Geometrical Stabilities and Electronic Structures of Ru3 Clusters on Rutile TiO2 for Green Hydrogen Production
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Isolated Ru3 Cluster
3.2. Ru3 Clusters Loaded on Perfect TiO2
3.3. Ru3 Cluster Loaded on Defective TiO2
4. Concluding Remarks
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. 2006. Available online: https://www.pnas.org/doi/10.1073/pnas.0603395103 (accessed on 1 December 2023).
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Xiaobo, C.; Lei, L.; Petrt, Y.Y.; Samuel, S.M. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Kim, C.; Choi, M.; Jang, J. Nitrogen-doped SiO2/TiO2 core/shell nanoparticles as highly efficient visible light photocatalyst. Catal. Commun. 2010, 11, 378–382. [Google Scholar] [CrossRef]
- Murphy, A.; Barnes, P.; Randeniya, L.; Plumb, I.; Grey, I.; Horne, M.D.; Glasscock, J. Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen Energy 2006, 31, 1999–2017. [Google Scholar] [CrossRef]
- Wu, J.; Lu, S.; Ge, D.; Zhang, L.; Chen, W.; Gu, H. Photocatalytic properties of Pd/TiO2 nanosheets for hydrogen evolution from water splitting. RSC Adv. 2016, 6, 67502–67508. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting-superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Morris, J.R. Surface chemistry of Au/TiO2: Thermally and photolytically activated reactions. Surf. Sci. Rep. 2016, 71, 77–271. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2002, 48, 53–229. