Triboelectric Nanogenerators for Preventive Health Monitoring
Abstract
:1. Introduction
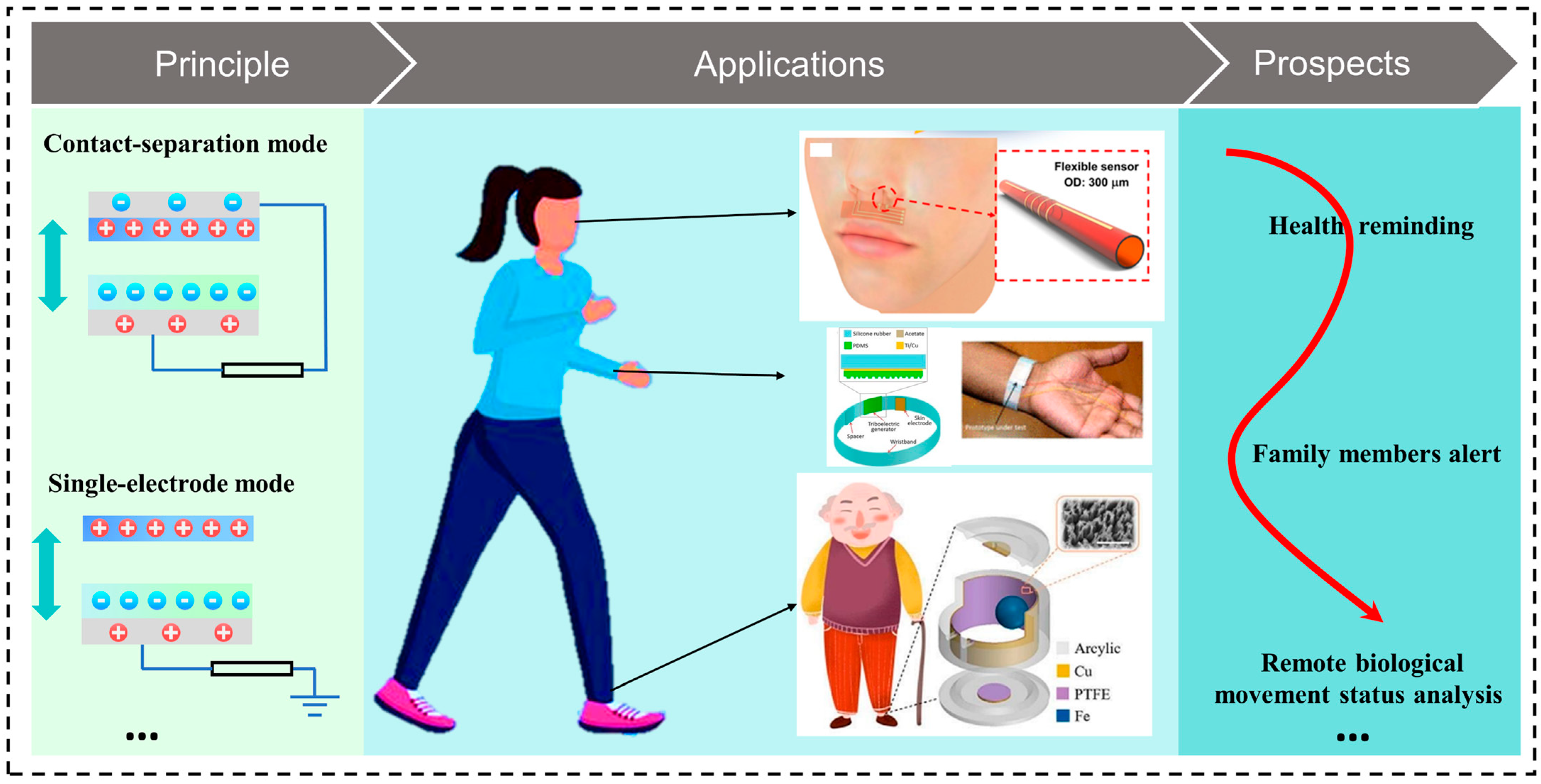
2. Working Principle and Nanomaterials of TENGs Applied in Preventive Health Monitoring
2.1. Contact–Separation Mode
2.2. Lateral-Sliding Mode
2.3. Freestanding Triboelectric-Layer Mode
2.4. Single-Electrode Mode
2.5. Nanomaterials Applied in TENGs
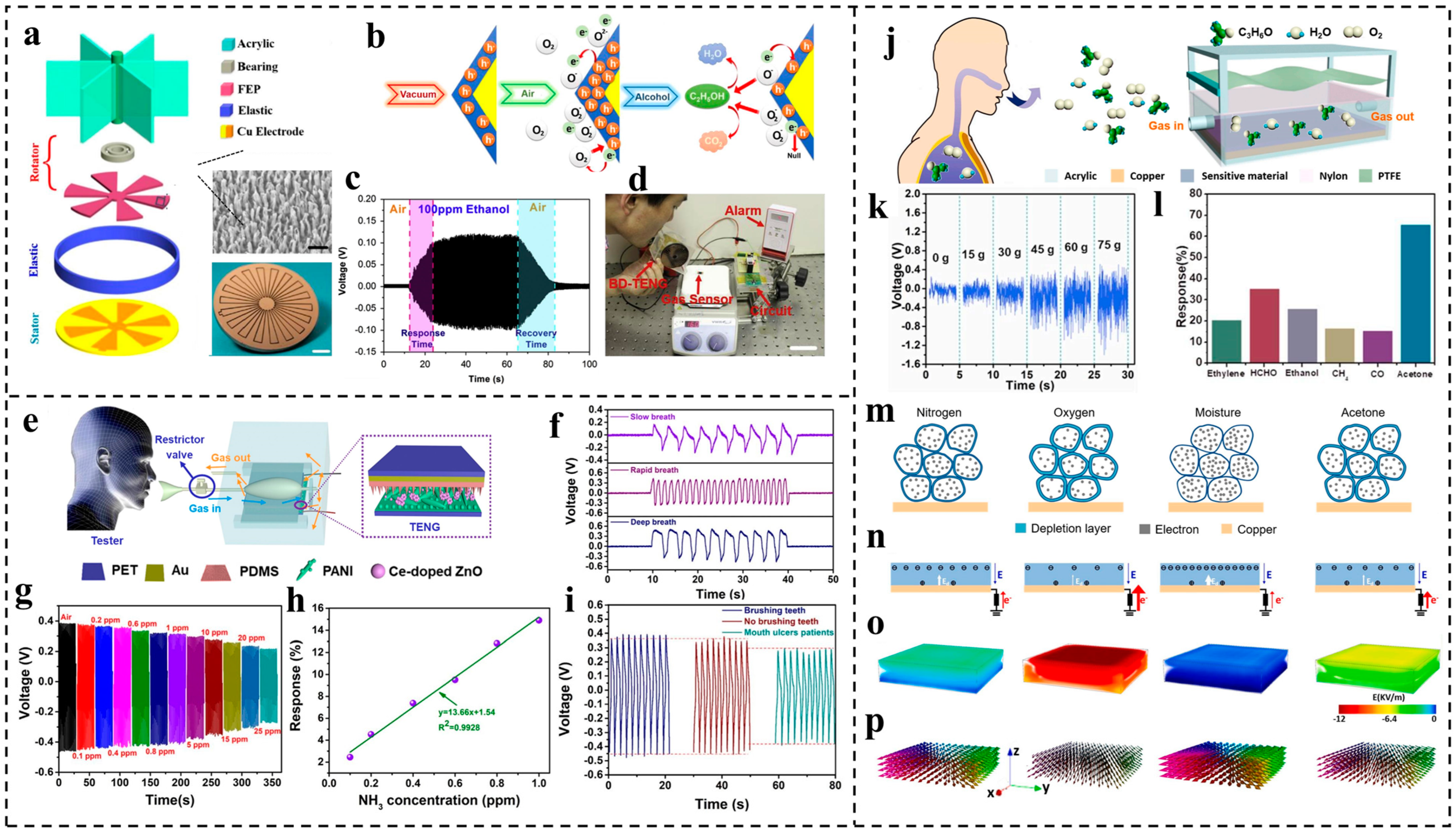
3. Applications of TENG-Based Preventive Health Monitoring
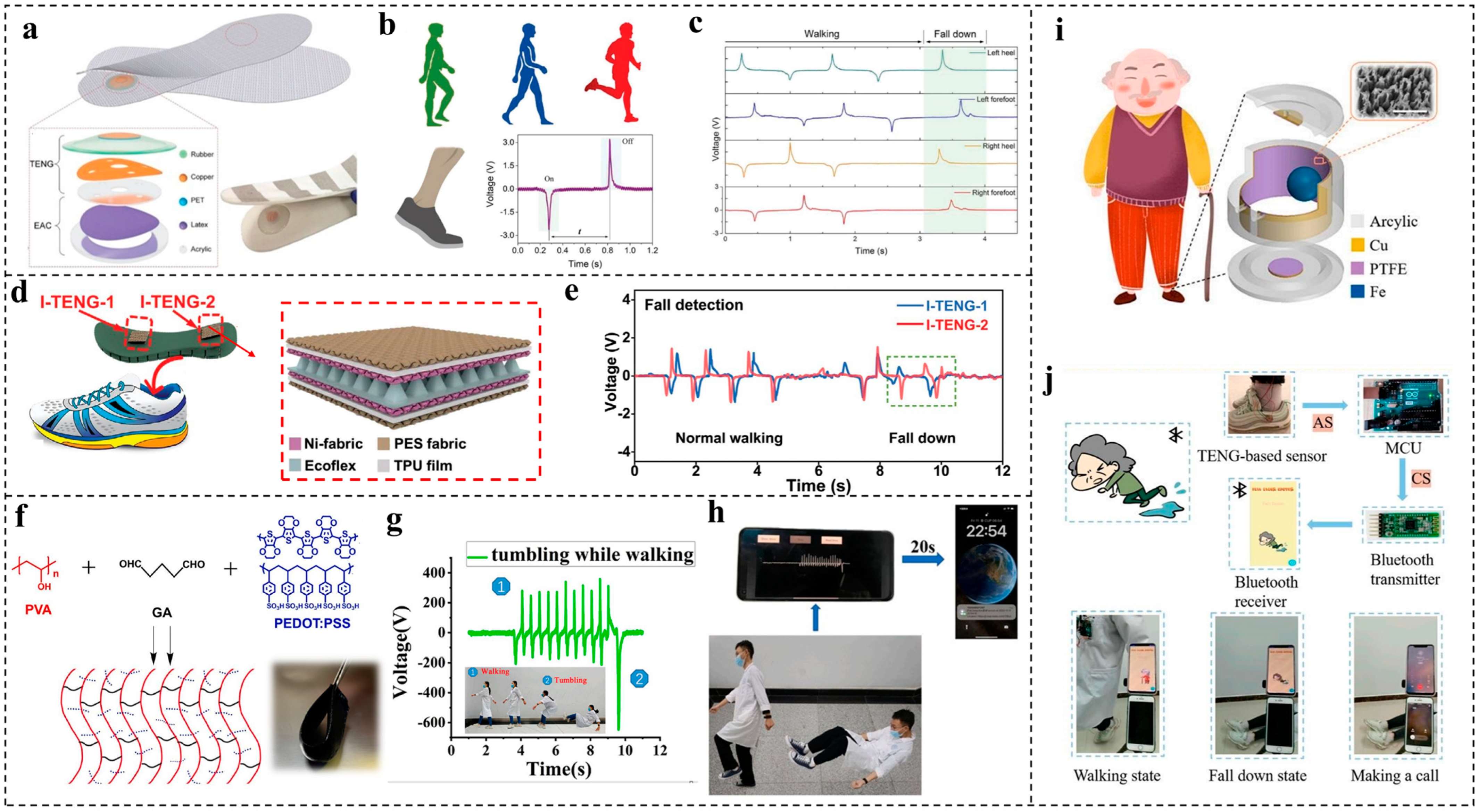
3.1. Fall Detection
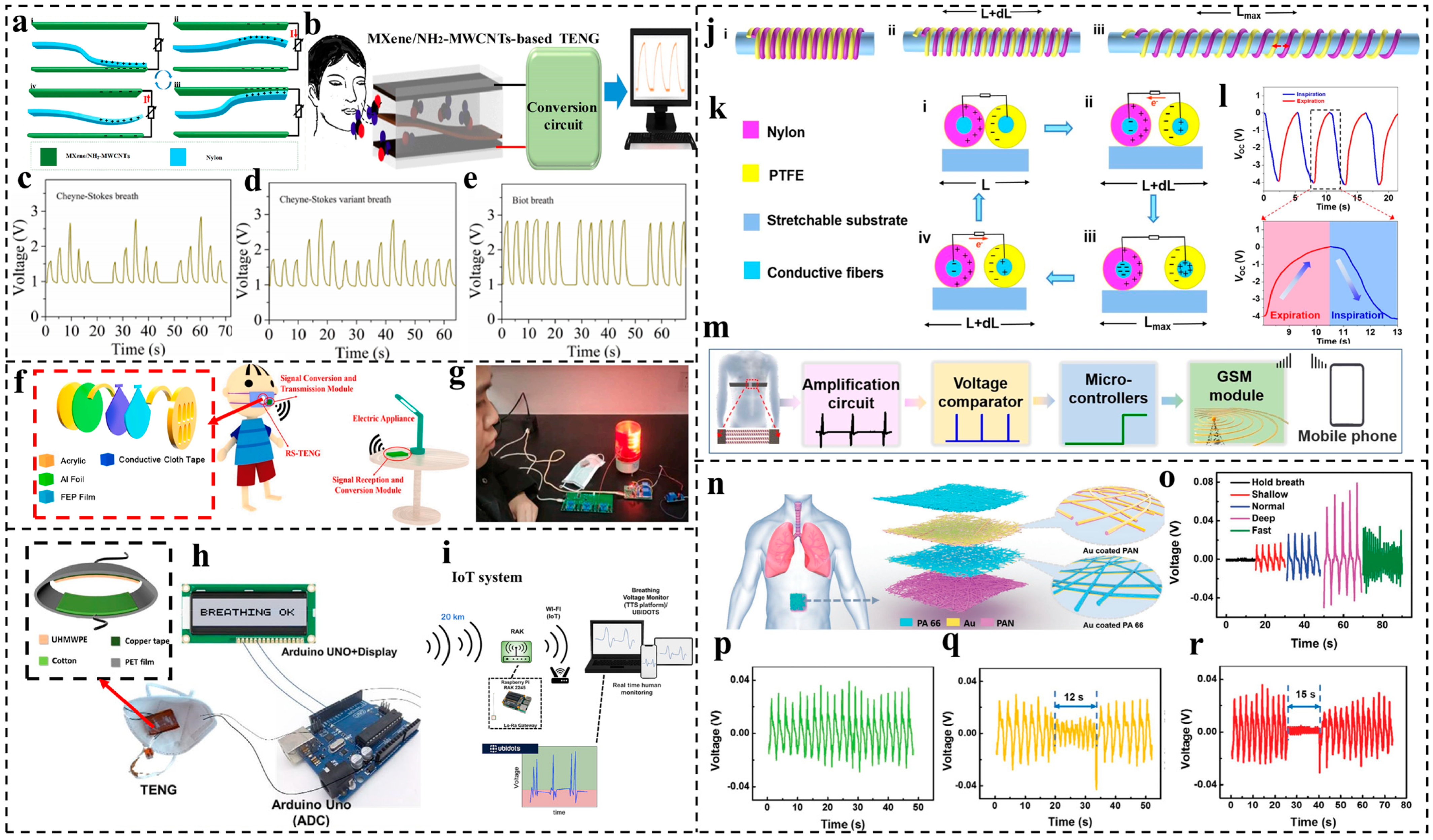
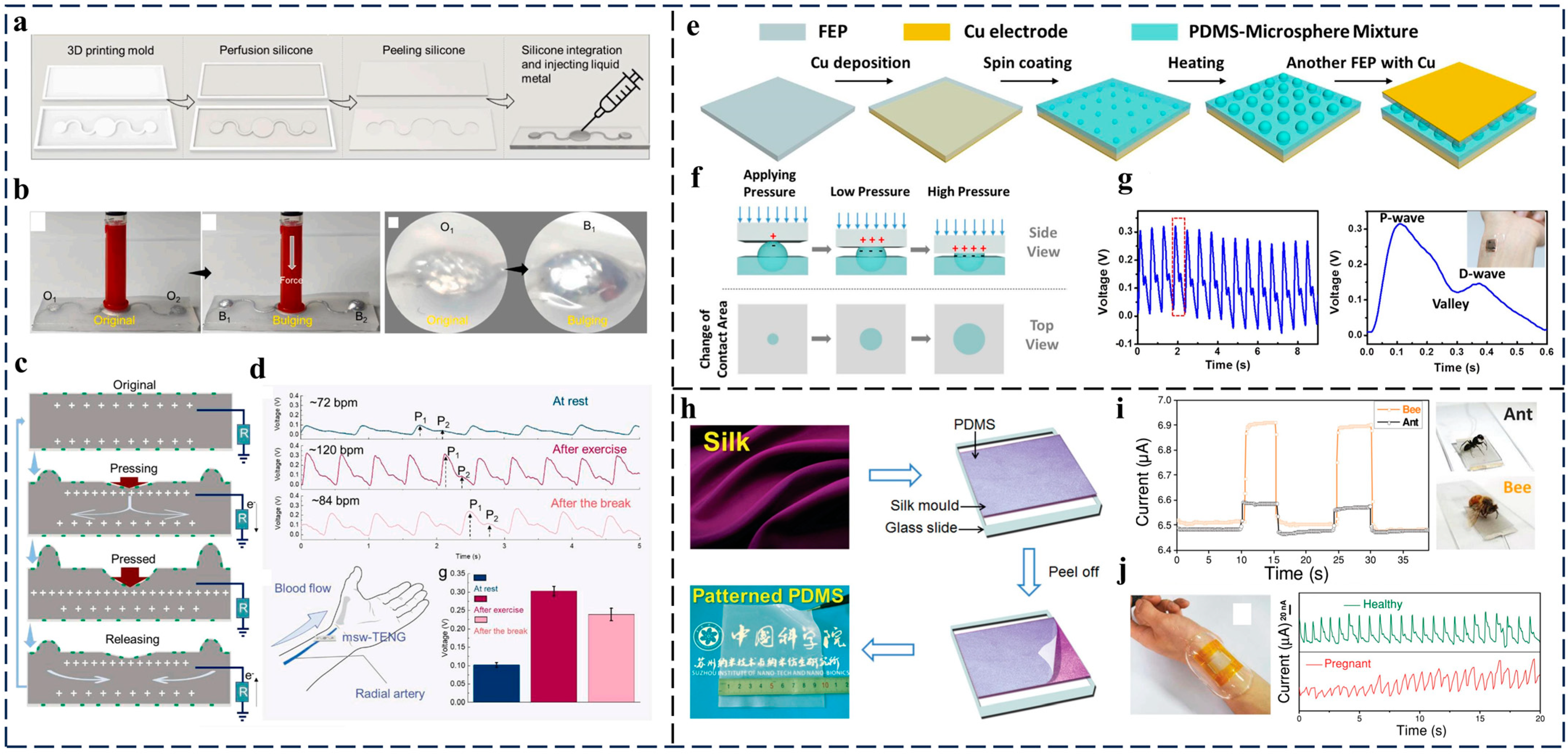
3.2. Respiration Monitoring
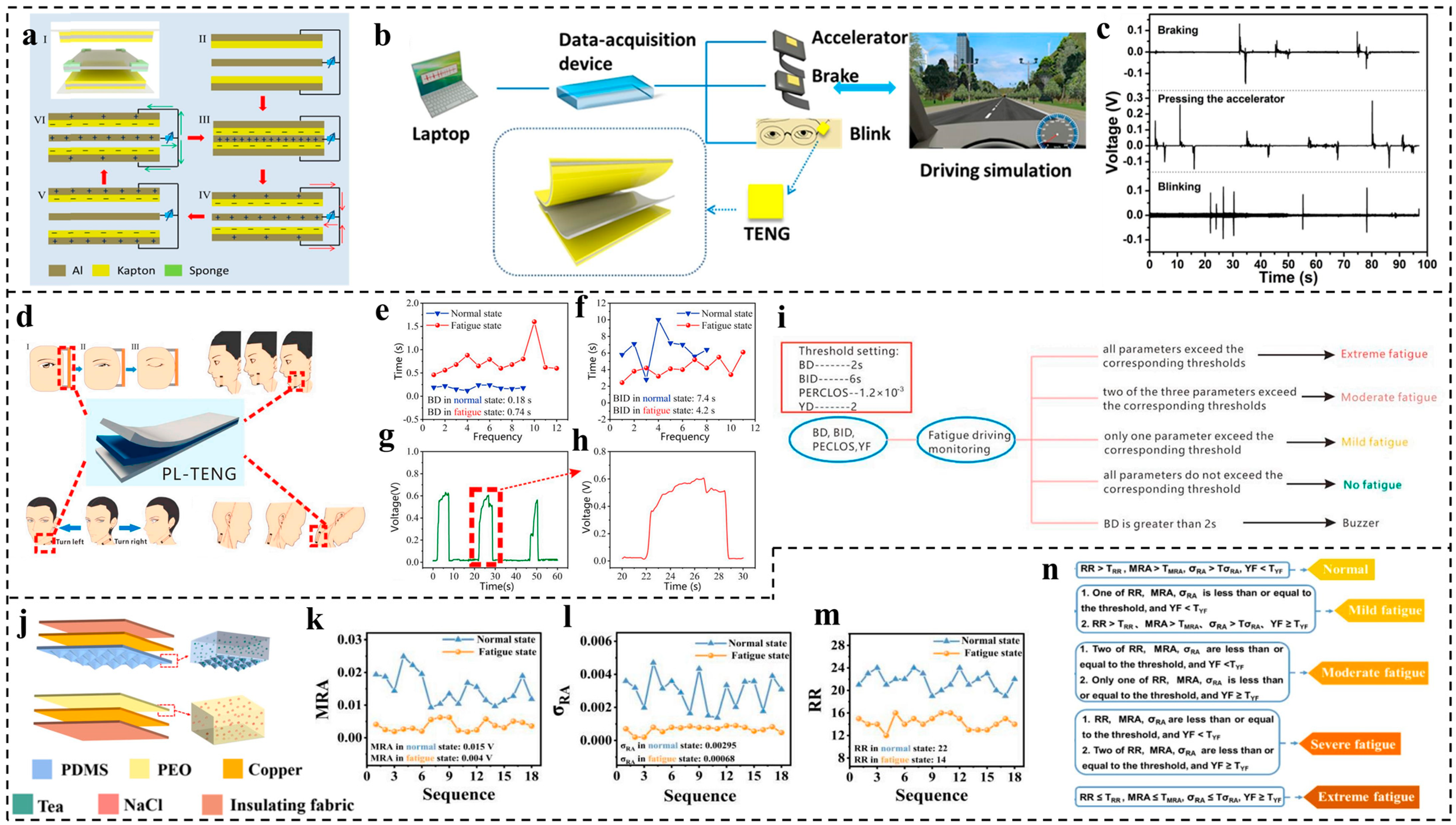
3.3. Fatigue Monitoring
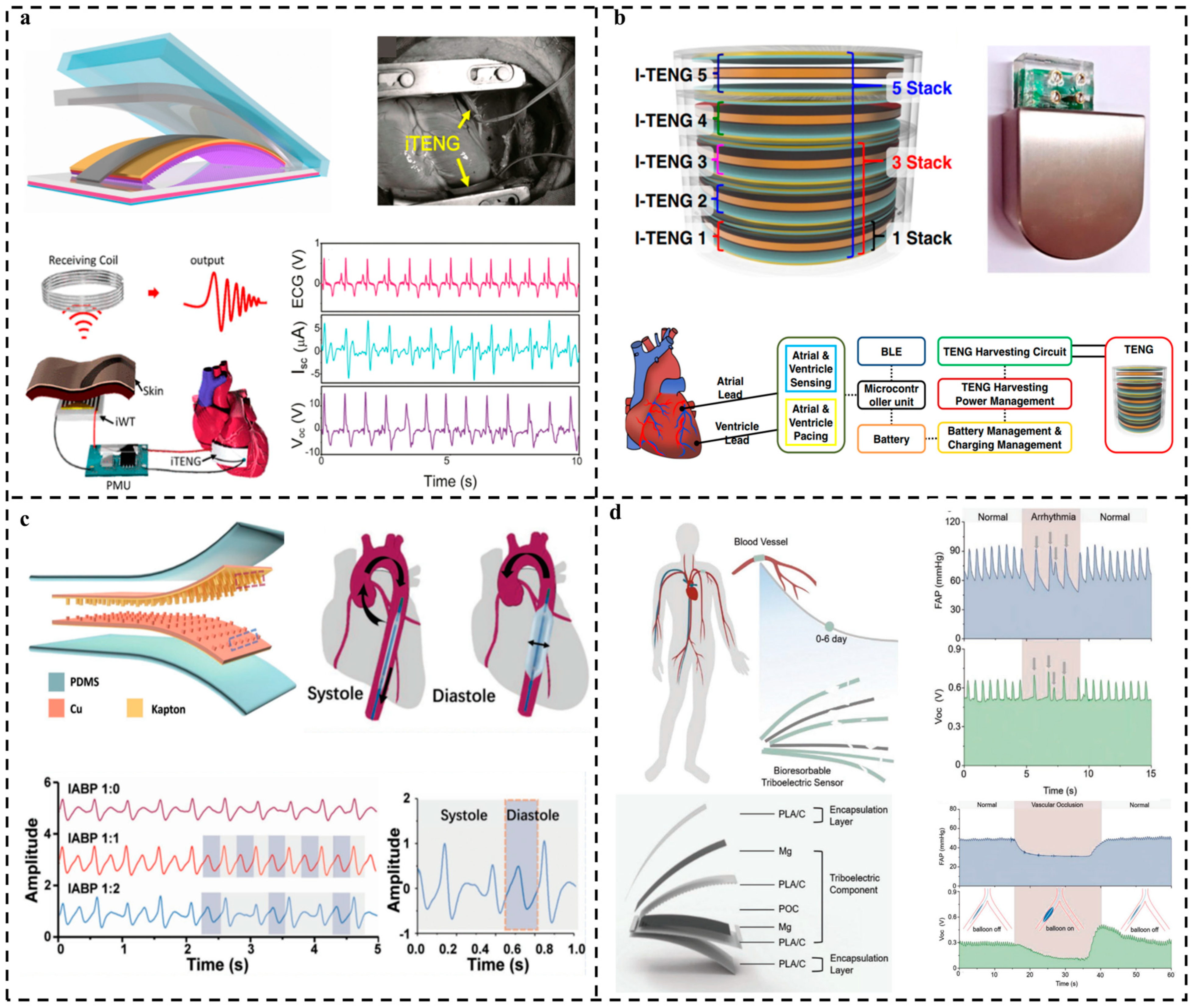
3.4. Preventive Monitoring for Cardiovascular Disease
4. Discussion and Prospects
- TENG-based sensors have many advantages when applied in fall detection for aging and disabled people due to their easy fabrication, low cost, real-time-monitoring ability, privacy proception, etc. Moreover, they can be easily integrated into a cane or shoes [71,122,123] to achieve the self-powered ability, which has the potential to eliminate the limitations of charging problems, giving aging and disabled people more convenience. In addition, they can be also applied to large healthcare centers and combined with carpets, which can protect the privacy of aging and disabled people [124]. However, according to the current research on TENG sensor systems in preventive health monitoring, they still need outside power sources, especially for ancillary systems, such as signal collection systems, signal transmission systems, and human–computer interaction devices. Therefore, the control circuit still needs to be further customized to make it suitable to the characteristics of TENGs, and low-power electronics should also be developed to cooperate with TENG-based sensors to achieve completely self-powered health-monitoring devices;
- The respiration-monitoring technologies based on TENGs can give a good early warning for many diseases, such as abnormalities in apnea, asthma, cardiac arrest, and even lung cancer, thereby leaving users and medical workers with more time to prevent the severity of the diseases. However, for real applications, portable TENG-based monitoring care systems for home environments are pressing. In addition to this, for clinical applications, a comparison of the research with that of medical researchers should also be conducted to achieve more accurate and practical applications;
- TENG-based fatigue monitoring has great potential for monitoring the driver’s status to avoid accidents and injuries and has the advantage of protecting the privacy of drivers compared with camera monitoring technologies. However, the response time is still very long for driving conditions [111], and thus a quick-response TENG should be developed, as accidents can happen in less than 1 s. Most of the fatigue monitoring based on triboelectric effects is used for driving monitoring, but it also has the potential to be applied in other dangerous-operation positions, such as high-altitude platform work;
- TENG-based pulse and cardiovascular monitoring is a very easy method for the early diagnosis of cardiovascular disease. However, because this kind of application requires that the TENG sensors are directly attached to the human body or even implanted inside of it, the biocompatibility of the materials used is crucial. Furthermore, because a long service life is always expected, some new materials with robust durability that can work stably in liquid environments (sweat, blood) for real applications need to be developed;
- Currently, most preventive-health-monitoring technologies are based on polymer materials, and the long-term durability is still a critical issue for practical applications. Some non-polymer materials can also be used as preventive-condition-monitoring materials that have good biocompatible abilities and high durability, such as DLC and Si-DLC [125,126];
