Abstract
Injectable colloidal solutions of lanthanide oxides (nanoparticles between 10 and 100 nm in size) have demonstrated high biocompatibility and no toxicity when the nanoparticulate units are functionalized with specific biomolecules that molecularly target various proteins in the tumor microenvironment. Among the proteins successfully targeted by functionalized lanthanide nanoparticles are folic receptors, fibroblast activation protein (FAP), gastrin-releasing peptide receptor (GRP-R), prostate-specific membrane antigen (PSMA), and integrins associated with tumor neovasculature. Lutetium, samarium, europium, holmium, and terbium, either as lanthanide oxide nanoparticles or as nanoparticles doped with lanthanide ions, have demonstrated their theranostic potential through their ability to generate molecular images by magnetic resonance, nuclear, optical, or computed tomography imaging. Likewise, photodynamic therapy, targeted radiotherapy (neutron-activated nanoparticles), drug delivery guidance, and image-guided tumor therapy are some examples of their potential therapeutic applications. This review provides an overview of cancer theranostics based on lanthanide nanoparticles coated with specific peptides, ligands, and proteins targeting the tumor microenvironment.
1. Introduction
The group of lanthanides has been of interest for decades because of their luminescent properties [1,2]. In contrast to the other metals used to prepare nanoparticles, lanthanides are mostly found as dopant ions (Ln3+) in core–shell nanostructures and as pure lanthanide oxides [2,3,4,5,6]. Lanthanide nanoparticles (LnNPs) are suitable to be used as pharmaceutical forms for theranostic applications in cancer. For this purpose, LnNPs must be colloidally stable in biological fluids, exhibit negligible uptake in healthy cells and tissues, and have functional groups on their surface attached to antibodies, peptides, biomolecules, or ligands that provide specific molecular targeting to proteins present in the tumor microenvironment (TME) [4,5,6].
In the pharmaceutical industry, a colloidal system is defined as a system consisting of two or more phases, one of which is a fluid (liquid), and the other is dispersed in the form of fine solid particles. The constant Brownian motion of each dispersed particle surrounding the solvent shell is responsible for the stability of such colloidal solutions. Nanocolloids are high-molecular-weight particles (size 10–100 nm) that have difficulty crossing capillary membranes in healthy tissues (with an opening between endothelial cells of 2 nm), making them ideal for accumulation in tumors, where the intercellular space of the vascular endothelium opens up to 400 nm [7].
The size and surface properties of LnNPs can be easily modified to obtain stable colloidal solutions that meet pharmaceutical requirements. The multifunctionalization of LnNPs is a relevant advantage when used as molecular targeting probes, since TME is formed by a complex network of proteins/peptides/small molecules and immune cells, fibroblasts and cancer cells, all of which are potential diagnostic/therapeutic targets. The functionalization of nanoparticles confers high molecular affinity by creating multiple ligand binding sites on the nanosurface, which is necessary for targeting specific proteins expressed in TME.
The surface of LnNPs determines their interaction with body systems when administered in vivo, so when coated with biomolecules, they are not recognized as foreign by the immune system and are not toxic. Recently, it has been demonstrated that lutetium oxide nanoparticles coated with inhibitory peptides of fibroblast activation protein (iFAP) and prostate-specific membrane antigen (iPSMA) accumulate exclusively in the TME. Additionally, they are taken up by Kupffer cells and macrophages of the endothelial reticulum system without being trapped in the healthy parenchyma of the liver, lung, spleen or bone marrow. In other words, the elimination of functionalized Lu2O3-NPs from the body via the lymphatic system does not affect healthy tissues [5,6]. Furthermore, LaNPs can be prepared using pharmaceutical processes that comply with good manufacturing practices, which provides the added value required for clinical translation in theranostic applications [6].
Lanthanide-based nanomaterials offer multimodal approaches to diagnostics and therapeutics due to their magnetic, relaxivity, optical, and nuclear emission properties. The multifunctionality of LnNPs lies in their ability to provide diagnostic imaging of primary and metastatic tumors and their utility in photodynamic therapy, targeted radiotherapy (neutron-activated nanoparticles), drug delivery, and image-guided tumor surgery [8,9,10,11,12].
This review provides an overview of cancer theranostics based on lanthanide nanoparticles coated with specific peptides, ligands, and proteins for targeting the tumor microenvironment.
2. Physical Properties of Lanthanide-Based Nanoparticles for Theranostics
Metal nanoparticles (1–100 nm) have an extensive range of applications in biomedicine due to their physicochemical properties. They are utilized as targeted drug delivery, biosensors, as well as diagnostic and therapeutic agents for diseases like cancer. Therefore, an essential aspect is the synthesis route to achieve both the desired size and morphology. Additionally, these features are the starting point for successful surface modification and functionalization. The most effective methods for synthesizing these compounds include coprecipitation, solvothermal synthesis, thermal decomposition, microemulsion techniques, and wet-based chemistry methods [4,13].
The rare earth elements, also known as the lanthanides, are members of group IIIB of the periodic table, from Ln to Lu. Their electronic configuration can be denoted as [Xe]4f(1−14)5d(0−1)6s2, which implies that the 4f orbital is partially filled, except for lanthanum ([Xe]4f0) and lutetium ([Xe]4f14). The 4f electrons are responsible for the special properties of rare earths, including enhanced electronic, magnetic, and optical properties at the nanometer scale. Ln3+ is the most stable and frequently observed oxidation state for these elements [4,13,14,15].
Optically, these properties are favored by the large quantum numbers (n = 4, l = 3), which create partially allowed intraconfigurations at the 4f level and are further shielded from environmental effects by the screening effect of the electrons at higher energy levels (5s and 5p). Therefore, these materials offer numerous advantages, such as numerous absorption bands with narrow emission profiles from the UV-Vis to the NIR, excellent photostability, large Stokes and anti-Stokes shifts, long luminescence half-lives (µm to ms), luminescence quantum efficiency, low background autofluorescence (practically zero), no photobleaching line-shaped emission, and low biotoxicity [2,15,16,17,18,19,20].
A theranostic molecule possesses unique and versatile properties that make it suitable for both therapy and diagnosis. Lanthanide-based nanoparticles (Ln-NPs) exhibit these characteristics. Depending on the nanoplatform design, one of the following scenarios may occur [5,6,21,22,23,24].
- Activation using near-infrared (NIR) light generates luminescence imaging for diagnostic purposes and triggers drug release for therapy.
- NIR activation also produces real-time luminescence imaging to evaluate the effectiveness of previously applied treatments for diagnosis and generates photothermal therapy (PTT) or photodynamic therapy (PDT).
- Neutron activation produces radioluminescence imaging with possible radiotherapy applications when beta particles are emitted.
Lanthanide nanoparticles include nanoparticles doped with lanthanide ions, alloys, metallic nanoparticles with varying percentages of lanthanides, lanthanide oxide nanoparticles, or core–shell nanoparticles [5,6,21,22,23,24].
2.1. Luminescence of Lanthanide-Doped Nanoparticles
Luminescence is a physical phenomenon generated by the movement of electrons contained in matter at different energy levels. It can be summarized as the absorption of energy to achieve an excited state which, upon returning to its basal state, releases this energy in the form of light, where the wavelength of the light emitted is a characteristic of the luminescent material and not of the incident radiation [17,21,25].
The luminescence of nanoparticles based on lanthanides occurs in the visible and NIR regions of the spectrum following stimulation by UV or NIR light. This phenomenon is further affected by both phonon energy and the strength of the crystal field that contains the Ln3+ ions. The use of Ln-NPs generates an optical image that enables visualization of subcellular morphological details and elucidation of signaling pathways and biological processes at the cellular level. Among the lanthanide ions, Eu3+, Dy3+, and Tb3+ exhibit the most effective luminescent characteristics [16,17,21,25].
2.2. Luminescence Emission Mechanisms
2.2.1. Downshifting
Downshift emission is a nonlinear Stokes-shift process. It is based on the absorption of a high energy, short wavelength photon (NIR-I photon), which is emitted as a lower energy, longer wavelength photon (NIR-II photon), producing luminescence. The mechanism is based on the direct excitation of photons to an E2 state and then to an E1 relaxation state with the emission of nonradiative energy. Finally, it reaches the E0 state, its initial state, with the emission of luminescence. The nonradiative relaxation (E2→E1) is a multiphonon-assisted process governed by phonon dynamics. The generated emissions fall in the optical window between 700–1100 nm. These emissions can be of two types:
- Conversion of UV into visible light: The lanthanide ions representative of this emission are Er3+ (red emitter) and Tb3+ (green emitter).
- Conversion from UV-Vis to NIR: The lanthanide ions representative of this emission are Yb3+, Nd3+ and Dy3+.
In this process, as in the upconversion process, the structure and composition of the material, such as size, distribution, shape, and crystal phase, have a direct relationship to the quality and quantity of emitted luminescence [26,27,28,29,30].
2.2.2. Upconversion
The upconversion process was first termed the infrared quantum counter in 1959. It is an anti-Stokes emission and a non-linear optical phenomenon. This process involves the absorption of two or more low-energy photons to produce the emission of higher-energy photons (shorter wavelength) to the incident photon via a high-duration energetic state [2,16,18,31].
Upconversion emissions result from four distinct energy transfer pathways: excited-state absorption (ESA), energy transfer upconversion (ETU), cooperative energy transfer (CET), and energy migration-mediated upconversion (EMU) [2,16,31,32,33].
- ESA: This process involves sequential absorption of two or more low-energy photons by a single type of Ln3+ ion with medium-length energy states.
- ETU: In this process, there are two different luminescent centers, a sensitizer, and an activator. After excitation with a photon pump, energy is transferred from the sensitizer to the activator.
- CET: The photons generated have energies almost twice the transition energy. The emission energy originates from a significant disparity between the basal and the first excited state of the Ln3+ ion.
- EMU: In the core–shell structures, this procedure implicates four luminescent centers, including the sensitizer, activator, accumulator, and migrator. Energy is transferred consecutively across the interface of the core–shell.
The efficiency and process of upconversion exhibit high variation due to the varying energy levels in the 4f-4f intra-configurations. The complexity of these energy differences arises from the potential orbital-spin couplings of electrons and their interaction with the crystal field, resulting in process variations. Another influencing factor is the macrometric size (bulk material), where the absorption cross-section of the Ln3+ ions is small, which also generates a limited emission upconversion efficiency [25,31].
Lanthanide-based upconversion nanoparticles (Ln-based UCNPs) have improved the emission process, taking into account critical factors: (a) The symmetry of the Ln3+ ion in the crystal structure has been exploited to facilitate intraconformational transitions, with ions of smaller ionic radius than the lanthanoids strategically placed in the crystal; (b) Passivation of the surface of core–shell structures in order to minimize the quenching effect due to surface defects; (c) Modulation of transfer energy to reduce non-radiative losses and enhance radiative emission is ideal. This can be achieved through the use of metals that have a d–d transition in their crystal structure [2,25].
Ln-based UCNPs effectively transform various photons from the near-infrared (NIR) region, with low energy, into high-energy NIR, visible, or ultraviolet photon emissions [19,21]. Excitation with near-infrared (NIR) light (980–808 nm) within the biological optical window (700–1000 nm) enables deeper penetration into biological tissues and subsequent imaging with high sensitivity. Excitation with this light produces minimal autofluorescence because the NIR light does not excite fluorophores in the organism, resulting in a near-zero signal-to-noise ratio [2,5,17,18,19,20,21,34]. These systems serve as therapeutics through three approaches:
- Photodynamic therapy (PDT): a non-invasive therapy for cancer treatment with three essential components—light, photosensitizer, and oxygen. PDT involves the NIR light irradiation of UCNPs to generate upconversion emission, which excites the photosensitizer (PS). Subsequently, the energy from the excited PS is transferred to nearby triplet oxygens (3O2), resulting in the creation of singlet-type reactive oxygen species (ROS) responsible for damaging cancer cells (O3). This therapy yields better effects at shorter distances between the activator donor (energy donor) and the PS (energy acceptor); it is low-cost, accurate, and has minimal long-term side effects [5,16,19,21].
- Photothermal therapy (PTT): Therapy that converts light into heat to generate local hyperthermia to cause cancer cell death. The therapy is typically generated using AuNPs, organic dyes, graphene oxides, or QDs [5]. Its mechanism is based on multiphoton relaxation of the excited states of trivalent Ln3+ ions, combined with emission quenching effects generated by nonradiative centers located in the periphery [5,21].
- Drug delivery and therapy: Ln-based UCNPs enable drug delivery and release from drug-carrying platforms by functioning as high-penetration probes without interfering with the therapeutic process of the drugs. Additionally, photoactivation or photorelease at specific sites following noninvasive stimulation of the UCNPs with light, triggers drug release. The major advantage of the therapy is the use of NIR light, which avoids unwanted phototoxic tissue damage, in contrast to the use of UV light [16].
