Merging of Bi-Modality of Ultrafast Laser Processing: Heating of Si/Au Nanocomposite Solutions with Controlled Chemical Content
Abstract
:1. Introduction
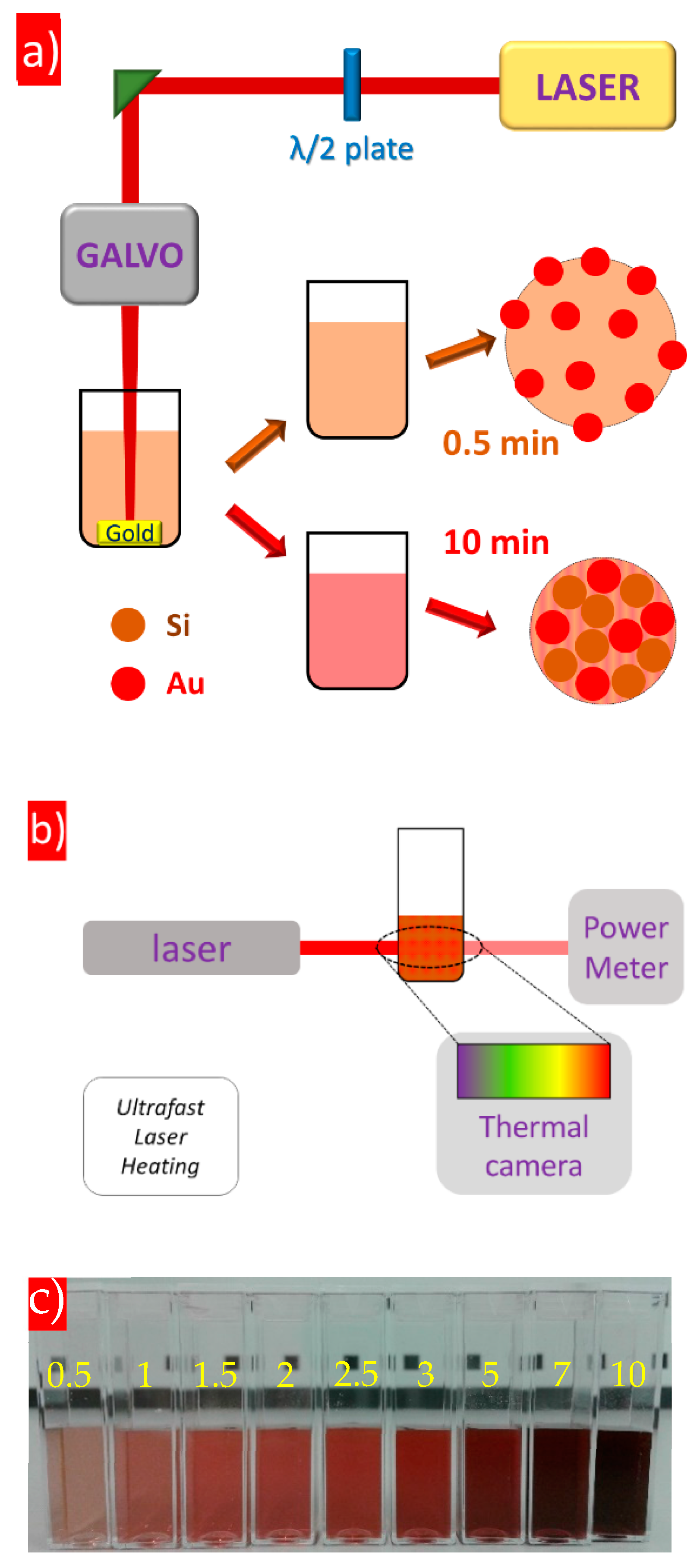
2. Materials and Experimental Methods
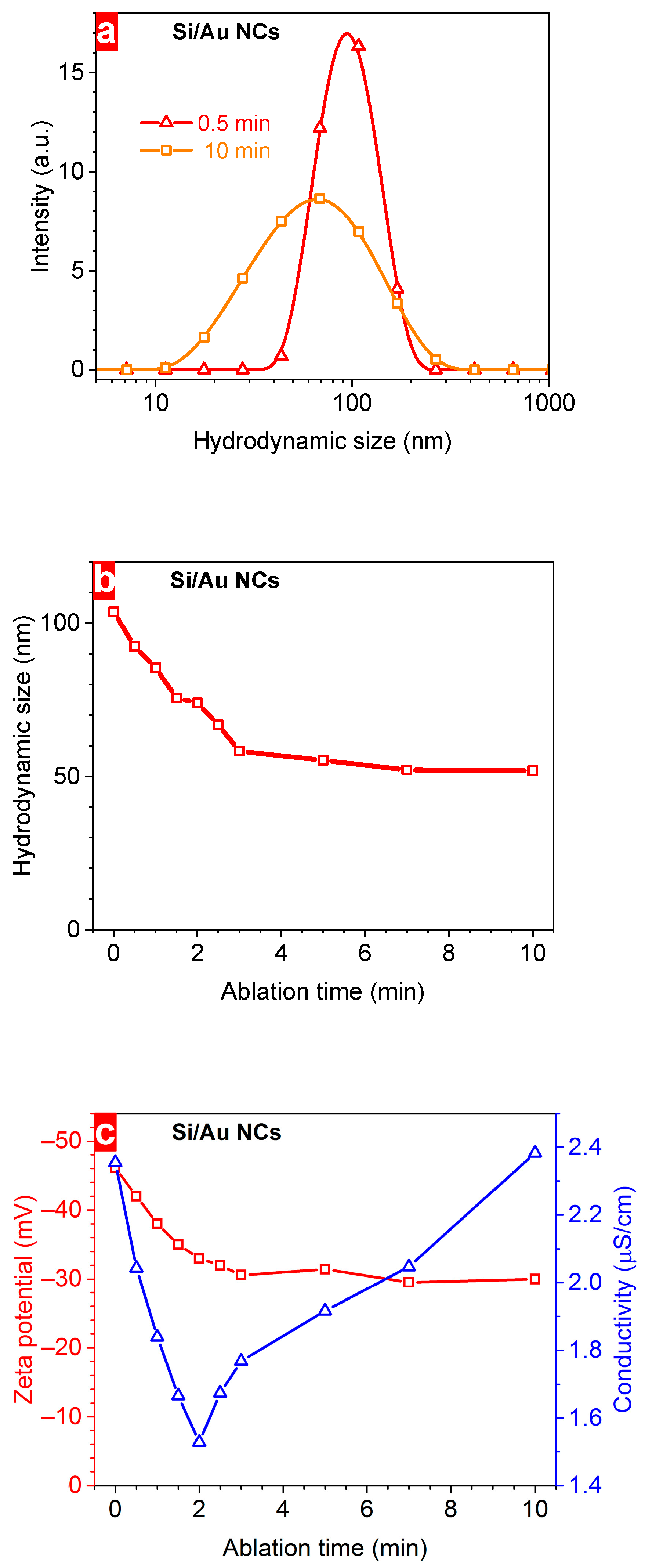
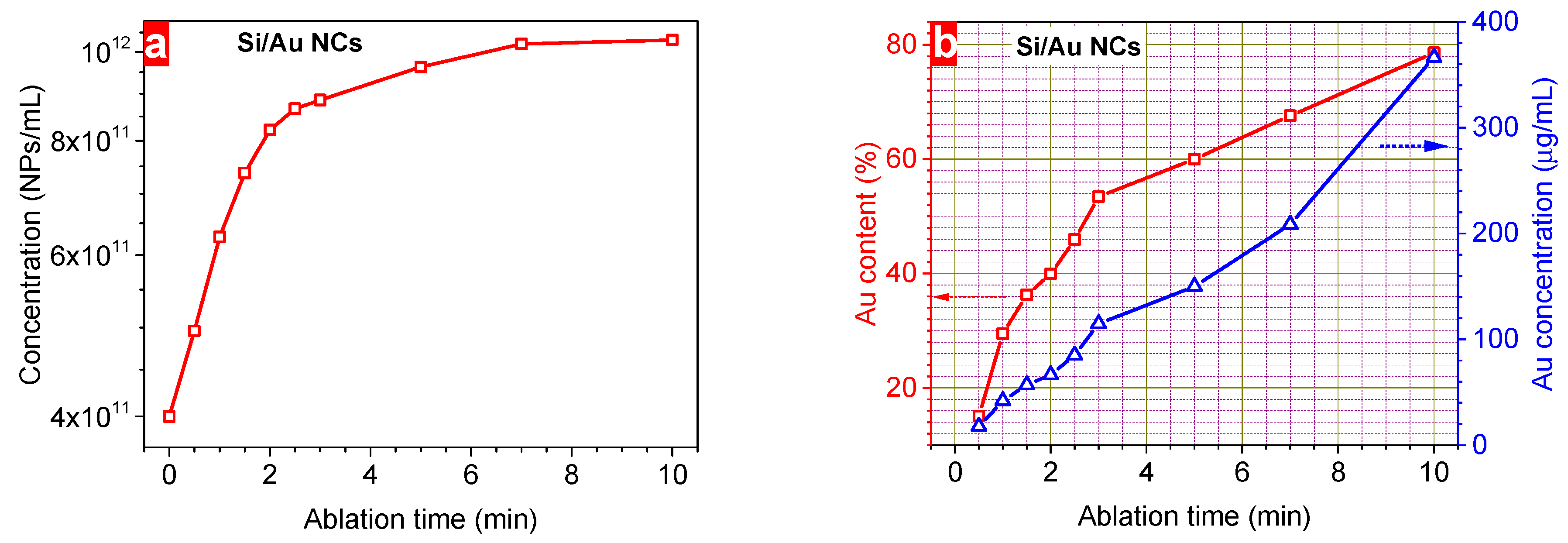
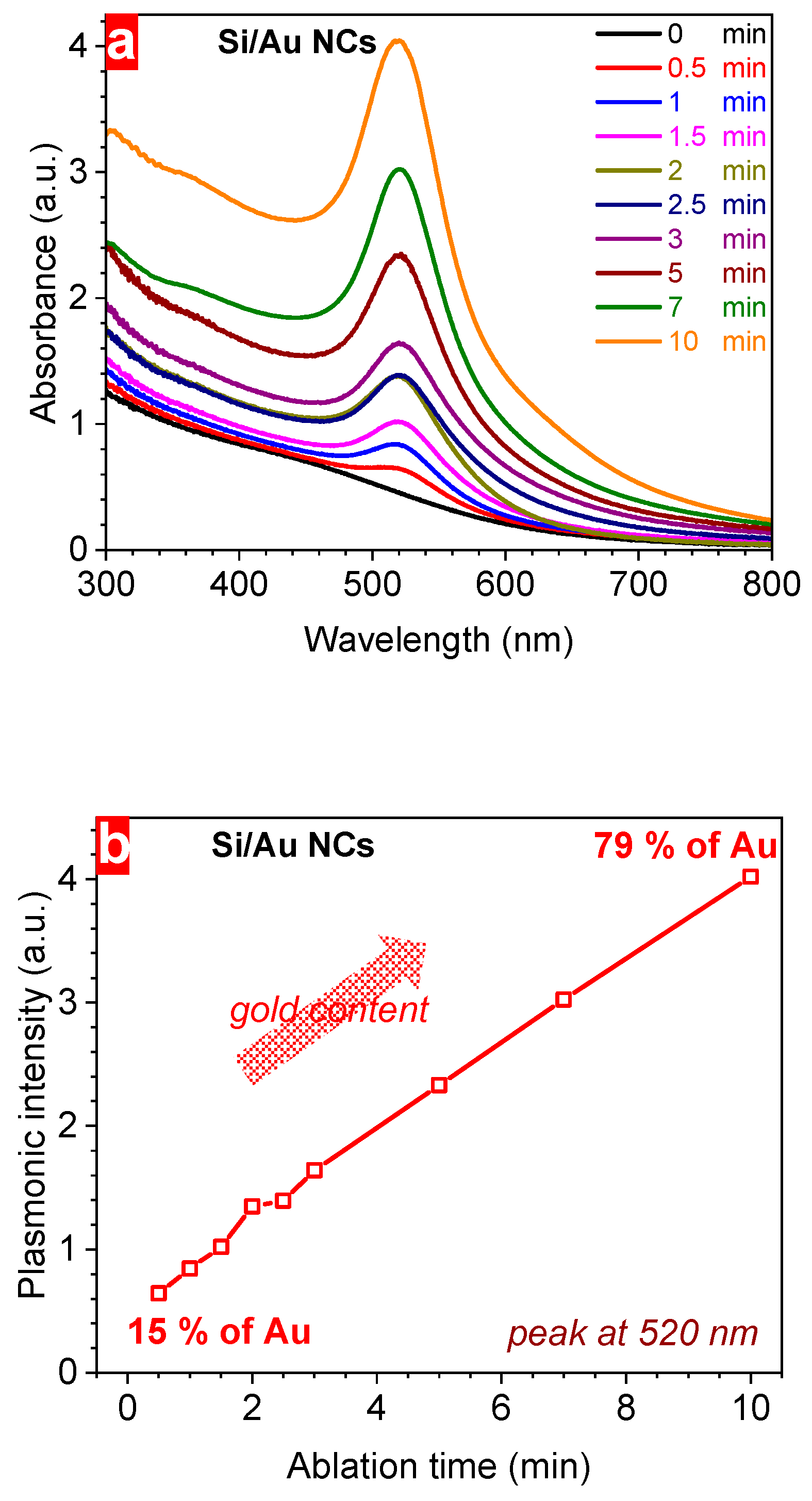
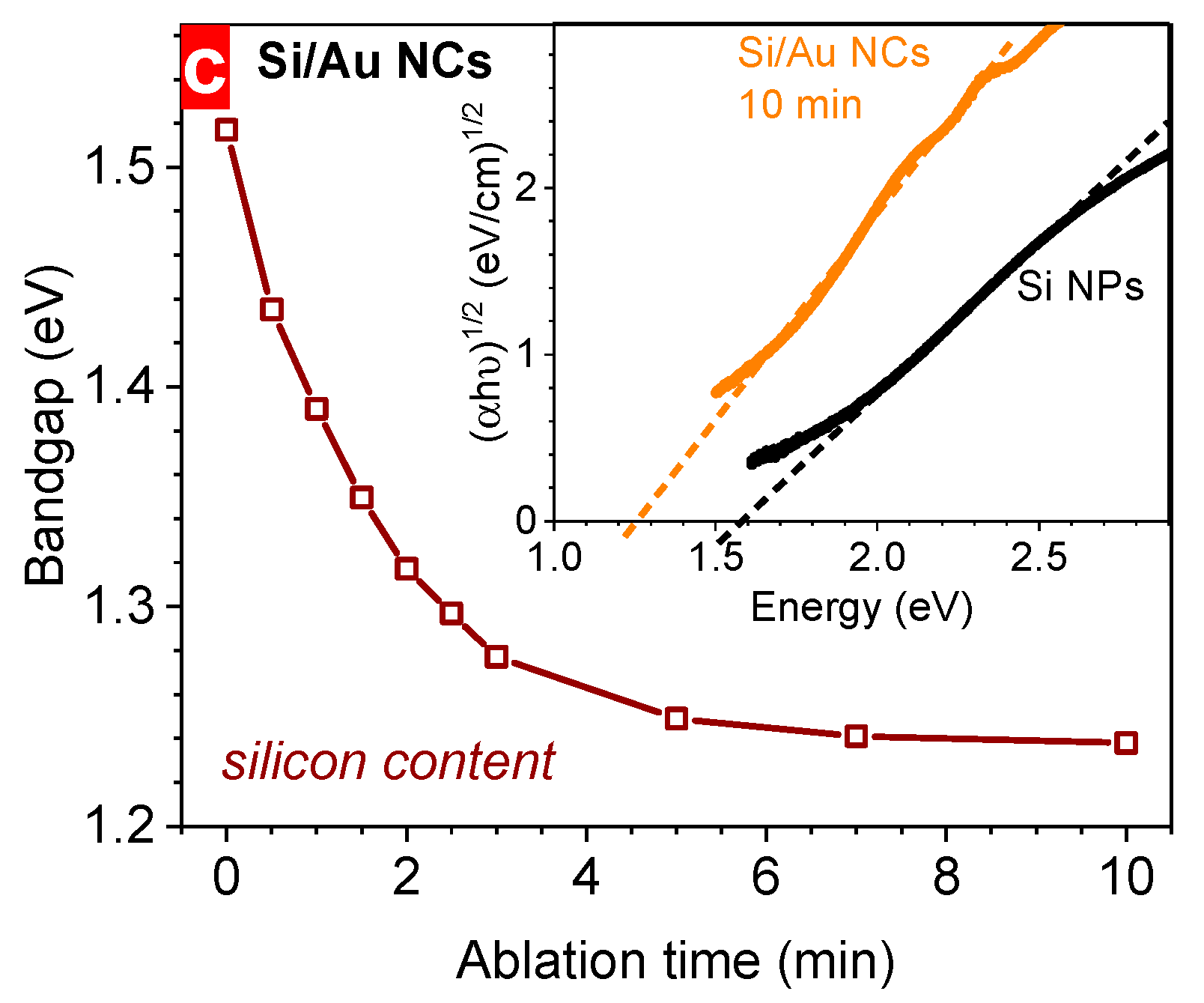
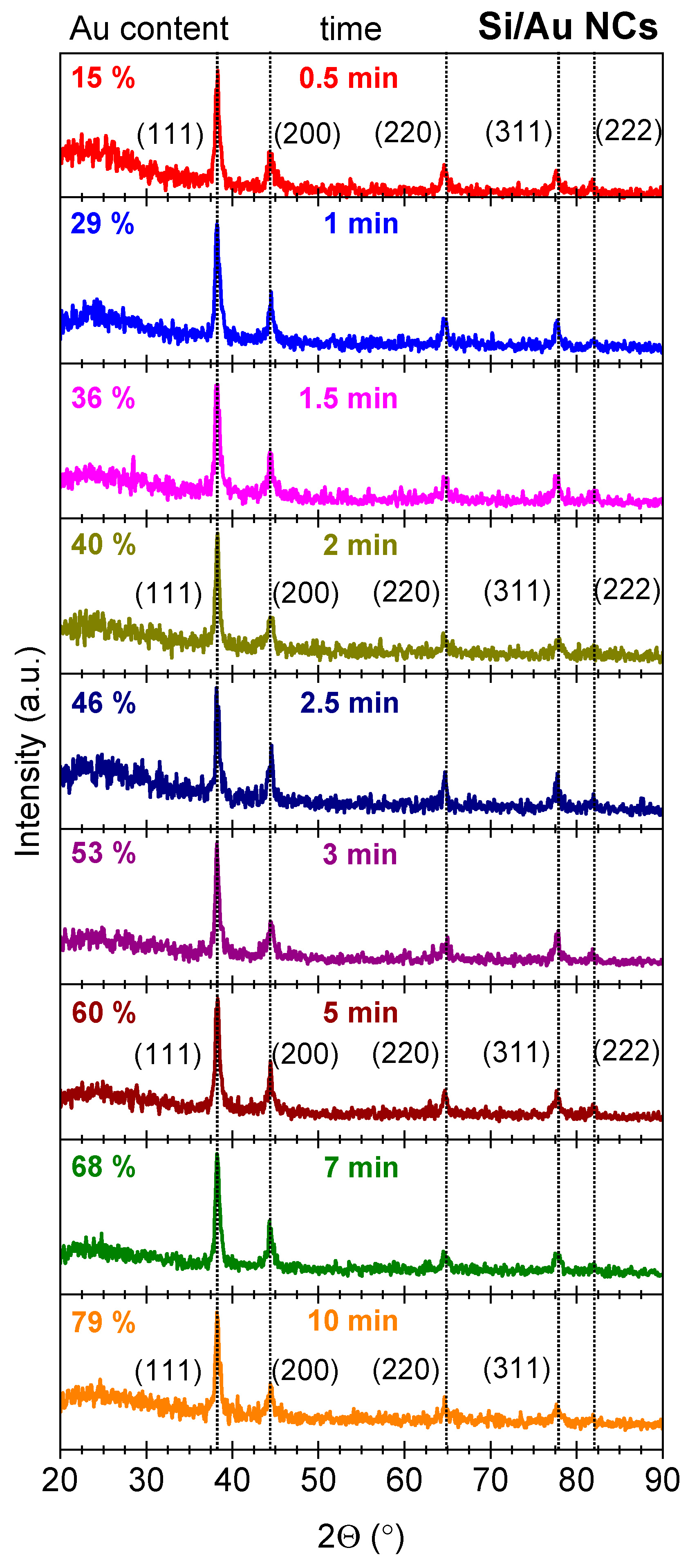
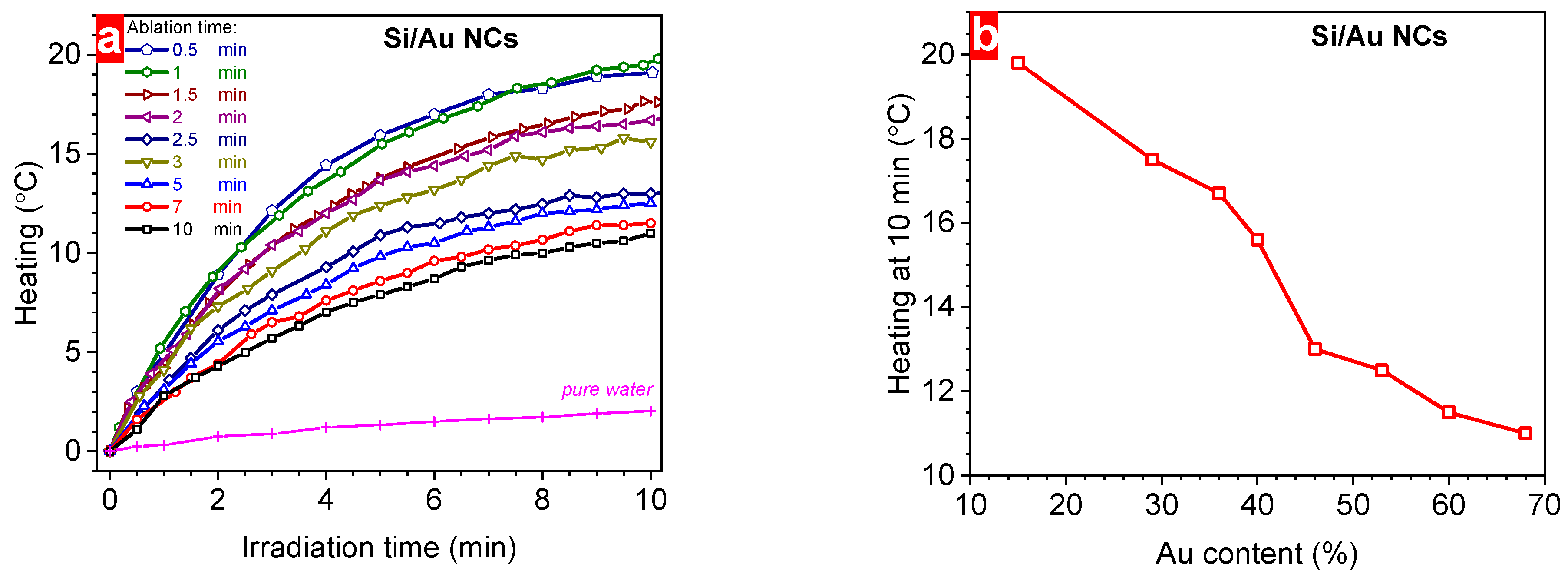
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Xing, F.; Zhou, Y.; Yu, P.; Xu, J.; Luo, R.; Xiang, Z.; Rommens, P.M.; Liu, M.; Ritz, U. Nanomaterials-based photothermal therapies for antibacterial applications. Mater. Design 2023, 233, 112231. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Zharov, V.; Melnikov, A.; Tuchin, V.; Khlebtsov, N. Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology 2006, 17, 5167. [Google Scholar] [CrossRef]
- Sharma, A.; Saini, A.K.; Kumar, N.; Tejwan, N.; Singh, T.A.; Thakur, V.K.; Das, J. Methods of preparation of metal-doped and hybrid tungsten oxide nanoparticles for anticancer, antibacterial, and biosensing applications. Surf. Interfaces 2022, 28, 101641. [Google Scholar] [CrossRef]
- Encarnación, C.; Aberasturi, D.J.; Liz-Marzán, L.M. Multifunctional plasmonic-magnetic nanoparticles for bioimaging and hyperthermia. Adv. Drug Deliver. Rev. 2022, 189, 114484. [Google Scholar] [CrossRef]
- Alheshibri, M.; Elsayed, K.; Haladu, S.A.; Magami, S.M.; Baroot, A.A.; Ercan, I.; Ercan, F.; Manda, A.A.; Çevik, E.; Kayed, T.S.; et al. Synthesis of Ag nanoparticles-decorated on CNTs/TiO2 nanocomposite as efficient photocatalysts via nanosecond pulsed laser ablation. Opt. Laser Technol. 2022, 155, 108443. [Google Scholar] [CrossRef]