- Preventive health monitoring will play a more and more important role in our daily lives in an aging society, as preventive health monitoring has the potential to reduce healthcare fees and prevent the severity of diseases and possible injuries and accidents. The flexible working mode and plentiful material selection of TENGs provide more possibilities for future preventive-health-monitoring sensing systems with the help of IoT and AI technologies. Currently, the research on TENG-based preventive health monitoring is still in the laboratory stage, and practical exploration cooperating with medicine and computer science should be on the agenda to make it smaller and more accurate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-enabled wearable sensors for healthcare. Adv. Healthc. Mater. 2018, 7, 1700889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Shi, B.; Fan, Y.; Wang, Z.L.; Li, Z. Wearable and Implantable Triboelectric Nanogenerators. Adv. Funct. Mater. 2019, 29, 1808820. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric nanogenerators as new energy technology for self-powered systems and as active mechanical and chemical sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, T.; Wang, Z.L. Self-Powered Sensors and Systems Based on Nanogenerators. Sensors 2020, 20, 2925. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Burman, S.R.; Khan, I.; Saha, S.; Choi, D.; Lee, S.; Lin, Z.H. Recent advancements in solid-liquid triboelectric nanogenerators for energy harvesting and self-powered applications. Nanoscale 2020, 12, 17663–17697. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xiong, Y.; Sun, J.; Xie, X.; Sun, Q.; Wang, Z.L. Multidiscipline Applications of Triboelectric Nanogenerators for the Intelligent Era of Internet of Things. Nano-Micro Lett. 2023, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mo, J.; Fu, Q.; Lu, Y.; Zhang, N.; Wang, S.; Nie, S. Enhancement of Triboelectric Charge Density by Chemical Functionalization. Adv. Funct. Mater. 2020, 30, 2004714. [Google Scholar] [CrossRef]
- Tat, T.; Libanori, A.; Au, C.; Yau, A.; Chen, J. Advances in triboelectric nanogenerators for biomedical sensing. Biosens. Bioelectron. 2021, 171, 112714. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Z.; Hu, G.-H.; Xiong, C.; Isogai, A.; Yang, Q. Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: A review. J. Mater. Chem. A 2021, 9, 1910–1937. [Google Scholar] [CrossRef]
- Miao, Q.; Liu, C.; Zhang, N.; Lu, K.; Gu, H.; Jiao, J.; Zhang, J.; Wang, Z.; Zhou, X. Toward Self-Powered Inertial Sensors Enabled by Triboelectric Effect. ACS Appl. Electron. Mater. 2020, 2, 3072–3087. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Li, Z.L.; Fan, X.; Zi, Y.L.; Jing, Q.S.; Guo, H.Y.; Wen, Z.; Pradel, K.C.; Niu, S.M.; et al. Networks of Triboelectric Nanogenerators for Harvesting Water Wave Energy: A Potential Approach toward Blue Energy. ACS Nano 2015, 9, 3324–3331. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, B.; Guo, H.; Wu, Z.; Zou, H.; Yang, J.; Wang, Z.L. Super-robust and frequency-multiplied triboelectric nanogenerator for efficient harvesting water and wind energy. Nano Energy 2019, 64, 103908. [Google Scholar] [CrossRef]
- An, J.; Wang, Z.M.; Jiang, T.; Liang, X.; Wang, Z.L. Whirling-Folded Triboelectric Nanogenerator with High Average Power for Water Wave Energy Harvesting. Adv. Funct. Mater. 2019, 29, 1904867. [Google Scholar] [CrossRef]
- Jiang, T.; Pang, H.; An, J.; Lu, P.; Feng, Y.; Liang, X.; Zhong, W.; Wang, Z.L. Robust Swing-Structured Triboelectric Nanogenerator for Efficient Blue Energy Harvesting. Adv. Energy Mater. 2020, 10, 2000064. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Song, Y.; Cao, R.; Wang, L.; Yan, Z.; Shan, G. Self-powered forest fire alarm system based on impedance matching effect between triboelectric nanogenerator and thermosensitive sensor. Nano Energy 2020, 73, 104843. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, L.; Guo, H.; Chen, C.; Wu, C.; Zhang, S.; Wang, Z.L. Signal Output of Triboelectric Nanogenerator at Oil-Water-Solid Multiphase Interfaces and its Application for Dual-Signal Chemical Sensing. Adv. Mater. 2019, 31, e1902793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Zhong, X.; Su, Y.; Zhou, Y.; Hu, C.; Wang, Z.L. Single-Electrode-Based Rotating Triboelectric Nanogenerator for Harvesting Energy from Tires. ACS Nano 2014, 8, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Z.; Xu, L.; Wang, A.C.; Han, K.; Jiang, T.; Lai, Q.; Bai, Y.; Tang, W.; Fan, F.R. Flexible and durable wood-based triboelectric nanogenerators for self-powered sensing in athletic big data analytics. Nat. Commun. 2019, 10, 5147. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, Z.; Zhao, D.; Ma, R.; Yi, W.; Zhang, C.; Liu, D.; Chen, X.; Yang, Y.; Wang, X.; et al. Tactile electronic skin to simultaneously detect and distinguish between temperature and pressure based on a triboelectric nanogenerator. Nano Energy 2020, 75, 105073. [Google Scholar] [CrossRef]