On the other hand, Ln-based UCNPs can function as multimodal platforms with the right design, as they are easily adjustable (Table 1).

Table 1.
Multimodal imaging and therapy platforms based on upconversion nanoparticles (UCNPs).
2.2.3. Quantum Cutting
This phenomenon is based on the absorption of high-energy photons emitted as two or more lower-energy photons. The efficiency of this phenomenon is greater than 100%. An example of this emission is presented by co-doped lanthanide compounds Gd3+ or Yb3+/Tb3+, Pr3+, Ho3+ or Dy3+.
Gd3+ or Yb3+ are the acceptor ions that emit luminescence by transferring energy to one of the complementary ions by multiphoton relaxation in the NIR. An option for this structure is hybrid NPs (organic–inorganic) with a sensitizer to absorb UV light. It can be said that this process is a mixture of downconversion and upconversion mechanisms. However, the clear difference between the first two and QC is that the latter has multiphoton emission [28,43].
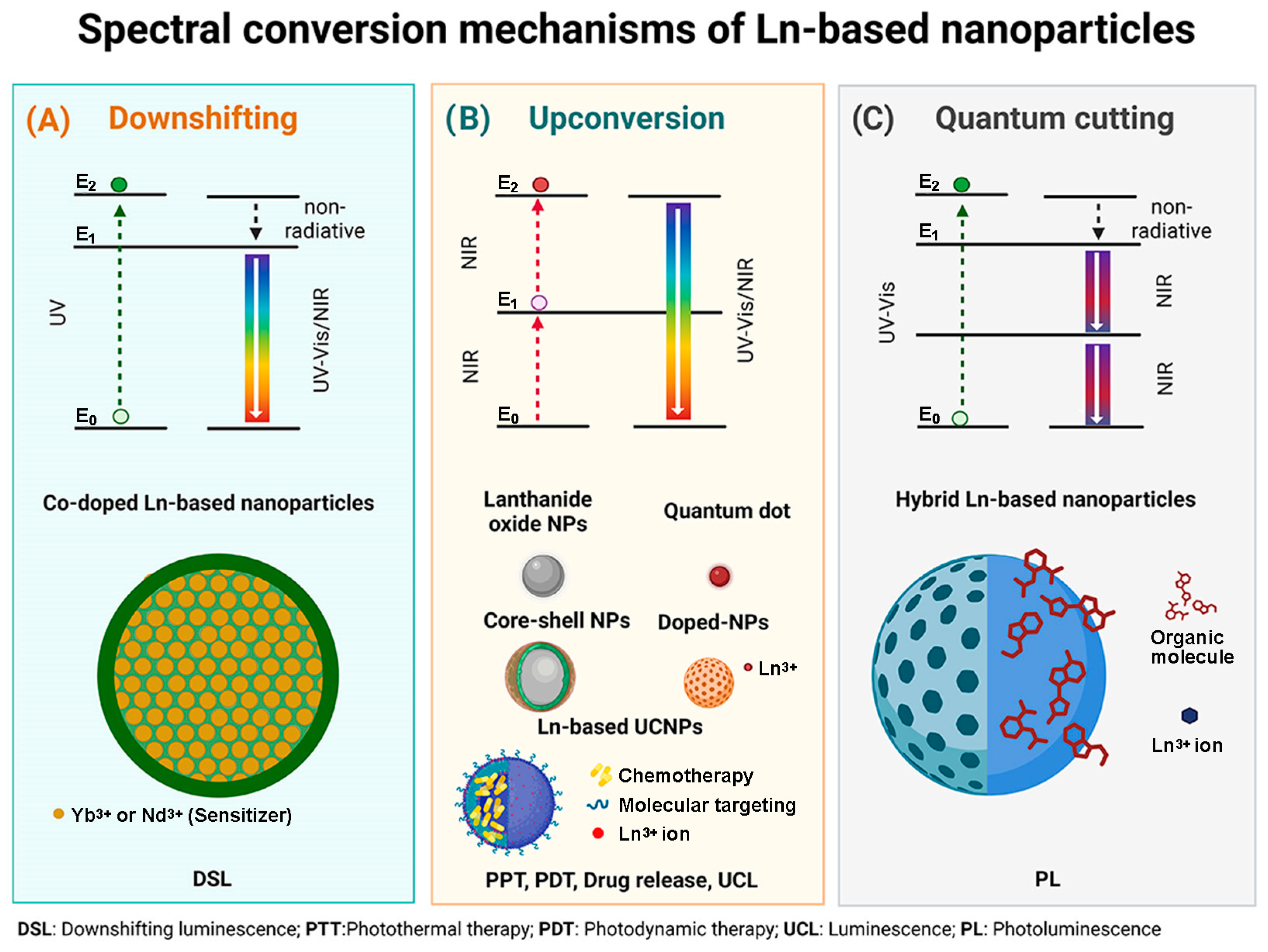
A schematic description of the spectral conversion mechanism of the different designs of Ln-based nanoparticles is shown in Figure 1.
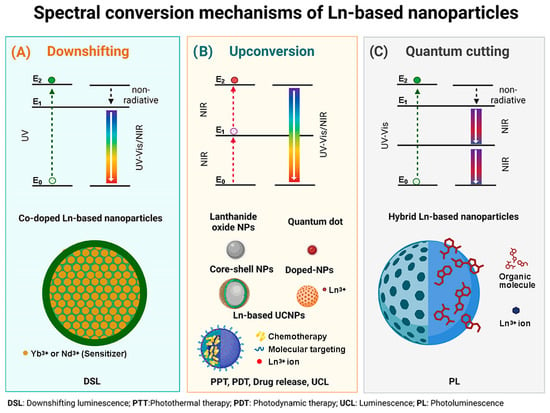
Figure 1.
(A) Downshifting mechanism associated with Co-doped Ln-based NPs; (B) Upconversion mechanism associated with Ln2O3 NPs, QDs, Core–shell Ln-based NPs, Ln-doped NPs and multimodal Ln-based platforms, and (C) Quantum cutting mechanism associated with hybrid Ln-based nanoparticles.
2.3. Photoluminescence
The primary restriction of Ln-based NPs for generating luminescence is the low absorption coefficient of lanthanide ions. While the proximity and concentration of the Ln3+ ions are enhanced by nanometer size, incorporating sensitizing molecules is the best approach to increase the absorption coefficient. These molecules usually form stable complexes by binding to the Ln3+ ions. They efficiently absorb excitation energy, which is then transferred to the lanthanide ion when it returns to its basal state. The lanthanide ion uses this transferred energy to generate luminescence [44,45].
If the sensitizing molecules are organic, their absorption efficiency is due to the presence of π bonds, and the energy is absorbed through π–π* transitions. Conversely, if they are inorganic molecules (e.g., phosphonates), their absorption efficiency is mainly due to dipole–dipole, dipole–magnetic, or dipole–electric transitions [44,46].
The most representative instance of this phenomenon is provided by UCNPs, which emit photoluminescence (PL) in both the visible and infrared (IR) regions upon being excited by IR light. The properties are favored by characteristics such as size, surface components, crystallinity, chemical composition, involved fluorophores, and the heterogeneity of electronic states among different components [27,47].
2.4. Radioluminescence
X-rays are high-energy electromagnetic radiation with short wavelengths and high penetrating power. They are currently used for X-ray imaging as a low-cost, high-availability diagnostic tool. However, one of its major drawbacks is that it provides a two-dimensional image, which results in loss or lack of relevant information. In other words, the technique has low sensitivity [48,49].
On the other hand, when ionizing radiation interacts with specific materials, it can produce radioluminescent optical photons. Materials with a high atomic number have demonstrated the greatest efficacy due to their ability to absorb X-rays. This interaction can generate Cerenkov radiation, interact with scintillators, and induce fluorescence, phosphorescence, and luminescence. Nanoparticles have several biomedical applications, including phototherapy, drug delivery, radiotherapy monitoring imaging, and molecular imaging [50,51].
Nanoparticles, particularly those based on lanthanides, have gained attention as materials with potential for radioluminescence imaging. They are influenced by the presence of a significant number of atoms on their surface, as well as by the quantum confinement of electronic states and the area-to-surface ratio. The aforementioned results in an increase in the interaction rate between ionizing radiation and ions, leading to more efficient radioluminescence generation despite their small size. Chemically, the high-Z, cross section, and high crystallinity of the ions are critical factors that enhance X-ray absorption and subsequent conversion into UV or NIR light. Last but not least, the radiation dose received or available to the nanoparticles in their vicinity is enhanced by the phenomenon known as radiosensitization [5,50,52]. However, the small size of nanoparticles when compared to ionizing particles has been deemed a challenge for achieving the desired interaction, resulting in the excitation of only a specific fraction of nanoparticles. Nevertheless, when the same nanoparticles (radionanoparticles) are the emitters of ionizing radiation, this gap is enhanced, as is the intensity of the luminescence emitted by the nanomaterials. The excitation is also “preserved” and influenced by the half-life of the emitting radionuclide [22,33,50,53].
Another benefit of Ln-based nanoparticles is the presence of their surface functionalization components, such as biomolecules, dyes, targeting ligands, and small peptides. These components typically have highly energetic absorption bands, allowing them to contribute to luminescence through energy transfer between their d electrons and the 4f electrons of the Ln3+ ions [22,23,50,53].
Regarding the therapeutic use of radioluminescence, some reports describe the combination of scintillator materials with radiotherapy to promote photodynamic therapy [53,54,55,56]. The approach proposes converting X-rays to light via the use of a scintillator to activate the photosensitizer and achieve a therapeutic effect. The phenomenon’s mechanism is described as relying on the transfer of Cerenkov radiation energy by a photon–photon or beta–photon emission interaction. The primary benefit of this approach is the restriction of additional dose radiation. However, further advancements and research are needed to address the current limitation in the energy transfer of the scintillators [50,57].
2.5. Fluorescence Imaging in the Second Near-Infrared Biological Window (NIR II 1000–1700 nm)
Optical stimulation in the near-infrared (NIR, 700–1700 nm) window has advantages in biomedical imaging because of its low absorption, low scattering, and minimal tissue autofluorescence, which is most evident in the NIR-II region (1000–1700 nm) [58,59].
NIR-II region is very close to zero at wavelengths longer than 1300 nm [60,61,62]. Chemically, the emitting and receiving electrons must conserve their spin moment to enable fluorescence emission. Physically, fluorescence is governed by the anti-Stokes shift phenomenon. NIR light excites the materials and produces emission in the NIR II region. In addition, by the same process, NIR light also excites sources for visible light-emitting fluorophores [24,54,55,63].
Some of the applications of fluorescence imaging with nanoparticles include gene detection, protein analysis, evaluation of enzyme activity, tracking of elements, diagnosis of early-stage diseases, and monitoring of therapeutic effects in real-time. The success of these applications is heavily reliant on several factors: (a) Employ excitation and emission wavelengths within the NIR region, preferably NIR-II, to enable deeper penetration of optical photons with less excitation damage to other components; (b) Implement systems with high biocompatibility and adequate functionalization and stability; (c) Ensure that fluorescence intensity and duration are maintained; (d) Avoid rapid scavenging when using nanoparticles. The appropriate size ranges from 5.5 to 150 nm, depending on the pathology to be analyzed [61,62]. Among the commonly used nanomaterials are fluorescent nanocrystals, or QDs, which emit light from 380 to 2000 nm. These QDs include Ag2S, Ag2Se, ZnS, PbS, PbSe, and CdHgTe. Single-walled carbon nanotubes (SWCNTs) and Ln-based NPs are also frequently utilized.
Ln-based nanoparticles (NPs) can emit in NIR II (Stokes-shift-based imaging agents). This phenomenon is due to the optical and electronic properties of electrons in the 4f orbital. Host matrixes are the primary components, along with sensitizers and activators, where the latter two contain Ln3+ ions. These systems provide benefits such as deep tissue penetration, reduced background noise, and lower toxicity. Other features include high temporal resolution (20 ms), high spatial resolution, and high tissue penetration (up to 3 cm) [28,54,56,60,63]. Moreover, the luminescence efficiency in Ln-based nanoparticles is determined by both internal and external factors. The internal factors are related to the Ln3+ ion, including the resonance level and intrinsic efficiency of the electric–dipole transitions of its 4f electrons. External factors include other competitive upconversion processes and non-radiative cross-relaxation, among others [28].
Examples of Ln-bases NPs include Yb3+-based nanoparticles with NIR-II luminescence at 1525 nm, Nd3+-based nanoparticles with NIR-II luminescence between 1300–1400 nm, Er3+-based nanoparticles with NIR-II luminescence between 1500–1700 nm, and core@shell type organic dye-based nanoparticles with Ln3+ dopant ions [28,59,64,65].