- Antwi-Baah, R.; Wang, Y.; Chen, X.; Liu, H.; Yu, K. Hybrid morphologies of paramagnetic manganese-based nanoparticles as theranostics. Chem. Eng. J. 2023, 466, 142970. [Google Scholar] [CrossRef]
- Li, T.; Guo, H.; Liu, Y.; Qi, W.; Wu, C.; Xi, L. All-in-One Photoacoustic Theranostics Using Multi-Functional Nanoparticles. Adv. Funct. Mat. 2022, 32, 2107624. [Google Scholar] [CrossRef]
- Karsakova, M.; Shchedrina, N.; Karamyants, A.; Ponkratova, E.; Odintsova, G.; Zuev, D. Eco-friendly Approach for Creation of Resonant Silicon Nanoparticle Colloids. Langmuir 2023, 39, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Song, H.; Li, Y.; Wang, P.; Liu, S. Fabrication of 4H–SiC nanoparticles using femtosecond pulsed laser ablation in deionized water. Opt. Mater. 2022, 132, 112817. [Google Scholar] [CrossRef]
- Mansour, Y.; Battie, Y.; Naciri, A.N.; Chaoui, N. In situ monitoring the productivity of ultra-small gold nanoparticles generated by pulsed-laser ablation of a high-speed rotating gold target in pure water. Nanotechnology 2023, 34, 075602. [Google Scholar] [CrossRef] [PubMed]
- Zwiehoff, S.; Johny, J.; Behrends, C.; Landmann, A.; Mentzel, F.; Bäumer, C.; Kröninger, K.; Rehbock, C.; Timmermann, B.; Barcikowski, S. Enhancement of Proton Therapy Efficiency by Noble Metal Nanoparticles Is Driven by the Number and Chemical Activity of Surface Atoms. Small 2022, 18, 2106383. [Google Scholar] [CrossRef] [PubMed]
- Homik, Z.; Kopniczky, J.; Smausz, T.; Berkesi, D.; Hopp, B. Formation of gold/silver composite nanoparticles by pulsed laser ablation of gold–silver layered films in liquid. Appl. Phys. A 2022, 128, 797. [Google Scholar] [CrossRef]