- Rong, X.; Zhao, J.; Guo, H.; Zhen, G.; Yu, J.; Zhang, C.; Dong, G. Material Recognition Sensor Array by Electrostatic Induction and Triboelectric Effects. Adv. Mater. Technol. 2020, 5, 2000641. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Z.; Liu, Z.; Wang, L.; Guo, H.; Li, L.; Li, S.; Chen, X.; Tang, W.; Wang, Z.L. Energy Harvesting from Breeze Wind (0.7–6 m s−1) Using Ultra-Stretchable Triboelectric Nanogenerator. Adv. Energy Mater. 2020, 10, 2001770. [Google Scholar] [CrossRef]
- Ding, W.; Wang, A.C.; Wu, C.; Guo, H.; Wang, Z.L. Human-Machine Interfacing Enabled by Triboelectric Nanogenerators and Tribotronics. Adv. Mater. Technol. 2019, 4, 1800487. [Google Scholar] [CrossRef]
- Jin, L.; Xiao, X.; Deng, W.; Nashalian, A.; He, D.; Raveendran, V.; Yan, C.; Hai, S.; Chu, X.; Yang, T.; et al. Manipulating Relative Permittivity for High-Performance Wearable Triboelectric Nanogenerators. Nano Lett. 2020, 20, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Ghayvat, H.; Pandya, S.; Patel, A. Deep learning model for acoustics signal based preventive healthcare monitoring and activity of daily living. In Proceedings of the 2nd International Conference on Data, Engineering and Applications (IDEA), Bhopal, India, 28–29 February 2020; pp. 1–7. [Google Scholar]
- Kadarina, T.; Priambodo, R. Monitoring heart rate and SpO2 using Thingsboard IoT platform for mother and child preventive healthcare. IOP Conf. Ser. Mater. Sci. Eng. 2018, 453, 012028. [Google Scholar] [CrossRef]
- Zois, D.-S. Sequential decision-making in healthcare IoT: Real-time health monitoring, treatments and interventions. In Proceedings of the 2016 IEEE 3rd World Forum on Internet of Things (WF-IoT), Reston, VA, USA, 12–14 December 2016; pp. 24–29. [Google Scholar]
- Bai, J.; Lian, S.; Liu, Z.; Wang, K.; Liu, D. Smart guiding glasses for visually impaired people in indoor environment. IEEE Trans. Consum. Electron. 2017, 63, 258–266. [Google Scholar] [CrossRef]
- Ramadhan, A.J. Wearable Smart System for Visually Impaired People. Sensors 2018, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Aladren, A.; Lopez-Nicolas, G.; Puig, L.; Guerrero, J.J. Navigation Assistance for the Visually Impaired Using RGB-D Sensor With Range Expansion. IEEE Syst. J. 2016, 10, 922–932. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.M. Fiber-based wearable electronics: A review of materials, fabrication, devices, and applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhu, G.; Zhang, F.; Tang, W.-L.; Jianping, S.; Yang, J.-Q.; Zhu, L.-Y. A review of flexible force sensors for human health monitoring. J. Adv. Res. 2020, 26, 53–68. [Google Scholar] [CrossRef]
- Chen, X.; Xie, X.; Liu, Y.; Zhao, C.; Wen, M.; Wen, Z. Advances in healthcare electronics enabled by triboelectric nanogenerators. Adv. Funct. Mater. 2020, 30, 2004673. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Wang, Z.; Ji, L.; Wang, Z.L. Triboelectric nanogenerators for human-health care. Sci. Bull. 2021, 66, 490–511. [Google Scholar] [CrossRef]
- Haghayegh, M.; Cao, R.; Zabihi, F.; Bagherzadeh, R.; Yang, S.; Zhu, M. Recent advances in stretchable, wearable and bio-compatible triboelectric nanogenerators. J. Mater. Chem. C 2022, 10, 11439–11471. [Google Scholar] [CrossRef]
- Wang, Z.L.; Jiang, T.; Xu, L. Toward the blue energy dream by triboelectric nanogenerator networks. Nano Energy 2017, 39, 9–23. [Google Scholar] [CrossRef]
- Wang, Z.L. On the first principle theory of nanogenerators from Maxwell’s equations. Nano Energy 2020, 68, 104272. [Google Scholar] [CrossRef]
- Wang, Z.L. From contact electrification to triboelectric nanogenerators. Rep. Prog. Phys. 2021, 84, 096502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Triboelectric nanogenerators as new energy technology and self-powered sensors–Principles, problems and perspectives. Faraday Discuss. 2015, 176, 447–458. [Google Scholar] [CrossRef]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk Factors for Falls among Elderly Persons Living in the Community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Jeon, S.-B.; Nho, Y.-H.; Park, S.-J.; Kim, W.-G.; Tcho, I.-W.; Kim, D.; Kwon, D.-S.; Choi, Y.-K. Self-powered fall detection system using pressure sensing triboelectric nanogenerators. Nano Energy 2017, 41, 139–147. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Cai, H.; Yang, Y.; Zhang, H.; Du, C. Performance enhancement of triboelectric nanogenerators using contact-separation mode in conjunction with the sliding mode and multifunctional application for motion monitoring. Nano Energy 2022, 102, 107719. [Google Scholar] [CrossRef]
