A Photoelectrochemical Sensor for the Sensitive Detection of Cysteine Based on Cadmium Sulfide/Tungsten Disulfide Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
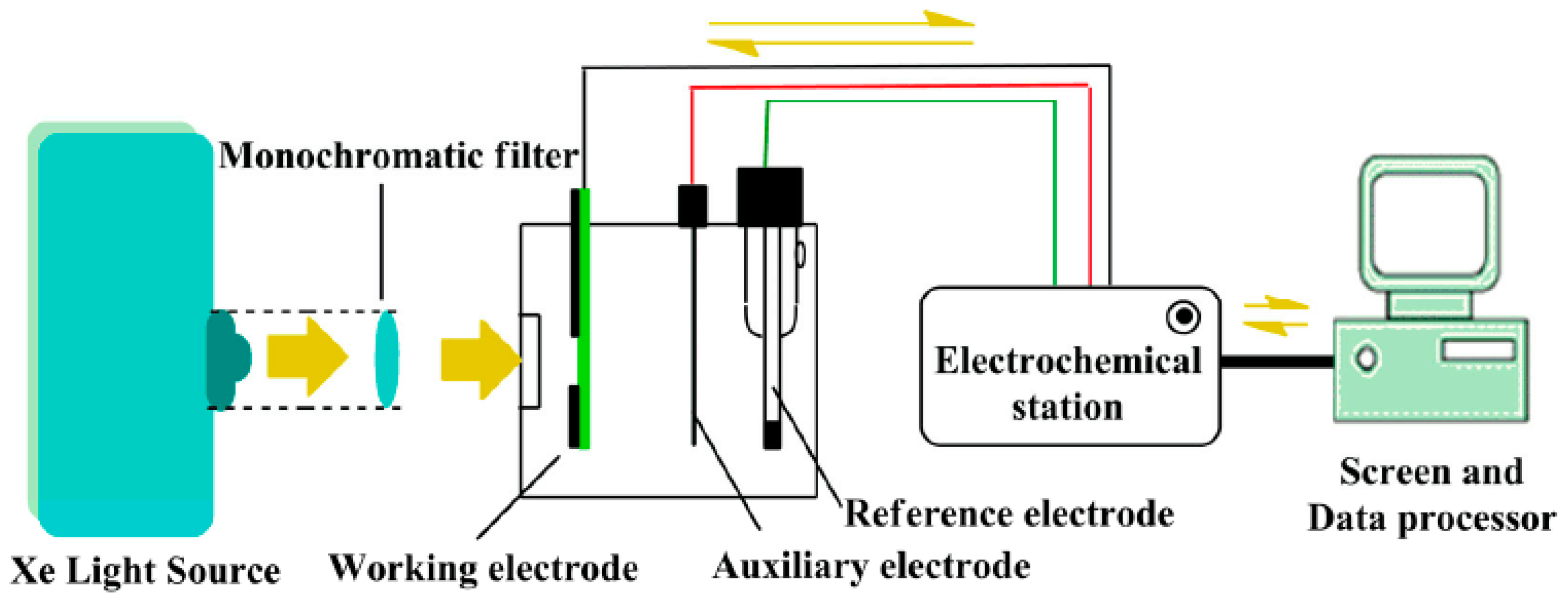
2.2. Apparatus
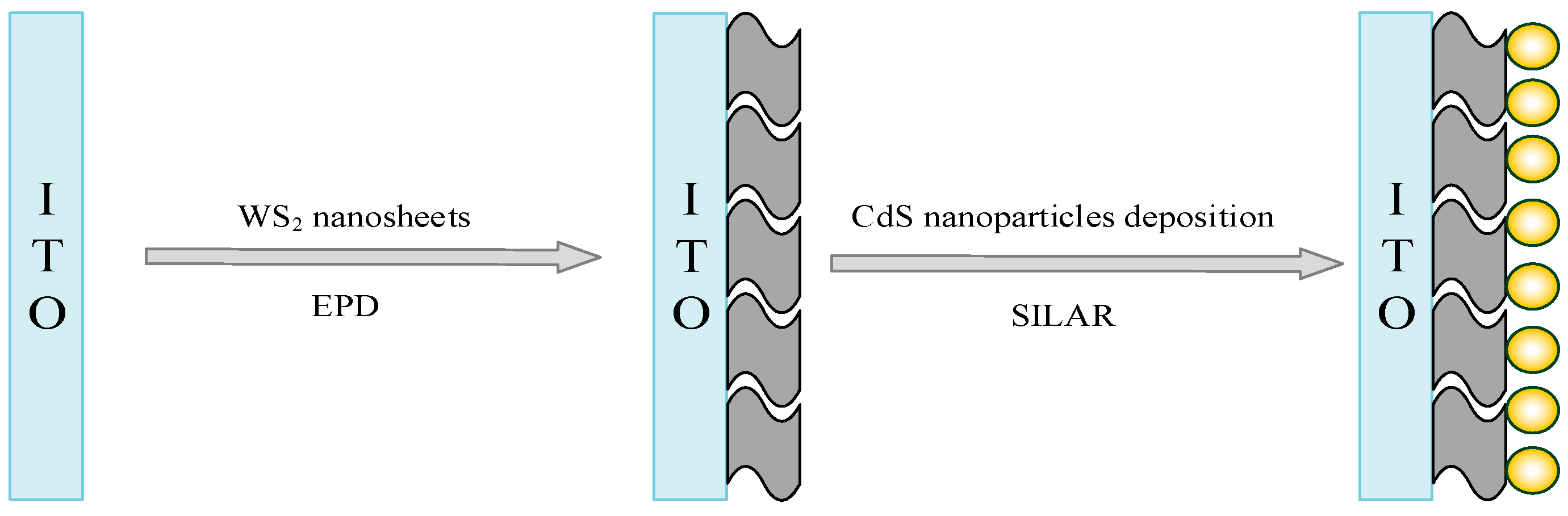
2.3. Construction of Photoelectrochemical Sensor
2.4. Photoelectrochemical Detection of Cysteine
3. Results and Discussion
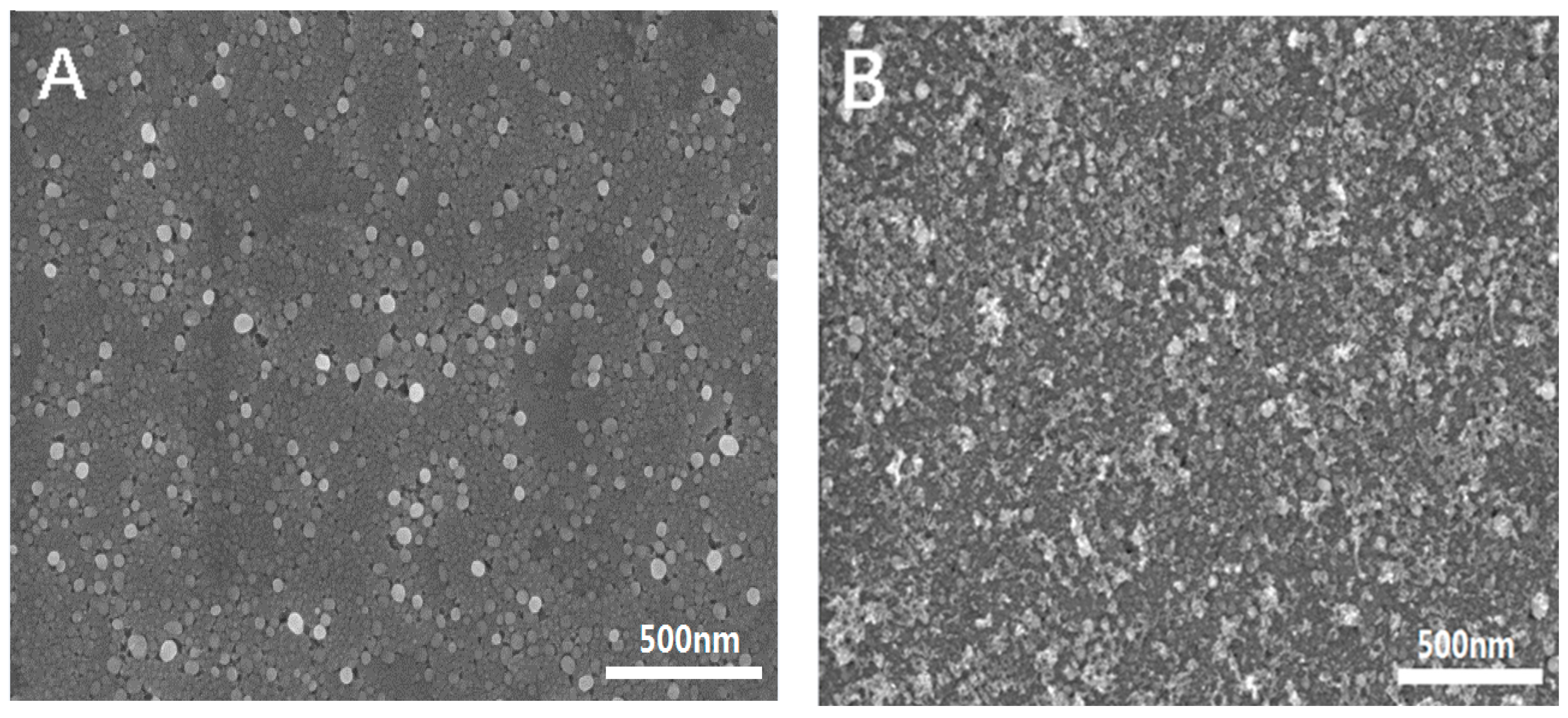
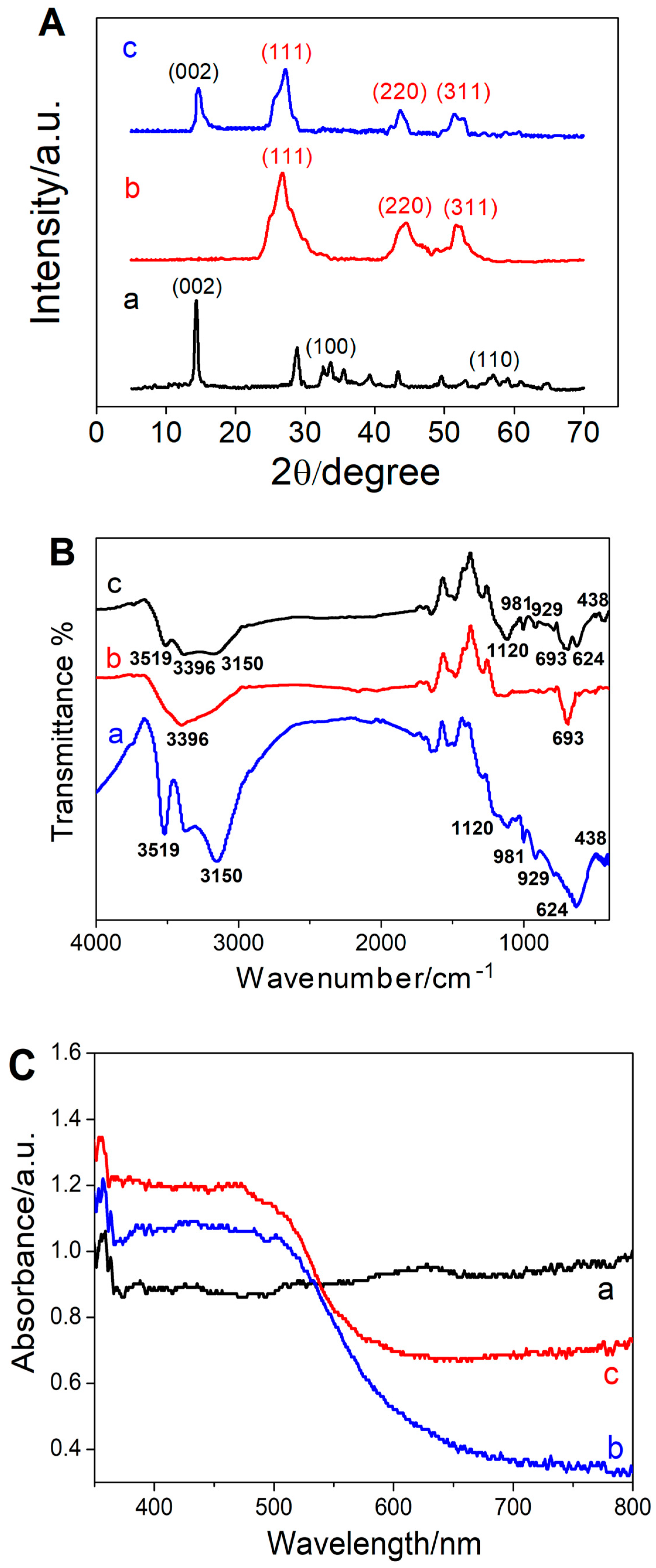
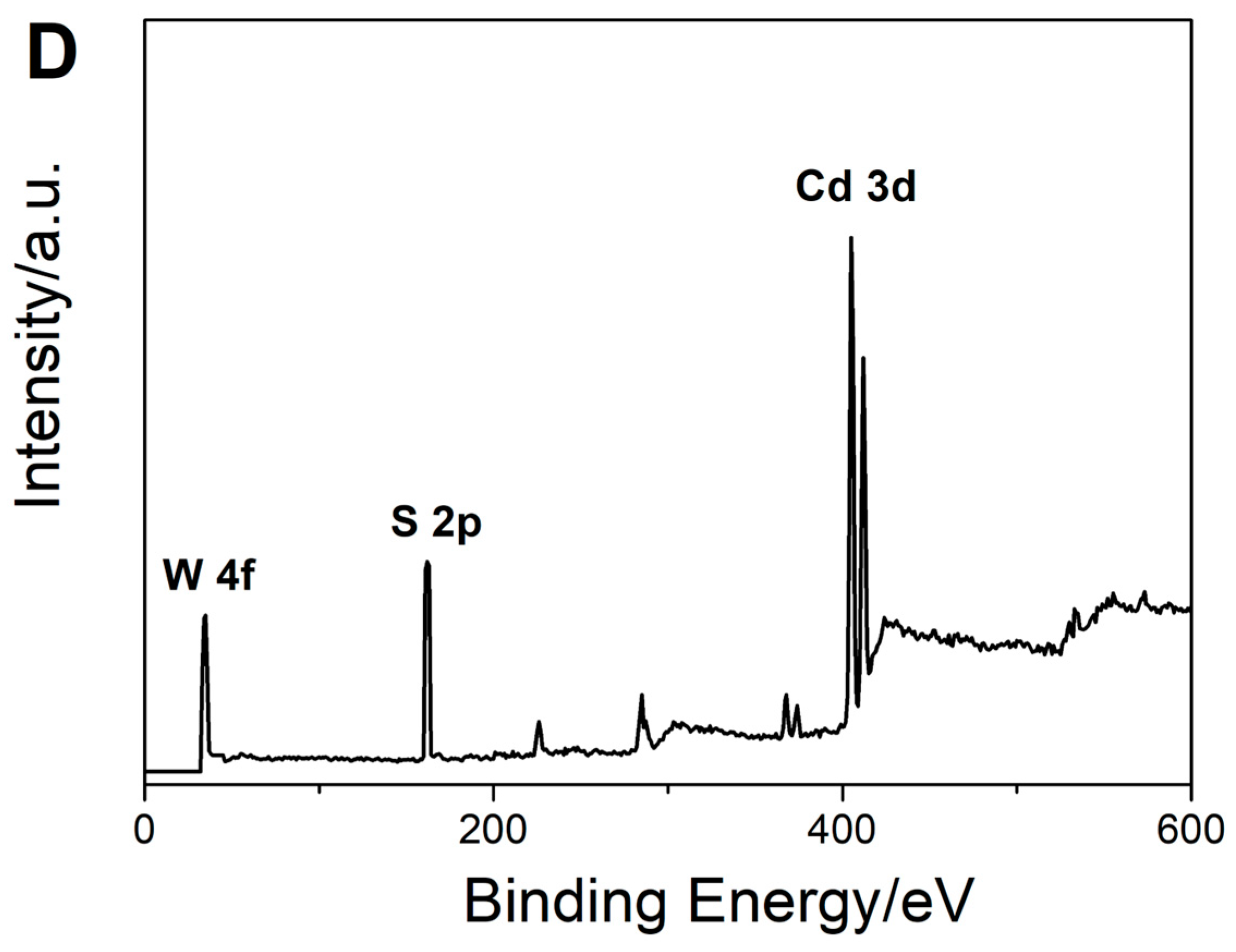
3.1. Morphological and Spectroscopic Characterization of the Prepared Nanomaterials
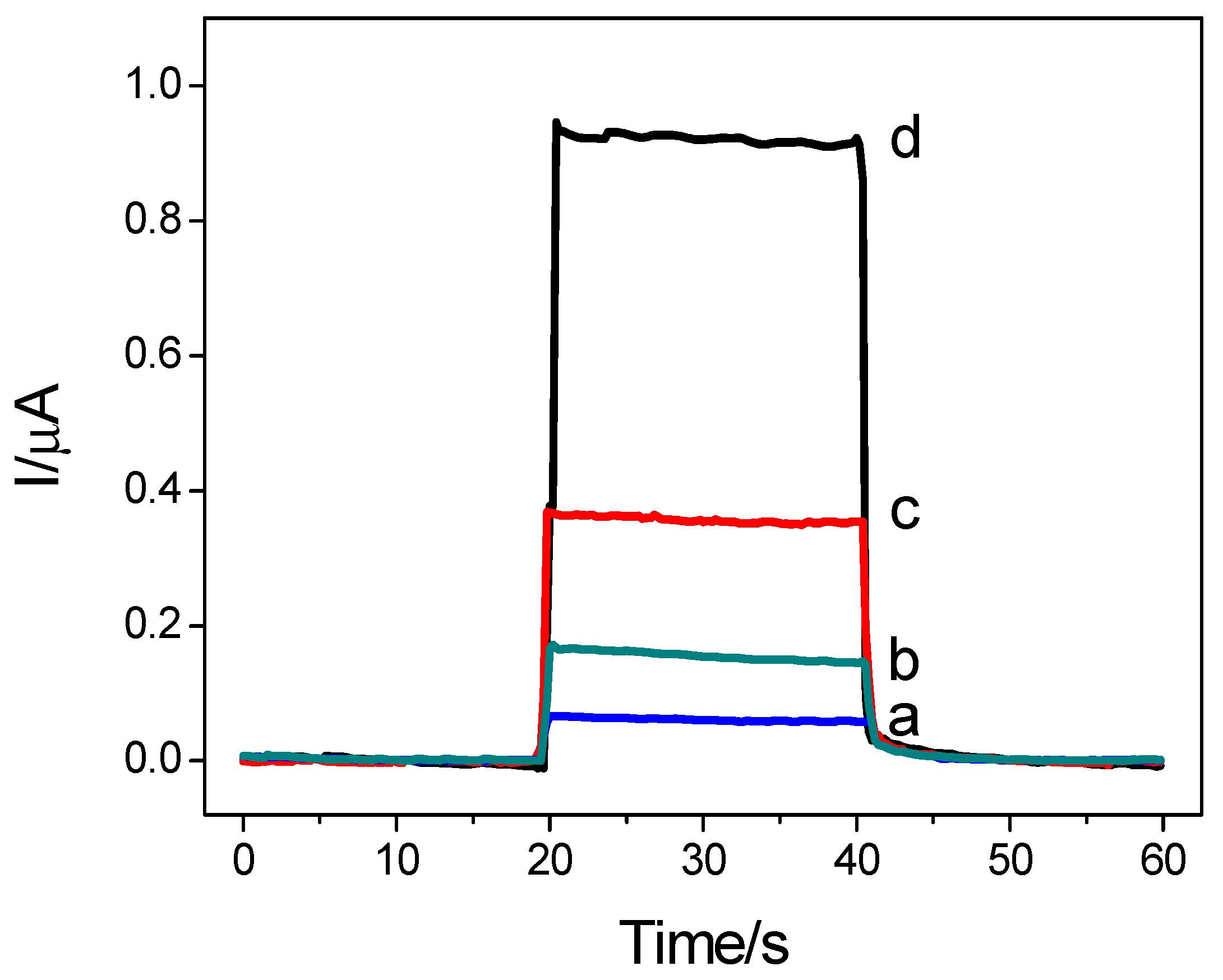
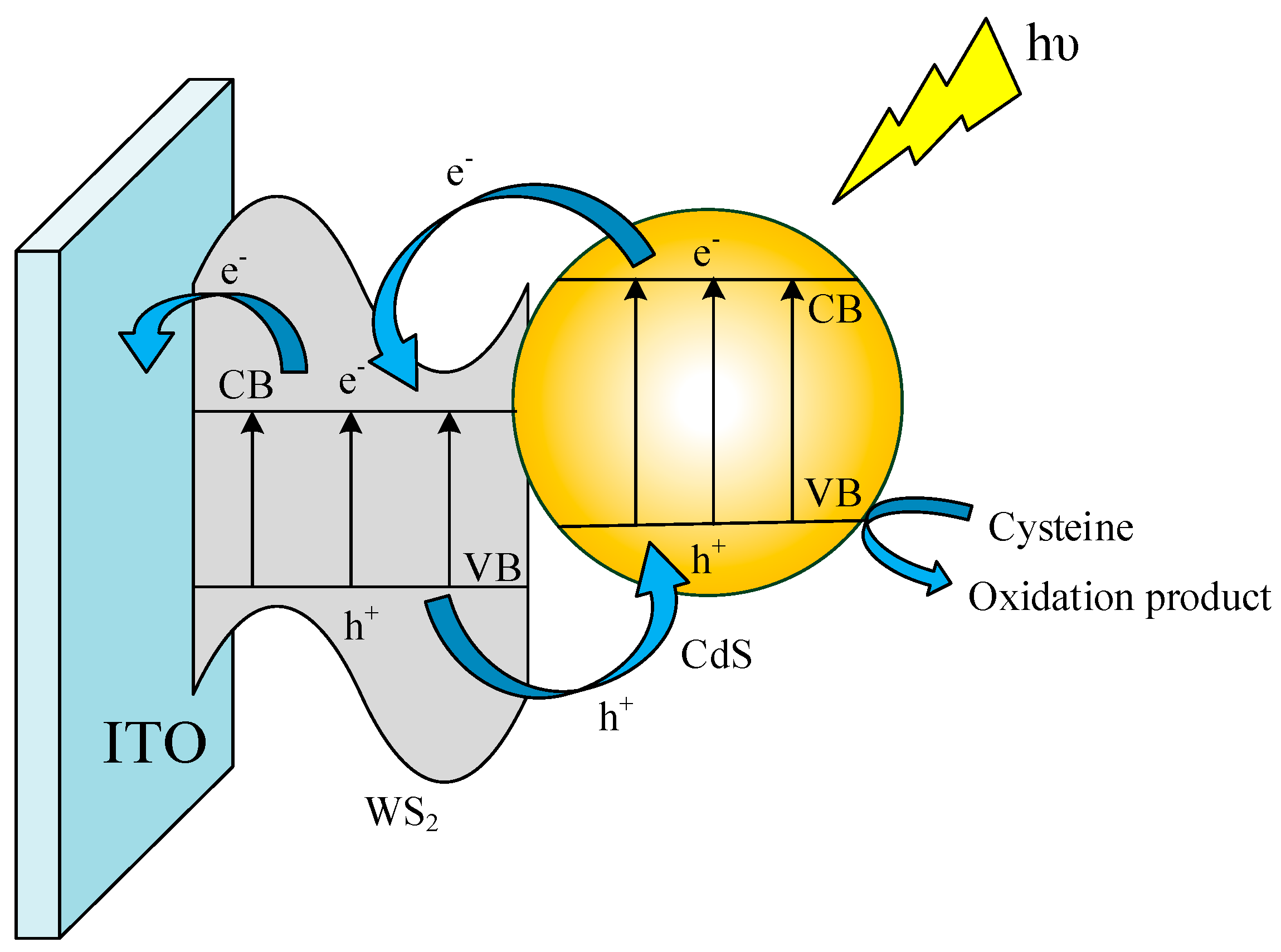
3.2. Photoelectrochemical Performance of the Sensor
3.3. Optimization of Experimental Conditions
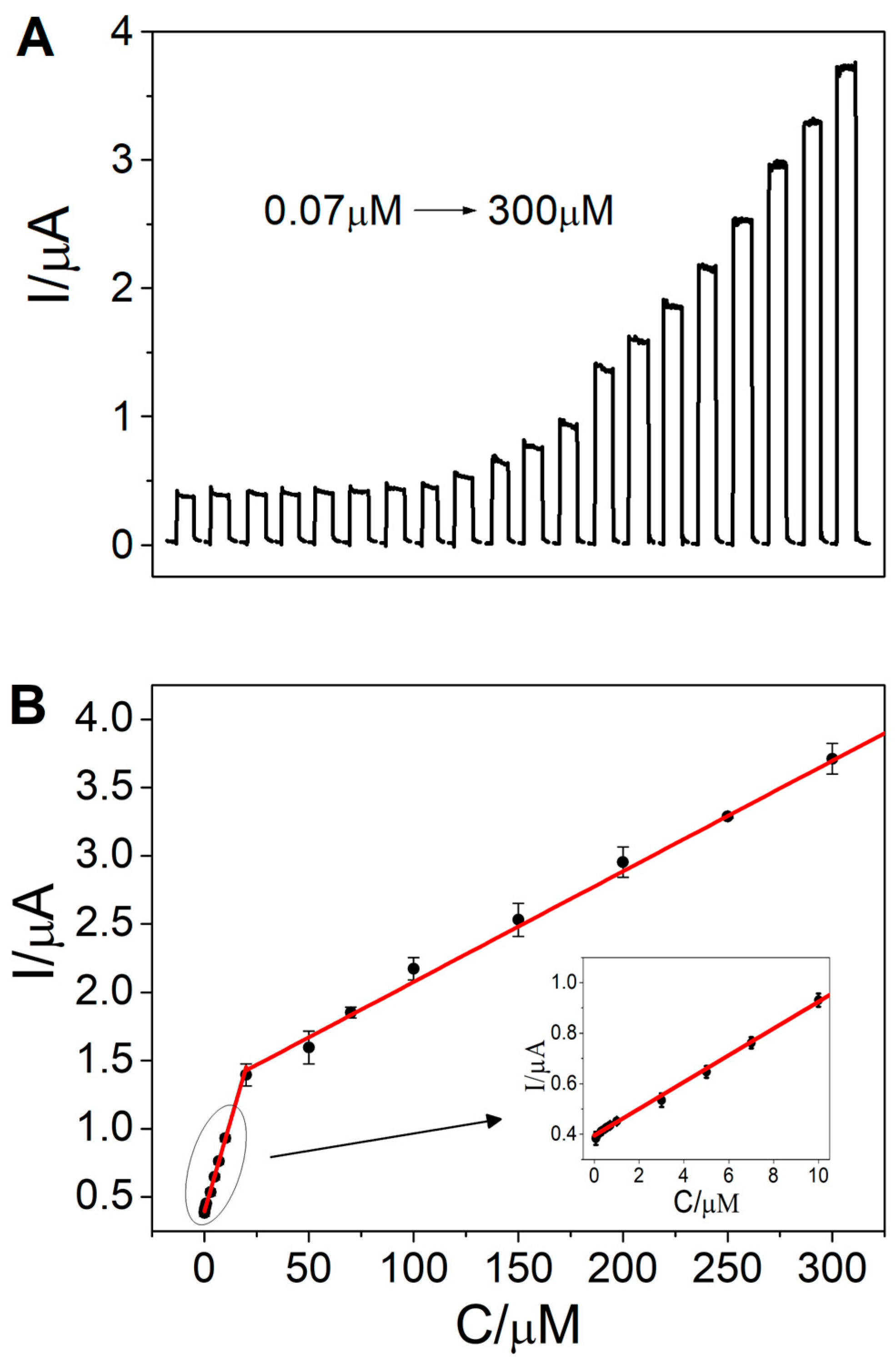
3.4. Photoelectrochemical Sensing of Cysteine
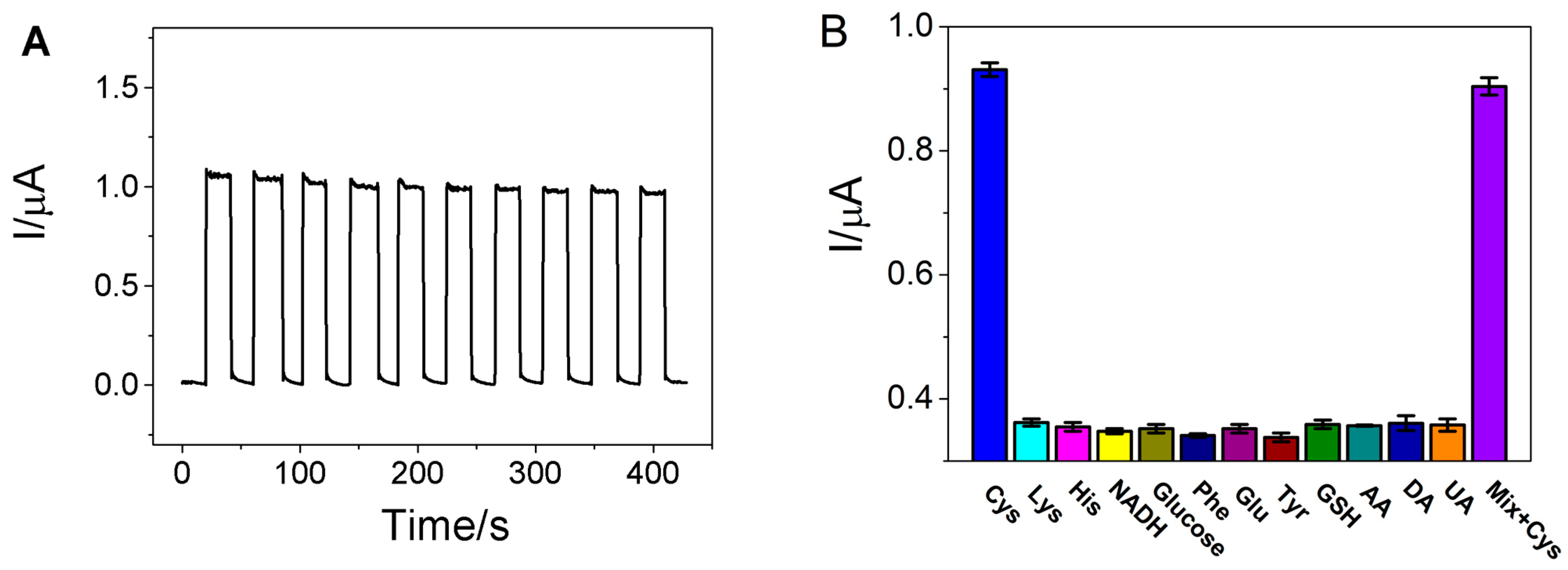
3.5. Reproducibility, Stability and Selectivity
3.6. Application in Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bertoldo, J.B.; Terenzi, H.S.; Huttelmaier, G.; Bernardes, J.L. Posttranslational chemical mutagenesis: To reveal the role of noncatalytic cysteine residues in pathogenic bacterial phosphatases. Biochemistry 2018, 57, 6144–6152. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.F.; Kurniawan, F.; Gunawan, S.H.; Chou, M.; Wang, J. Disposable electrochemical sensor based on copper-electro deposited screen-printed gold electrode and its application in sensing L-cysteine. Electrochim. Acta 2019, 293, 318–327. [Google Scholar] [CrossRef]