2.6. Magnetic Resonance Imaging
MRI is based on the net polarization of the nuclear spins of water protons in the presence of an intense magnetic field of 1.5 to 3T. Therefore, the magnetic properties of the contrast agent require the insertion of one or more metal centers with unpaired electrons [66]. Gadolinium, terbium, dysprosium, and holmium oxide nanoparticles are of particular interest because they have appreciable magnetic moments, which is useful for MRI [67]. Gd3+ ions have been widely used to produce molecular T1 contrast enhancement [66]. Many papers have been published on Gd3+-based nanostructures of different composition, shape, and size. However, only a few of them have been functionalized with small molecules, such as folic acid [50,54], RGD, chlorotoxin and transactivator of transcription (TAT) peptides [68,69,70], and the anti-thrombomodulin antibody [71]. Other Ln3+ ions increase the magnetic moment as the magnetic field strength increases before reaching saturation, resulting in higher transverse relaxivities [72].
Tb2O3, Ho2O3, and Dy2O3 nanoparticles have been reported as useful as T2 MRI contrast agents at high MR fields in vivo [71,72]. Although the lanthanide ion Eu2+ also has seven unpaired electrons in its outer electron shell, it has a larger ionic radius than Gd3+, which results in a faster water exchange rate. This characteristic ensures that Eu-based contrast agents have relatively high relaxivity values [66]. However, to date, no functionalized nanoparticles containing Tb+3, Ho+3, Dy+3, and Eu2+ ions have been described for molecularly targeted MRI.
3. Modification of Lanthanide-Based Nanoparticles with Active Molecules for Theranostics
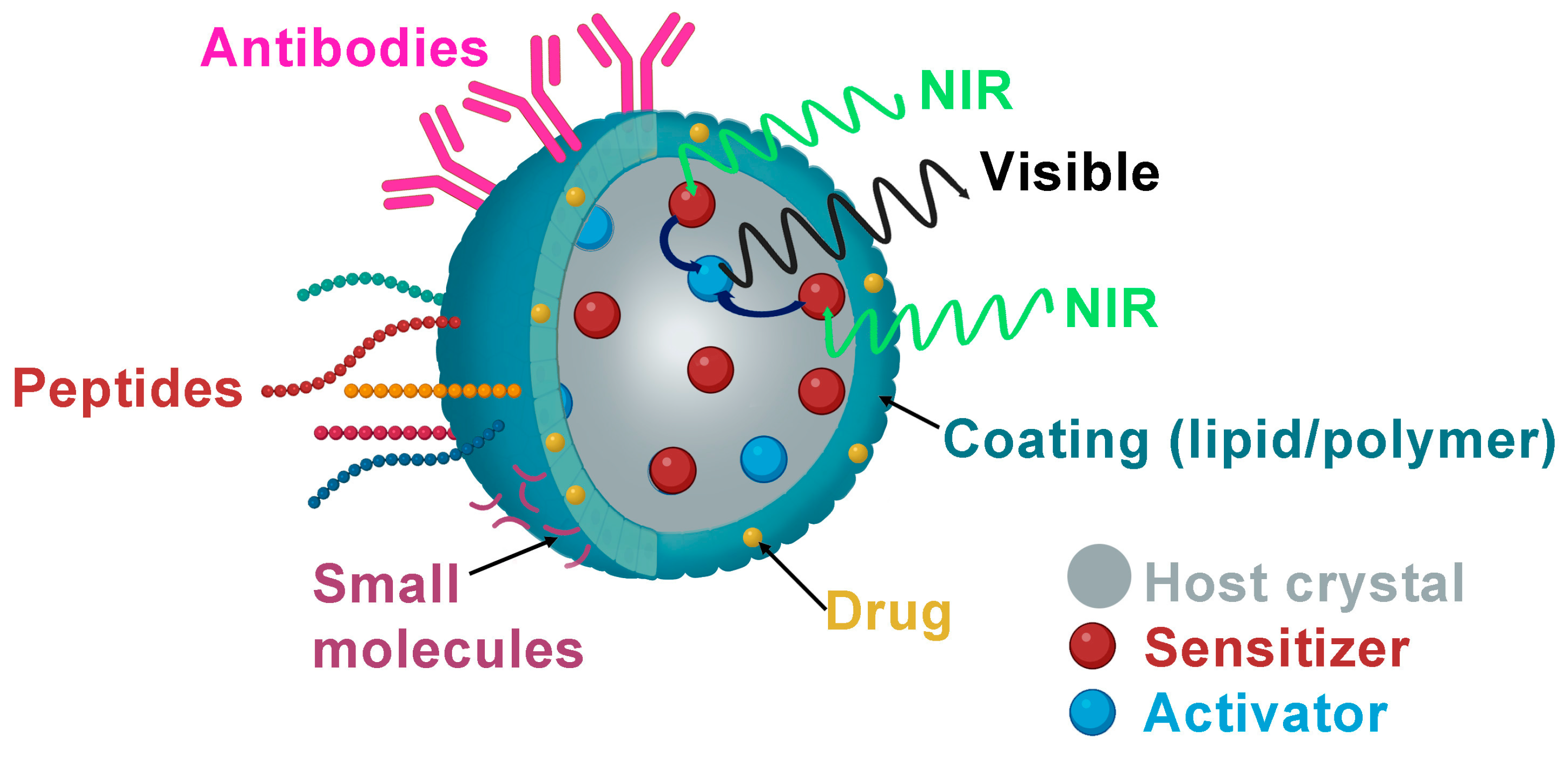
Lanthanide nanoparticles have been functionalized with various biomolecules to molecularly target different specific sites in the tumor microenvironment (Table 2 and Figure 2).

Table 2.
Targeted lanthanide-based nanoparticles for molecular imaging and therapy 1.
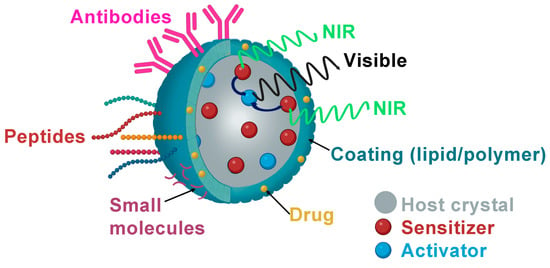
Figure 2.
Schematic illustration of biological ligand-mediated targeted UCNPs that could be loaded with anticancer drugs as DOX and functionalized with small molecules as FA, peptides or antibodies.
3.1. Folic Acid (Small Molecules)
The first attempt to produce targeted NPs with biological molecules was made using stable and non-immunogenic small molecules such as folate or folic acid (FA), a water-soluble vitamin with a low molecular weight (411.4 Da). Folate receptors are overexpressed on rapidly proliferating cells, such as cancer cells, due to their increased need for folate compared to normal cells. This overexpression in human tumors such as endometrial, breast, ovarian, and brain cancers can be used to target these tumors [65].
Cao et al. were the first to report water-soluble UCNPs functionalized with FA by introducing 6-aminohexanoic acid (AA) and oleate to control generation and crystal growth. AA was attached to the lanthanide ions through the carboxylic acid group, providing free amine groups that made the NPs dispersible in water and allowed conjugation with the FA. The UCNPs showed intense luminescence and helped obtain mouse lymphatic capillary imaging with a high signal-to-noise ratio. Their FA-functionalized UCNPs showed specific internalization in KB (FR-positive) cells, but the author did not test them in vivo [73]. The first FA-functionalized UCNPs used in vivo were reported by Idris et al., who used them as nanotransducers to convert NIR light to visible wavelengths and also as PS. The authors showed that irradiation of these UCNPs with a 980 nm laser produces green and red light and activates the coloaded PS cargo, increasing the generation of cytotoxic singlet oxygen and reducing cell viability in vitro, and also demonstrated growth inhibition of melanoma tumors in mouse models [74].
Sun et al. prepared three kinds of FA-functionalized UCNPs (UCNC-FA) by doping NaYbF4 with Er3+ and Tm3+ ions (UCNC-Tm-FA, UCNC-Er-FA and UCNC-Er, Tm-FA) and demonstrated that UCNC-FA NPs were able to efficiently target HELA cells in vitro with low toxicity and good biocompatibility, and thus could be used as a potential fluorescent contrast agent [75]. Similar results were reported by Gainer et al. using NaYbF4 doped with Er3+ and Yb3+ and functionalized with FA; they were able to obtain luminescence images of ovarian cancer by multiphoton scanning microscopy, demonstrating the potential application of lanthanide nanoparticles to detect relevant receptors in tissues [76]. NaYbF4 doped with Yb3+, Tm3+ were also used by An et al., but they incorporated the Fe3+, and demonstrated that the addition of 20% of the non-luminescent Fe3+ increased the NIR UCL intensity 20-fold. The authors also add NaGdF4 to the nanoparticle to develop multimodal in vivo bioimaging, as Gd3+ ions exhibit high X-ray attenuation and strong paramagnetic properties. They demonstrate that FA functionalization of this NP can successfully target tumors in vivo and obtain trimodal imaging (fluorescence, MRI, and X-ray) [77]. Recently, NaYbF4 core shells doped with Nd3+ and functionalized with FA have also been shown to be promising dual-PS nanoconstructs for the treatment of deep-seated tumors, as they can maximize the antitumor efficacy of PDT through the 808 nm laser excitation capability [78]. Zeng et al. also enhanced the therapeutic efficacy in deep tumor sites producing ROS and PTT by irradiation of 980 nm laser irradiation of a Linde Type A (LTA) zeolite-derived UCNPs dopped with ER3+ and Yb3+ functionalized with FA-PEG [79].
Metal-organic frameworks (MOFs) are also promising candidates for drug delivery due to their microporous structure. UCNPs have been used to increase drug concentration at the tumor site by encapsulating molecules and rendering them biologically inert, thereby increasing chemotherapeutic efficacy and reducing toxicity and side effects for patients. By physical adsorption, Liu et al. synthesized PeGylated UCNPs loaded with the anticancer drug doxorubicin (DOX). They conjugated them with folic acid to evaluate the loading and release of DOX at different pH values. They found that 20% of DOX was released at physiological pH (7.4), with this rate increasing at lower pH values. In vitro studies showed that this nanoconstruct produced cytotoxic effects in folate receptor-positive cells due to the enhanced cellular uptake of DOX caused by the specific FA targeting [80]. Chien et al. reported another folate-PEGylated UCNP as a delivery agent for bioimaging and chemotherapy. Still, in this case, DOX was thiolated on the surface of these NPs by disulfide bonds that can be cleaved by the lysosomal enzymes present in the cell [81]. When UCNPs were irradiated with a 980 nm laser, they emitted upconversion luminescence at 360 nm, which uncaged the photolabile o-nitrobenzyl group and allowed FA to target the tumor cells and deliver the DOX via FR-mediated endocytosis. This nanostructure’s therapeutic efficacy and in vivo, photoluminescence imaging was demonstrated in mice bearing a HeLa tumor model. The brightest NIR emission in the tumor was found 5 h after injection, and mice showed significant inhibition of tumor growth compared to control groups. Recently, Cong et al. used a new layer-by-layer self-assembly method to synthesize core–shell microspheres NaYF4:Yb,Er@NaYF4:Nd@MIL-53, and encapsulated the FA into the structure to be used as DOX delivery carriers [82]. The in vitro test showed that the core–shell was non-toxic to cells, and the functionalized version could recognize the target cells.
Cao et al. synthesized a MoS2 quantum dots (QDs) decorated UCNPs nanoplatform, modified it with PEG and FA, loaded it with DOX (UMPFD), and evaluated it as a tumor-targeting agent for chemo-photodynamic synergistic therapy [83]. These constructed UCNPs were designed to convert 980 nm NIR into visible light, which can activate the MoS2QDs to produce oxygen species by fluorescence resonance energy transfer. UMPFDs exhibited good biocompatibility and tumor targeting with pH-responsive drug release and abundant ROS generation.
Mesoporous silica nanospheres (MSNs) have also been functionalized with FA for tumor targeting. Shi et al. reported MSNs loaded with Zn1.1Ga1.8Ge0.1O4:Cr3+, Eu3+ to obtain persistent luminescent nanoparticles (PLNPs), which were modified with FA and subsequently loaded with DOX [84]. The DOX-NLPLNPs@MSNs-FA was evaluated as a real-time monitoring drug delivery agent. The results showed that this construct exhibited long afterglow NIR luminescence and high sensitivity to target tumors in vitro and in vivo with a sustained release of DOX.