- Guo, X.; He, T.; Zhang, Z.; Luo, A.; Wang, F.; Ng, E.J.; Zhu, Y.; Liu, H.; Lee, C. Artificial Intelligence-Enabled Caregiving Walking Stick Powered by Ultra-Low-Frequency Human Motion. ACS Nano 2021, 15, 19054–19069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, T.; Cai, J.; Xu, L.; He, T.; Wang, T.; Tian, Y.; Li, L.; Peng, Y.; Lee, C. Wearable Triboelectric Sensors Enabled Gait Analysis and Waist Motion Capture for IoT-Based Smart Healthcare Applications. Adv. Sci. 2022, 9, e2103694. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lopez, A.; Del Rio Saez, J.S.; de la Vega, J.; Ao, X.; Wang, D.Y. All-Fabric Triboelectric Nanogenerator (AF-TENG) Smart Face Mask: Remote Long-Rate Breathing Monitoring and Apnea Alarm. ACS Sens. 2023, 8, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zou, Y.; Rao, W.; Gai, Y.; Xue, J.; Wu, L.; Qu, X.; Liu, Y.; Xu, G.; et al. A multi-mode triboelectric nanogenerator for energy harvesting and biomedical monitoring. Nano Energy 2022, 92, 106715. [Google Scholar] [CrossRef]
- Li, R.; Wei, X.; Xu, J.; Chen, J.; Li, B.; Wu, Z.; Wang, Z.L. Smart Wearable Sensors Based on Triboelectric Nanogenerator for Personal Healthcare Monitoring. Micromachines 2021, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Akram, W.; Chen, Q.; Xia, G.; Fang, J. A review of single electrode triboelectric nanogenerators. Nano Energy 2022, 106, 108043. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Lu, F.; Wang, F.; You, Z.; Huang, M.; Fang, W.; Liu, X.; Li, Y.; Liu, Y. Human motion recognition by a shoes-floor triboelectric nanogenerator and its application in fall detection. Nano Energy 2023, 108, 108230. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Guo, L.; Wang, P.; He, X.; Dai, G.; Zheng, H.; Chen, C.; Wang, A.C.; Xu, C.; et al. Quantifying the triboelectric series. Nat. Commun. 2019, 10, 1427. [Google Scholar] [CrossRef]
- Kou, H.; Wang, H.; Cheng, R.; Liao, Y.; Shi, X.; Luo, J.; Li, D.; Wang, Z.L. Smart Pillow Based on Flexible and Breathable Triboelectric Nanogenerator Arrays for Head Movement Monitoring during Sleep. ACS Appl. Mater. Interfaces 2022, 14, 23998–24007. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Zeng, X.; Fu, X.; Hu, Y. Expandable microsphere-based triboelectric nanogenerators as ultrasensitive pressure sensors for respiratory and pulse monitoring. Nano Energy 2019, 59, 295–301. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef]
- Wang, S.; Tai, H.; Liu, B.; Duan, Z.; Yuan, Z.; Pan, H.; Su, Y.; Xie, G.; Du, X.; Jiang, Y. A facile respiration-driven triboelectric nanogenerator for multifunctional respiratory monitoring. Nano Energy 2019, 58, 312–321. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, Z.; Gu, M.; Hu, Y.; Xu, L.; Jiang, D.; Cheng, S.; Zou, Y.; Deng, Y.; Shi, B.; et al. A Bioresorbable Dynamic Pressure Sensor for Cardiovascular Postoperative Care. Adv. Mater. 2021, 33, 2102302. [Google Scholar] [CrossRef]
- Hawranik, P. A Clinical Possibility: PREVENTING HEALTH PROBLEMS After the Age of 65. J. Gerontol. Nurs. 1991, 17, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.D.; Krauss, M.J.; Dunagan, W.C.; Birge, S.; Hitcho, E.; Johnson, S.; Costantinou, E.; Fraser, V.J. Patterns and Predictors of Inpatient Falls and Fall-Related Injuries in a Large Academic Hospital. Infect. Control Hosp. Epidemiol. 2005, 26, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Sadigh, S.; Reimers, A.; Andersson, R.; Laflamme, L. Falls and fall-related injuries among the elderly: A survey of residential-care facilities in a Swedish municipality. J. Community Health 2004, 29, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Hong, S.; Kim, J.; Lee, S.; Hyeon, T.; Lee, M.; Kim, D.-H. Wearable fall detector using integrated sensors and energy devices. Sci. Rep. 2015, 5, 17081. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.C. Wearable sensors for human activity monitoring: A review. IEEE Sens. J. 2014, 15, 1321–1330. [Google Scholar] [CrossRef]
- Min-Seok, L.; Jong-Gwan, L.; Ki-Ru, P.; Dong-Soo, K. Unsupervised clustering for abnormality detection based on the tri-axial accelerometer. In Proceedings of the 2009 ICCAS-SICE, Fukuoka, Japan, 18–21 August 2009; pp. 134–137. [Google Scholar]
- Bourke, A.K.; Lyons, G.M. A threshold-based fall-detection algorithm using a bi-axial gyroscope sensor. Med. Eng. Phys. 2008, 30, 84–90. [Google Scholar] [CrossRef]