- Gazit, V.; Ben-Abraham, R.; Coleman, R. Cysteine-induced hypoglycemic brain damage: An alternative mechanism to excitotox icity. Amino Acids 2004, 26, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Deng, J.J.; Wang, D.L.; Yang, L.F.; Yu, P.; Mao, L.Q. Aspartic acid-promoted highly selective and sensitive colorimetric sensing of cysteine in rat brain. Anal. Chem. 2012, 84, 9579–9584. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, X.; Kang, X.; Song, Y. Determination of glutathione and cysteine in human breast milk by high-performance liquid chromatography with chemiluminescence detection for evaluating the oxidative stress and exposure to heavy metals of lac-tating women. Anal. Lett. 2020, 53, 2607–2618. [Google Scholar] [CrossRef]
- Ummadi, M.; Weimer, B.C. Use of capillary electrophoresis and laser-induced fluorescence for attomole detection of amino acids. J. Chromatogr. A 2002, 964, 243–253. [Google Scholar] [CrossRef]
- Lu, S.; Li, Z.; Fu, X. Carbon dots-based fluorescence and UV–vis absorption dual-modal sensors for Ag+ and L-cysteine de-tection. Dyes Pigm. 2021, 187, 109126. [Google Scholar] [CrossRef]
- Li, H.; Ye, L.; Wang, Y. A glassy carbon electrode modified with hollow cubic cuprous oxide for voltammetric sensing of L-cysteine. Microchim. Acta 2017, 185, 5. [Google Scholar] [CrossRef]
- Zare, H.R.; Jahangiri-Dehaghani, F.; Shekari, Z.; Benvidi, A. Electrocatalytic simultaneous determination of ascorbic acid, uric acid and L-cysteine in real samples using quercetin silver nanoparticles graphene nanosheets modified glassy carbon electrode. Appl. Surf. Sci. 2016, 375, 169–178. [Google Scholar] [CrossRef]