Lanthanum oxide nanoparticles have also been used as optical devices in medicine. Recently, hafnium dioxide (HfO2) NPs doped with Eu3+, Gd3+, and Tb3+ ions and functionalized with FA were synthesized by different routes to evaluate their potential as a matrix for multimodal theranostic agents for image-guided radiotherapy. The results showed that sol–gel-based synthesis was the best method to prepare uniformly doped particles with good size control. It was also demonstrated that introducing lanthanide ions could tune the luminescence, MR, and CT properties. Gd:HfO2 NPs showed the best properties, such as the lowest degradation rate and no relevant in vitro cytotoxicity [85]. Recently, Cai et al. reported a versatile nanosystem for real-time monitoring of cancer treatment efficacy. They developed gadolinium oxyfluoride (GdOF) nanoparticles doped with Yr and Er, which were used to encapsulate Au nanosheets, then decorated with both DOX and the photosensitizer rose bengal (RB), and functionalized with FA [54]. Thanks to doped lanthanide ions and encapsulated Au nanosheets, trimodal fluorescence, CT, and MRI imaging were obtained simultaneously. Moreover, the fluorescence resonance energy transfer from GdOF to RB in this nanosystem contributed to the high PDT efficacy, which acted synergistically with the chemotherapy in the targeted tumor microenvironment, resulting in tumor eradication after 14 days of administration in mice with cervical carcinoma.
3.2. Peptides
Peptides are cancer-specific ligands with moderate size, high stability, and low immunogenicity but still possess a high specificity towards their target. Moreover, they are easy to synthesize on a large scale and modify; therefore, some peptides have been used to functionalize the NPs to be applied as image-guided tracking and also to direct chemical drug-loaded lanthanide nanoprobes to the tumor sites, achieving higher tumor inhibition than pristine drugs [9].
The most commonly used peptide to functionalize NPs is arginine-glycine-aspartic acid (RGD) because it can recognize the adhesion receptor integrin αvβ3, which is overexpressed on tumor endothelial cells [104]. Xiong et al. reported the first UCNPs doped with Yb, Er, and Tm and functionalized with RGD for fluorescence-targeted imaging in vivo. To improve the blood circulation of the nanosystem, the authors added a PEG linkage [91]. Confocal imaging of tissue sections at high penetration depths of these nanosystems showed that UCL imaging without an autofluorescence signal was possible. In addition, the experimental results in mice bearing human glioblastoma (U87MG) showed that the functionalization of this nanoplatform enables target-specific imaging of tumors with a high signal-to-noise ratio, making this technique also suitable for tracking in vivo components of biological systems. Jin et al. added Ga3+ DOTA to RGD-functionalized UCNP to perform in vivo fluorescence upconversion studies and MRI of glioblastoma with excellent results in mice bearing xenografted tumors [68]. Tang et al. used similar nanosystems to deliver the chlorin e6, a photosensitizer, and a chelator for Mn2+ loaded in human serum albumin, conjugated to the UCNP. The results demonstrated these NPs’ potential for MRI and glioma PDT [92]. Recently, a NIR-regulated nanoplataform called UCNP@TTD-cRGD was synthesized by using an amphiphilic polymer to encapsulate UCNPs and the TTD luminogen and functionalized with cyclic RGD (cRGD) to be used as an antitumor and bioimaging agent. The results showed that this nanosystem could selectively target MDA-MB-231 cancer cells in mice bearing subcutaneous tumors and inhibit their growth due to two main facts: the NIR-regulated PDT treatment in vivo and also the ROS generation by the close matching of UCNP emission and the absorption of the aggregation-induced emission [93]. CsLu2F7-based UCNPs doped with Yb, Er, and TM were also functionalized with RGD and loaded with the chemotherapeutic drug α-tocopheryl succinate (alpha-TOS) and the photothermal agent ICG for multifunctional imaging (UCL and CT) and targeted synergistic therapy. In vivo studies in mice bearing U87MG tumors showed a significant tumor reduction after treatment with this nanoplatform [94].
Prostate-specific membrane antigen (PSMA protein) is an important molecular target due to its overexpression in various cancer cells, including advanced and metastatic prostate cancer. This has led to successfully generating radiolabeled PSMA inhibitor peptide (iPSMA) based systems as agents for molecular imaging and radiotherapy [23,105,106]. Another protein overexpressed in several neoplastic cells, including early prostate cancer, is the gastrin-releasing peptide receptor (GRPr), which recognizes the bombesin peptide selectively and specifically [107]. Therefore, a heterodimeric peptide of iPSMA and bombesin has been reported in the functionalization of [153Sm]Sm2O3 nanoparticles with radioluminescent and radiotherapeutic features (NIR at 750 nm), allowing in vivo optical imaging of their biodistribution and high affinity with respect to PSMA protein and GRP receptors [22].
The iPSMA peptide has also been used to functionalize [166Dy]Dy2O3, 166Ho2O3, and [177Lu]Lu2O3 nanoparticles with utility as targeted radiotherapy systems [23,105,106]. Dysprosium and holmium oxide nanoparticles were reported as a novel in vivo generator. That is, [166Dy]Dy2O3 NPs were obtained by neutron irradiation of 164Dy2O3 NPs, which decay to 166Ho2O3 NPs under a nuclear transient equilibrium. After functionalization with the iPSMA peptide, an in vivo [166Dy]Dy2O3-iPSMA/166Ho2O3-iPSMA generator for medical applications was obtained. In vitro and in vivo studies demonstrated the potential of this molecularly targeted lanthanide generator to deliver ablative radiation doses to HepG2 liver cancer cells [89].
On the other hand, chemical analysis of Lu2O3-iPSMA also resulted in a well-defined hemispherical shape with a uniform and monodispersed size distribution (30–45 nm) and characteristic radioluminescence features after neutron activation. The in vitro studies showed a high affinity of 177Lu2O3-PSMA for PSMA-expressing cells. Preclinical biodistribution in murine models demonstrated the nanosystem’s potential for in vivo nuclear and optical imaging and its utility for targeted radiotherapy [22,23].
Fibroblast activation protein (FAP) has become one of the most important molecular targets involved in the tumor microenvironment [108]. Cancer-associated fibroblasts induce the cancer phenotype, account for 90% of the macroscopic tumor mass, and strongly express FAP in >90% of carcinomas. As a result, FAP is overexpressed in the neoplastic microenvironment of more than ninety percent of epithelial tumors and is a major contributor to cancer progression [108]. This is why Lu2O3 nanoparticles activated by neutron activation have also been functionalized with FAP inhibitor peptides (iFAP). Additionally, the iPSMA and iFAP peptides were concomitantly attached to the lutetium oxide nanoparticles to be used as a dual-targeted radiotherapy nanosystem [5,6]. The quality control results showed that the nanoparticles with the appropriate pharmaceutical properties were produced reproducibly under good manufacturing practices [6].
Bombesin can also be recognized by GRPr overexpressed in breast, prostate, lung, CNS, colon, and pancreatic tumors, where it functions as an autocrine growth factor [109]. Tang et al. first used bombesin to functionalize Gd3+-loaded UCNPs and used it to obtain in vivo MRI, CT, and UCL images of prostate tumors [88].
Another peptide that has been used to functionalize lanthanide nanoparticles is Angiopep-2 (Ang2), a peptide designed from the Kunitz domain that binds to low-density lipoprotein receptor-related protein 1 (LRP1) at the blood–brain barrier (BBB) and has been shown to enhance nanosystem accumulation in brain tumors [110]. UCNPs were coated with oleic acid and functionalized with PEG-conjugated Ang2 peptide to deliver hydrophobic photosensitizers to brain tumors in mouse models [87]. In vivo studies showed that ANG-IMNPs could cross the BBB and selectively deliver synergistic PDT and PTT to brain astrocytoma tumors, significantly improving mouse survival. Ang et al. developed a multi-shell Nd-UCNPs, loaded with DOX and chlorin e6 (Ce6) and functionalized with an Ang2 peptide for a synergistic metronomic chemo-photodynamic therapy of glioblastoma [86]. This study provided evidence that these nanosystems could cross the BBB and be endocytosed by the lysosomes of glioblastomas in mouse models. In addition, they demonstrated a 3.5-fold higher antitumor efficacy compared to standard chemotherapy and inhibited angiogenesis in the tumor microenvironment. Ren et al. used down-conversion nanoparticles (DCNPs) modified with a dye-brush polymer and functionalized with Ang2 peptide. They delivered them into the glioma by temporally opening the BBB using ultrasound sonication [64]. The authors obtained a targeted NIR IIb fluorescence imaging with a very high tumor-to-background ratio (TBR = 12.5) for small orthotopic gliomas. They found that the glioma size obtained by this method was very close to the size obtained from T2-weighted MRI images.
KE108, a somatostatin analog that binds with high affinity to all five somatostatin receptors, has also been used to specifically deliver nanoplatforms to neuroendocrine tumors [111]. Chem et al. developed theranostic micelles formed by UCNPs functionalized with photosensitive copolymer (PNBMA-PEG) and the photosensitizer RB. The micelles were loaded with the anticancer drug AB3 and conjugated with the KE180 peptide. These UCNP micelles proved to be good UCL imaging agents without apparent systemic toxicity. Moreover, they offer the possibility of selectively targeting neuroendocrine tumors in vivo, producing synergistic effects of chemotherapy and PDT, resulting in higher antitumor efficacy than a single technique [90].
TP53 is one of the most frequently mutated genes in human cancer; therefore, small molecules capable of reacting with the mutant p53 function have recently been used as an anticancer strategy [112]. The p53-activating peptide PMI is one of them. He et al. designed a nanorod using lanthanide-doped nanocrystals functionalized with PEG and PMI to treat human colon cancer in mouse models. This LProd proved to be useful for real-time disease tracking and produced tumor growth inhibition by activating the tumor suppressor p53, showing improved tumor targeting compared to its spherical counterpart [113].
It has been reported that the dual-targeted radiotherapy strategy increases the affinity of the nanosystem for the tumor sites. Proof of this fact is the lanthanide oxide nanoparticles functionalized with heterodimeric peptides such as bombesin/iPSMA or iFAP/iPSMA described above [5,6,23]. Recently, a dual-targeting nanoplatform (UCNP@P-RGD-NGR) has also been developed using polydopamine-coated UCNPs conjugated with two peptides, the RGD and the asparagine-glycine-arginine (NGR), which were used to target the integrin and aminopeptidase N receptors present in tumor cells. This nanoplatform was shown to be non-toxic, biocompatible, and highly specific for lung tumor cells in vitro and in vivo, as well as be able to discriminate tumors from the surrounding normal tissue by UCL [95]. Zha et al. also used this dual targeting to monitor and inhibit cancer associated with the Epstein–Barr virus using UCNPs functionalized with two peptides that specifically bind to EBNA1 and LMP1, two overexpressed EBV oncoproteins. This nanosystem was used as a pH-sensitive imaging agent for cancer differentiation, showing enhanced specific uptake in EBV-positive tumors, resulting in high therapeutic efficacy demonstrated by in vivo tumor inhibition [96].
3.3. Antibodies
Several antibodies or antigens have been attached to lanthanide nanoparticles to produce luminescent images and/or targeted radiotherapy. In these approaches, the role of antibodies is primarily to target approaches to the interested tissue through specific recognition. In these terms, antibody-functionalized lanthanide-doped CaF2 biolabel for cancer cell targeting was synthesized by Sasidharan et al. They prepared monodispersed lanthanide (Eu3+) doped CaF2 nanoparticles and functionalized them with anti-EGFR through EDC-NHS coupling chemistry for specific binding to EGFR-overexpressing cells. The nanoparticles exhibited strong fluorescence emission at 612 nm [97]. EGFR overexpression has also been used to enable specific binding of functionalized UNPs (anti-EGFR-UNPs) for imaging modalities to achieve three-dimensional functional tissue imaging based on laser scanning in cancer cells [98].
Lanthanide nanoparticles functionalized with polyethyleneimine (PEI) and an antibody to detect alpha-fetoprotein (AFP) were also prepared. The positively charged PEI-PLNPs were bound to the negatively charged antibody-functionalized gold nanoparticles, forming an FRET inhibition probe. The persistent luminescence of the PEI-PLNPs was quenched in the presence of AFP but recovered when the AFP-specific antibodies were desorbed from the nanoparticles [99]. In a different nanoparticle–antibody system for targeted radiotherapy, the mAb-201b was conjugated to Au-coated lanthanide phosphate nanoparticles containing 177Lu to target thrombomodulin receptors for pulmonary metastatic disease. This interesting approach resulted in up to 95% of the injected dose being delivered to the lungs [71].
Fluorescent lanthanide oxyfluoride nanoparticles conjugated with anti-CD-33 antibody for potential treatment of acute myeloid leukemia by conjugating PMI, a dodecameric peptide antagonist of MDM2 and MDMX and a CD-33-targeted humanized monoclonal antibody to nanoparticles via metal thiolate bonds. NP emitted fluorescence at 545 nm with minor red emission peaks at 585 and 620 nm. The lanthanide nanoparticles used in this study were composed of LaOF, Ce, and Tb and were designed to have specific properties for their application in drug delivery for acute myeloid leukemia (AML) therapy through binding to CD33 surface receptor overexpressed in AML, which allows the intracellular drug delivery of PMI. This peptide antagonizes MDM2 and MDMX to activate the p53 pathway, leading to apoptosis of AML cells. This nanosystem also enables real-time visualization of apoptotic events in AML cells [100].
