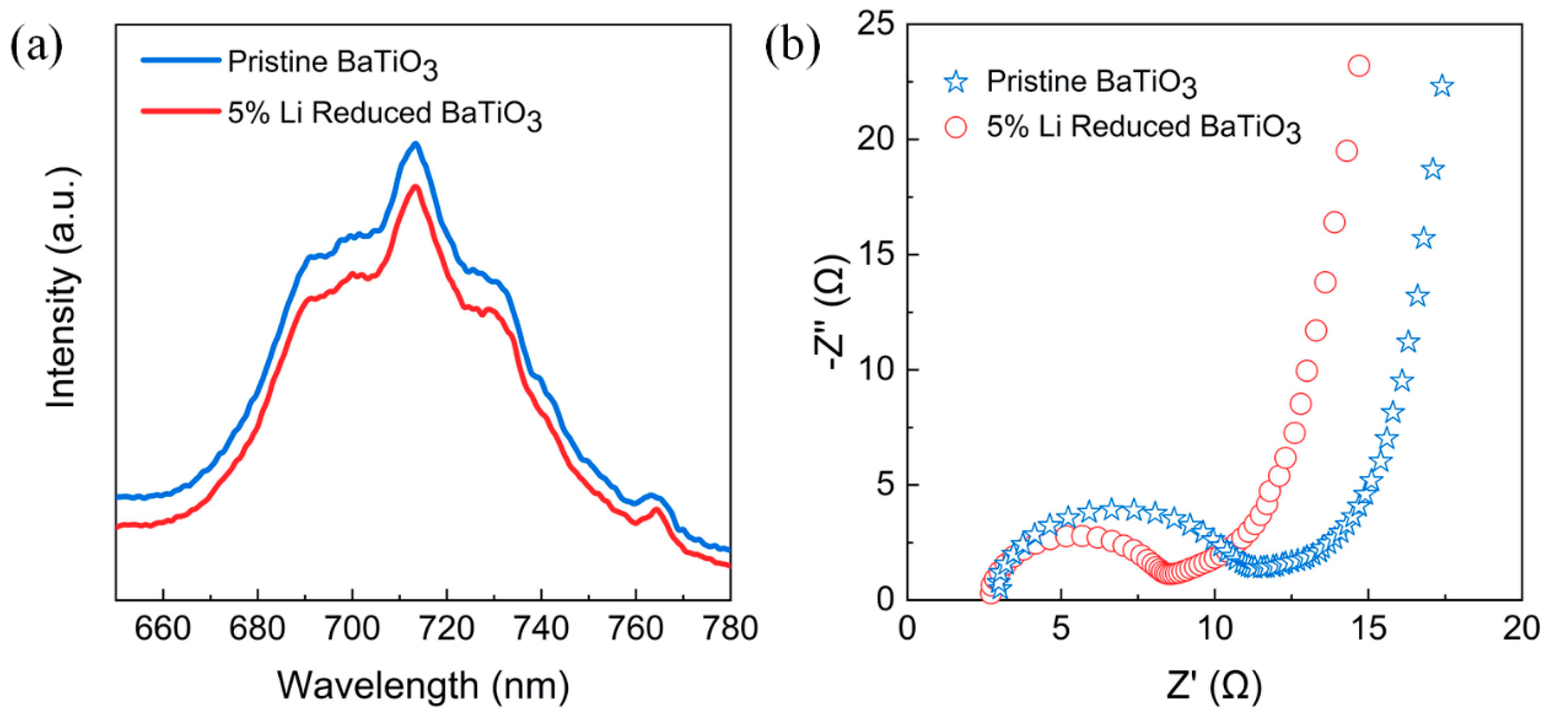
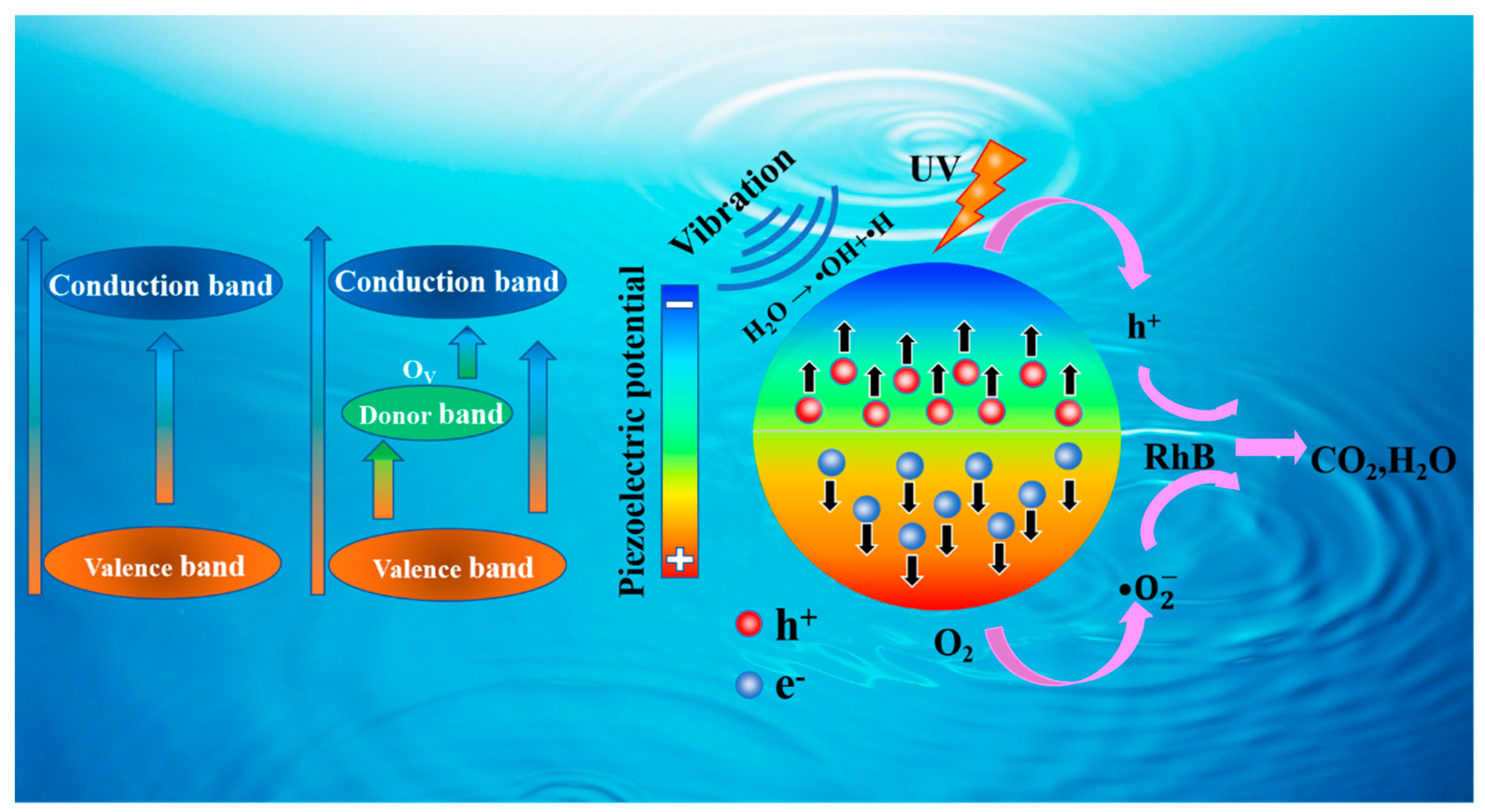
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, G. Electrocatalytic H2O2 generation for disinfection. Chin. J. Catal. 2021, 42, 2149–2163. [Google Scholar] [CrossRef]
- Wang, M.; Wang, B.; Huang, F.; Lin, Z. Enabling PIEZO potential in PIEZO electric Semiconductors for Enhanced Catalytic Activities. Angew. Chem. Int. Ed. 2019, 58, 7526–7536. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Jia, Y.; Huang, M.; Wang, F.; Zhang, X.; Bai, Y.; Yuan, G.; Wang, Y. Piezo-catalysis for nondestructive tooth whitening. Nat. Commun. 2020, 11, 1328. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E.; Chang, Y.T.; Chang, C.K. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single- and few-layers MoS2 nanoflowers. Adv. Mater. 2016, 28, 3718–3725. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Z.; Xie, B.; Lu, J.; Guo, K.; Ke, S.; Shu, L.; Fan, H. Vibration catalysis of eco-friendly Na0.5K0.5NbO3-based piezoelectric: An efficient phase boundary catalyst. Appl. Catal. B Environ. 2020, 279, 119353. [Google Scholar] [CrossRef]
- Xu, S.; Hansen, B.J.; Wang, Z.L. Piezoelectric-nanowire-enabled power source for driving wireless microelectronics. Nat. Commun. 2010, 1, 93. [Google Scholar] [CrossRef]
- Peng, F.; Yin, R.; Liao, Y.; Xie, X.; Sun, J.; Xia, D.; He, C. Kinetics and mechanisms of enhanced degradation of ibuprofen by piezo-catalytic activation of persulfate. Chem. Eng. J. 2020, 392, 123818. [Google Scholar] [CrossRef]
- Xia, D.; Tang, Z.; Wang, Y.; Yin, R.; He, H.; Xie, X.; Sun, J.; He, C.; Wong, P.K.; Zhang, G. Piezo-catalytic persulfate activation system for water advanced disinfection: Process efficiency and inactivation mechanisms. Chem. Eng. J. 2020, 400, 125894. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Tian, Y.; Song, C.; Qiu, H.; Xue, H. Piezotronic effect boosted photocatalytic performance of heterostructured BaTiO3/TiO2 nanofibers for degradation of organic pollutants. Nano Energy 2020, 77, 105122. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Wang, F.; Hu, S.; Wang, X.; Ma, B.; Liu, H.; Wang, Z.; Sang, Y. BaTiO3 nanocrystal-mediated micro pseudo-electrochemical cells with ultrasound-driven piezotronic enhancement for polymerization. Nano Energy 2017, 39, 461–469. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Su, R.; Hsain, H.A.; Wu, M.; Zhang, D.; Hu, X.; Wang, Z.; Wang, X.; Li, F.