Enhancing Bone Cement Efficacy with Hydrogel Beads Synthesized by Droplet Microfluidics
Abstract
1. Introduction
2. Materials and Methods
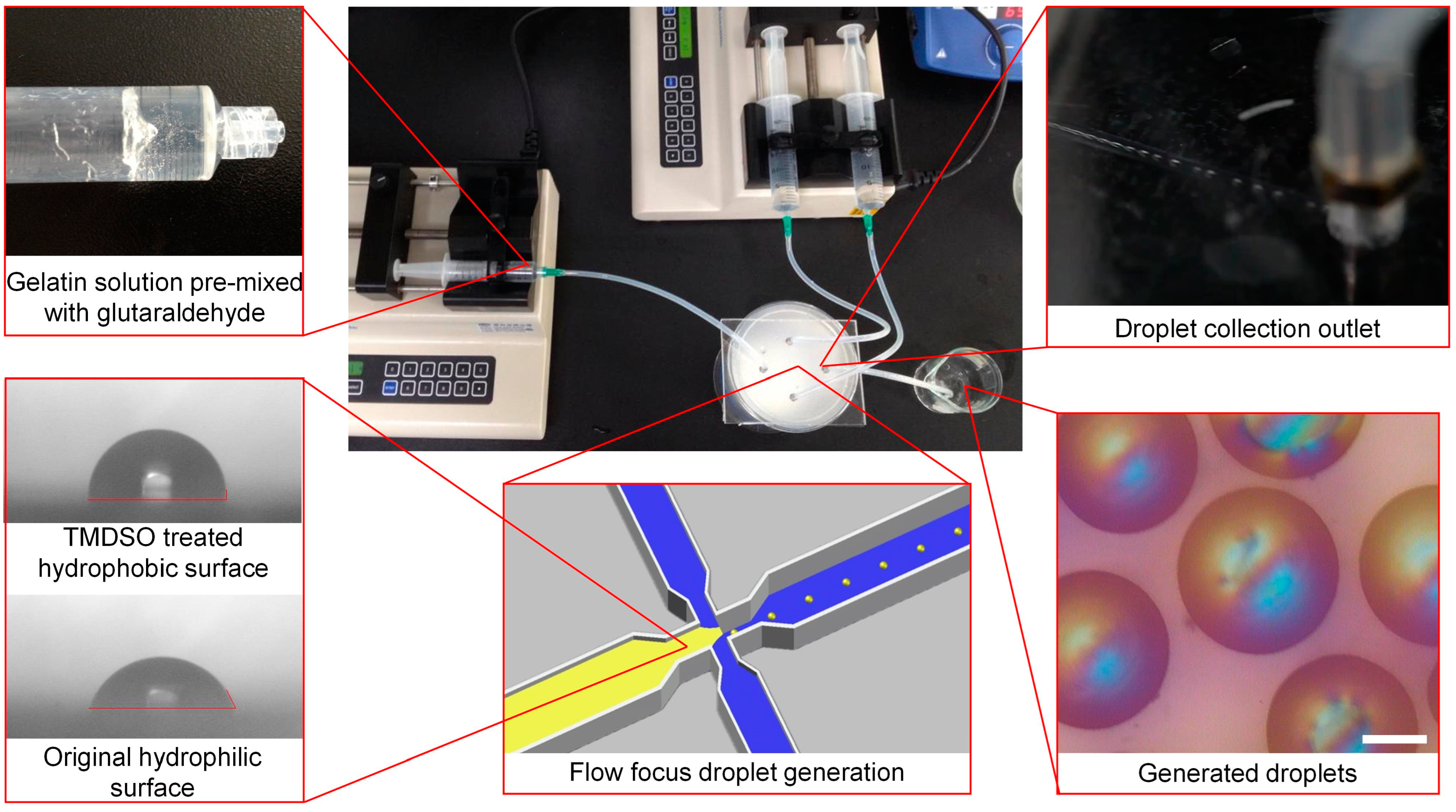
2.1. Microfluidic Chip Fabrication and Droplet Synthesis
2.2. Hydrogel Bead Validation and Drug Release Evaluation
2.3. Bacterial Culture and Proliferation Validation
2.4. Cell Culture and Viability Validation
2.5. Creating and Validating Gelatin Bead–CPC Cement Complex
3. Results
3.1. Device Design and Droplet Generation
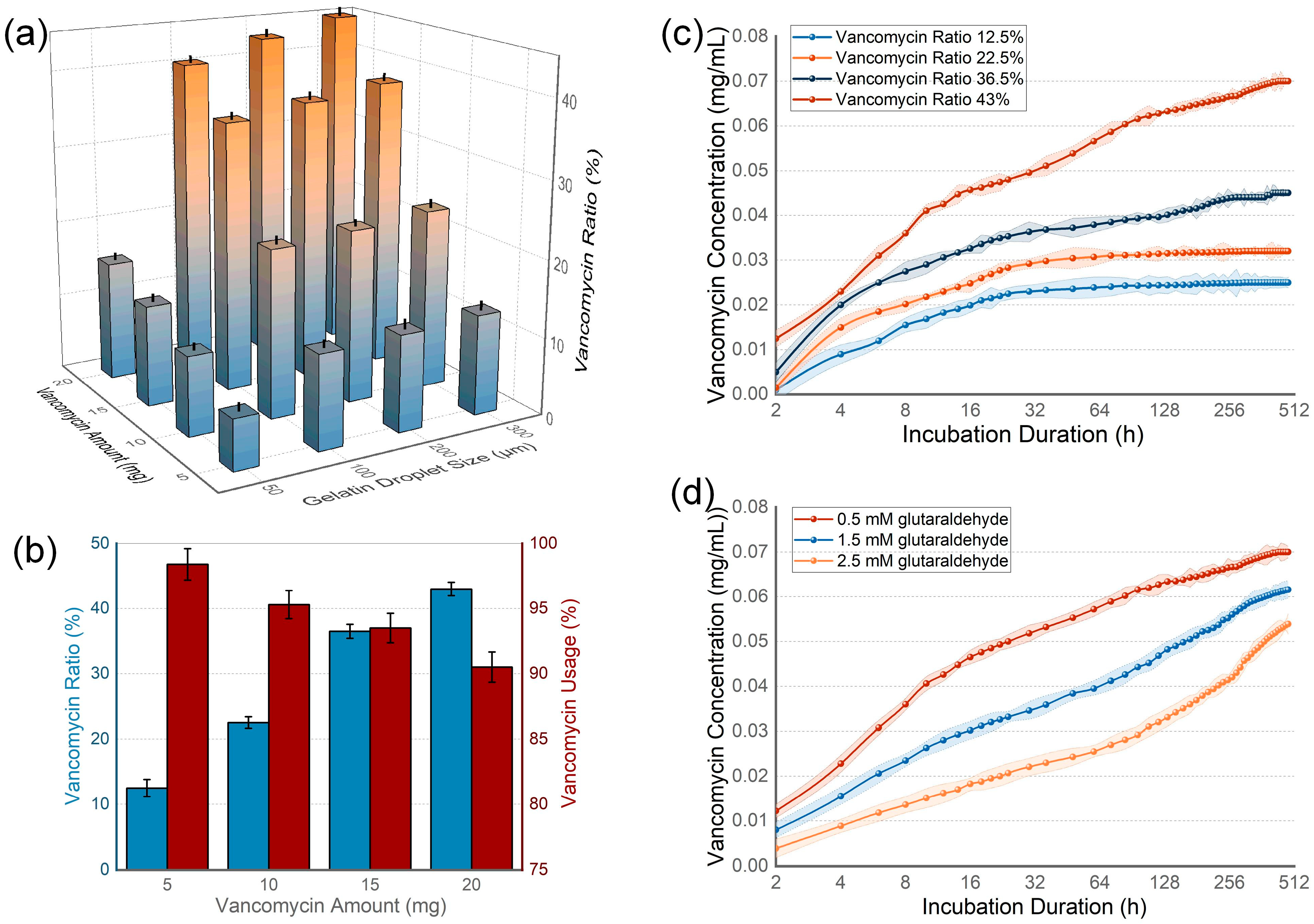
3.2. Drug Loading Capacity and Releasing Kinetics of Hydrogel Beads
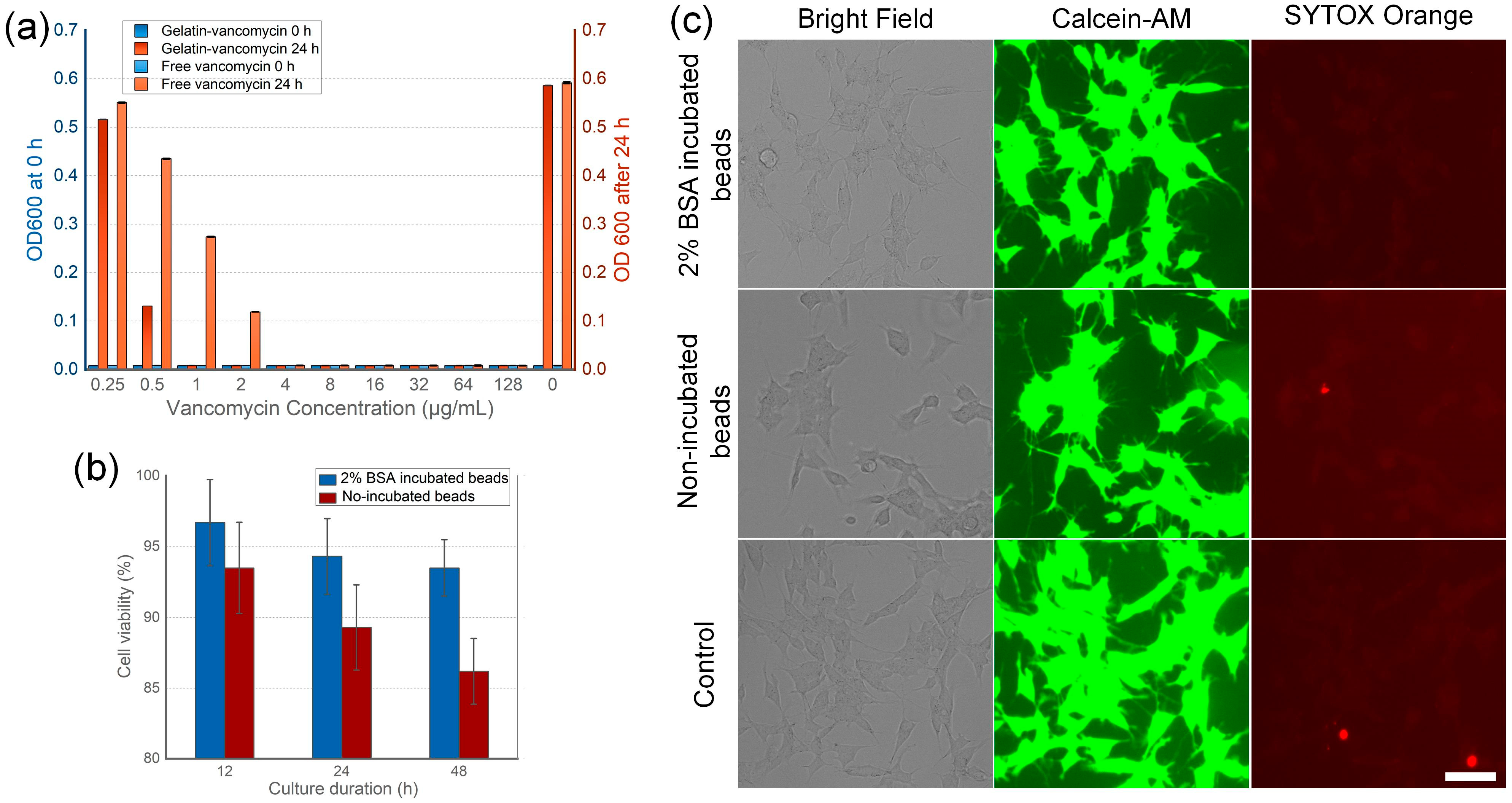
3.3. Antibiotic Efficiency and Tissue Toxicity of the Beads
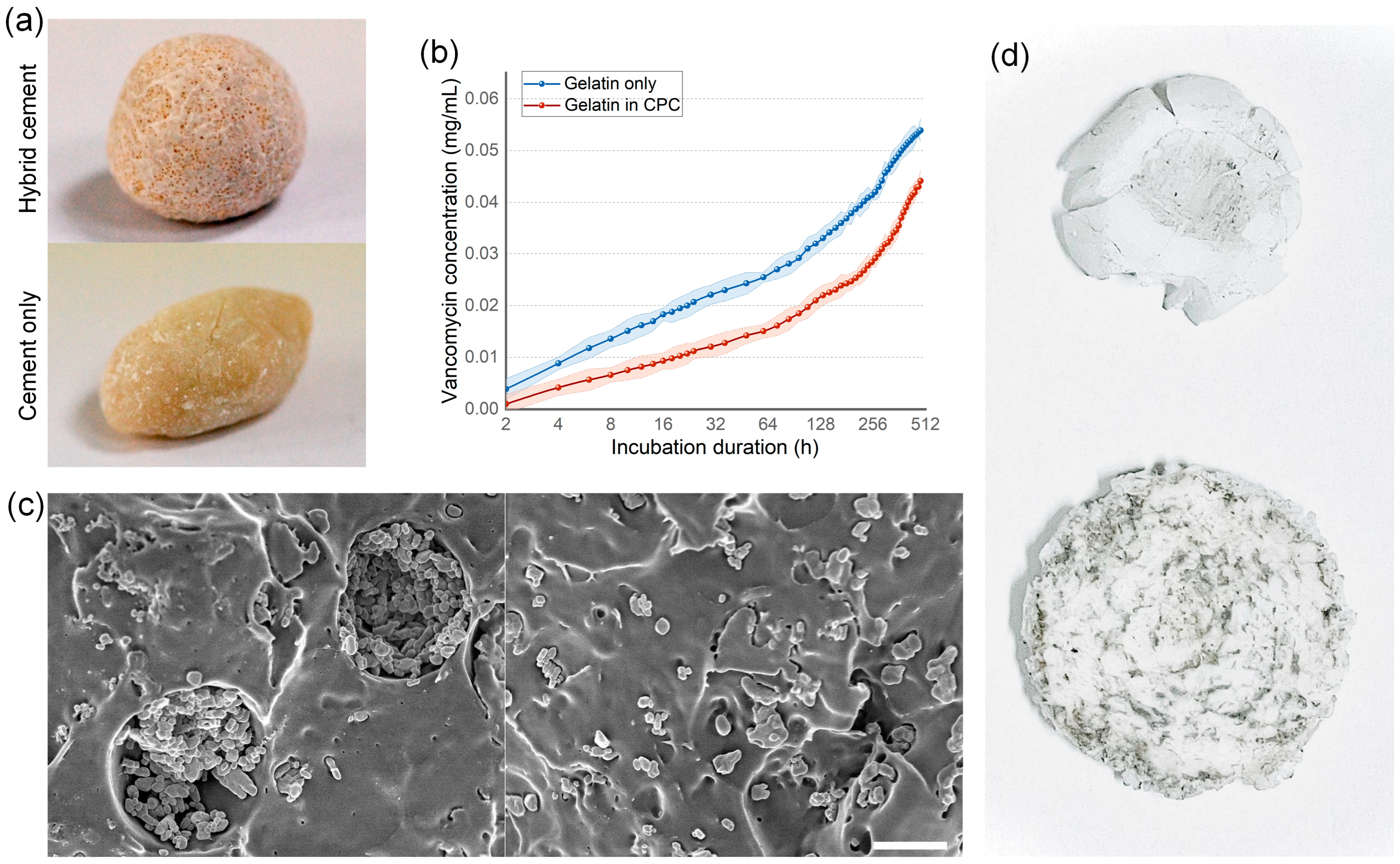
3.4. Bone Cement Loading Ability
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadegh, A.B.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Montufar, E.B. Cements as bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–271. [Google Scholar]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-based bone cements and the problem of joint arthroplasty infections: Status and new perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef]
- Hines, C.B. Understanding Bone Cement Implantation Syndrome. AANA J. 2018, 86, 433–441. [Google Scholar]
- Robo, C.; Hulsart-Billström, G.; Nilsson, M.; Persson, C. In vivo response to a low-modulus PMMA bone cement in an ovine model. Acta Biomater. 2018, 72, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Dong, L.; Yuan, Y.; Jiang, Q. In vitro and in vivo analysis of the biocompatibility of two novel and injectable calcium phosphate cements. Regen. Biomater. 2019, 6, 13–19. [Google Scholar] [CrossRef]