Biocompatible cubic-phase erbium-based rare-earth nanoparticles (ErNPs) with downconversion luminescence at 1600 nm for dynamic imaging of cancer immunotherapy were also prepared. Functionalization was performed with cross-linked hydrophilic polymer layers decorated with anti-PD-L1 antibodies for molecular imaging of PD-L1 in a colon cancer model, achieving a tumor-to-normal tissue ratio of approximately 40. The PD-L1 antibody enabled the specific targeting and imaging of PD-L1 in the tumor microenvironment to evaluate the immune checkpoint blockade therapy and the response to immunotherapy [101].
NP-avidin and NP-IgG were synthesized by Eliseeva et. al. The lanthanide (Eu, Gd) binuclear helicate [Ln2(LC2)3] was embedded into silica nanoparticles Ln2(LC2)3]@SiO2/NH2 used to detect and mark cancerous cells in immunocytochemical assays. Ln2(LC2)3]@SiO2 was then conjugated with either avidin or goat anti-mouse IgG antibody to target mucin-like antigens expressed specifically on the cell membrane. The nanoparticles display red emission and have a high quantum yield, making them effective markers for cancer cells. The bioconjugates of the nanoparticles with the IgG antibody show a higher luminescent signal-to-noise ratio compared to the bioconjugates of the molecular probe with IgG [114].
Biosensors traditionally focus on the use of antibodies for antigen detection. However, antibody detection is now increasingly utilized in biosensor research. Antibody detection has been applied to monitor immune responses to biotoxins based on the antigen–antibody recognition systems in HIV infections, tuberculosis, human papillomavirus, and breast cancer or to measure the vaccine-induced immune response. Covalent conjugation of okadaic acid to LaF3:Ce, Tb nanoparticles was performed to detect anti-okadaic acid antibodies. This system allows luminescence resonance energy transfer (LRET) to detect anti-okadaic acid rabbit polyclonal IgG for new antigen–antibody recognition applications [115].
3.4. DNA/RNA and Aptamers
Attaching DNA/RNA to nanomaterials will enable nucleic acid-based assembly and drug delivery. RNA interference (RNAi) is a natural cellular process of post-transcriptional gene regulation that uses small, double-stranded RNAs to induce the controlled silencing of genes (siRNA). Therefore, by introducing siRNAs, we are able to harness the RNAi machinery for the therapeutic control of genes and the treatment of a variety of diseases [116,117,118].
For siRNA delivery, nanoparticles have been recently proposed as excellent approaches. siRNA can be incorporated into an NP formulation by covalently binding to NP components or by electrostatic interaction with the NP surface [119]. Coordination of lanthanides with phosphate groups and nucleobases is used to construct lanthanide-doped nanoparticles. The siRNA/NaGdF4 nanoparticle system allowed siRNA to escape from the endosome for efficient gene silencing in vivo. In addition, siRNA targeting PD-L1 (siPD-L1) complexed with NaGdF4 was able to block PD-L1 expression and thereby suppress tumor growth in mouse models of breast and colon cancer [102].
Aptamers are single-stranded DNA or RNA sequences between 20 and 80 nucleotides in length that bind to their target with high affinity. The AS1411 aptamer (dsDNA) binds nucleolin overexpressed in the nucleus of cancer cells and was employed to synthesize UCNPs@PDL@dsDNA/DOX for doxorubicin delivery into the nucleus of cancer cells [103].
4. Theranostic Lanthanide Nanoparticles with Potential for Clinical Translation
The stability of the colloidal solution is the first requirement for the clinical translation of the different theranostic nanosystems of LnNPs as stable, effective, and safe drug forms [115].
For the stability of LnNPs, repulsive forces must be generated by similar charges to prevent the coagulation of the colloidal particles. After preparing a nanocolloidal solution from which all ions are removed by dialysis, the particles agglomerate because the total surface area is reduced, the particle size increases, and the particles settle rapidly. Therefore, the presence of electrolytes that provide some electrical charge is always necessary. However, the number of electrolytes must be limited to the amount that the colloids can adsorb, since an excess reduces the zeta potential by accumulating ions opposite to the charge of the LnNPs. Therefore, the number of electrolytes should be sufficient to keep the zeta potential below a critical level (Table 3) [120].

Table 3.
Values of the zeta potential for the stability of nanocolloids.
In this context, most of the lanthanide nanoparticles functionalized with small molecules, peptides, antibodies, and oligonucleotides mentioned in Table 2, have demonstrated that they can be prepared as isotonic solutions with high colloidal stability, potentially satisfying the first requirement for a stable dosage form.
The stability of nanoparticles as a pharmaceutical form with high zeta potential values is a prerequisite for their in vivo application. However, interactions with components of biological fluids, such as proteins, electrolytes, and small molecules, influence their electrical potential and reduce the absolute value of the zeta potential, although without affecting the stability of the nanosystems when coated with biomolecules covalently bound to their surface, which guarantees colloidal stability [23].
The development of efficient processes that meet the good manufacturing practices (GMP) requirements of regulatory authorities is one of the most important current challenges in the production of theranostic nanoparticles for use in patients. Biomolecule-coated LnNPs (Table 2) can be produced under GMP procedures as sterilized colloidal liquids with the quality characteristics of a clinical-grade formulation [6].
The toxicity associated with nanoparticles varies depending on their intrinsic composition, shape and size, surface area, surface layer, and in vivo stability [121]. The mechanism of nanoparticle toxicity is based on their ability to generate cytotoxic and genotoxic reactive species related to immune-mediated effects (inflammation) and oxidative stress [122]. Among the most studied are the toxic effects of metal nanoparticles on tissues. Since the main uptake of nanoparticles is by hepatic Kupffer cells and splenic macrophages, they have been described to induce hepatic dysfunction and structural changes related to, for example, increased levels of interleukin-1β and interleukin-6, pro-inflammatory cytokines, as well as increased alkaline phosphatase, liver enzyme aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [123,124,125,126]. In addition, pyroptosis, lipid peroxidation (induced by reactive oxygen species), autophagy, and necrosis are pathways of hepatocyte damage and death attributed to nanoparticle toxicity in various studies [122,124,126].
However, specific differences make LnNPs a valuable and safe option to use in theranostics for cancer patients. First, the immune system does not recognize LnNPs as foreign elements because of the biocompatibility of their surface due to the use of biomolecules (Table 2). Second, chemical interactions that could form reactive oxygen species are minimized due to the steric effect of the biomolecules covering the nanoparticle. As previously demonstrated [6], the administration of functionalized lanthanide nanoparticles in mice does not show signs of inflammation (lymphocytic infiltration), hemorrhage, granuloma formation, fibrotic processes, or necrosis in healthy tissues [6]. Spleen and liver macrophages have been reported as the only mechanisms of cellular uptake of functionalized lanthanide nanoparticles [5]. Notably, macrophages are highly resistant to the point that they are able to repair double-strand breaks in DNA [124,127]. Increased transcriptional activation of NF-κB and Bcl-xL expression have been described as pro-survival mechanisms of macrophages [128]. In addition, macrophages damaged by irradiation or oxidative stress continue being metabolically active, viable, exhibit a reduced anti-inflammatory profile (decreased expression of mannose receptor C-type 1, versican, interleukin 10, and the cluster of differentiation 163, CD163) and increased phagocytic activity [127]. Phagocytosis of certain metal nanoparticles by macrophages has been reported to induce pro-inflammatory mechanisms [128]. However, in the case of functionalized lanthanides (e.g., Lu2O3-iPSMA/iFAP), no damage is observed in healthy tissues, which is attributed to biocompatibility mediated by ligands coating the nanoparticle surface [6].
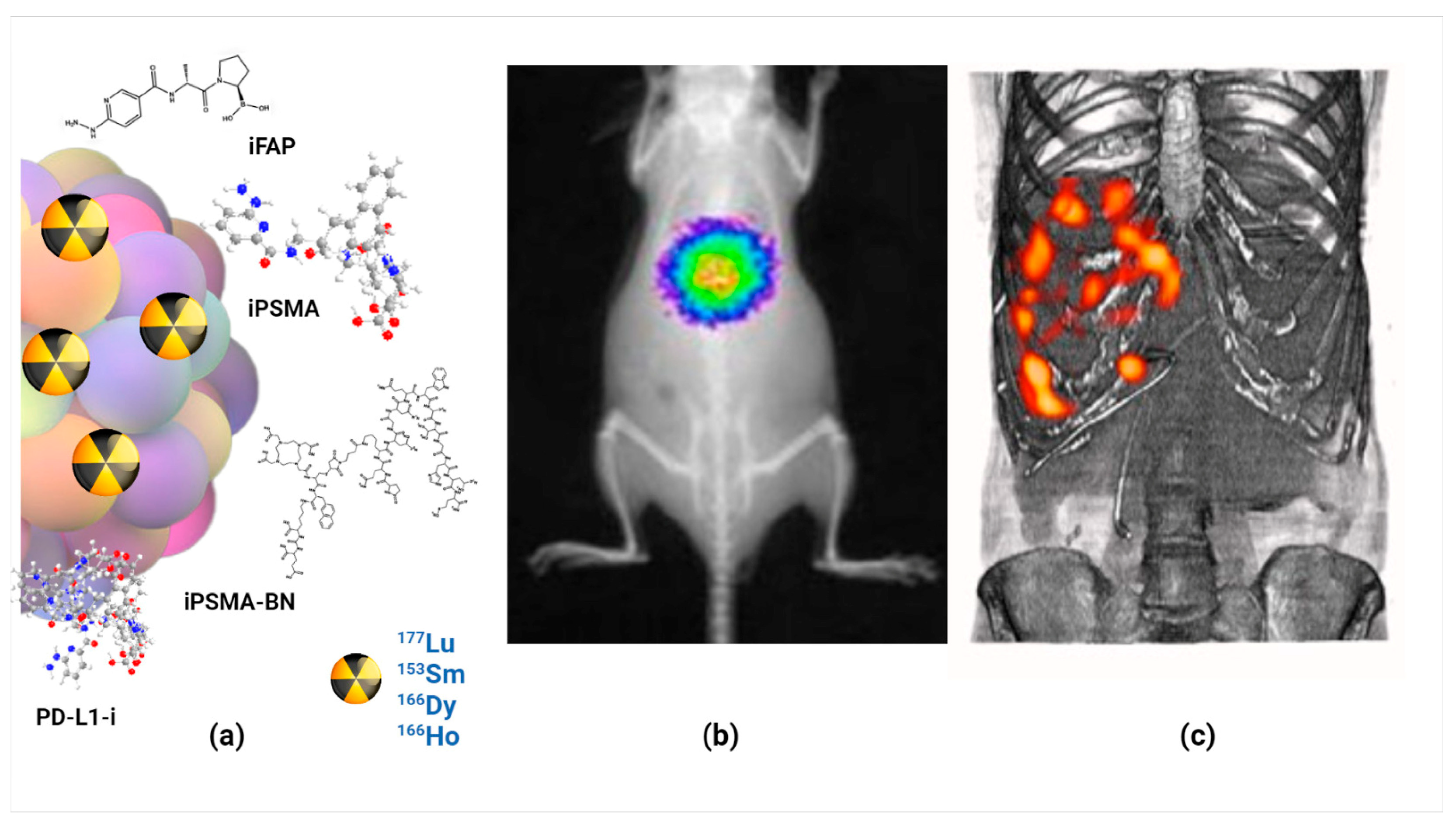
As an interesting case, preclinical results have shown that Lu2O3-iPSMA/-iFAP nanoparticles activated by neutron irradiation inhibit colorectal HCT116 tumor progression due to a combination of radiotherapy, prolonged tumor retention, and molecular recognition of iPSMA and iFAP, which made possible the in vivo tumor optical and nuclear imaging. There was no liver and renal toxicity evidence since negligible uptake values in non-target tissues were observed. The first clinical case of a patient with colorectal liver metastases was the proof-of-concept showing the selective uptake of the LnNPs by liver metastases but not in the liver parenchyma (Figure 3) [5].
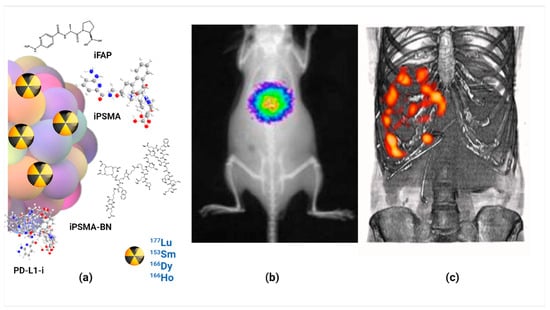
Figure 3.
Schematic illustration of (a) molecularly targeted lanthanide nanoparticles for (b) optical imaging, (c) nuclear imaging and targeted radiotherapy (theranostics) of malignant tumors; observe the selective uptake of radiolanthanide lutetium oxide nanoparticles in colorectal liver metastases with negligible uptake in the liver parenchyma.