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface science studies of the photoactivation of TIO2—New photochemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Klein, M.; Nadolna, J.; Gołąbiewska, A.; Mazierski, P.; Klimczuk, T.; Remita, H.; Zaleska-Medynska, A. The effect of metal cluster deposition route on structure and photocatalytic activity of mono- and bimetallic nanoparticles supported on TiO2 by radiolytic method. Appl. Surf. Sci. 2016, 378, 37–48. [Google Scholar] [CrossRef]
- Zhang, H.; Ming, H.; Lian, S.; Huang, H.; Li, H.; Zhang, L.; Liu, Y.; Kang, Z.; Lee, S.-T. Fe2O3/carbon quantum dots complex photocatalysts and their enhanced photocatalytic activity under visible light. Dalt. Trans. 2011, 40, 10822–10825. [Google Scholar] [CrossRef] [PubMed]
- Chainarong, S.; Sikong, L.; Pavasupree, S.; Niyomwas, S. Synthesis and characterization of nitrogen-doped TiO2 nanomaterials for photocatalytic activities under visible light. Energy Procedia 2011, 9, 418–427. [Google Scholar] [CrossRef]
- Fujii, H.; Inata, K.; Ohtaki, M.; Eguchi, K.; Arai, H. Synthesis of TiO2/CdS nanocomposite via TiO2 coating on CdS nanoparticles by compartmentalized hydrolysis of Ti alkoxide. J. Mater. Sci. 2001, 36, 527–532. [Google Scholar] [CrossRef]
- Ismael, M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Huang, X.; Cao, J.; Huo, Q.; Mi, L.; Yang, H.; Hu, Q.; He, C. Confining ultrafine Ru clusters into TiO2 lattice frameworks to yield efficient and ultrastable electrocatalysts towards practical hydrogen evolution. Chem. Eng. J. 2022, 446, 137248. [Google Scholar] [CrossRef]
- Gao, P.; Yang, L.; Xiao, S.; Wang, L.; Guo, W.; Lu, J. Effect of Ru, Rh, Mo, and Pd adsorption on the electronic and optical properties of anatase TiO2(101): A DFT investigation. Materials 2019, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Han, Q.; Su, Q.; Yang, J.; Zhao, Y.; Wen, H.; Jiang, Z. Effects of 4d transition metals doping on the photocatalytic activities of anatase TiO2 (101) surface. Int. J. Quantum Chem. 2021, 121, e26683. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.-W.; Zhang, S.; Li, G.-L.; Ju, W.; Li, T.; Li, L. H2O splitting on Run/TiO2(101) surface: Lowered energy barrier due to charge transfer at interface. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 131, 114730. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metalamorphous- semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, D.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Seriani, N.; Pinilla, C.; Crespo, Y. Presence of gap states at Cu/TiO2 anatase surfaces: Consequences for the photocatalytic activity. J. Phys. Chem. C 2015, 119, 6696–6702. [Google Scholar] [CrossRef]
- Morgan, B.J.; Watson, G.W. A DFT + U description of oxygen vacancies at the TiO2 rutile (110) surface. Surf. Sci. 2007, 601, 5034–5041. [Google Scholar] [CrossRef]
- Morgan, B.J.; Watson, G.W. A density functional theory + u study of Oxygen vacancy formation at the (110), (100), (101), and (001) surfaces of rutile TiO2. J. Phys. Chem. C 2009, 113, 7322–7328. [Google Scholar] [CrossRef]
- Antony, A.; Hakanoglu, C.; Asthagiri, A.; Weaver, J.F. Dispersion-corrected density functional theory calculations of the molecular binding of n-alkanes on Pd(111) and PdO(101). J. Chem. Phys. 2012, 136, 054702. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Zhao, X.; Li, Y.L.; Fan, W.L. Correlating the surface structure and hydration of a γ-Al2O3 support with the Run (n = 1–4) cluster adsorption behavior: A density functional theory study. RSC Adv. 2016, 6, 40459–40473. [Google Scholar] [CrossRef]