- Kuang, G.M.; Yau, W.; Wu, J.; Yeung, K.W.; Pan, H.; Lam, W.; Lu, W.; Chiu, K. Strontium exerts dual effects on calcium phosphate cement: Accelerating the degradation and enhancing the osteoconductivity both in vitro and in vivo. J. Biomed. Mater. Res. Part A 2015, 103, 1613–1621. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- de Lacerda Schickert, S.; Pinto, J.C.; Jansen, J.; Leeuwenburgh, S.C.; van den Beucken, J.J. Tough and injectable fiber reinforced calcium phosphate cement as an alternative to polymethylmethacrylate cement for vertebral augmentation: A biomechanical study. Biomater. Sci. 2020, 8, 4239–4250. [Google Scholar] [CrossRef]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef]
- Loca, D.; Sokolova, M.; Locs, J.; Smirnova, A.; Irbe, Z. Calcium phosphate bone cements for local vancomycin delivery. Mater. Sci. Eng. C 2015, 49, 106–113. [Google Scholar] [CrossRef]
- Rau, J.; Fosca, M.; Graziani, V.; Egorov, A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.; Komlev, V. Silver-doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, T.H.; Kim, M.; Jang, J.H.; Ginebra, M.P.; Kim, H.W. Calcium phosphate cements loaded with basic fibroblast growth factor: Delivery and in vitro cell response. J. Biomed. Mater. Res. Part A 2013, 101, 923–931. [Google Scholar] [CrossRef]
- Ko, C.L.; Chen, J.C.; Tien, Y.C.; Hung, C.C.; Wang, J.C.; Chen, W.C. Osteoregenerative capacities of dicalcium phosphate-rich calcium phosphate bone cement. J. Biomed. Mater. Res. Part A 2015, 103, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Fan, J.J.; Li, Z.Q.; Liu, Y.W.; Wu, Y.P.; Liu, J. Effects of pore size on the osteoconductivity and mechanical properties of calcium phosphate cement in a rabbit model. Artif. Organs 2017, 41, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Akkineni, A.R.; Förster, Y.; Köhler, T.; Knaack, S.; Gelinsky, M.; Lode, A. Design and fabrication of complex scaffolds for bone defect healing: Combined 3D plotting of a calcium phosphate cement and a growth factor-loaded hydrogel. Ann. Biomed. Eng. 2017, 45, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef]