- Long, Y.T.; Kong, C.; Li, D.W. Ultrasensitive determination of cysteine based on the photocurrent of nafion-functionalized CdS-MV quantum dots on an ITO electrode. Small 2011, 7, 1624–1628. [Google Scholar] [CrossRef]
- Shen, Q.M.; Jiang, J.Y.; Liu, S.L. Facile synthesis of Au–SnO2 hybrid nanospheres with enhanced photoelectrochemical biosens ing performance. Nanoscale 2014, 6, 6315–6321. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.F.; Wang, Q.Q.; Geng, L.P.; Shu, X.L.; Wang, Y. A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 2019, 197, 28–35. [Google Scholar] [CrossRef]
- Qin, X.F.; Geng, L.P.; Wang, Q.Q.; Wang, Y. Photoelectrochemical aptasensing of ofloxacin based on the use of a TiO2 nanotube array co-sensitized with a nanocomposite prepared from polydopamine and Ag2S nanoparticles. Microchim. Acta 2019, 186, 430. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef]
- Yang, J.; Shin, H.S. Recent advances in layered transition metal dichalcogenides for hydrogen evolution reaction. J. Mater. Chem. A 2014, 2, 5979–5985. [Google Scholar] [CrossRef]
- Feng, C.Q.; Huang, L.F.; Guo, Z.P.; Liu, H.K. Synthesis of tungsten disulfide (WS2) nanoflakes for lithium-ion battery application. Electrochem. Commun. 2007, 9, 119–122. [Google Scholar] [CrossRef]
- Teo, W.Z.; Chng, E.L.K.; Sofer, Z.; Pumera, M. Cytotoxicity of exfoliated transitionmetal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of grapheme and its analogues. Chem. Eur. J. 2014, 20, 9627–9632. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ji, G.; Ding, B.; Ma, Y.; Qu, B.; Chen, W.; Lee, J.Y. In situ nitrogenated graphene–few-layer WS2 composites for fast and reversible Li+ storage. Nanoscale 2013, 5, 7890–7896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qin, X.; Geng, L.; Wang, Y. Label-free electrochemical aptasensor for sensitive detection of malachite green based on Au nanoparticle/graphene quantum dots/tungsten disulfide nanocomposites. Nanomaterials 2019, 9, 229. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, Y.; Xu, Y.; Wang, X. Photocatalytic hydrogen production over carbon nitride loaded with WS2 as cocatalyst under visible light. Appl. Catal. B-Environ. 2014, 156, 122–127. [Google Scholar] [CrossRef]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two dimensional hybrid nanosheets of tungsten disulfide and reduced grapheme oxide as catalysts for enhanced hydrogen evolution. Angew. Chem. Int. Edit. 2013, 52, 13751–13754. [Google Scholar] [CrossRef]
- Chen, G.; Li, F.; Fan, Y.; Luo, Y.; Li, D.; Meng, Q. A novel noble metal-free ZnS–WS2/CdS composite photocatalyst for H2 evlution under visible light irradiation. Catal. Commun. 2013, 40, 51–54. [Google Scholar] [CrossRef]
- Ashfaq, M.; Talreja, N.; Chauhanc, D.; Viswanathan, M.R. Synthesis of Cu-doped 2D-WS2 nanosheet-based nano-antibiotic materials for inhibiting E. coli and S. aureus bacterial strains. New J. Chem. 2022, 46, 5581–5587. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, F.; Qin, X.F.; Wang, Q.Q. Visible light photoelectrochemical aptasensor for sensitive detection of chloramphen icol based on trivalent Eu-doped CdS quantum dots-sensitized TiO2 nanorod array. Microchim. Acta 2018, 185, 161. [Google Scholar] [CrossRef]