-T.; Chen, X.; Zhu, L.; et al. Nano-ferroelectric for high efficiency overall water splitting under ultrasonic vibration. Angew. Chem. Int. Ed. 2019, 58, 15076–15081. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z.; Zhang, J.; Li, S.; Zhao, Z.; Zhu, L.; Liu, W.; Lin, Y.; Liu, H.; Zhang, Z. Enhanced catalytic performance by multi-field coupling in KNbO3 nanostructures: Piezo-photocatalytic and ferro-photoelectrochemical effects. Nano Energy 2019, 58, 695–705. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, J.; Qin, N.; Lin, E.; Bao, D. Enhanced piezocatalytic performance of (Ba,Sr)TiO3 nanowires to degrade organic pollutants. ACS Appl. Nano Mater. 2018, 1, 5119–5127. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, J.M. Effect of controlled oxygen vacancy on H2-production through the piezocatalysis and piezophototronics of ferroelectric R3C ZnSnO3 Nanowires. Adv. Funct. Mater. 2020, 30, 1907619. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, C.; Yang, S.; Yang, Z.; Yang, Y. Ferroelectric polarization effect promoting the bulk charge separation for enhance the efficiency of photocatalytic degradation. Chem. Eng. J. 2021, 410, 128430. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, K.; Li, D.; Li, N.; Xu, J.; Bahnemann, D.W.; Wang, C. Polarization-enhanced photocatalytic activity in non-centrosymmetric materials based photocatalysis: A review. Chem. Eng. J. 2021, 426, 131681. [Google Scholar] [CrossRef]
- Lan, S.; Zeng, X.; Rather, R.A.; Lo, I.M.C. Enhanced trimethoxypyrimidine degradation by piezophotocatalysis of BaTiO3/Ag3PO4 using mechanical vibration and visible light simultaneously. Environ. Sci. Nano 2019, 6, 554–564. [Google Scholar] [CrossRef]
- Li, H.; Sang, Y.; Chang, S.; Huang, X.; Zhang, Y.; Yang, R.; Jiang, H.; Liu, H.; Wang, Z.L. Enhanced ferroelectric-nanocrystal-based hybrid photocatalysis by ultrasonic-wave-generated piezophototronic effect. Nano Lett. 2015, 15, 2372–2379. [Google Scholar] [CrossRef]
- Xu, S.; Guo, L.; Sun, Q.; Wang, Z.L. Piezotronic effect enhanced plasmonic photocatalysis by AuNPs/BaTiO3 heterostructures. Adv. Funct. Mater. 2019, 29, 1808737. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Mo, S.; Li, J.; Liao, R.; Peng, P.; Li, J.; Wu, J.; Fu, M.; Liao, L.; Shen, T.; Xie, Q.; et al. Unraveling the decisive role of surface CeO2 nanoparticles in the Pt-CeO2/MnO2 hetero-catalysts for boosting toluene oxidation: Synergistic effect of surface decorated and intrinsic O-vacancies. Chem. Eng. J. 2021, 418, 129399. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Gao, S.; Shen, Q.; Xue, J.; Chen, K.; Li, Q. Creation of rich oxygen vacancies in bismuth molybdate nanosheets to boost the photocatalytic nitrogen fixation performance under visible light illumination. Chem. Eng. J. 2021, 404, 127115. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Fan, S.; Chen, X.; Qin, M.; Long, D.; Tadé, M.O.; Liu, S. Impact of oxygen vacancy occupancy on piezo-catalytic activity of BaTiO3 nanobelt. Appl. Catal. B Environ. 2020, 279, 119340. [Google Scholar] [CrossRef]
- Mao, C.; Cheng, H.; Tian, H.; Li, H.; Xiao, W.-J.; Xu, H.; Zhao, J.; Zhang, L. Visible light driven selective oxidation of amines to imines with BiOCl: Does oxygen vacancy concentration matter? Appl. Catal. B Environ. 2018, 228, 87–96. [Google Scholar] [CrossRef]
- Wang, S.; Hai, X.; Ding, X.; Chang, K.; Xiang, Y.; Meng, X.; Yang, Z.; Chen, H.; Ye, J. Light-switchable oxygen vacancies in ultrafine Bi5O7Br nanotubes for boosting solar-driven nitrogen fixation in pure water. Adv. Mater. 2017, 29, 1701774. [Google Scholar] [CrossRef]
- Kang, Z.; Lin, E.; Qin, N.; Wu, J.; Yuan, B.; Bao, D. Effect of oxygen vacancies and crystal symmetry on piezocatalytic properties of Bi2WO6 ferroelectric nanosheets for wastewater decontamination. Environ. Sci. Nano 2021, 8, 1376–1388. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Fan, S.; Yin, Z.; Wang, L.; Tadé, M.O.; Liu, S. Piezotronic effect and oxygen vacancies boosted photocatalysis C‒N coupling of benzylamine. Nano Energy 2021, 83, 105831. [Google Scholar] [CrossRef]
- Jin, C.; Ai, J.-D.; Liu, D.-M.; Tan, L.; Cao, L.; Shen, B.; Qiu, X.; Zhang, L. Significantly enhanced piezocatalytic activity of BaTiO3 by regulating the quenching process. J. Mater. Chem. A 2023, 11, 10360–10370. [Google Scholar] [CrossRef]
- Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Huang, K.; Zu, D.; et al. Tuning defects in oxides at room temperature by lithium reduction. Nat. Commun. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Freedman, D.A.; Roundy, D.; Arias, T.A. Elastic effects of vacancies in strontium titanate: Short- and long-range strain fields, elastic dipole tensors, and chemical strain. Phys. Rev. B 2009, 80, 064108. [Google Scholar] [CrossRef]