- Nho, Y.H.; Lim, J.G.; Kim, D.E.; Kwon, D.S. User-adaptive fall detection for patients using wristband. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Republic of Korea, 9–14 October 2016; pp. 480–486. [Google Scholar]
- Ozcan, K.; Mahabalagiri, A.K.; Casares, M.; Velipasalar, S. Automatic Fall Detection and Activity Classification by a Wearable Embedded Smart Camera. IEEE J. Emerg. Sel. Top. Circuits Syst. 2013, 3, 125–136. [Google Scholar] [CrossRef]
- Yu, M.; Rhuma, A.; Naqvi, S.M.; Wang, L.; Chambers, J. A Posture Recognition-Based Fall Detection System for Monitoring an Elderly Person in a Smart Home Environment. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, X.; Lu, S.; Zhou, H.; Xie, W.; Chen, J.; Qi, M.; Yu, H.; Mu, X.; Wang, Z.L.; et al. Enhancing the Output Performance of Triboelectric Nanogenerator via Grating-Electrode-Enabled Surface Plasmon Excitation. Adv. Energy Mater. 2019, 9, 1902725. [Google Scholar] [CrossRef]
- Silva de Lima, A.L.; Smits, T.; Darweesh, S.K.L.; Valenti, G.; Milosevic, M.; Pijl, M.; Baldus, H.; de Vries, N.M.; Meinders, M.J.; Bloem, B.R. Home-Based Monitoring of Falls Using Wearable Sensors in Parkinson’s Disease. Mov. Disord. 2020, 35, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Nishikawa, Y.; Shima, S.; Uchiyama, Y.; Kobayashi, E.; Takamura, E.; Sakamoto, H. Omni-directional detectable textile brush-based triboelectric nanogenerators. Sens. Actuators A Phys. 2022, 345, 113803. [Google Scholar] [CrossRef]
- Komatsu, T.; Uejima, R.; Shima, S.; Uchiyama, Y.; Kobayashi, E.; Takamura, E.; Sakamoto, H. Output Behavior of Fall Detection Sensor Using Brush-Based Triboelectric Nanogenerator Depending on Fiber Characteristics. Integr. Ferroelectr. 2023, 237, 47–57. [Google Scholar] [CrossRef]
- Martins, M.M.; Santos, C.P.; Frizera-Neto, A.; Ceres, R. Assistive mobility devices focusing on smart walkers: Classification and review. Robot. Auton. Syst. 2012, 60, 548–562. [Google Scholar] [CrossRef]
- Peng, S.; Feng, Y.; Liu, Y.; Feng, M.; Wu, Z.; Cheng, J.; Zhang, Z.; Liu, Y.; Shen, R.; Wang, D. New blind navigation sensor based on triboelectrification and electrostatic induction. Nano Energy 2022, 104, 107899. [Google Scholar] [CrossRef]
- Huang, X.; Li, B.; Wang, L.; Lai, X.; Xue, H.; Gao, J. Superhydrophilic, Underwater Superoleophobic, and Highly Stretchable Humidity and Chemical Vapor Sensors for Human Breath Detection. ACS Appl. Mater. Interfaces 2019, 11, 24533–24543. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yu, Q.; Wang, S.; Sun, P.; Lu, H.; Liu, F.; Yan, X.; Lu, G. Novel Self-Assembly Route Assisted Ultra-Fast Trace Volatile Organic Compounds Gas Sensing Based on Three-Dimensional Opal Microspheres Composites for Diabetes Diagnosis. ACS Appl. Mater. Interfaces 2018, 10, 32913–32921. [Google Scholar] [CrossRef]
- Zhang, S.; Bick, M.; Xiao, X.; Chen, G.; Nashalian, A.; Chen, J.J.M. Leveraging triboelectric nanogenerators for bioengineering. Matter 2021, 4, 845–887. [Google Scholar] [CrossRef]
- Cannac, O.; Martinez-Almoyna, L.; Hraiech, S. Critical illness–associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology 2020, 95, 498–499. [Google Scholar] [CrossRef]
- George Kerry, R.; Ukhurebor, K.E.; Kumari, S.; Maurya, G.K.; Patra, S.; Panigrahi, B.; Majhi, S.; Rout, J.R.; Rodriguez-Torres, M.d.P.; Das, G.; et al. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Biomater. Sci. 2021, 9, 3576–3602. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C.J.A.M. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Yu, Y.; Ye, L.; Song, Y.; Guan, Y.; Zang, J. Wrinkled nitrile rubber films for stretchable and ultra-sensitive respiration sensors. Extrem. Mech. Lett. 2017, 11, 128–136. [Google Scholar] [CrossRef]
- de Figueiredo, C.S.; Sandre, P.C.; Portugal, L.C.L.; Mázala-de-Oliveira, T.; da Silva Chagas, L.; Raony, Í.; Ferreira, E.S.; Giestal-de-Araujo, E.; dos Santos, A.A.; Bomfim, P.O.-S. COVID-19 pandemic impact on children and adolescents’ mental health: Biological, environmental, and social factors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110171. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, H.A.S.; Asif, H.M. Early detection and assessment of COVID-19. Front. Med. 2020, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.; Litterst, P.; Freitag, L.; Urfer, W.; Bader, S.; Baumbach, J.I. Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: Results of a pilot study. Thorax 2009, 64, 744. [Google Scholar] [CrossRef] [PubMed]
