Polymer-Clay Nanocomposites for the Uptake of Hazardous Anions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
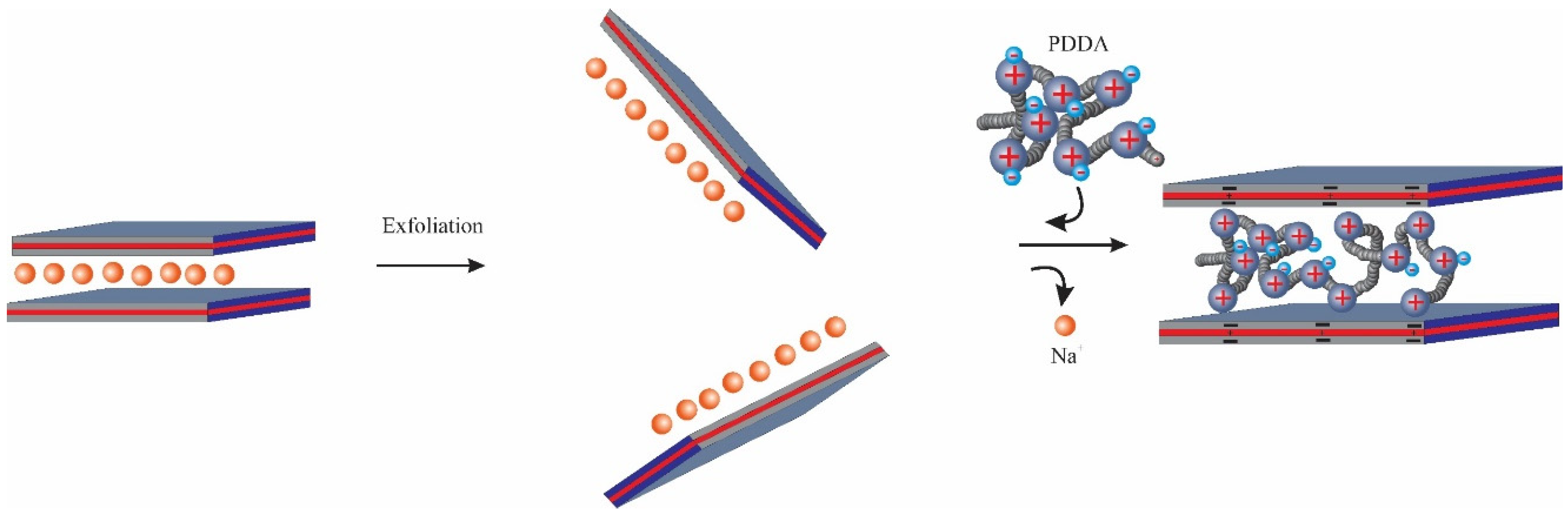
2.2. Preparation of PDDA Intercalated Montmorillonites (Mt)
2.3. Sample Characterization
2.3.1. Powder X-ray Diffraction
2.3.2. Infrared Spectroscopy
2.4. Anion Uptake Studies
2.5. Kinetics Studies
2.6. Isotherm Studies
2.7. Effect of Competitive Anions and Determination of Values
3. Results and Discussion
3.1. X-ray Diffraction
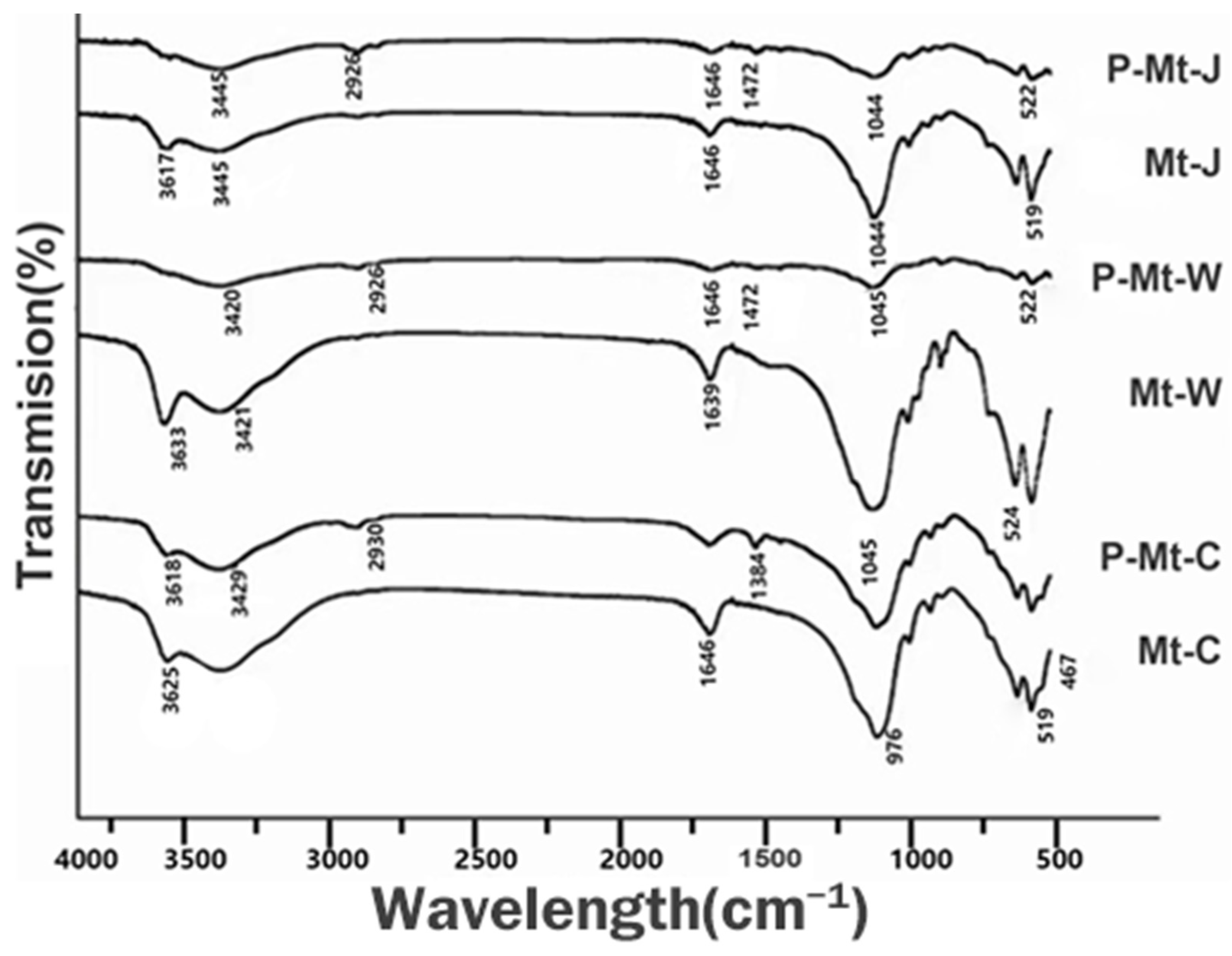
3.2. Infrared Spectroscopy
3.3. Perchlorate, Chromate, and Nitrate Uptake by Mt-PDDA Nanocomposites