- Herklotz, A.; Lee, D.; Guo, E.-J.; Meyer, T.L.; Petrie, J.R.; Lee, H.N. Strain coupling of oxygen non-stoichiometry in perovskite thin films. J. Phys. Condens. Matter 2017, 29, 493001. [Google Scholar] [CrossRef]
- Madhan, K.; Jagadeeshwaran, C.; Murugaraj, R. Enhancement of electrical and magnetic properties in acceptor-doped BaTiO3 ferroelectric ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 2953–2965. [Google Scholar] [CrossRef]
- Joshi, U.A.; Yoon, S.; Baik, S.; Lee, J.S. Surfactant-free hydrothermal synthesis of highly tetragonal barium titanate nanowires: A structural investigation. J. Phys. Chem. B 2006, 110, 12249–12256. [Google Scholar] [CrossRef]
- EI Marssi, M.; Le Marrec, F.; Lukyanchuk, I.A.; Karkut, M.G. Ferroelectric transition in an epitaxial barium titanate thin film: Raman spectroscopy and x-ray diffraction study. J. Appl. Phys. 2003, 94, 3307–3312. [Google Scholar] [CrossRef]
- Asiaie, R.; Zhu, W.; Akbar, S.A.; Dutta, P.K. Characterization of submicron particles of tetragonal BaTiO3. Chem. Mater. 1996, 8, 226–234. [Google Scholar] [CrossRef]
- Adireddy, S.; Lin, C.; Cao, B.; Zhou, W.; Caruntu, G. Solution-based growth of monodisperse cube-like BaTiO3 colloidal nanocrystals. Chem. Mater. 2010, 22, 1946–1948. [Google Scholar] [CrossRef]
- Jin, C.; Liu, D.; Hu, J.; Wang, Y.; Zhang, Q.; Lv, L.; Zhuge, F. The role of microstructure in piezocatalytic degradation of organic dye pollutants in wastewater. Nano Energy 2019, 59, 372–379. [Google Scholar] [CrossRef]
- Lan, S.; Feng, J.; Xiong, Y.; Tian, S.; Liu, S.; Kong, L. Performance and mechanism of piezo-catalytic degradation of 4-Chlorophenol: Finding of effective piezo-dichlorination. Environ. Sci. Technol. 2017, 51, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, Q.; Lin, E.; Yuan, B.; Qin, N.; Thatikonda, S.K.; Bao, D. Insights into the role of ferroelectric polarization in piezocatalysis of nanocrystalline BaTiO3. ACS Appl. Mater. Interfaces 2018, 10, 17842–17849. [Google Scholar] [CrossRef]
- Muller, D.A.; Nakagawa, N.; Ohtomo, A.; Grazul, J.L.; Hwang, H.Y. Atomic-scale imaging of nanoengineered oxygen vacancy profiles in SrTiO3. Nature 2004, 430, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, C.; Xu, S.; Liu, J.; Liang, C.; Chen, L.; Wang, P.; Ivey, D.G.; Deng, Y.; Wei, W. Stable heteroepitaxial interface of Li-rich layered oxide cathodes with enhanced lithium storage. Energy Storage Mater. 2019, 21, 69–76. [Google Scholar] [CrossRef]