As mentioned above, luminescence imaging and drug delivery using LnNPs is another area where preclinical studies have demonstrated their theranostic potential. For example, the UCNPs@polyHPMA-5FU nanosystem is used for luminescence imaging and selective cancer drug delivery [37]. Also relevant is the development of target-specific biomolecules coated on Yb/Er/Ho enriched NaGdF4 nanoparticles as NIR-II systems for luminescence imaging, MR imaging, X-ray CT imaging, and photodynamic therapy (Table 2).
5. Conclusions and Outlook
Lanthanide-based nanoparticles for multimodal imaging and multifunctional therapy in cancer have been in discussion as potential nanomedicine tools for about two decades. However, to achieve their application in cancer patients, molecular targeting is mandatory. Functionalization must ensure that LnNPs selectively target the tumor microenvironment by directing them to one or more molecular targets. Once accumulated around or inside cancer cells, lanthanide nanosystems must be retained to effectively exert the therapy for which they were designed (photothermal therapy, radiotherapy, photodynamic therapy, chemotherapeutic drug release, etc.), as well as to detect, in time and space, molecular processes by different imaging modalities (optical imaging, nuclear imaging, magnetic resonance imaging, etc.).
Likewise, LnNPs should have a particle size between 10 and 100 nm, so that most of them can be captured and retained by tumors without crossing the endothelium of healthy tissues, while those NPs captured in a smaller percentage by macrophages (endothelial reticulum system) can be eliminated over time through the lymphatic system, avoiding damage to normal organs.
Analyzing the advances presented in this review, we can conclude and establish the following outlook:
- Many lanthanide-based nanosystems have successfully completed the preclinical phases, so their preparation under good manufacturing practices as stable colloidal dosage forms should be carried out to accelerate their clinical translation.
- Considering recent advances in cancer molecular biology, a greater number of different LnNPs, designed as multimodal and multifunctional nanosystems, should be functionalized with molecular targeting biomolecules associated with immunosuppression checkpoints and with those associated with tumor microenvironment remodeling. For example, functionalization with peptide inhibitors of PD-L1, PD-1, and FAP proteins would allow monitoring of disease prognosis and follow-up, as well as determining whether anti-PD-L1 immunotherapy can be combined with radiotherapy using neutron-activated lanthanide nanoparticles. It would also allow a better understanding of disease progression, therapeutic resistance, and metastasis in patients in a personalized manner.
- There is a need to increase the number of preclinical studies of lanthanide-based nanosystems whose imaging and therapeutic features could be evaluated with the imaging equipment and technology most widely available in different medical centers. This would allow LnNPs to become clinically translated in less time.
- Nevertheless, recent advances in the synthesis of monodisperse, biocompatible, and surface-functionalized LnNPs have been of paramount importance in demonstrating their usefulness in cancer theranostics, from which clinical applications could become a reality in the short and medium term.
Author Contributions
Conceptualization, G.F.-F.; methodology, A.A.-C., B.O.-G., L.M.-A. and G.F.-F.; formal analysis, A.A.-C., B.O.-G., L.M.-A. and G.F.-F.; writing—original draft preparation, A.A.-C., B.O.-G., L.M.-A. and G.F.-F.; writing—review and editing, L.M.-A. All authors are responsible for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the 5x1000 (2019) for the financial support of the project Development of 67Cu-Radiopharmaceuticals for Theranostic of Prostate Cancer (DECURTA) to LMA. This research was funded by the Italian Ministry of Health Ricerca Corrente.
Data Availability Statement
Not applicable.
Acknowledgments
This study was conducted as a component of the activities of the “Laboratorio Nacional de Investigación y Desarrollo de Radiofármacos, ININ”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, S.-Y.; Guo, X.-Q.; Zhou, L.-P.; Sun, Q.-F. Fine-tuned visible and near-infrared luminescence on self-assembled lanthanide-organic tetrahedral cages with triazole-based chelates. Inorg. Chem. 2019, 58, 7091–7098. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide nanoparticles: From design toward bioimaging and therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Tegafaw, T.; Liu, Y.; Ho, S.L.; Liu, S.; Ahmad, M.Y.; Al Saidi, A.K.A.; Zhao, D.; Ahn, D.; Nam, H.; Chae, W.-S. High-Quantum-Yield Ultrasmall Ln2O3 (Ln = Eu, Tb, or Dy) Nanoparticle Colloids in Aqueous Media Obtained via Photosensitization. Langmuir 2023, 39, 15338–15342. [Google Scholar] [CrossRef]
- Luna-Gutiérrez, M.; Ocampo-García, B.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Trujillo-Benítez, D.; Hernández-Jiménez, T.; Ramírez-Nava, G.; Hernández-Ramírez, R.; Santos-Cuevas, C.; Ferro-Flores, G. Targeted Endoradiotherapy with Lu2O3-iPSMA/-iFAP Nanoparticles Activated by Neutron Irradiation: Preclinical Evaluation and First Patient Image. Pharmaceutics 2022, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, T.; Cruz-Nova, P.; Ancira-Cortez, A.; Gibbens-Bandala, B.; Lara-Almazán, N.; Ocampo-García, B.; Santos-Cuevas, C.; Morales-Avila, E.; Ferro-Flores, G. Toxicity Assessment of [177Lu] Lu− iFAP/iPSMA Nanoparticles Prepared under GMP-Compliant Radiopharmaceutical Processes. Nanomaterials 2022, 12, 4181. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Wei, Z.; Chao, Z.; Zhang, X.; Yu, J.; Xiao, F.; Zhang, X.; Tian, L. NIR-II Luminescent and Multi-Responsive Rare Earth Nanocrystals for Improved Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2023, 15, 11575–11585. [Google Scholar] [CrossRef]
- Luo, Z.; Yi, Z.; Liu, X. Surface Engineering of Lanthanide Nanoparticles for Oncotherapy. Acc. Chem. Res. 2023, 56, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; O’Brien, S.; Grimm, J. Biomedical applications of lanthanide nanomaterials, for imaging, sensing and therapy. Nanotheranostics 2022, 6, 184. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, M.M.; Kaszewski, J.; Kielbik, P.; Olszewski, J.; Lipinski, W.; Slonska-Zielonka, A.; Rosowska, J.; Witkowski, B.S.; Gralak, M.A.; Gajewski, Z.; et al. New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics. Nanotechnol. Rev. 2020, 9, 274–302. [Google Scholar] [CrossRef]
- Atabaev, T.S. Chapter 8—Multimodal inorganic nanoparticles for biomedical applications. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 253–278. [Google Scholar]
- Pokhrel, M.; Burger, A.; Groza, M.; Mao, Y. Enhance the photoluminescence and radioluminescence of La2Zr2O7: Eu3+ core nanoparticles by coating with a thin Y2O3 shell. Opt. Mater. 2017, 68, 35–41. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, C.; Yang, P.; Hou, Z.; Lin, J. 808-nm-Light-excited lanthanide-doped nanoparticles: Rational design, luminescence control and theranostic applications. Adv. Mater. 2017, 29, 1605434. [Google Scholar] [CrossRef]
- Kalyani, N.T.; Swart, H.C.; Dhoble, S.J. Principles and Applications of Organic Light Emitting Diodes (OLEDs); Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Pallares, R.M.; Abergel, R.J. Transforming lanthanide and actinide chemistry with nanoparticles. Nanoscale 2020, 12, 1339–1348. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Zhang, S.; Zhang, H. Stimuli-responsive nanotheranostics based on lanthanide-doped upconversion nanoparticles for cancer imaging and therapy: Current advances and future challenges. Nano Today 2019, 25, 38–67. [Google Scholar] [CrossRef]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-activated nanoparticles: A toolbox for bioimaging, therapeutics, and neuromodulation. Acc. Chem. Res. 2020, 53, 2692–2704. [Google Scholar] [CrossRef]
- Trujillo-Benítez, D.; Ferro-Flores, G.; Morales-Avila, E.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M.; Azorín-Vega, E. Synthesis and biochemical evaluation of samarium-153 oxide nanoparticles functionalized with iPSMA-bombesin heterodimeric peptide. J. Biomed. Nanotechnol. 2020, 16, 689–701. [Google Scholar] [CrossRef]
- Ancira-Cortez, A.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Morales-Avila, E.; Trujillo-Benítez, D.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M. Synthesis, chemical and biochemical characterization of Lu2O3-iPSMA nanoparticles activated by neutron irradiation. Mater. Sci. Eng. C 2020, 117, 111335. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Cent. Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef]
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing luminescence in lanthanide-doped upconversion nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 11702–11715. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.F.-C.; Chien, Y.-H.; Yin, F.; Kong, S.-K.; Ho, H.-P.; Yong, K.-T. Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coord. Chem. Rev. 2019, 400, 213042. [Google Scholar] [CrossRef]
- Yan, J.; Li, B.; Yang, P.; Lin, J.; Dai, Y. Progress in light-responsive lanthanide nanoparticles toward deep tumor theranostics. Adv. Funct. Mater. 2021, 31, 2104325. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Tu, D.; Zheng, W.; Chen, X. Luminescent lanthanide metal–organic framework nanoprobes: From fundamentals to bioapplications. Nanoscale 2020, 12, 15021–15035. [Google Scholar] [CrossRef]
- Sudheendra, L.; Das, G.K.; Li, C.; Cherry, S.R.; Kennedy, I.M. Lanthanide-doped nanoparticles for hybrid x-ray/optical imaging. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V, San Francisco, CA, USA, 4–6 February 2013; pp. 76–83. [Google Scholar]
- Cheignon, C.; Kassir, A.A.; Soro, L.K.; Charbonnière, L.J. Dye-sensitized lanthanide containing nanoparticles for luminescence based applications. Nanoscale 2022, 14, 13915–13949. [Google Scholar] [CrossRef]
- Kolobkova, E.; Mironov, L.Y.; Apanasevich, P.; Khodasevich, I.; Grabtchikov, A. Cooperative energy transfer as a probe of clustering in Yb3+ doped fluoroaluminate glasses. J. Lumin. 2023, 257, 119755. [Google Scholar] [CrossRef]
- Huang, H.; Wang, T.; Zhou, H.; Huang, D.; Wu, Y.; Zhou, G.; Hu, J.; Zhan, J. Luminescence, energy transfer, and up-conversion mechanisms of Yb3+ and Tb3+ co-doped LaNbO4. J. Alloys Compd. 2017, 702, 209–215. [Google Scholar] [CrossRef]
- Boschi, F.; Spinelli, A.E. Nanoparticles for Cerenkov and radioluminescent light enhancement for imaging and radiotherapy. Nanomaterials 2020, 10, 1771. [Google Scholar] [CrossRef]
- Lu, S.; Ke, J.; Li, X.; Tu, D.; Chen, X. Luminescent nano-bioprobes based on NIR dye/lanthanide nanoparticle composites. Aggregate 2021, 2, e59. [Google Scholar] [CrossRef]
- He, Y.; Guo, S.; Ju, H.; Liu, Y. Photo-Cleavable Polycations-Wrapped Upconversion Nanoparticles for Efficient siRNA Delivery and Cancer Therapy. Targets 2023, 1, 63–78. [Google Scholar] [CrossRef]