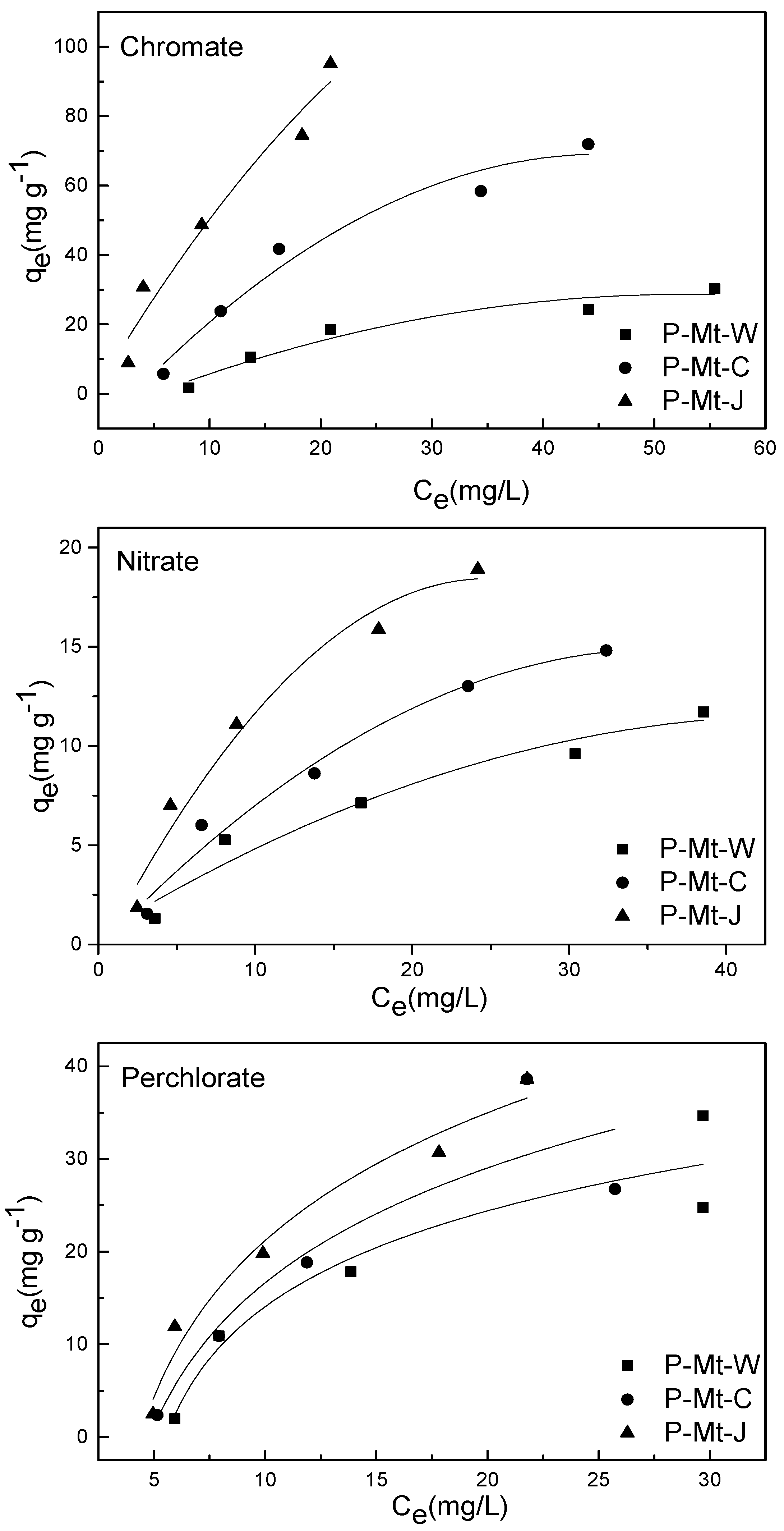
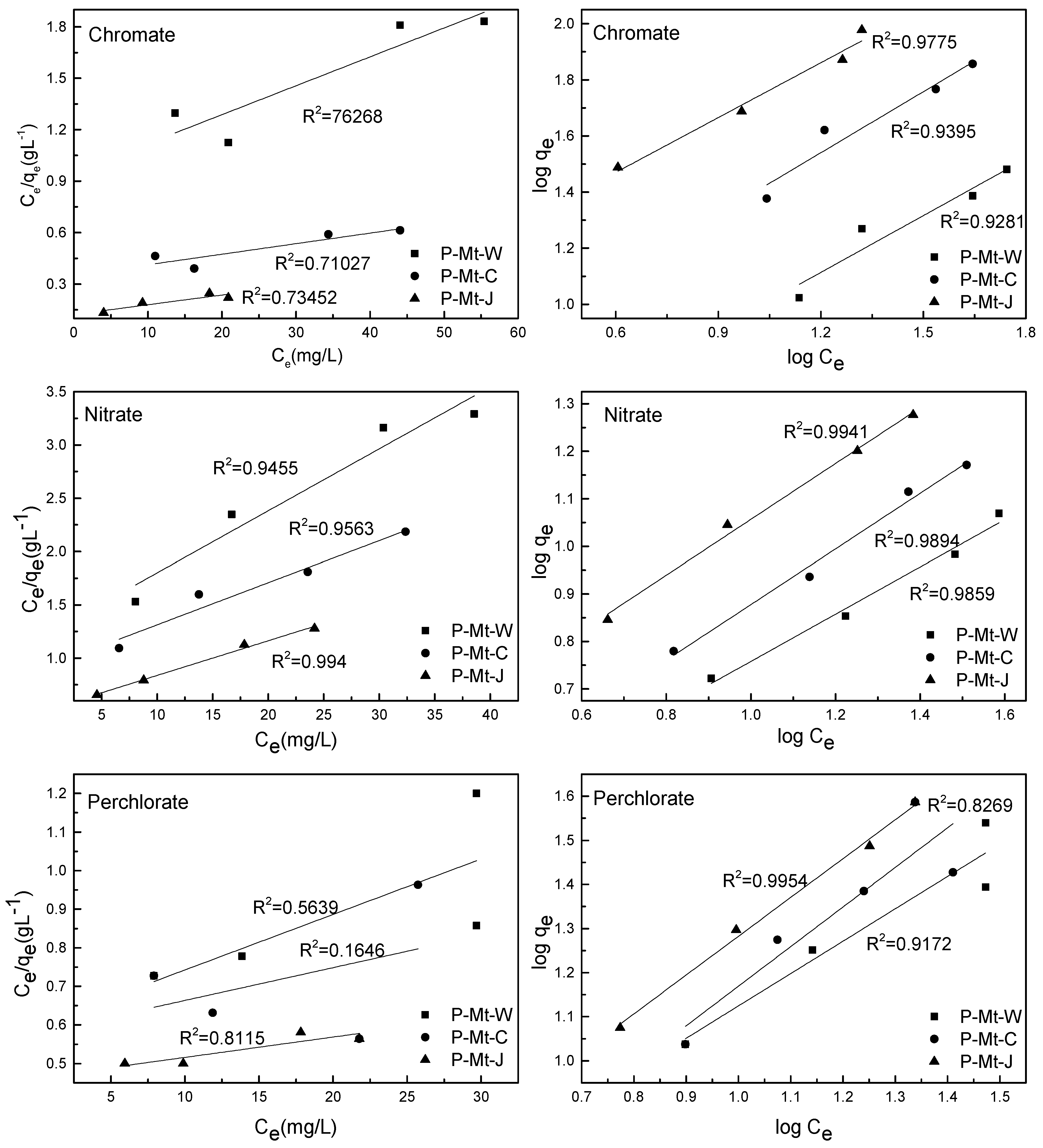
3.4. Adsorption Equilibrium Isotherms
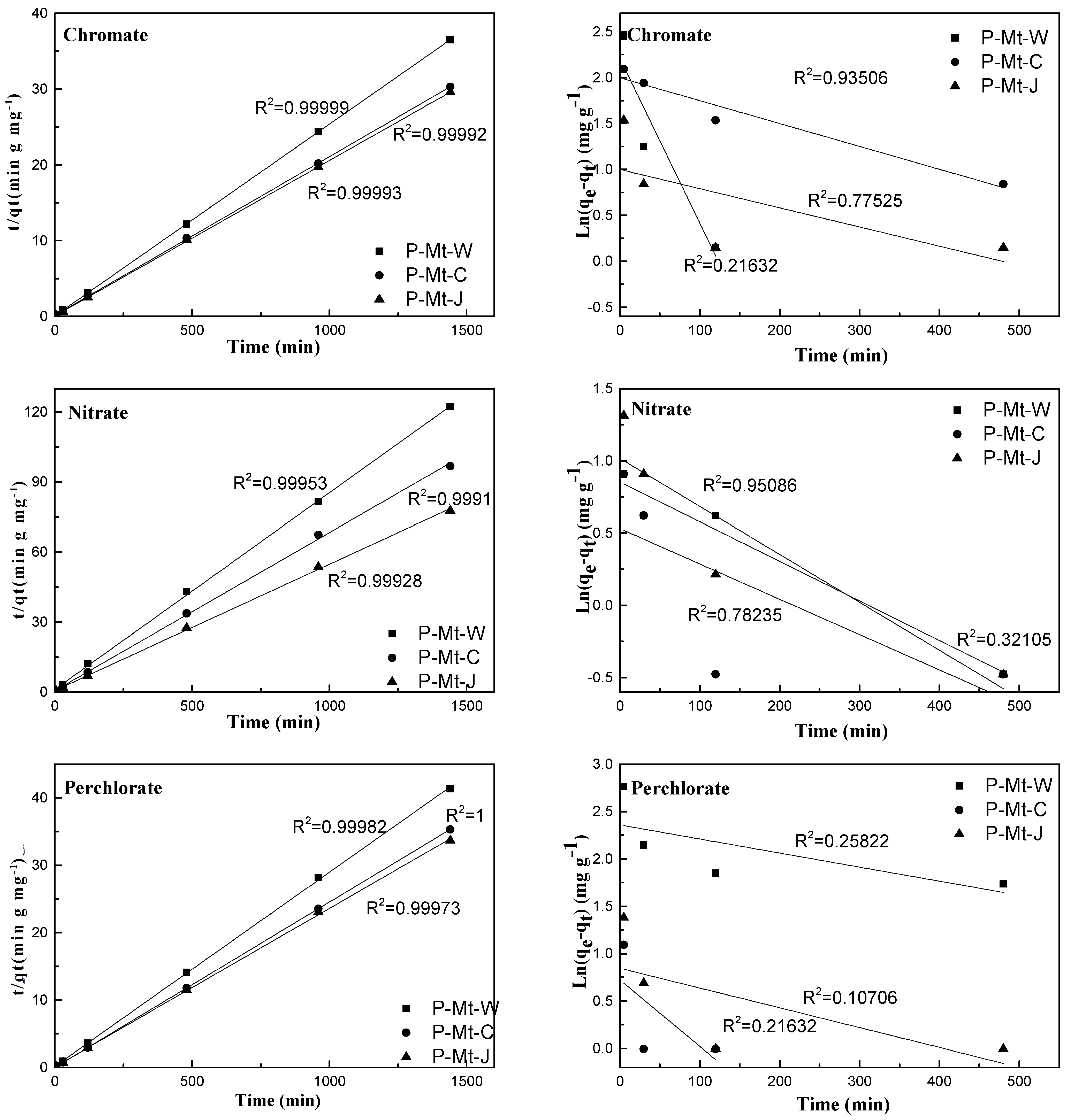
3.5. Kinetics Studies
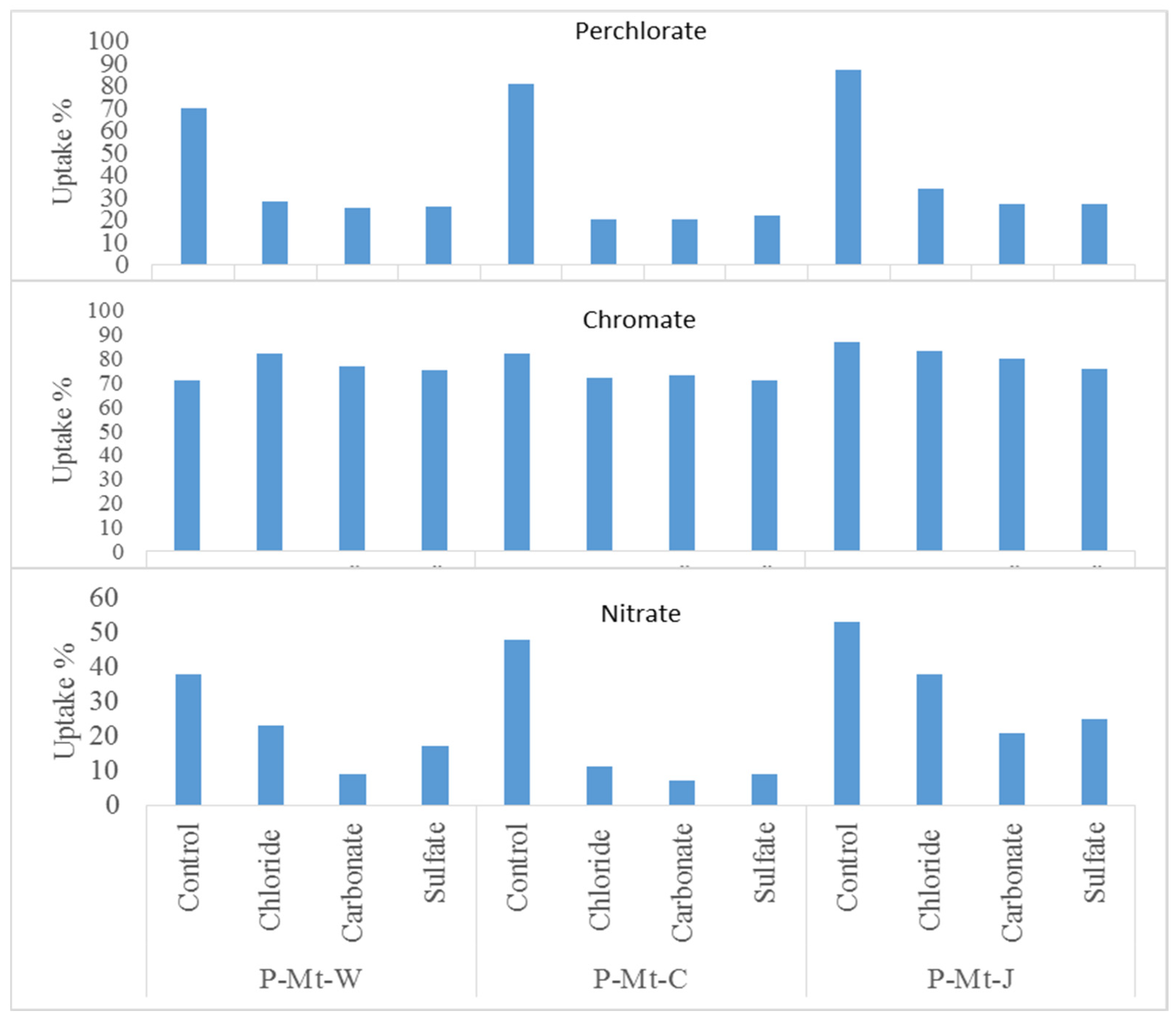
3.6. The Effects of Competitive Anions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrilli, F.L.; De Flora, S. Toxicity and mutagenicity of hexavalent chromium on Salmonella typhimurium. Appl. Environ. Microbiol. 1977, 33, 805–809. [Google Scholar] [CrossRef]
- Sharma, D.; Chatterjee, C.; Sharma, C. Chromium accumulation and its effects on wheat (Triticum