- Chen, J.Z.; Wu, X.J.; Yin, L.S.; Li, B.; Hong, X.; Fan, Z.X.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-laye transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Edit. 2015, 54, 1210–1214. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, S.S.; Zhang, Z.; Li, M. Multiscale and multicomponent layer by layer assembly of optical thin films triggered by electrochemical coupling reactions of N-alkylcarbazoles. Chin. Chem. Lett. 2016, 27, 487–491. [Google Scholar] [CrossRef]
- Sciortino, F.; Rydzek, G.; Grasset, F.; Ariga, K. Electro-click construction of hybrid nanocapsule films with triggered delivery properties. Phys. Chem. Chem. Phys. 2018, 20, 2761–2770. [Google Scholar] [CrossRef]
- Hu, S.S.; Li, W.Y.; Finklea, H.; Liu, X.B. A review of electrophoretic deposition of metal oxides and its application in solid oxide fuel cells. Adv. Colloid Interface Sci. 2020, 276, 102102. [Google Scholar] [CrossRef]
- Zhou, K.G.; Mao, N.N.; Wang, H.X.; Peng, Y.; Zhang, H.L. A mixed-solvent strategy for efficient exfoliation of inorganic graphene analogues. Angew. Chem. Int. Ed. 2011, 50, 10839–10842. [Google Scholar] [CrossRef]
- Zirak, M.; Zhao, M.; Moradlou, O.; Samadi, M.; Sarikhani, N.; Wang, Q.; Zhang, H.L.; Moshfegh, A.Z. Controlled engineering of WS2 nanosheets–CdS nanoparticle heterojunction with enhanced photoelectrochemical activity. Sol. Energy Mater. Sol. Cells 2015, 141, 260–269. [Google Scholar] [CrossRef]
- Zirak, M.; Moradlou, O.; Bayati, M.R.; Nien, Y.T.; Moshfegh, A.Z. On the growth and photocatalytic activity of the verticall aligned ZnO nanorods grafted by CdS shells. Appl. Surf. Sci. 2013, 273, 391–398. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C.; Chitturi, V. Selective hydrothermally synthesis of hexagonal WS2 platelets and their photocatalytic performance under visible light irradiation. Superlattices Microstruct. 2016, 94, 39–50. [Google Scholar] [CrossRef]