- Zhou, C.; Chu, Z.; Hou, W.; Wang, X. Lanthanide-Doped Upconversion-Linked Immunosorbent Assay for the Sensitive Detection of Carbohydrate Antigen 19-9. Front. Chem. 2021, 8, 592445. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, M.; Xie, Y.; Sun, Q.; Gao, M.; Li, C. Rationally Integrated Precise ER-Targeted and Oxygen-Compensated Photodynamic Immunostimulant for Immunogenicity-Boosted Tumor Therapy. Adv. Healthc. Mater. 2023, 12, 2301728. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shi, J.; Gao, Y.; Yang, W.; Liu, P.; Liu, Q.; He, F.; Wang, C.; Li, T.; Xie, R. 808 nm near-infrared light-excited UCNPs@ mSiO2-Ce6-GPC3 nanocomposites for photodynamic therapy in liver cancer. Int. J. Nanomed. 2019, 14, 10009–10021. [Google Scholar] [CrossRef] [PubMed]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Tuning polymers grafted on upconversion nanoparticles for the delivery of 5-fluorouracil. Eur. Polym. J. 2020, 137, 109935. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Zang, Y.; Han, J.; Xiong, Q.; Xiong, J. SiO2 coated up-conversion nanomaterial doped with ag nanoparticles for micro-CT imaging. Nanomaterials 2021, 11, 3395. [Google Scholar] [CrossRef] [PubMed]
- Ghazyani, N.; Majles Ara, M.H. Effect of Protein Corona Formation on Photonic Response of Upconverting Nanoparticles. Int. J. Biophotonics Biomed. Eng. 2023, 3, 49–56. [Google Scholar]
- Chen, H.; Yang, X.; Liu, Y.; Wang, L. Turn-on detection of glutathione S-transferase based on luminescence resonance energy transfer between near-infrared to near-infrared core-shell upconversion nanoparticles and organic dye. Anal. Bioanal. Chem. 2020, 412, 5843–5851. [Google Scholar] [CrossRef]
- He, H.S.; Wong, W.-K.; Li, K.-F.; Cheah, K.-W. Synthesis, characterization and photoluminescence properties of monoporphyrinate lanthanide complexes. Synth. Met. 2004, 143, 81–87. [Google Scholar] [CrossRef]
- Kumar, P.; Creason, T.D.; Fattal, H.; Sharma, M.; Du, M.H.; Saparov, B. Composition-Dependent Photoluminescence Properties and Anti-Counterfeiting Applications of A2AgX3 (A = Rb, Cs; X = Cl, Br, I). Adv. Funct. Mater. 2021, 31, 2104941. [Google Scholar] [CrossRef]
- Liu, F.-Y.; Roces, L.; Sa, F.R.A.; Garca-Granda, S.; Garca, J.R.; Carlos, L.D.; Rocha, J. Crystal structure and photoluminescence properties of lanthanide diphosphonates. J. Mater. Chem. 2007, 17, 3696–3701. [Google Scholar] [CrossRef]
- Guryev, E.L.; Smyshlyaeva, A.S.; Shilyagina, N.Y.; Sokolova, E.A.; Shanwar, S.; Kostyuk, A.B.; Lyubeshkin, A.V.; Schulga, A.A.; Konovalova, E.V.; Lin, Q. UCNP-Based photoluminescent nanomedicines for targeted imaging and theranostics of cancer. Molecules 2020, 25, 4302. [Google Scholar] [CrossRef] [PubMed]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon dots as promising tools for cancer diagnosis and therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Yang, B.; Zhu, J.; Niu, G.; Wu, H.; Yin, L.; Du, X.; Niu, M.; Ge, Y. Metal halide scintillators with fast and self-absorption-free defect-bound excitonic radioluminescence for dynamic X-ray imaging. Adv. Funct. Mater. 2021, 31, 2007921. [Google Scholar] [CrossRef]
- Klein, J.S.; Sun, C.; Pratx, G. Radioluminescence in biomedicine: Physics, applications, and models. Phys. Med. Biol. 2019, 64, 04TR01. [Google Scholar] [CrossRef]
- Cooper, D.R.; Capobianco, J.A.; Seuntjens, J. Radioluminescence studies of colloidal oleate-capped β-Na (Gd, Lu) F4: Ln3+ nanoparticles (Ln = Ce, Eu, Tb). Nanoscale 2018, 10, 7821–7832. [Google Scholar] [CrossRef]
- Zhang, E.; Bandera, Y.; Dickey, A.; Foulger, I.; Kolis, J.W.; Foulger, S.H. Development of dispersible radioluminescent silicate nanoparticles through a sacrificial layer approach. J. Colloid Interface Sci. 2021, 582, 1128–1135. [Google Scholar] [CrossRef]
- Maurizio, S.L.; Mandl, G.A.; Long, M.D.; Capobianco, J.A. Investigating the Fundamental Material Properties That Influence the Radioluminescence of Lanthanide-Doped Nanoparticles. Chem. Mater. 2022, 34, 10123–10132. [Google Scholar] [CrossRef]
- Ancira-Cortez, A.; Trujillo-Benitez, D.; Jiménez-Mancilla, N.; Santos-Cuevas, C.; Morales-Avila, E.; Ferro-Flores, G. Synthesis and physicochemical characterization of Lu and Sm sesquioxide nanoparticles by precipitation-calcination and pulsed laser ablation in liquids. Mater. Chem. Phys. 2022, 275, 125229. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, C.; Gai, S.; Yang, P. Integration of Au nanosheets and GdOF: Yb, Er for NIR-I and NIR-II light-activated synergistic theranostics. ACS Appl. Mater. Interfaces 2022, 14, 3809–3824. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, J.; Peng, S.; Zhong, H.; Lin, P.; Wang, J.; Sun, X.; Song, L.; Yuan, Q.; Zhang, Y. Biodegradable near-infrared-IIb lanthanide-doped inorganic nanoparticles with red up-conversion luminescence for bioimaging and photodynamic therapy. Sci. China Mater. 2023, 66, 2893–2901. [Google Scholar] [CrossRef]
- Zhong, Y.; Dai, H. A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 2020, 13, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, S.; Oliveira, J.; Guidelli, E.J.; Araujo, J.F.; Wiekhorst, F.; Baffa, O. Synthesis of radioluminescent iron oxide nanoparticles functionalized by anthracene for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125105. [Google Scholar] [CrossRef]
- Kudinov, K.; Bekah, D.; Cooper, D.; Shastry, S.; Hill, C.; Bradforth, S.; Nadeau, J. Lanthanum fluoride nanoparticles for radiosensitization of tumors. In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XI, San Francisco, CA, USA, 13–15 February 2016; SPIE: Paris, France, 2016; pp. 109–116. [Google Scholar]
- Jia, T.; Chen, G. Lanthanide nanoparticles for near-infrared II theranostics. Coord. Chem. Rev. 2022, 471, 214724. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, C.; Hu, B.; Wang, Q. Recent advances of lanthanide nanomaterials in Tumor NIR fluorescence detection and treatment. Mater. Today Bio 2023, 20, 100646. [Google Scholar] [CrossRef]
- Ma, L.; Huang, S.; He, S.; Wang, Z.; Cheng, Z. Polydopamine-coated downconversion nanoparticle as an efficient dual-modal near-infrared-II fluorescence and photoacoustic contrast agent for non-invasive visualization of gastrointestinal tract in vivo. Biosens. Bioelectron. 2020, 151, 112000. [Google Scholar] [CrossRef]
- Kim, D.; Lee, N.; Park, Y.I.; Hyeon, T. Recent Advances in Inorganic Nanoparticle-Based NIR Luminescence Imaging: Semiconductor Nanoparticles and Lanthanide Nanoparticles. Bioconjugate Chem. 2017, 28, 115–123. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide luminescence: From a mystery to rationalization, understanding, and applications. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 141–176. [Google Scholar]
- Ren, F.; Liu, H.; Zhang, H.; Jiang, Z.; Xia, B.; Genevois, C.; He, T.; Allix, M.; Sun, Q.; Li, Z. Engineering NIR-IIb fluorescence of Er-based lanthanide nanoparticles for through-skull targeted imaging and imaging-guided surgery of orthotopic glioma. Nano Today 2020, 34, 100905. [Google Scholar] [CrossRef]
- Sabu, A.; Lin, J.-Y.; Doong, R.-A.; Huang, Y.-F.; Chiu, H.-C. Prospects of an engineered tumor-targeted nanotheranostic platform based on NIR-responsive upconversion nanoparticles. Mater. Adv. 2021, 2, 7101–7117. [Google Scholar] [CrossRef]
- Banerjee, A.; Blasiak, B.; Dash, A.; Tomanek, B.; van Veggel, F.; Trudel, S. High-field magnetic resonance imaging: Challenges, advantages, and opportunities for novel contrast agents. Chem. Phys. Rev. 2022, 3, 011304. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Yue, H.; Tegafaw, T.; Liu, S.W.; Ho, S.L.; Lee, G.H.; Nam, S.W.; Chang, Y.M. Functionalized Lanthanide Oxide Nanoparticles for Tumor Targeting, Medical Imaging, and Therapy. Pharmaceutics 2021, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.F.; Xu, Z.H.; Zhang, Y.; Gu, Y.J.; Lam, M.H.W.; Wong, W.T. Upconversion Nanoparticles Conjugated with Gd3+-DOTA and RGD for Targeted Dual-Modality Imaging of Brain Tumor Xenografts. Adv. Healthc. Mater. 2013, 2, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Y.; Ahmad, M.W.; Cha, H.; Oh, I.T.; Tegafaw, T.; Miao, X.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Yue, H.; et al. Cyclic RGD-Coated Ultrasmall Gd2O3 Nanoparticles as Tumor-Targeting Positive Magnetic Resonance Imaging Contrast Agents. Eur. J. Inorg. Chem. 2018, 2018, 3070–3079. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Cha, H.; Oh, I.T.; Tegafaw, T.; Miao, X.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Yue, H.; Chae, K.S. Synthesis, Characterization, and Enhanced Cancer-Imaging Application of Trans-activator of Transcription Peptide-conjugated Ultrasmall Gadolinium Oxide Nanoparticles. Bull. Korean Chem. Soc. 2018, 39, 435–441. [Google Scholar] [CrossRef]
- Sobol, N.; Sutherlin, L.; Cedrowska, E.; Schorp, J.; Rodríguez-Rodríguez, C.; Sossi, V.; Lattimer, J.; Miller, D.C.; Pevsner, P.; Robertson, J.D. Synthesis and targeting of gold-coated 177Lu-containing lanthanide phosphate nanoparticles—A potential theranostic agent for pulmonary metastatic disease. APL Bioeng. 2018, 2, 016101. [Google Scholar] [CrossRef]
- Biju, S.; Parac-Vogt, T.N. Recent Advances in Lanthanide Based Nano-Architectures as Probes for Ultra High-Field Magnetic Resonance Imaging. Curr. Med. Chem. 2020, 27, 352–361. [Google Scholar] [CrossRef]
- Cao, T.Y.; Yang, Y.; Gao, Y.A.; Zhou, J.; Li, Z.Q.; Li, F.Y. High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging. Biomaterials 2011, 32, 2959–2968. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef]
- Sun, L.N.; Wei, Z.W.; Chen, H.G.; Liu, J.T.; Guo, J.J.; Cao, M.; Wen, T.Q.; Shi, L.Y. Folic acid-functionalized up-conversion nanoparticles: Toxicity studies in vivo and in vitro and targeted imaging applications. Nanoscale 2014, 6, 8878–8883. [Google Scholar] [CrossRef]
- Gainer, C.F.; Romanowski, M. Multiphoton Imaging of Upconverting Lanthanide Nanoparticles in Three Dimensional Models of Cancer. In Proceedings of the Conference on Colloidal Nanocrystals for Biomedical Applications VIII, San Francisco, CA, USA, 2–4 February 2013. [Google Scholar]
- An, Z.; Wang, L.; Gao, C.; He, N.; Zhu, B.; Liu, Y.; Cai, Q. Fe3+-Enhanced NIR-to-NIR upconversion nanocrystals for tumor-targeted trimodal bioimaging. New J. Chem. 2018, 42, 17073–17082. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Wang, Z.H.; Han, Z.H.; Gu, Y.Q. A Nd3+ sensitized upconversion nanosystem with dual photosensitizers for improving photodynamic therapy efficacy. Biomater. Sci. 2019, 7, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Zhang, Y.L.; Lin, H.; Kang, S.F.; Li, Y.H.; Sun, D.; Chen, M.Y.; Wang, Z.X.; Jiao, Z.; Wang, Y.W.; et al. Ultrasound and Near-Infrared Light Dual-Triggered Upconversion Zeolite-Based Nanocomposite for Hyperthermia-Enhanced Multimodal Melanoma Therapy via a Precise Apoptotic Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 32420–32431. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.A.; Liu, Z.A. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chou, Y.L.; Wang, S.W.; Hung, S.T.; Liau, M.C.; Chao, Y.J.; Su, C.H.; Yeh, C.S. Near-Infrared Light Photocontrolled Targeting, Bioimaging, and Chemotherapy with Caged Upconversion Nanoparticles in Vitro and in Vivo. ACS Nano 2013, 7, 8516–8528. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.L.; Jia, F.F.; Wang, S.; Yu, M.T.; Shen, Y.Q.; Yu, B. Core-Shell Upconversion Nanoparticle@Metal-Organic Framework Nanoprobes for Targeting and Drug Delivery. Integr. Ferroelectr. 2020, 206, 66–78. [Google Scholar] [CrossRef]