- Gibbs, D.M.; Black, C.R.; Dawson, J.I.; Oreffo, R.O. A review of hydrogel use in fracture healing and bone regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 187–198. [Google Scholar] [CrossRef]
- Joensson, H.N.; Andersson Svahn, H. Droplet microfluidics—A tool for single-cell analysis. Angew. Chem. Int. Ed. 2012, 51, 12176–12192. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic devices for drug delivery systems and drug screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef]
- Riche, C.T.; Roberts, E.J.; Gupta, M.; Brutchey, R.L.; Malmstadt, N. Flow invariant droplet formation for stable parallel microreactors. Nat. Commun. 2016, 7, 10780. [Google Scholar] [CrossRef] [PubMed]
- Kashani, S.Y.; Afzalian, A.; Shirinichi, F.; Moraveji, M.K. Microfluidics for core–shell drug carrier particles—A review. RSC Adv. 2021, 11, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.H.; Lee, W.; Pun, S.H.; Kim, J.; Kim, D.-H. Microfluidics-assisted in vitro drug screening and carrier production. Adv. Drug Deliv. Rev. 2013, 65, 1575–1588. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab A Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab A Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, S.; Moraveji, M.K. Droplet microfluidics: Fundamentals and its advanced applications. RSC Adv. 2020, 10, 27560. [Google Scholar] [CrossRef]