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry. PLoS ONE 2014, 9, e114555. [Google Scholar] [CrossRef] [PubMed]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Anttalainen, O.; Puton, J.; Kontunen, A.; Karjalainen, M.; Kumpulainen, P.; Oksala, N.; Safaei, Z.; Roine, A. Possible strategy to use differential mobility spectrometry in real time applications. Int. J. Ion Mobil. Spectrom. 2020, 23, 1–8. [Google Scholar] [CrossRef]
- Chuang, M.-Y.; Lin, Y.-T.; Tung, T.-W.; Chang, L.-Y.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J.; Tao, Y.-T. Room-temperature-operated organic-based acetone gas sensor for breath analysis. Sens. Actuators B Chem. 2018, 260, 593–600. [Google Scholar] [CrossRef]
- Chuang, M.-Y.; Chen, C.-C.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J. Organic Gas Sensor with an Improved Lifetime for Detecting Breath Ammonia in Hemodialysis Patients. ACS Sens. 2017, 2, 1788–1795. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, C.; Gong, Q.; Xie, G.; Yao, M.; Tai, H.; Jiang, Y.; Chen, J. Self-powered respiration monitoring enabled by a triboelectric nanogenerator. Adv. Mater. 2021, 33, 2101262. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Chen, X.; Zhang, H.; Tang, M.; Wang, J. Multifunctional respiration-driven triboelectric nanogenerator for self-powered detection of formaldehyde in exhaled gas and respiratory behavior. Nano Energy 2022, 102, 107711. [Google Scholar] [CrossRef]
- Hanly, P.J.; Zuberi-Khokhar, N.S. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am. J. Respir. Crit. Care Med. 1996, 153, 272–276. [Google Scholar] [CrossRef]
- Yumino, D.; Bradley, T.D. Central Sleep Apnea and Cheyne-Stokes Respiration. Proc. Am. Thorac. Soc. 2008, 5, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Tang, Y.; Li, J.; Zhang, B.; Liang, E.; Mao, Y.; Wang, X. Air-flow-driven triboelectric nanogenerators for self-powered real-time respiratory monitoring. ACS Nano 2018, 12, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, H.; Zeng, Y.; Xue, J.; Cao, X.; Wang, N.; Wang, Z. Intelligent facemask based on triboelectric nanogenerator for respiratory monitoring. Nano Energy 2022, 91, 106612. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Cheng, R.; Jiang, Y.; Sheng, F.; Yi, J.; Shen, S.; Zhang, Y.; Peng, X.; Dong, K.; Wang, Z.L. Helical Fiber Strain Sensors Based on Triboelectric Nanogenerators for Self-Powered Human Respiratory Monitoring. ACS Nano 2022, 16, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Dong, K.; Ning, C.; Cheng, R.; Yi, J.; Zhang, Y.; Sheng, F.; Wu, Z.; Wang, Z.L. All-Nanofiber Self-Powered Skin-Interfaced Real-Time Respiratory Monitoring System for Obstructive Sleep Apnea-Hypopnea Syndrome Diagnosing. Adv. Funct. Mater. 2021, 31, 2103559. [Google Scholar] [CrossRef]
- Zamanzad Ghavidel, M.; Rahman, M.R.; Easton, E.B. Fuel cell-based breath alcohol sensors utilizing Pt-alloy electrocatalysts. Sens. Actuators B Chem. 2018, 273, 574–584. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Brinkman, P.; van de Pol, M.A.; Gerritsen, M.G.; Bos, L.D.; Dekker, T.; Smids, B.S.; Sinha, A.; Majoor, C.J.; Sneeboer, M.M.; Knobel, H.H.; et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin. Exp. Allergy 2017, 47, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fei, T.; Guan, X.; Lin, X.; Zhao, H.; Zhang, T. Room temperature ammonia gas sensor based on ionic conductive biomass hydrogels. Sens. Actuators B Chem. 2020, 320, 128318. [Google Scholar] [CrossRef]
- Liao, Y.S.; Lee, L.W.; Yang, P.H.; Kuo, L.M.; Kuan, L.Y.; Tseng, W.Y.I.; Hwang, D.W. Assessment of liver cirrhosis for patients with Child’s A classification before hepatectomy using dynamic contrast-enhanced MRI. Clin. Radiol. 2019, 74, 407.e411–407.e417. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yang, T.; Zhao, X.; Cai, Z.; Chen, G.; Yao, M.; Chen, K.; Bick, M.; Wang, J.; Li, S.; et al. A wireless energy transmission enabled wearable active acetone biosensor for non-invasive prediabetes diagnosis. Nano Energy 2020, 74, 104941. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, C.; Fan, X.; Chen, J. Progress in triboelectric nanogenerators as self-powered smart sensors. J. Mater. Res. 2017, 32, 1628–1646. [Google Scholar] [CrossRef]
- Brazil, N.; Kirk, D. Ridehailing and alcohol-involved traffic fatalities in the United States: The average and heterogeneous association of uber. PLoS ONE 2020, 15, e0238744. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, J.; Yeh, M.-H.; Guo, H.; Li, Z.; Fan, X.; Zhang, T.; Zhu, L.; Wang, Z.L. Blow-driven triboelectric nanogenerator as an active alcohol breath analyzer. Nano Energy 2015, 16, 38–46. [Google Scholar] [CrossRef]