- Coviello, V.; Forrer, D.; Amendola, V. Recent Developments in Plasmonic Alloy Nanoparticles: Synthesis, Modelling, Properties and Applications. ChemPhysChem 2022, 23, e202200136. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Guadagnini, A.; Agnoli, S.; Badocco, D.; Pastore, P.; Fracasso, G.; Gerosa, M.; Vurro, F.; Busato, A.; Marzola, P. Polymer-coated silver-iron nanoparticles as efficient and biodegradable MRI contrast agents. J. Colloid Interface Sci. 2021, 596, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Ryabchikov, Y.V. Multi-Modal Laser-Fabricated Nanocomposites with Non-Invasive Tracking Modality and Tuned Plasmonic Properties. Crystals 2023, 13, 1381. [Google Scholar] [CrossRef]
- Gurbatov, S.O.; Puzikov, V.; Storozhenko, D.; Modin, E.; Mitsai, E.; Cherepakhin, A.; Shevlyagin, A.; Gerasimenko, A.V.; Kulinich, S.A.; Kuchmizhak, A.A. Multigram-Scale Production of Hybrid Au-Si Nanomaterial by Laser Ablation in Liquid (LAL) for Temperature-Feedback Optical Nanosensing, Light-to-Heat Conversion, and Anticounterfeit Labeling. ACS Appl. Mater. Interfaces 2023, 15, 3336–3347. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V. Facile laser synthesis of multimodal composite silicon/gold nanoparticles with variable chemical composition. J. Nanoparticle Res. 2019, 21, 85. [Google Scholar] [CrossRef]
- Nasiri, P.; Doranian, D.; Sari, A.H. Synthesis of Au/Si nanocomposite using laser ablation method. Opt. Laser Technol. 2019, 113, 217–224. [Google Scholar] [CrossRef]
- Saraeva, I.N.; Luong, N.V.; Kudryashov, S.I.; Rudenko, A.A.; Khmelnitskiy, R.A.; Shakhmin, A.L.; Kharin, A.Y.; Ionin, A.A.; Zayarny, D.A.; Tung, D.H.; et al. Laser synthesis of colloidal Si@Au and Si@Ag nanoparticles in water via plasma-assisted reduction. J. Photochem. Photobiol. 2018, 360, 125–131. [Google Scholar] [CrossRef]
- Kutrovskaya, S.; Arekelian, S.; Kucherik, A.; Osipov, A.; Evlyukhin, A.; Kavokin, A.V. The synthesis of hybrid gold-silicon nano particles in a liquid. Sci. Rep. 2017, 7, 10284. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, M.S.; Salim, E.T.; Saimon, J.A. Structural, morphological and optical properties of tungsten trioxide nanoparticle synthesis by pulsed laser ablation in water: Effect of laser fluence. J. Opt. 2023, 1–16. [Google Scholar] [CrossRef]