- Cao, Y.T.; Wang, K.Q.; Zhu, P.Y.; Zou, X.W.; Ma, G.Q.; Zhang, W.X.; Wang, D.Q.; Wan, J.P.; Ma, Y.L.; Sun, X.; et al. A near-infrared triggered upconversion/MoS2 nanoplatform for tumour-targeted chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2022, 213, 112393. [Google Scholar] [CrossRef]
- Shi, J.P.; Sun, X.; Li, J.L.; Man, H.Z.; Shen, J.S.; Yu, Y.K.; Zhang, H.W. Multifunctional near infrared-emitting long-persistence luminescent nanoprobes for drug delivery and targeted tumor imaging. Biomaterials 2015, 37, 260–270. [Google Scholar] [CrossRef]
- Gerken, L.R.H.; Keevend, K.; Zhang, Y.C.; Starsich, F.H.L.; Eberhardt, C.; Panzarasa, G.; Matter, M.T.; Wichser, A.; Boss, A.; Neels, A.; et al. Lanthanide-Doped Hafnia Nanoparticles for Multimodal Theranostics: Tailoring the Physicochemical Properties and Interactions with Biological Entities. Acs Appl. Mater. Interfaces 2019, 11, 437–448. [Google Scholar] [CrossRef]
- Ang, M.J.Y.; Yoon, J.; Zhou, M.Z.; Wei, H.L.; Goh, Y.Y.; Li, Z.L.; Feng, J.; Wang, H.F.; Su, Q.Q.; Ong, D.S.T.; et al. Deciphering Nanoparticle Trafficking into Glioblastomas Uncovers an Augmented Antitumor Effect of Metronomic Chemotherapy. Adv. Mater. 2022, 34, 2106194. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Vijayaraghavan, P.; Chiang, W.H.; Chen, H.H.; Liu, T.I.; Shen, M.Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.C. Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.H.; Wang, J.N.; Yang, C.X.; Dong, L.X.; Kong, D.L.; Yan, X.P. Ultrasonic assisted preparation of lanthanide-oleate complexes for the synthesis of multifunctional monodisperse upconversion nanoparticles for multimodal imaging. Nanoscale 2014, 6, 8037–8044. [Google Scholar] [CrossRef] [PubMed]
- Canseco-Hernández, O.; Ferro-Flores, G.; Jimenez-Mancilla, N.; Aranda-Lara, L.; Ocampo-Garcia, B.; Trujillo-Benitez, D.; Ancira-Cortés, A.; Morales-Avila, E.; Santos-Cuevas, C. Preparation and Dosimetry Assessment of 166Dy2O3/166Ho2O3-iPSMA Nanoparticles for Targeted Hepatocarcinoma Radiotherapy. J. Nanosci. Nanotechnol. 2021, 21, 5449–5458. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Jaskula-Sztul, R.; Esquibel, C.R.; Lou, I.; Zheng, Q.F.; Dammalapati, A.; Harrison, A.; Eliceiri, K.W.; Tang, W.P.; Chen, H.; et al. Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging. Adv. Funct. Mater. 2017, 27, 1604671. [Google Scholar] [CrossRef]
- Xiong, L.Q.; Chen, Z.G.; Tian, Q.W.; Cao, T.Y.; Xu, C.J.; Li, F.Y. High Contrast Upconversion Luminescence Targeted Imaging in Vivo Using Peptide-Labeled Nanophosphors. Anal. Chem. 2009, 81, 8687–8694. [Google Scholar] [CrossRef]
- Tang, X.L.; Wu, J.; Li, B.L.; Cui, S.; Liu, H.M.; Yu, R.T.; Shen, X.D.; Wang, T.W.; Xia, W. Near-infrared light-activated red-emitting upconverting nanoplatform for T1-weighted magnetic resonance imaging and photodynamic therapy. Acta Biomater. 2018, 74, 360–373. [Google Scholar] [CrossRef]
- Jin, G.R.; He, R.Y.; Liu, Q.; Lin, M.; Dong, Y.Q.; Li, K.; Tang, B.Z.; Liu, B.; Xu, F. Near-infrared light-regulated cancer theranostic nanoplatform based on aggregation-induced emission luminogen encapsulated upconversion nanoparticles. Theranostics 2019, 9, 246–264. [Google Scholar] [CrossRef]
- Liu, Y.X.; Li, L.Y.; Guo, Q.W.; Wang, L.; Liu, D.D.; Wei, Z.W.; Zhou, J. Novel Cs-Based Upconversion Nanoparticles as Dual-Modal CT and UCL Imaging Agents for Chemo-Photothermal Synergistic Therapy. Theranostics 2016, 6, 1491–1505. [Google Scholar] [CrossRef]
- Cao, J.X.; Zhang, L.; Ding, X.; Liu, D.; Su, B.; Shi, J.Y. Dual-Targeting Peptides RGD10-NGR9-Conjugated Lanthanide Nanoparticle@Polydopamine as Upconversion Nanoprobes for In Vivo Imaging of Lung Cancer. Small Methods 2020, 4, 2000648. [Google Scholar] [CrossRef]
- Zha, S.; Chau, H.F.; Chau, W.Y.; Chan, L.S.; Lin, J.; Lo, K.W.; Cho, W.C.S.; Yip, Y.L.; Tsao, S.W.; Farrell, P.J.; et al. Dual-Targeting Peptide-Guided Approach for Precision Delivery and Cancer Monitoring by Using a Safe Upconversion Nanoplatform. Adv. Sci. 2021, 8, 2002919. [Google Scholar] [CrossRef]
- Sasidharan, S.; Jayasree, A.; Fazal, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Ambient temperature synthesis of citrate stabilized and biofunctionalized, fluorescent calcium fluoride nanocrystals for targeted labeling of cancer cells. Biomater. Sci. 2013, 1, 294–305. [Google Scholar] [CrossRef]
- Gainer, C.F.; Utzinger, U.; Romanowski, M. Scanning two-photon microscopy with upconverting lanthanide nanoparticles via Richardson-Lucy deconvolution. J. Biomed. Opt. 2012, 17, 076003. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Wang, H.-F.; Chen, J.-T.; Yan, X.-P. Fluorescence resonance energy transfer inhibition assay for α-fetoprotein excreted during cancer cell growth using functionalized persistent luminescence nanoparticles. J. Am. Chem. Soc. 2011, 133, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Yan, J.; Ma, B.H.; Li, S.C.; Shao, Y.P.; He, P.C.; Zhang, W.G.; He, W.X.; Ma, P.X.; Lu, W.Y. Lanthanide-doped nanoparticles conjugated with an anti-CD33 antibody and a p53-activating peptide for acute myeloid leukemia therapy. Biomaterials 2018, 167, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.T.; Ma, Z.R.; Wang, F.F.; Wang, X.; Yang, Y.J.; Liu, Y.L.; Zhao, X.; Li, J.C.; Du, H.T.; Zhang, M.X.; et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019, 37, 1322–1331. [Google Scholar] [CrossRef]
- Yu, C.; Li, K.; Xu, L.; Li, B.; Li, C.; Guo, S.; Li, Z.; Zhang, Y.; Hussain, A.; Tan, H.; et al. siRNA-functionalized lanthanide nanoparticle enables efficient endosomal escape and cancer treatment. Nano Res. 2022, 15, 9160–9168. [Google Scholar] [CrossRef]
- Song, X.; Yan, T.; Tian, F.; Li, F.; Ren, L.; Li, Q.; Zhang, S. Aptamer functionalized upconversion nanotheranostic agent with nuclear targeting as the highly localized drug-delivery system of doxorubicin. Front. Bioeng. Biotechnol. 2021, 9, 639487. [Google Scholar] [CrossRef]
- Bolzati, C.; Salvarese, N.; Carpanese, D.; Seraglia, R.; Meléndez-Alafort, L.; Rosato, A.; Capasso, D.; Saviano, M.; Del Gatto, A.; Comegna, D.; et al. 99mTc Tc(N)PNP43 -Labeled RGD Peptides As New Probes for a Selective Detection of αvβ3 Integrin: Synthesis, Structure-Activity and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 9596–9610. [Google Scholar] [CrossRef] [PubMed]
- Brunello, S.; Salvarese, N.; Carpanese, D.; Gobbi, C.; Melendez-Alafort, L.; Bolzati, C. A review on the current state and future perspectives of [99mTc] Tc-housed PSMA-i in prostate cancer. Molecules 2022, 27, 2617. [Google Scholar] [CrossRef]
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef]
- Vallejo-Armenta, P.; Ferro-Flores, G.; Santos-Cuevas, C.; García-Pérez, F.O.; Casanova-Triviño, P.; Sandoval-Bonilla, B.; Ocampo-García, B.; Azorín-Vega, E.; Luna-Gutiérrez, M. [99mTc] Tc-iFAP/SPECT tumor stroma imaging: Acquisition and analysis of clinical images in six different cancer entities. Pharmaceuticals 2022, 15, 729. [Google Scholar] [CrossRef]
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef]
- Tapia-Arellano, A.; Gallardo-Toledo, E.; Ortiz, C.; Henríquez, J.; Feijóo, C.G.; Araya, E.; Sierpe, R.; Kogan, M.J. Functionalization with PEG/Angiopep-2 peptide to improve the delivery of gold nanoprisms to central nervous system: In Vitro and in vivo studies. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 121, 111785. [Google Scholar] [CrossRef]
- Chen, G.; Jaskula–Sztul, R.; Harrison, A.; Dammalapati, A.; Xu, W.; Cheng, Y.; Chen, H.; Gong, S. KE108-conjugated unimolecular micelles loaded with a novel HDAC inhibitor thailandepsin-A for targeted neuroendocrine cancer therapy. Biomaterials 2016, 97, 22–33. [Google Scholar] [CrossRef]
- Hiraki, M.; Hwang, S.Y.; Cao, S.G.; Ramadhar, T.R.; Byun, S.; Yoon, K.W.; Lee, J.H.; Chu, K.; Gurkar, A.U.; Kolev, V.; et al. Small-Molecule Reactivation of Mutant p53 to Wild-Type-like p53 through the p53-Hsp40 Regulatory Axis. Chem. Biol. 2015, 22, 1206–1216. [Google Scholar] [CrossRef]
- He, W.X.; Yan, J.; Wang, L.J.; Lei, B.; Hou, P.; Lu, W.Y.; Ma, P.X. A lanthanide-peptide-derived bacterium-like nanotheranostic with high tumor-targeting, -imaging and -killing properties. Biomaterials 2019, 206, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Song, B.; Vandevyver, C.D.; Chauvin, A.-S.; Wacker, J.B.; Bünzli, J.-C.G. Increasing the efficiency of lanthanide luminescent bioprobes: Bioconjugated silica nanoparticles as markers for cancerous cells. New J. Chem. 2010, 34, 2915–2921. [Google Scholar] [CrossRef]
- Stipic, F.; Pletikapić, G.; Jaksic, Z.; Frkanec, L.; Zgrablic, G.; Buric, P.; Lyons, D.M. Application of functionalized lanthanide-based nanoparticles for the detection of okadaic acid-specific immunoglobulin G. J. Phys. Chem. B 2015, 119, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A.; Viard, M.; Kagiampakis, I.; Case, C.L.; Dobrovolskaia, M.A.; Hofmann, J.; Vrzak, A.; Kireeva, M.; Kasprzak, W.K.; KewalRamani, V.N. Triggering of RNA Interference with RNA–RNA, RNA–DNA, and DNA–RNA Nanoparticles. In Therapeutic RNA Nanotechnology; Jenny Stanford Publishing: Singapore, 2021; pp. 681–705. [Google Scholar]
- Chakraborty, C. Potentiality of small interfering RNAs (siRNA) as recent therapeutic targets for gene-silencing. Curr. Drug Targets 2007, 8, 469–482. [Google Scholar] [CrossRef]
- Senapati, D.; Patra, B.C.; Kar, A.; Chini, D.S.; Ghosh, S.; Patra, S.; Bhattacharya, M. Promising approaches of small interfering RNAs (siRNAs) mediated cancer gene therapy. Gene 2019, 719, 144071. [Google Scholar] [CrossRef]
- Morales-Becerril, A.; Aranda-Lara, L.; Isaac-Olivé, K.; Ocampo-García, B.E.; Morales-Ávila, E. Nanocarriers for delivery of siRNA as gene silencing mediator. EXCLI J. 2022, 21, 1028–1052. [Google Scholar]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef]
- Ahmad, A. Safety and Toxicity Implications of Multifunctional Drug Delivery Nanocarriers on Reproductive Systems In Vitro and In Vivo. Front. Toxicol. 2022, 4, 895667. [Google Scholar] [CrossRef]
- Liu, F.; Chang, X.; Tian, M.; Zhu, A.; Zou, L.; Han, A.; Su, L.; Li, S.; Sun, Y. Nano NiO induced liver toxicity via activating the NF-κB signaling pathway in rats. Toxicol. Res. 2017, 6, 242–250. [Google Scholar] [CrossRef]
- Sha, B.; Gao, W.; Wang, S.; Gou, X.; Li, W.; Liang, X.; Qu, Z.; Xu, F.; Lu, T.J. Oxidative stress increased hepatotoxicity induced by nano-titanium dioxide in BRL-3A cells and Sprague–Dawley rats. J. Appl. Toxicol. 2014, 34, 345–356. [Google Scholar] [CrossRef]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed]
- Sha, B.; Gao, W.; Wang, S.; Li, W.; Liang, X.; Xu, F.; Lu, T.J. Nano-titanium dioxide induced cardiac injury in rat under oxidative stress. Food Chem. Toxicol. 2013, 58, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, A.; Vilhelmsson Timmermand, O.; Altai, M.; Strand, J.; Strand, S.-E.; Åkerström, B.; Örbom, A. Hematological toxicity in mice after high activity injections of 177Lu-PSMA-617. Pharmaceutics 2022, 14, 731. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).