- Seo, J.; Jun, Y.; Park, S.; Nah, H.; Moon, T.; Park, B.; Kim, J.G.; Kim, Y.J.; Cheon, J. Two-dimensional nanosheet crystals. Angew. Chem. Int. Ed. 2007, 46, 8828–8831. [Google Scholar] [CrossRef]
- Zhang, K.X.; Yu, Y.X.; Sun, S.Q. Influence of Eu doping on the microstructure and photoluminescence of CdS nanocrystals. Appl. Surf. Sci. 2012, 258, 7658–7663. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Ngo, I.L.; Byon, C. Physicochemcial characteristic of CdS-anchored porous WS2 hybrid in the photocatalytic degradation of crystal violet under UV and visible light irradiation. Solid State Sci. 2016, 61, 121–130. [Google Scholar] [CrossRef]
- Winkler, U.; Eich, D.; Chen, Z.H.; Fink, R.; Kulkarni, S.K.; Umbach, E. Detailed investigation of CdS nanoparticle surfaces by high resolution photoelectron spectroscopy. Chem. Phys. Lett. 1999, 306, 95–102. [Google Scholar] [CrossRef]
- Raza, F.; Park, J.H.; Lee, H.R.; Kim, H.I.; Jeon, S.J.; Kim, J.H. Visible-light-driven oxidative coupling reactions of amines by photoactive WS2 nanosheets. ACS Catal. 2016, 6, 2754–2759. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.; Jang, H.W.; Kim, S.Y. Size-dependent properties of two-dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, S.; Chu, W.; Wei, T.; Tao, H.; Zhang, C.; Sun, Y. Enhanced photoelectrochemical detection of L-cysteine based on the ultrathin polythiophene layer sensitized anatase TiO2 on F-doped tin oxide substrates. Sens. Actuators B Chem. 2016, 232, 448–453. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Xu, Z.W.; Yan, K.; Zhao, H.B.; Zhang, J.D. One-step synthesis of CuO-Cu2O heterojunction by flame spray pyrolysis for cathodic photoelectrochemical sensing of L-cysteine. ACS Appl. Mater. Inter. 2017, 9, 40452–40460. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, B.; Zhang, W.B. A novel photoelectrochemical assay for cysteine based on the target-induced generation of pho tosensitizer. Int. J. Electrochem. Sci. 2018, 13, 7183–7192. [Google Scholar] [CrossRef]
- Peng, J.Y.; Huang, Q.; Liu, Y.X.; Huang, Y.Y.; Xiang, G. Photoelectrochemical detection of L-cysteine with a covalently grafted ZnTAPc-Gr-based probe. Electroanalysis 2020, 32, 1237–1242. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, G.; Ji, D.Z.; Zhang, F.N.; Liu, Q.G. Smartphone-based label-free photoelectrochemical sensing of cysteine with cadmium ion chelation. Analyst 2022, 147, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Yao, C.F.; Cui, H.; Cang, Y.G.; Zhang, Z.H.; Miao, Y.Q.; Xin, Y.M. A nano-enzymatic photoelectrochemical L-cysteine biosensor based on Bi2MoO6 modified honeycomb TiO2 nanotube arrays composite. Microchem. J. 2022, 175, 107200. [Google Scholar] [CrossRef]
- Xin, Y.M.; Wang, Z.; Yao, H.Z.; Liu, W.T.; Miao, Y.Q.; Zhang, Z.G.; Wu, D. Au-mediated Z-scheme TiO2-Au-BiOI photoelec trode for sensitive and selective photoelectrochemical detection of L-cysteine. Sens. Actuators B Chem. 2023, 393, 134285. [Google Scholar] [CrossRef]