- Lazar, O.A.; Moise, C.C.; Nikolov, A.S.; Enache, L.-B.; Mihai, G.V.; Enachescu, M. The Water-Based Synthesis of Platinum Nanoparticles Using KrF Excimer Laser Ablation. Nanomaterials 2022, 12, 348. [Google Scholar] [CrossRef]
- Moura, C.G.; Pereira, R.S.F.; Andritschky, M.; Lopes, A.L.B.; Grilo, J.P.F.; do Nascimento, R.M.; Silva, F.S. Effects of laser fluence and liquid media on preparation of small Ag nanoparticles by laser ablation in liquid. Opt. Laser Technol. 2017, 97, 20–28. [Google Scholar] [CrossRef]
- Ghaem, E.N.; Dorranian, D.; Sari, A.H. Characterization of cobalt oxide nanoparticles produced by laser ablation method: Effects of laser fluence. Physica E 2020, 115, 113670. [Google Scholar] [CrossRef]
- Maximova, K.; Aristov, A.; Sentis, M.; Kabashin, A.V. Size-controllable synthesis of bare gold nanoparticles by femtosecond laser fragmentation in water. Nanotechnology 2015, 26, 065601. [Google Scholar] [CrossRef]
- Gerasimova, E.N.; Uvarov, E.; Yaroshenko, V.V.; Epifanovskaya, O.; Shakirova, A.; Logunov, L.S.; Vlasova, O.; Parodi, A.; Zamyatnin, A.A.; Timin, A.S.; et al. Single-Step Fabrication of Resonant Silicon–Gold Hybrid Nanoparticles for Efficient Optical Heating and Nanothermometry in Cells. ACS Appl. Nano Mater. 2023, 6, 18848–18857. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V.; Lysenko, V.; Nychyporuk, T. Enhanced Thermal Sensitivity of Silicon Nanoparticles Embedded in (nano–Ag)/SiNx for Luminescent Thermometry. J. Phys. Chem. C 2014, 118, 12515–12519. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V.; Alekseev, S.A.; Lysenko, V.; Bremond, G.; Bluet, J.