- Ray, S.; Kolen’ko, Y.V.; Kovnir, K.A.; Lebedev, O.I.; Turner, S.; Chakraborty, T.; Erni, R.; Watanabe, T.; Van Tendeloo, G.; Yoshimura, M.; et al. Defect controlled room temperature ferromagnetism in Co-doped barium titanate nanocrystals. Nanotechnology 2012, 23, 025702. [Google Scholar] [CrossRef]
- Baek, K.; Lee, S.-Y.; Doh, S.-G.; Kim, M.; Hyun, J.K. Axial oxygen vacancy-regulated microwave absorption in micron-sized tetragonal BaTiO3 particles. J. Mater. Chem. C 2018, 6, 9749–9755. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, M.; Tai, X.; Li, H.; Han, X.; Yu, J. Analytical transmission electron microscopy for emerging advanced materials. Matter 2021, 4, 2309–2339. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef]
- Cai, J.; Cao, A.; Huang, J.; Jin, W.; Zhang, J.; Jiang, Z.; Li, X. Understanding oxygen vacancies in disorder-engineered surface and subsurface of CaTiO3 nanosheets on photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 267, 118378. [Google Scholar] [CrossRef]
- Elia, V.; Niccoli, M. New physico-chemical properties of water induced by mechanical treatments. A calorimetric study at 25 °C. J. Therm. Anal. Calorim. 2000, 61, 527–537. [Google Scholar] [CrossRef]
- Hu, Y.H. A highly efficient photocatalyst—Hydrogenated black TiO2 for the photocatalytic splitting of water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef]
- Lin, T.; Yang, C.; Wang, Z.; Yin, H.; Lü, X.; Huang, F.; Lin, J.; Xie, X.; Jiang, M. Effective nonmetal incorporation in black titania with enhanced solar energy utilization. Energy Environ. Sci. 2014, 7, 967–972. [Google Scholar] [CrossRef]
- Ning, X.; Hao, A.; Cao, Y.; lv, N.; Jia, D. Boosting piezocatalytic performance of Ag decorated ZnO by piezo-electrochemical synergistic coupling strategy. Appl. Surf. Sci. 2021, 566, 150730. [Google Scholar] [CrossRef]
- Yu, C.; Tan, M.; Li, Y.; Liu, C.; Yin, R.; Meng, H.; Su, Y.; Qiao, L.; Bai, Y. Ultrahigh piezocatalytic capability in eco-friendly BaTiO3 nanosheets promoted by 2D morphology engineering. J. Colloid Interface Sci. 2021, 596, 288–296. [Google Scholar] [CrossRef]