aestivum L. cv. HD 2204) metabolism. Plant Sci. 1995, 111, 145–151. [Google Scholar] [CrossRef]
- Nishioka, H. Mutagenic activities of metal compounds in bacteria. Mutat. Res. 1975, 31, 185–189. [Google Scholar] [CrossRef]
- Venitt, S.; Levy, L. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature 1974, 250, 493–495. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.-Y.; Yue, W.-W.; Yue, Q.-Y. Adsorption kinetics of nitrate from aqueous solutions onto modified wheat residue. Colloids Surf. A Physicochem. Eng. Asp. 2007, 308, 1–5. [Google Scholar] [CrossRef]
- Neşe, Ö.; Ennil, K.T. A kinetic study of nitrite adsorption onto sepiolite and powdered activated carbon. Desalination 2008, 223, 174–179. [Google Scholar] [CrossRef]
- Afkhami, A. Adsorption and electrosorption of nitrate and nitrite on high-area carbon cloth: An approach to purification of water and waste-water samples. Carbon 2003, 41, 1320–1322. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Schock, M. Issues in managing the risks associated with perchlorate in drinking water. J. Environ. Manag. 1999, 56, 79–95. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Martinelango, P.K.; Jackson, W.A.; Anderson, T.A.; Tian, K.; Tock, R.W.; Rajagopalan, S. The origin of naturally occurring perchlorate: The role of atmospheric processes. Environ. Sci. Technol. 2005, 39, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Borjan, M.; Marcella, S.; Blount, B.; Greenberg, M.; Zhang, J.J.; Murphy, E.; Valentin-Blasini, L.; Robson, M. Perchlorate exposure in lactating women in an urban community in New Jersey. Sci. Total Environ. 2011, 409, 460–464. [Google Scholar] [CrossRef]
- Agency USEP. EPA’s Report on the Environment (2003 Draft). Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=56830 (accessed on 28 February 2024).