-M. Photoluminescence thermometry with alkyl–terminated silicon nanoparticles dispersed in low–polar liquids. Phys. Status Solidi-RRL 2013, 7, 414–417. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, Z.; Li, M.; Zhang, H.; Li, T.; Qin, X.; Li, S.; Wu, C.; You, F.; Liao, X.; et al. Mesoporous Silica Nanoparticles-Based Nanoplatforms: Basic Construction, Current State, and Emerging Applications in Anticancer Therapeutics. Adv. Healthc. Mater. 2023, 12, 2201884. [Google Scholar] [CrossRef] [PubMed]
- Ryabchikov, Y.V.; Belogorokhov, I.A.; Vorontsov, A.S.; Osminkina, L.A.; Timoshenko, V.Y.; Kashkarov, P.K. Dependence of the Singlet Oxygen Photosensitization Efficiency on Morphology of Porous Silicon. Phys. Status Solidi A 2007, 204, 1271–1275. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Demin, V.A.; Vorontzov, A.S.; Ryabchikov, Y.V.; Belogorokhov, I.A.; Osminkina, L.A.; Forsh, P.A.; Kashkarov, P.K.; Timoshenko, V.Y. Electron Paramagnetic Resonance and Photoluminescence Study of Si Nanocrystals—Photosensitizers of Singlet Oxygen Molecules. J. Non–Cryst. Solids 2006, 352, 1156–1159. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V.; Belogorokhov, I.A.; Gongalskiy, M.B.; Osminkina, L.A.; Timoshenko, V.Y. Photosensitized Generation of Singlet Oxygen in Powders and Aqueous Suspensions of Silicon Nanocrystals. Semiconductors 2011, 45, 1059–1063. [Google Scholar] [CrossRef]
- Hamza, S.; Ignaszak, A.; Kiani, A. Synthesis of electrical conductive silica nanofiber/gold nanoparticle composite by laser pulses and sputtering technique. Nanoscale Res. Lett. 2017, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Y.; Wang, M.L. On-chip highly sensitive saliva glucose sensing using multilayer films composed of single-walled carbon nanotubes, gold nanoparticles, and glucose oxidase. Sens. Bio-Sens. Res. 2015, 4, 96–102. [Google Scholar] [CrossRef]