- Liu, D.; Jin, C.; Shan, F.; He, J.; Wang, F. Synthesizing BaTiO3 nanostructures to explore morphological influence, kinetics, and mechanism of piezocatalytic dye degradation. ACS Appl. Mater. Interfaces 2020, 12, 17443–17451. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.; Li, C.; Yan, F.; Bai, H.; Shen, B.; Zeng, H.; Zhai, J. Piezophototronic effect in enhancing charge carrier separation and transfer in ZnO/BaTiO3 heterostructures for high-efficiency catalytic oxidation. Nano Energy 2019, 66, 104127. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Starr, M.B.; Wang, X. Fundamental analysis of piezocatalysis process on the surfaces of strained piezoelectric materials. Sci. Rep. 2013, 3, 2160. [Google Scholar] [CrossRef]
| Piezocatalyst | Dye Species | Vibration Source | Light Source | Initial Dye Concentration | Catalyst Dosage | Rate Constant | Ref. |
|---|---|---|---|---|---|---|---|
| BTO nanoparticles | RhB | 150 W, 40 kHz | 125 W, 365 nm | 5 mg/L | 1 g/L | 0.1721 min−1 | This work |
| BTO nanoparticles | RhB | 150 W, 40 kHz | dark | 5 mg/L | 1 g/L | 0.1189 min−1 | This work |
| BTO nanobelts | RhB | 100 W, 50 kHz | dark | 10 mg/L | 1 g/L | 0.0253 min−1 | [26] |
| BTO nanosheets | MO | 100 W, 40 kHz | / | 5 mg/L | 1 g/L | 0.1279 min−1 | [54] |
| BTO nanowires | RhB | 80 W, | / | 5 mg/L | / | <0.016 min−1 | [12] |
| BTO/TiO2 nanofibers | RhB | 300 W, 40 kHz | 250 W, 365 nm | 5 mg/L | 1 g/L | 0.0967 min−1 | [10] |
| BTO nanofibers | RhB | 100 W, 40 kHz | / | 7.5 mg/L | 1 g/L | 0.0736 min−1 | [55] |
| ZnO/BTO heterostructures | RhB | 120 W, 40 kHz | 150 W | 10 mg/L | 1 g/L | 0.118 min−1 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, D.; Liang, R.; Liu, H.; Zhou, C.; Ye, M.; Zheng, R.; Li, H.; Ke, S. Boosting Piezocatalytic Performance of BaTiO3 by Tuning Defects at Room Temperature. Nanomaterials 2024, 14, 276. https://doi.org/10.3390/nano14030276
An D, Liang R, Liu H, Zhou C, Ye M, Zheng R, Li H, Ke S. Boosting Piezocatalytic Performance of BaTiO3 by Tuning Defects at Room Temperature. Nanomaterials. 2024; 14(3):276. https://doi.org/10.3390/nano14030276
Chicago/Turabian StyleAn, Donghui, Renhong Liang, Hua Liu, Chao Zhou, Mao Ye, Renkui Zheng, Han Li, and Shanming Ke. 2024. "Boosting Piezocatalytic Performance of BaTiO3 by Tuning Defects at Room Temperature" Nanomaterials 14, no. 3: 276. https://doi.org/10.3390/nano14030276
APA StyleAn, D., Liang, R., Liu, H., Zhou, C., Ye, M., Zheng, R., Li, H., & Ke, S. (2024). Boosting Piezocatalytic Performance of BaTiO3 by Tuning Defects at Room Temperature. Nanomaterials, 14(3), 276. https://doi.org/10.3390/nano14030276