- Tezuka, Y.; Shin, S.; Ishii, T.; Ejima, T.; Suzuki, S.; Sato, S. Photoemission and Bremsstrahlung Isochromat Spectroscopy Studies of Ti02 (Rutile) and SrTi03. J. Phys. Soc. Jpn. 1994, 63, 347–357. [Google Scholar] [CrossRef]
- Alotaibi, M.; Wu, Q.; Lambert, C. Computational Studies of Ag 5 Atomic Quantum Clusters Deposited on Anatase and Rutile TiO2 Surfaces. Appl. Surf. Sci. 2022, 613, 156054. [Google Scholar] [CrossRef]
- Alotaibi, M. Geometrical Stabilities and Electronic Structures of Rh5 Nanoclusters on Rutile TiO2 (110) for Green Hydrogen Production. Nanomaterials 2024, 14, 191. [Google Scholar] [CrossRef]
- Akamaru, S.; Shimazaki, T.; Kubo, M.; Abe, T. Density functional theory analysis of methanation reaction of CO2 on Ru nanoparticle supported on TiO2 (101). Appl. Catal. A Gen. 2014, 470, 405–411. [Google Scholar] [CrossRef]
- Zhang, S.T.; Li, C.M.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Density functional theory study on the metal-support interaction between Ru cluster and anatase TiO2(101) surface. J. Phys. Chem. C 2014, 118, 3514–3522. [Google Scholar] [CrossRef]
- López-Caballero, P.; Hauser, A.W.; Pilar De Lara-Castells, M. Exploring the Catalytic Properties of Unsupported and TiO2-Supported Cu5 Clusters: CO2 Decomposition to CO and CO2 Photoactivation. J. Phys. Chem. C 2019, 123, 23064–23074. [Google Scholar] [CrossRef] [PubMed]
- López-Caballero, P.; Miret-Artés, S.; Mitrushchenkov, A.O.; De Lara-Castells, M.P. Ag5-induced stabilization of multiple surface polarons on perfect and reduced TiO2 rutile (110). J. Chem. Phys. 2020, 153, 164702. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.J.; Thornton, G. TiO2 Polarons in the Time Domain: Implications for Photocatalysis. J. Phys. Chem. Lett. 2022, 13, 559–566. [Google Scholar] [CrossRef]