- Láng, G.G.; Sas, N.S.; Ujvári, M.; Horányi, G. The kinetics of the electrochemical reduction of perchlorate ions on rhodium. Electrochim. Acta 2008, 53, 7436–7444. [Google Scholar] [CrossRef]
- Wang, D.; Lin, H.; Shah, S.I.; Ni, C.; Huang, C. Indirect electrochemical reduction of perchlorate and nitrate in dilute aqueous solutions at the Ti–water interface. Sep. Purif. Technol. 2009, 67, 127–134. [Google Scholar] [CrossRef]
- Almeida, C.; Giannetti, B.; Rabockai, T. Electrochemical study of perchlorate reduction at tin electrodes. J. Electroanal. Chem. 1997, 422, 185–189. [Google Scholar] [CrossRef]
- Rusanova, M.Y.; Polášková, P.; Muzikař, M.; Fawcett, W.R. Electrochemical reduction of perchlorate ions on platinum-activated nickel. Electrochim. Acta 2006, 51, 3097–3101. [Google Scholar] [CrossRef]
- Kimura, K.; Nakamura, M.; Watanabe, Y. Nitrate removal by a combination of elemental sulfur-based denitrification and membrane filtration. Water Res. 2002, 36, 1758–1766. [Google Scholar] [CrossRef]
- Ergas, S.J.; Rheinheimer, D.E. Drinking water denitrification using a membrane bioreactor. Water Res. 2004, 38, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Keskinler, B.; Danis, U.; Çakici, A.; Akay, G. Chromate removal from water using surfactant-enhanced crossflow filtration. Sep. Sci. Technol. 1997, 32, 1899–1920. [Google Scholar] [CrossRef]
- Yoon, Y.; Amy, G.; Cho, J.; Her, N.; Pellegrino, J. Transport of perchlorate (ClO4−) through NF and UF membranes. Desalination 2002, 147, 11–17. [Google Scholar] [CrossRef]
- Rabah, F.K.; Dahab, M.F. Nitrate removal characteristics of high performance fluidized-bed biofilm reactors. Water Res. 2004, 38, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Jianping, W.; Wei, P.L.H.; Liping, D.; Guozhu, M. The denitrification of nitrate contained wastewater in a gas–liquid–solid three-phase flow airlift loop bioreactor. Biochem. Eng. J. 2003, 15, 153–157. [Google Scholar] [CrossRef]
- Parette, R.; Cannon, F.S.; Weeks, K. Removing low ppb level perchlorate, RDX, and HMX from groundwater with cetyltrimethylammonium chloride (CTAC) pre-loaded activated carbon. Water Res. 2005, 39, 4683–4692. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Parette, R.; Cannon, F.S. The removal of perchlorate from groundwater by activated carbon tailored with cationic surfactants. Water Res. 2005, 39, 4020–4028. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of Cr (VI) from aqueous solution by two Lewatit-anion exchange resins. J. Hazard. Mater. 2005, 119, 175–182. [Google Scholar] [CrossRef]
- Shi, T.; Wang, Z.; Liu, Y.; Jia, S.; Changming, D. Removal of hexavalent chromium from aqueous solutions by D301, D314 and D354 anion-exchange resins. J. Hazard. Mater. 2009, 161, 900–906. [Google Scholar] [CrossRef]
- Gu, B.; Ku, Y.-K.; Brown, G.M. Sorption and desorption of perchlorate and U (VI) by strong-base anion-exchange resins. Environ. Sci. Technol. 2005, 39, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Brown, G.M.; Bonnesen, P.V.; Liang, L.; Moyer, B.A.; Ober, R.; Alexandratos, S.D. Development of novel bifunctional anion-exchange resins with improved selectivity for pertechnetate sorption from contaminated groundwater. Environ. Sci. Technol. 2000, 34, 1075–1080. [Google Scholar] [CrossRef]