- Rathi, R. Road traffic accidents–burden on society. EC Orthop. 2018, 9, 30–33. [Google Scholar]
- Anjuman, T.; Hasanat-E.-Rabbi, S.; Siddiqui, C.K.A.; Hoque, M.M. Road traffic accident: A leading cause of the global burden of public health injuries and fatalities. In Proceedings of the International Conference on Mechanical Engineering 2007 (ICME2007), Dhaka, Bangladesh, 29–31 December 2007; pp. 29–31. [Google Scholar]
- Savaş, B.K.; Becerikli, Y. Real Time Driver Fatigue Detection System Based on Multi-Task ConNN. IEEE Access 2020, 8, 12491–12498. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.; Hu, G. Traffic safety in China: Challenges and countermeasures. Accid. Anal. Prev. 2016, 95, 305–307. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, T.; Leo, M.; Guaragnella, C.; Distante, A. A visual approach for driver inattention detection. Pattern Recognit. 2007, 40, 2341–2355. [Google Scholar] [CrossRef]
- You, F.; Li, Y.-H.; Huang, L.; Chen, K.; Zhang, R.-H.; Xu, J.-M. Monitoring drivers’ sleepy status at night based on machine vision. Multimed. Tools Appl. 2017, 76, 14869–14886. [Google Scholar] [CrossRef]
- Meng, X.; Cheng, Q.; Jiang, X.; Fang, Z.; Chen, X.; Li, S.; Li, C.; Sun, C.; Wang, W.; Wang, Z.L. Triboelectric nanogenerator as a highly sensitive self-powered sensor for driver behavior monitoring. Nano Energy 2018, 51, 721–727. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, L.; Zhang, H.; Wang, W.; Wang, Z.L.; Sun, C. Stretchable, transparent triboelectric nanogenerator as a highly sensitive self-powered sensor for driver fatigue and distraction monitoring. Nano Energy 2020, 78, 105359. [Google Scholar] [CrossRef]
- Luo, F.; Chen, B.; Ran, X.; Ouyang, W.; Shang, L. PEO-PDMS-based triboelectric nanogenerators as self-powered sensors for driver status monitoring. Chem. Eng. J. 2023, 451, 138961. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.; Yu, X.; Li, H.; Cheng, T.; Lu, X.; Wang, Z.L. Real-time monitoring system of automobile driver status and intelligent fatigue warning based on triboelectric nanogenerator. ACS Nano 2021, 15, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, Q.; Lu, X.; Wang, W.; Wang, Z.L.; Sun, C. Detection of driving actions on steering wheel using triboelectric nanogenerator via machine learning. Nano Energy 2021, 79, 105455. [Google Scholar] [CrossRef]
- Marcinkevics, Z.; Greve, M.; Aivars, J.I.; Erts, R.; Zehtabi, A.H.J.A.U.L.B. Relationship between arterial pressure and pulse wave velocity using photoplethysmography during the post-exercise recovery period. Acta Univesitatis Latv. Biol. 2009, 753, 59–68. [Google Scholar]
- O’Rourke, M.F. The arterial pulse in health and disease. Am. Heart J. 1971, 82, 687–702. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Kelly, R.P. Wave reflection in the systemic circulation and its implications in ventricular function. J. Hypertens. 1993, 11, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Au, C.; Chen, J. Textile triboelectric nanogenerators for wearable pulse wave monitoring. Trends Biotechnol. 2021, 39, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, H.; Shi, B.; Xue, X.; Liu, Z.; Jin, Y.; Ma, Y.; Zou, Y.; Wang, X.; An, Z. In vivo self-powered wireless cardiac monitoring via implantable triboelectric nanogenerator. ACS Nano 2016, 10, 6510–6518. [Google Scholar] [CrossRef]
- Ryu, H.; Park, H.-M.; Kim, M.-K.; Kim, B.; Myoung, H.S.; Kim, T.Y.; Yoon, H.-J.; Kwak, S.S.; Kim, J.; Hwang, T.H.; et al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat. Commun. 2021, 12, 4374. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Tian, J.; Sun, G.; Zou, Y.; Liu, Z.; Li, H.; Zhao, L.; Shi, B.; Fan, Y.; Fan, Y.; et al. Self-Powered Pulse Sensor for Antidiastole of Cardiovascular Disease. Adv. Mater. 2017, 29, 1703456. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.; Zhang, B.; Wang, Y.C.; Guo, H.; Liu, G.; Chen, C.; Chen, Y.; Yang, J.; Wang, Z.L. A triboelectric nanogenerator-based smart insole for multifunctional gait monitoring. Adv. Mater. Technol. 2019, 4, 1800360. [Google Scholar] [CrossRef]
- So, M.Y.; Xu, B.; Li, Z.; Lai, C.L.; Jiang, C. Flexible corrugated triboelectric nanogenerators for efficient biomechanical energy harvesting and human motion monitoring. Nano Energy 2023, 106, 108033. [Google Scholar] [CrossRef]
- Jiang, C.; Lai, C.L.; Xu, B.; So, M.Y.; Li, Z. Fabric-rebound triboelectric nanogenerators with loops and layered structures for energy harvesting and intelligent wireless monitoring of human motions. Nano Energy 2022, 93, 106807. [Google Scholar] [CrossRef]