- De Lara-Castells, M.P.; Cabrillo, C.; Micha, D.A.; Mitrushchenkov, A.O.; Vazhappilly, T. Ab initio design of light absorption through silver atomic cluster decoration of TiO2. Phys. Chem. Chem. Phys. 2018, 20, 19110–19119. [Google Scholar] [CrossRef]
- Gomes Silva, C.; Juarez, R.; Marino, T.; Molinari, R.; Garcia, H. Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef]
- Pabisiak, T.; Kiejna, A. Energetics of oxygen vacancies at rutile TiO2(110) surface. Solid State Commun. 2007, 144, 324–328. [Google Scholar] [CrossRef]
- Oviedo, J.; Miguel, M.A.S.; Sanz, J.F. Oxygen vacancies on TiO2 (110) from first principles calculations. J. Chem. Phys. 2004, 121, 7427–7433. [Google Scholar] [CrossRef]
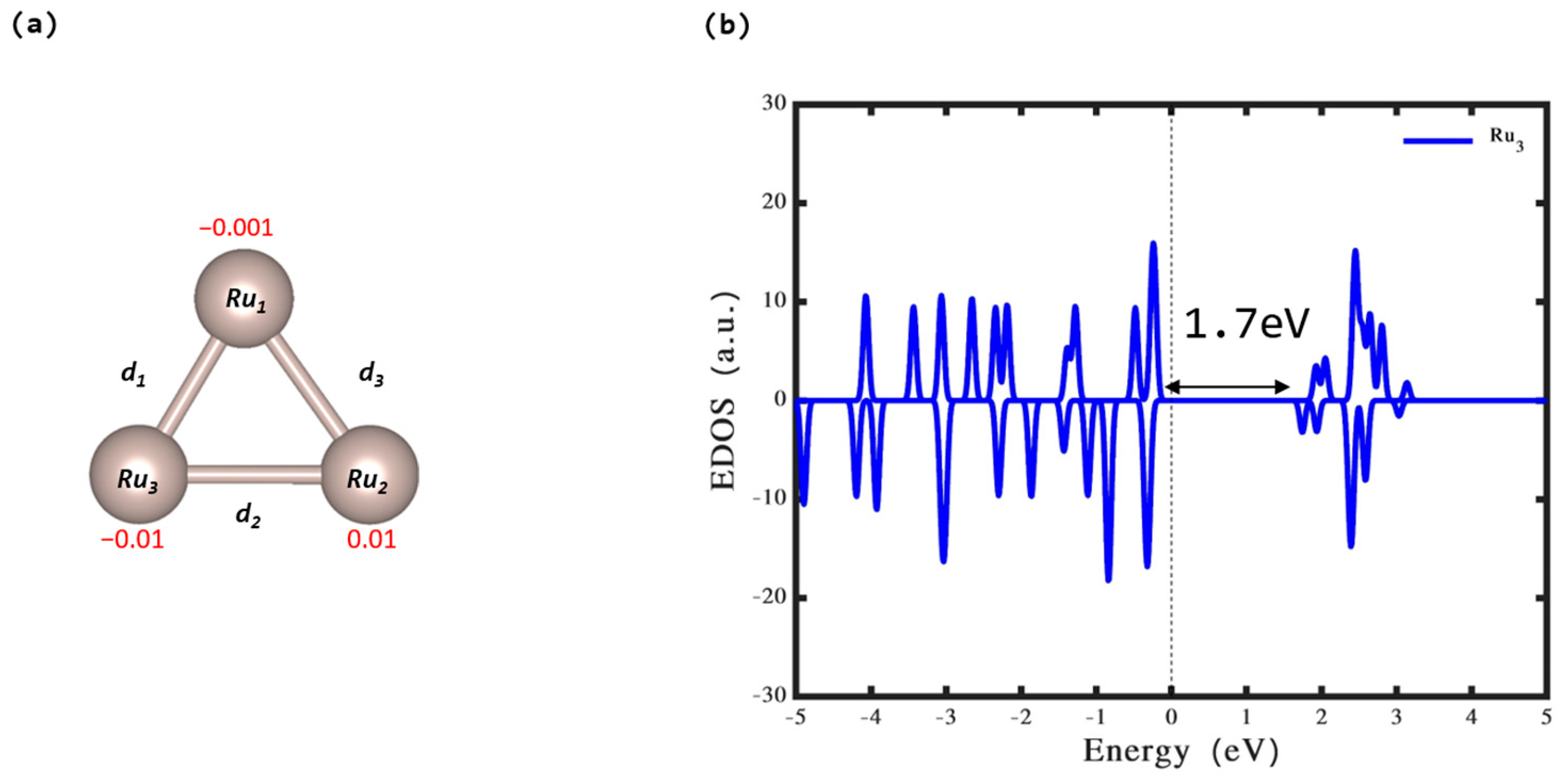

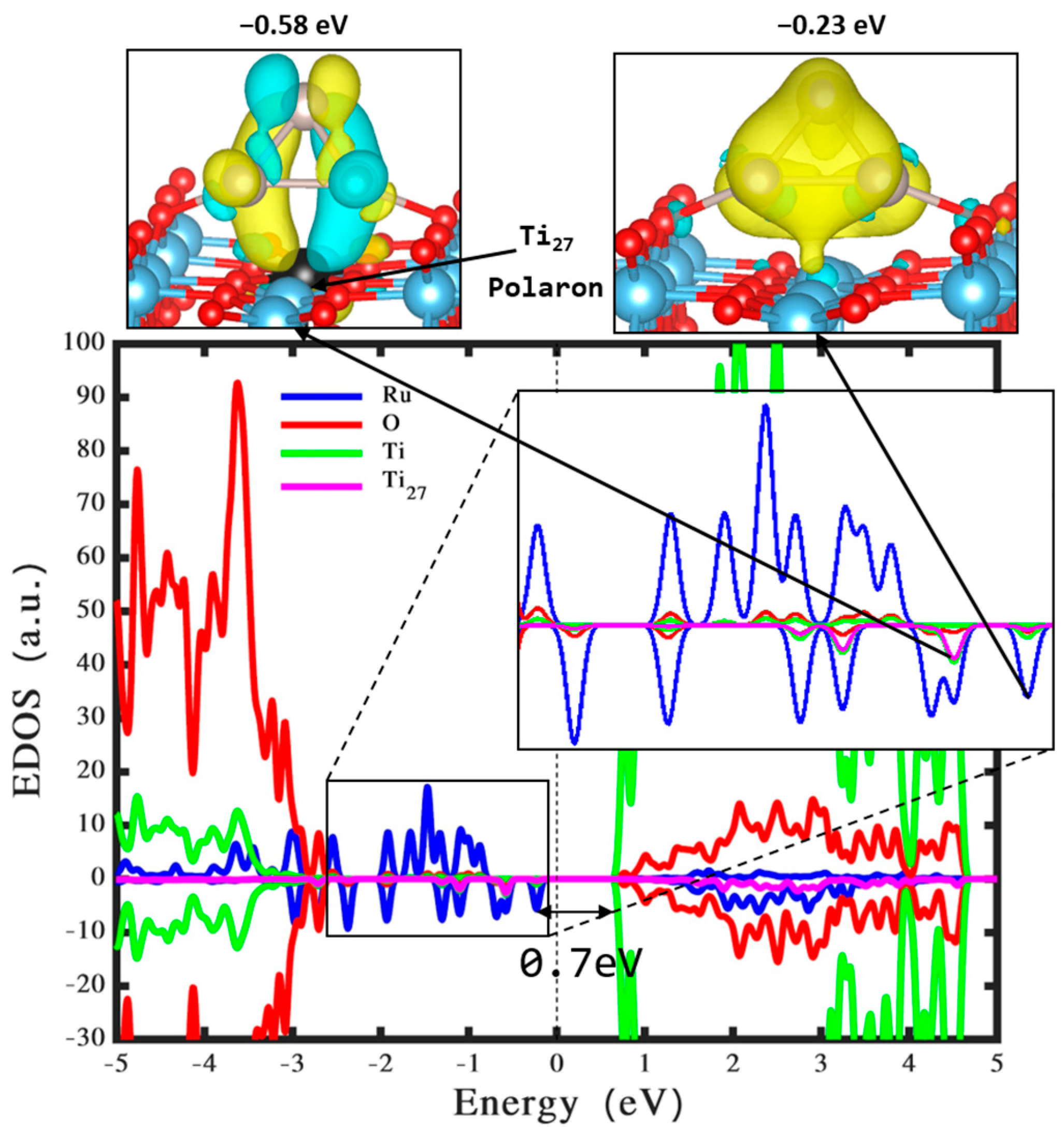
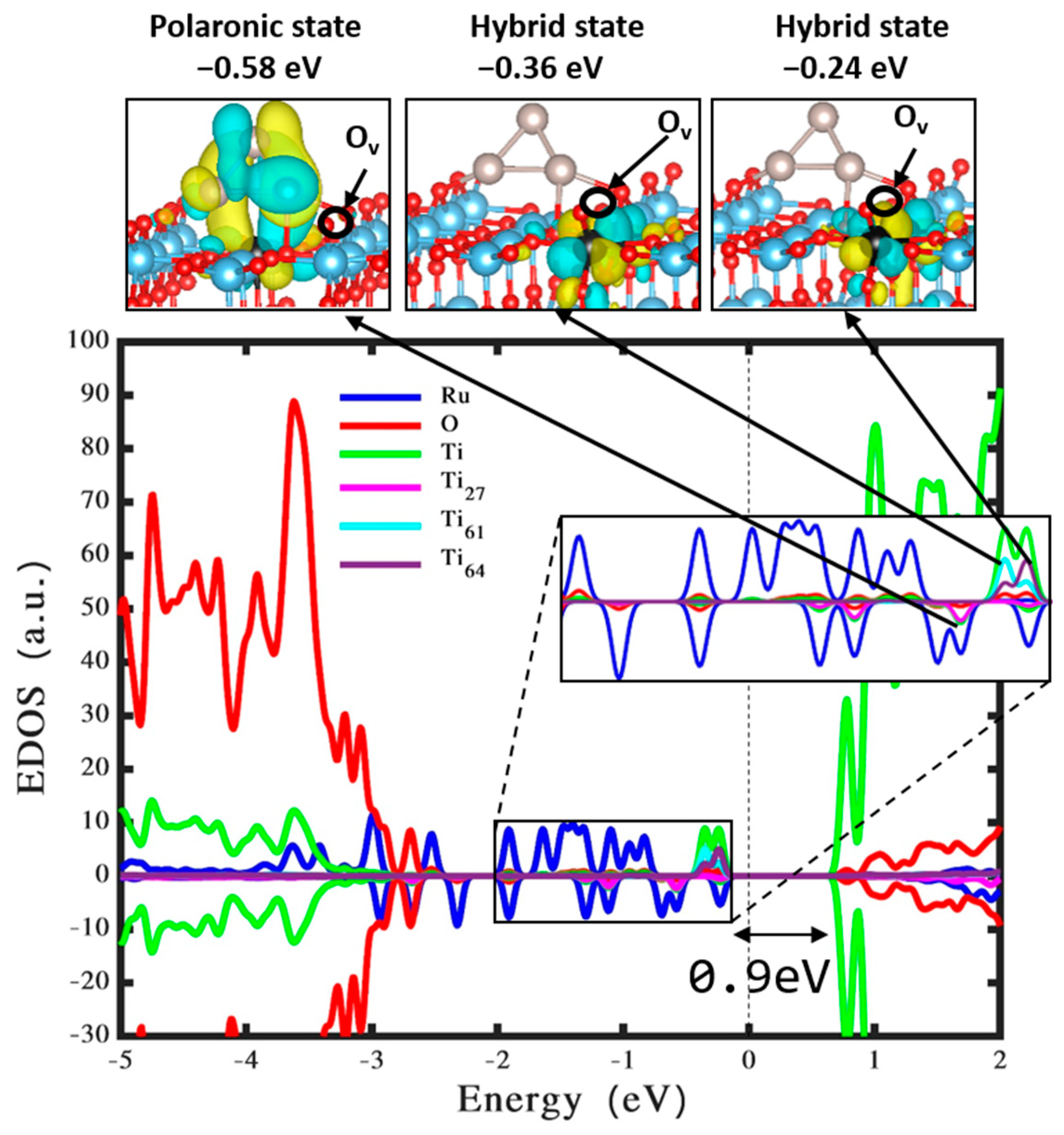
| Bond Length (Å) | Ru3 |
|---|---|
| d1 | 2.20 |
| d2 | 2.33 |
| d3 | 2.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M. Geometrical Stabilities and Electronic Structures of Ru3 Clusters on Rutile TiO2 for Green Hydrogen Production. Nanomaterials 2024, 14, 396. https://doi.org/10.3390/nano14050396
Alotaibi M. Geometrical Stabilities and Electronic Structures of Ru3 Clusters on Rutile TiO2 for Green Hydrogen Production. Nanomaterials. 2024; 14(5):396. https://doi.org/10.3390/nano14050396
Chicago/Turabian StyleAlotaibi, Moteb. 2024. "Geometrical Stabilities and Electronic Structures of Ru3 Clusters on Rutile TiO2 for Green Hydrogen Production" Nanomaterials 14, no. 5: 396. https://doi.org/10.3390/nano14050396