- Wen-Ge, D.; Jing, Y.; Ling-Hai, M.; Shu-Jie, W.; Wei, Y.; Guang-Sheng, F. Dependence of Optical Absorption in Silicon Nanostructures on Size of Silicon Nanoparticles. Commun. Theor. Phys. 2011, 55, 688–692. [Google Scholar] [CrossRef]
- Ghobadi, N. Band gap determination using absorption spectrum fitting procedure. Int. Nano Lett. 2013, 3, 2. [Google Scholar] [CrossRef]
- Theiβ, W. Optical properties of porous silicon. Surf. Sci. Rep. 1997, 29, 91–192. [Google Scholar] [CrossRef]
- Rustamov, F.A.; Darvishov, N.H.; Bagiev, V.E.; Mamedov, M.Z.; Bobrova, E.Y.; Qafarova, H.O. Determination of size and bandgap distributions of Si nanoparticles from photoluminescence excitation and emission spectra in n-type stain etched porous silicon. J. Lumin. 2014, 154, 224–228. [Google Scholar] [CrossRef]
- Ryabchikov, Y.V.; Behrends, J. Expedient paramagnetic properties of surfactant-free plasmonic silicon-based nanoparticles. Opt. Quant. Electron. 2020, 52, 177. [Google Scholar] [CrossRef]
- Forbes, L. Gold in Silicon: Characterisation and Infra-red Detector Applications. Gold Bull. 1977, 10, 49–53. [Google Scholar] [CrossRef]
- Utzig, J.; Schroter, W. Donor and acceptor behavior of gold in silicon. Appl. Phys. Lett. 1984, 45, 761–763. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Z.; Li, W.; Shen, X. Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement. Nanomaterials 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Svetlichnyi, V.A.; Izaak, T.I.; Lapin, I.N.; Martynova, D.O.; Stonkus, O.A.; Stadnichenko, A.I.; Boronin, A.I. Physicochemical investigation of nanopowders prepared by laser ablation of crystalline silicon in water. Adv. Powder Technol. 2015, 26, 478–486. [Google Scholar] [CrossRef]