- Ding, Y.; Howes, P.D.; deMello, A.J. Recent advances in droplet microfluidics. Anal. Chem. 2019, 92, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Campos, J.; Miranda, J. High viscosity polymeric fluid droplet formation in a flow focusing microfluidic device—Experimental and numerical study. Chem. Eng. Sci. 2019, 195, 442–454. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Feng, L.; Lin, T. Fluid mixing in droplet-based microfluidics with a serpentine microchannel. RSC Adv. 2015, 5, 104138–104144. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samlali, K.; Vo, P.Q.; Shih, S.C. An integrated droplet-digital microfluidic system for on-demand droplet creation, mixing, incubation, and sorting. Lab A Chip 2019, 19, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, C.J. Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc. Chem. Res. 2017, 50, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, S.; Yamada, M.; Hori, A.; Seki, M. Microfluidic production of single micrometer-sized hydrogel beads utilizing droplet dissolution in a polar solvent. Biomicrofluidics 2013, 7, 54120. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Chen, K.-R.; Lin, Y.-C. Developing heatable microfluidic chip to generate gelatin emulsions and microcapsules. Microfluid. Nanofluidics 2013, 15, 775–784. [Google Scholar] [CrossRef]
- Samanipour, R.; Wang, Z.; Ahmadi, A.; Kim, K. Experimental and computational study of microfluidic flow—focusing generation of gelatin methacrylate hydrogel droplets. J. Appl. Polym. Sci. 2016, 133, 43701. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Rosli, N.A.; Ahmad, I.; Ramli, S.; Amin, M.C.I.M. Drug delivery and in vitro biocompatibility studies of gelatin-nanocellulose smart hydrogels cross-linked with gamma radiation. J. Mater. Res. Technol. 2021, 15, 7145–7157. [Google Scholar] [CrossRef]
- Mathew, S.A.; Arumainathan, S. Crosslinked chitosan–gelatin biocompatible nanocomposite as a neuro drug carrier. ACS Omega 2022, 7, 18732–18744. [Google Scholar] [CrossRef]