- Chen, S.; Gao, H.L.; Shen, W.W.; Lu, C.; Yuan, Q.P. Colorimetric detection of cysteine using noncrosslinking aggregation of fluorosurfactant-capped silver nanoparticles. Sens. Actuators B Chem. 2014, 190, 673–678. [Google Scholar] [CrossRef]
| Working Electrode | Linear Range (μM) | Detection Limit (nM) | Reference |
|---|---|---|---|
| Nafion/CdS-MV/ITO | 0.2–2.8 | 100 | [10] |
| Au-SnO2/CdS/ITO | 0.4–120 | 100 | [11] |
| PTh/TiO2/FTO | 10–800 | 12,800 | [39] |
| CuO–Cu2O/GCE | 0.2–10 | 50 | [40] |
| CA-TiO2/FTO | 2.0–100 | 650 | [41] |
| ZnTAPc-Gr/ITO | 0.25–113 | 11.4 | [42] |
| ITO/g-C3N4/Au | 10–40 | 9200 | [43] |
| Bi2MoO6/TiO2 | 0.5–600 | 150 | [44] |
| TiO2-Au-BiOI | 0.8–200 | 70 | [45] |
| CdS/WS2/ITO | 0.07–300 | 5.29 | This work |
| Sample | Original (µM) | Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Serum 1 | 3.86 | 5.00 | 8.99 | 101.5 | 2.6 |
| Serum 2 | 3.95 | 5.00 | 8.83 | 98.7 | 1.8 |
| Urine 1 | 4.81 | 5.00 | 9.64 | 98.3 | 3.0 |
| Urine 2 | 5.56 | 5.00 | 10.78 | 102.1 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, J.; Lin, F. A Photoelectrochemical Sensor for the Sensitive Detection of Cysteine Based on Cadmium Sulfide/Tungsten Disulfide Nanocomposites. Nanomaterials 2024, 14, 427. https://doi.org/10.3390/nano14050427
Wang Y, Liu J, Lin F. A Photoelectrochemical Sensor for the Sensitive Detection of Cysteine Based on Cadmium Sulfide/Tungsten Disulfide Nanocomposites. Nanomaterials. 2024; 14(5):427. https://doi.org/10.3390/nano14050427
Chicago/Turabian StyleWang, Yan, Jiaxin Liu, and Fancheng Lin. 2024. "A Photoelectrochemical Sensor for the Sensitive Detection of Cysteine Based on Cadmium Sulfide/Tungsten Disulfide Nanocomposites" Nanomaterials 14, no. 5: 427. https://doi.org/10.3390/nano14050427