- Burge, S.; Halden, R. Nitrate and Perchlorate Removal from Groundwater by Ion Exchange; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1999. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey. J. Hazard. Mater. 2006, 137, 332–335. [Google Scholar] [CrossRef]
- Ghiaci, M.; Kia, R.; Abbaspur, A.; Seyedeyn-Azad, F. Adsorption of chromate by surfactant-modified zeolites and MCM-41 molecular sieve. Sep. Purif. Technol. 2004, 40, 285–295. [Google Scholar] [CrossRef]
- Zhang, P.; Avudzega, D.M.; Bowman, R.S. Removal of perchlorate from contaminated waters using surfactant-modified zeolite. J. Environ. Qual. 2007, 36, 1069–1075. [Google Scholar] [CrossRef]
- Guan, H.; Bestland, E.; Zhu, C.; Zhu, H.; Albertsdottir, D.; Hutson, J.; Simmons, C.T.; Ginic-Markovic, M.; Tao, X.; Ellis, A.V. Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites. J. Hazard. Mater. 2010, 183, 616–621. [Google Scholar] [CrossRef]
- Mahadevaiah, N.; Venkataramani, B.; Jai Prakash, B. Interaction of chromate on surfactant modified montmorillonite: Breakthrough curve study in fixed bed columns. Ind. Eng. Chem. Res. 2008, 47, 1755–1759. [Google Scholar] [CrossRef]
- Krishna, B.; Murty, D.; Prakash, B.J. Surfactant-modified clay as adsorbent for chromate. Appl. Clay Sci. 2001, 20, 65–71. [Google Scholar] [CrossRef]
- Dultz, S.; An, J.-H.; Riebe, B. Organic cation exchanged montmorillonite and vermiculite as adsorbents for Cr (VI): Effect of layer charge on adsorption properties. Appl. Clay Sci. 2012, 67–68, 125–133. [Google Scholar] [CrossRef]
- Rathnayake, S.I.; Martens, W.N.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Remediation of Cr(VI) by inorganic-organic clay. J. Colloid. Interface Sci. 2017, 490, 163–173. [Google Scholar] [CrossRef]
- Rafiei, H.R.; Shirvani, M. Utilization of Poly (acrylic acid)–Bentonite Composite for the Sorption of Chromium from Aqueous Solutions. Nashrieh Shimi Va Mohandesi Shimi Iran 2017, 36, 127–139. [Google Scholar]
- Hata, H.; Kobayashi, Y.; Mallouk, T.E. Encapsulation of anionic dye molecules by a swelling fluoromica through intercalation of cationic polyelectrolytes. Chem. Mater. 2007, 19, 79–87. [Google Scholar] [CrossRef]
- Bagherifam, S.; Komarneni, S.; Lakzian, A.; Fotovat, A.; Khorasani, R.; Huang, W.; Ma, J.; Hong, S.; Cannon, F.S.; Wang, Y. Highly selective removal of nitrate and perchlorate by organoclay. Appl. Clay Sci. 2014, 95, 126–132. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, S.; Byrne, T.; Cannon, F.; Shahien, M.; Khalil, A.; El-Gaid, I.A. Removal of nitrate by synthetic organosilicas and organoclay: Kinetic and isotherm studies. Sep. Purif. Technol. 2013, 110, 181–187. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Wei, S.; Mizaikoff, B.; Taylor, A.E.; Favero, C.; Huang, C.-H. Degradation of amine-based water treatment polymers during chloramination as N-nitrosodimethylamine (NDMA) precursors. Environ. Sci. Technol. 2009, 43, 1360–1366. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Sanusi, N.; Ismail, H.; Othman, N.; Ariffin, A. Application of response surface methodology (RSM) for optimization of cassava starch grafted polyDADMAC synthesis for cationic properties. Starch-Stärke 2012, 64, 935–943. [Google Scholar] [CrossRef]
- Bagherifam, S.; Lakzian, A.; Ahmadi, S.J.; Rahimi, M.F.; Halajnia, A. Uranium removal from aqueous solutions by wood powder and wheat straw. J. Radioanal. Nucl. Chem. 2010, 283, 289–296. [Google Scholar] [CrossRef]