- Sivasamy, J.; Wong, T.-N.; Nguyen, N.-T.; Kao, L.T.-H. An investigation on the mechanism of droplet formation in a microfluidic T-junction. Microfluid. Nanofluidics 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R. Effect of geometry on droplet formation in the squeezing regime in a microfluidic T-junction. Microfluid. Nanofluidics 2010, 8, 799–812. [Google Scholar] [CrossRef]
- Medvecky, L.; Giretova, M.; Stulajterova, R.; Danko, J.; Vdoviakova, K.; Kresakova, L.; Zert, Z.; Petrovova, E.; Holovska, K.; Varga, M. Characterization of properties, in vitro and in vivo evaluation of calcium phosphate/amino acid cements for treatment of osteochondral defects. Materials 2021, 14, 436. [Google Scholar] [CrossRef]
- Hisatome, T.; Yasunaga, Y.; Ikuta, Y.; Fujimoto, Y. Effects on articular cartilage of subchondral replacement with polymethylmethacrylate and calcium phosphate cement. J. Biomed. Mater. Res. 2002, 59, 490–498. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Z.; Liu, B.; Xiang, J.; Qiu, D.; Tian, Y.; Qu, X.; Yang, Z. An injectable strong hydrogel for bone reconstruction. Adv. Healthc. Mater. 2019, 8, 1900709. [Google Scholar] [CrossRef]
- Ly, O.; Monchau, F.; Rémond, S.; Lors, C.; Jouanneaux, A.; Debarre, É.; Damidot, D. Optimization of the formulation of an original hydrogel-based bone cement using a mixture design. J. Mech. Behav. Biomed. Mater. 2020, 110, 103886. [Google Scholar] [CrossRef]
- Pastorino, D.; Canal, C.; Ginebra, M.-P. Multiple characterization study on porosity and pore structure of calcium phosphate cements. Acta Biomater. 2015, 28, 205–214. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Sariibrahimoglu, K.; Yang, W.; Bohner, M.; Yamashita, D.; Kunstar, A.; Van Apeldoorn, A.A.; Bronkhorst, E.M.; Lanao, R.P.F.; Leeuwenburgh, S.C. Influence of the pore generator on the evolution of the mechanical properties and the porosity and interconnectivity of a calcium phosphate cement. Acta Biomater. 