- Kuzmin, P.G.; Shafeev, G.A.; Bukin, V.V.; Garnov, S.V.; Farcau, C.; Carles, R.; Warot-Fontrose, B.; Guieu, V.; Viau, G. Silicon Nanoparticles Produced by Femtosecond Laser Ablation in Ethanol: Size Control, Structural Characterization, and Optical Properties. J. Phys. Chem. C 2010, 114, 15266–15273. [Google Scholar] [CrossRef]
- Ferrah, D.; Penuelas, J.; Boudaa, F.; Botella, C.; Silly, M.; Sirotti, F.; Grenet, G. A Photoemission Analysis of Gold on Silicon Regarding the Initial Stages of Nanowire Metal-Catalyzed Vapor–Liquid–Solid Growth. J. Phys. Chem. C 2022, 126, 18692–18703. [Google Scholar] [CrossRef]
- Lu, Z.H.; Sham, T.K.; Norton, P.R. Interaction of Au on Si(100) studied by core level binding energy shifts. Sol. State Comm. 1993, 85, 957. [Google Scholar] [CrossRef]
- Khalakhan, I.; Vorokhta, M.; Chundak, M.; Matolín, V. Au-CeO2 nanoporous films/carbon nanotubes composites prepared by magnetron sputtering. Appl. Surf. Sci. 2013, 267, 150–153. [Google Scholar] [CrossRef]
- Higo, M.; Mitsushio, M.; Yoshidome, T.; Nakatake, S. Characterization and preservation of gold oxides prepared by an oxygen-dc glow discharge from gold films and studied by X-ray photoelectron spectroscopy. Gold Bull. 2020, 53, 77–92. [Google Scholar] [CrossRef]
- Sivakumar, M.; Venkatakrishnan, K.; Tan, B. Characterization of MHz pulse repetition rate femtosecond laser-irradiated gold-coated silicon surfaces. Nanoscale Res. Lett. 2011, 6, 78. [Google Scholar] [CrossRef]
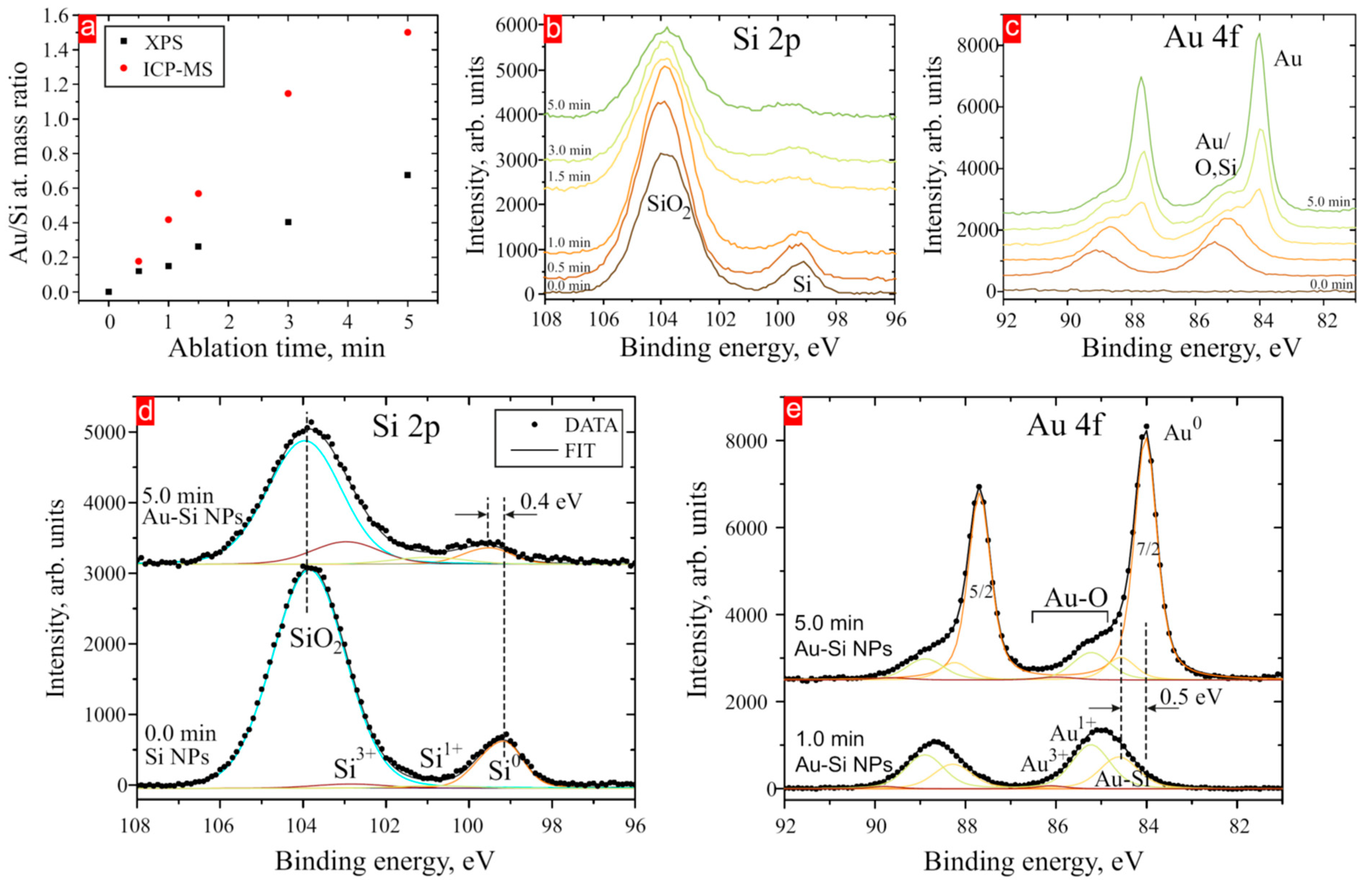

| BE Au 4f7/2, eV | BE Si 2p3/2, eV | |||||||
|---|---|---|---|---|---|---|---|---|
| Time, min | Au0 | Au-Si | Au1+ | Au3+ | Si0 | Si1+ | Si3+ | Si4+/SiO2 |
| 0.0 | --- | --- | --- | --- | 99.1 | 100.7 | 102.7 | 103.7 |
| 0.5 | --- | 84.9 | 85.5 | 86.4 | 99.2 | 100.8 | 102.8 | 103.8 |
| 1.0 | --- | 84.6 | 85.2 | 86.1 | 99.0 | 100.7 | 102.7 | 103.7 |
| 1.5 | 84.0 | 84.5 | 85.2 | 86.0 | 99.2 | 100.7 | 102.7 | 103.7 |