- Okpalugo, T.; Ogwu, A.; Maguire, P.; McLaughlin, J.; Hirst, D. In-vitro blood compatibility of aC:H:Si and aC:H thin films. Diam. Relat. Mater. 2004, 13, 1088–1092. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lee, J.-S.; Kheradmandfard, M.; Kim, C.-L.; Cho, S.-W.; Kim, D.-E. Highly durable and biocompatible periodical Si/DLC nanocomposite coatings. Nanoscale 2018, 10, 4852–4860. [Google Scholar] [CrossRef] [PubMed]




| Year | Researchers | TENG Application | Used Materials | Function | Performance |
|---|---|---|---|---|---|
| 2017 | Jeon et al. [41] | Sensor arrays | PTFE and Kapton | Fall detection by evaluating the triggered number of TENG arrays | Voc of 162 V Isc of 22 µA 95.75% classification accuracy |
| 2018 | Lin et al. [66] | Smart insole | Rubber and copper | Fall detection by monitoring the signals generated by walking | Response time less than 56 ms |
| 2021 | Zhang et al. [44] | Smart insole | TPU-coated PES and conductive nickel fabric | Fall detection by monitoring the signals generated by walking | Sensing range more than 245 Kpa Durability more than 5000 cycles |
| 2022 | Kou et al. [51] | Smart pillow | PDMS and FEP | Fall detection on bed by monitoring the position of head | Sensitivity of 2.1 mV/Pa Durability more than 14,000 cycles |
| 2022 | Guo et al. [43] | Smart walking stick | Ecoflex, Nitrile, Al, and PTFE | Mobility disability evaluation motion status determination and fall detection enabled by AI | Average power density of 0.137 mW cm−3 at 0.083 Hz 100% classification accuracy |
| 2022 | Lu et al. [92] | Smart mask | FEP film and Al foil | Apnea alarming and switching on/off the operation of household appliances by monitoring respiration | Light weight of 4.7567 g |
| 2023 | Wang et al. [45] | Smart mask | Cotton fabric and polyethylene | Remote respiration monitoring by using advanced IoT technology | Maximum communication distance of 20 km |
| 2015 | Wen et al. [103] | Ethanol sensor | FEP and copper | Self-powered ethanol detection enabled by connecting a gas-sensing, rhombus-shaped Co3O4 nanorod array with TENG | Detection limit of 10 ppm Sensitivity around 0.15 ppm−1 11 s of response time 20 s of recovery time |
| 2019 | Wang et al. [54] | Ammonia sensor | PDMS and Ce-doped ZnO-PANI film | Self-powered ammonia detection enabled by the chemical reaction between PANI and ammonia, which leads to the output change | Detection limit of 0.1 ppm Sensitivity of 1.1 ppm−1 109 s of response time 233 s of recovery time |
| 2020 | Su et al. [100] | Acetone sensor | PTFE and nylon | Self-powered acetone detection by using chitosan and reduced graphene oxide (RGO) as sensitive materials on electrode, which can react with acetone | Detection limit lower than 2 ppm Sensitivity of 2.71 ppm−1 |
| 2018 | Meng et al. [110] | Wearable sensor | Al foil and Kapton | Fatigue detection by monitoring the blinking and braking behavior of driver | Voc of 14 V Isc of 1.2 µA |
| 2020 | Lu et al. [111] | Wearable sensor | PDMS and human skin | Fatigue detection by attaching TENG sensors on different parts of driver for monitoring physiological signals | Durability more than 10,000 cycles Voc more than 200 V |
| 2022 | Wu et al. [46] | Pressure sensor | Silicone and EGaIn | Pulse information collection by attaching TENG on arterial pulse | Stretchability around 300% Durability more than 10,000 cycles |
| 2021 | Ouyang et al. [55] | Pressure sensor | PLA/C and Mg | Cardiovascular event identification by attaching TENG sensors on vascular wall | Durability more than 45,000 cycles 99% sterilization Sensitivity of 11 mV mmHg−1 Service life more than 5 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Yang, Z.; Choi, J.; Wang, C.; Dai, G.; Yang, J. Triboelectric Nanogenerators for Preventive Health Monitoring. Nanomaterials 2024, 14, 336. https://doi.org/10.3390/nano14040336
Gao M, Yang Z, Choi J, Wang C, Dai G, Yang J. Triboelectric Nanogenerators for Preventive Health Monitoring. Nanomaterials. 2024; 14(4):336. https://doi.org/10.3390/nano14040336
Chicago/Turabian StyleGao, Mang, Zhiyuan Yang, Junho Choi, Chan Wang, Guozhang Dai, and Junliang Yang. 2024. "Triboelectric Nanogenerators for Preventive Health Monitoring" Nanomaterials 14, no. 4: 336. https://doi.org/10.3390/nano14040336
APA StyleGao, M., Yang, Z., Choi, J., Wang, C., Dai, G., & Yang, J. (2024). Triboelectric Nanogenerators for Preventive Health Monitoring. Nanomaterials, 14(4), 336. https://doi.org/10.3390/nano14040336