2012, 8, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sherwood, J.M.; Huck, W.T.; Balabani, S. On the flow topology inside droplets moving in rectangular microchannels. Lab A Chip 2014, 14, 3611–3620. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels… A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Zhang, C.; Grossier, R.; Candoni, N.; Veesler, S. Preparation of alginate hydrogel microparticles by gelation introducing cross-linkers using droplet-based microfluidics: A review of methods. Biomater. Res. 2021, 25, 41. [Google Scholar] [CrossRef]
- Baah, D.; Floyd-Smith, T. Microfluidics for particle synthesis from photocrosslinkable materials. Microfluid. Nanofluidics 2014, 17, 431–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, S.; He, C.; Li, C.; Louh, R.-F. Enhancing Bone Cement Efficacy with Hydrogel Beads Synthesized by Droplet Microfluidics. Nanomaterials 2024, 14, 302. https://doi.org/10.3390/nano14030302
Wang Z, Yang S, He C, Li C, Louh R-F. Enhancing Bone Cement Efficacy with Hydrogel Beads Synthesized by Droplet Microfluidics. Nanomaterials. 2024; 14(3):302. https://doi.org/10.3390/nano14030302
Chicago/Turabian StyleWang, Zeyu, Sherwin Yang, Chunjie He, Chaoqiang Li, and Rong-Fuh Louh. 2024. "Enhancing Bone Cement Efficacy with Hydrogel Beads Synthesized by Droplet Microfluidics" Nanomaterials 14, no. 3: 302. https://doi.org/10.3390/nano14030302
APA StyleWang, Z., Yang, S., He, C., Li, C., & Louh, R.-F. (2024). Enhancing Bone Cement Efficacy with Hydrogel Beads Synthesized by Droplet Microfluidics. Nanomaterials, 14(3), 302. https://doi.org/10.3390/nano14030302