| 3.0 | 83.9 | 84.5 | 85.1 | 85.9 | 99.1 | 100.7 | 102.7 | 103.7 |
| 5.0 | 84.0 | 84.5 | 85.2 | 86.0 | 99.4 | 100.8 | 102.8 | 103.8 |
| Ablation Time (min) | Au Mass (µg) | Au Content (%) | Plasmonic Intensity (a.u.) | Bandgap (eV) | Hydrodynamic Size (nm) | ξ-Potential (mV) | Concentration (1011 NPs/mL) | Conductivity (µS/cm) | Maximum Heating at 10 min (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 18 | 15 | 0.64 | 1.44 | 92.4 | –42 | 4.96 | 2.04 | 18.1 |
| 1 | 42 | 29 | 0.84 | 1.39 | 85.5 | –38 | 6.28 | 1.84 | 19.8 |
| 1.5 | 57 | 36 | 1.02 | 1.35 | 75.6 | –35 | 7.37 | 1.67 | 17.5 |
| 2 | 66 | 40 | 1.35 | 1.32 | 73.9 | –33 | 8.22 | 1.53 | 16.7 |
| 2.5 | 85 | 46 | 1.39 | 1.30 | 66.8 | –32 | 8.66 | 1.67 | 13.0 |
| 3 | 115 | 53 | 1.64 | 1.28 | 58.2 | –31 | 8.86 | 1.77 | 15.6 |
| 5 | 150 | 60 | 2.33 | 1.25 | 55.3 | –30 | 9.63 | 1.92 | 12.5 |
| 7 | 208 | 68 | 3.02 | 1.24 | 52.1 | –30 | 10.2 | 2.05 | 11.5 |
| 10 | 366 | 79 | 4.02 | 1.24 | 51.9 | –30 | 10.3 | 2.38 | 11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryabchikov, Y.V.; Mirza, I.; Flimelová, M.; Kana, A.; Romanyuk, O. Merging of Bi-Modality of Ultrafast Laser Processing: Heating of Si/Au Nanocomposite Solutions with Controlled Chemical Content. Nanomaterials 2024, 14, 321. https://doi.org/10.3390/nano14040321
Ryabchikov YV, Mirza I, Flimelová M, Kana A, Romanyuk O. Merging of Bi-Modality of Ultrafast Laser Processing: Heating of Si/Au Nanocomposite Solutions with Controlled Chemical Content. Nanomaterials. 2024; 14(4):321. https://doi.org/10.3390/nano14040321
Chicago/Turabian StyleRyabchikov, Yury V., Inam Mirza, Miroslava Flimelová, Antonin Kana, and Oleksandr Romanyuk. 2024. "Merging of Bi-Modality of Ultrafast Laser Processing: Heating of Si/Au Nanocomposite Solutions with Controlled Chemical Content" Nanomaterials 14, no. 4: 321. https://doi.org/10.3390/nano14040321