- Akyil, S.; Aslani, M.; Eral, M. Sorption characteristics of uranium onto composite ion exchangers. J. Radioanal. Nucl. Chem. 2003, 256, 45–51. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Gammoudi, S.; Frini-Srasra, N.; Srasra, E. Nitrate sorption by organosmectites. Eng. Geol. 2012, 124, 119–129. [Google Scholar] [CrossRef]
- Öztürk, N.; Bektaş, T. Nitrate removal from aqueous solution by adsorption onto various materials. J. Hazard. Mater. 2004, 112, 155–162. [Google Scholar] [CrossRef]
- Šćiban, M.; Radetić, B.; Kevrešan, Ž.; Klašnja, M. Adsorption of heavy metals from electroplating wastewater by wood sawdust. Bioresour. Technol. 2007, 98, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Yusan, S.D.; Akyil, S. Sorption of uranium (VI) from aqueous solutions by akaganeite. J. Hazard. Mater. 2008, 160, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Park, H.-S. Ammonium and potassium removal for anaerobically digested wastewater using natural clinoptilolite followed by membrane pretreatment. J. Hazard. Mater. 2008, 151, 125–133. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Komarneni, S.; Aref, A.R.; Hong, S.; Noh, Y.D.; Cannon, F.; Wang, Y. Organoclays of high-charge synthetic clays and alumina pillared natural clays: Perchlorate uptake. Appl. Clay Sci. 2013, 80, 340–345. [Google Scholar] [CrossRef]



| Sample | Solution | Removal (%) | Uptake () |
|---|---|---|---|
| P-Mt-W | 1 Perchlorate | 71 | 0.350 ± 0.020 |
| P-Mt-C | 1 Perchlorate | 81 | 0.400 ± 0.010 |
| P-Mt-J | 1 Perchlorate | 87 | 0.440 ± 0.001 |
| P-Mt-W | 1 Chromate | 66 | 0.338 ± 0.009 |
| P-Mt-C | 1 Chromate | 82 | 0.413 ± 0.017 |
| P-Mt-J | 1 Chromate | 84 | 0.423 ± 0.005 |
| P-Mt-W | 1 Nitrate | 38 | 0.189 ± 0.002 |
| P-Mt-C | 1 Nitrate | 48 | 0.239 ± 0.004 |
| P-Mt-J | 1 Nitrate | 53 | 0.299 ± 0.002 |
| Anion | Material | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| Perchlorate | P-Mt-W | 0.56 | 69.44 | 0.024 | 0.92 | 2.46 | 0.73 |
| P-Mt-C | 0.16 | 117.6 | 0.015 | 0.82 | 1.87 | 0.89 | |
| P-Mt-J | 0.81 | 188.7 | 0.011 | 0.99 | 2.52 | 0.88 | |
| Chromate | P-Mt-W | 0.84 | 59.17 | 0.018 | 0.93 | 2.01 | 0.67 |
| P-Mt-C | 0.81 | 161.3 | 0.017 | 0.94 | 4.69 | 0.72 | |
| P-Mt-J | 0.82 | 175.4 | 0.047 | 0.98 | 11.0 | 0.65 | |
| Nitrate | P-Mt-W | 0.95 | 17.18 | 0.048 | 0.99 | 1.82 | 0.49 |
| P-Mt-C | 0.97 | 25.32 | 0.043 | 0.99 | 1.96 | 0.58 | |
| P-Mt-J | 0.99 | 30.67 | 0.064 | 0.99 | 2.94 | 0.59 | |
| Material | Anion | Pseudo-Second Order Model | Pseudo-First Order Model | |||
|---|---|---|---|---|---|---|
| P-Mt-W | Nitrate | 0.99 | 0.0250 | 39.78 | 0.26 | 0.0031 |
| Perchlorate | 0.99 | 0.0254 | 40.77 | 0.22 | 0.0179 | |
| Chromate | 0.99 | 0.0855 | 42.76 | 0.96 | 0.0027 | |
| P-Mt-C | Nitrate | 0.99 | 0.0251 | 76.84 | 0.21 | 0.007 |
| Perchlorate | 0.99 | 0.0212 | 92.66 | 0.94 | 0.0025 | |
| Chromate | 0.99 | 0.0666 | 94.92 | 0.32 | 0.0024 | |
| P-Mt-J | Nitrate | 0.99 | 0.233 | 11.78 | 0.10 | 0.0109 |
| Perchlorate | 0.99 | 0.0206 | 14.88 | 0.77 | 0.0021 | |
| Chromate | 0.99 | 0.0383 | 26.04 | 0.78 | 0.0033 | |
| Ion | ||||
|---|---|---|---|---|
| 0.200 | 0.033 | 1.8 | −280 | |
| 0.179 | 0.044 | 2.0 | −300 | |
| 0.181 | 0.043 | 2.0 | −340 | |
| 0.240 | 0.039 | 3.0 | −950 | |
| 0.230 | 0.043 | 3.1 | −1080 | |
| 0.178 | 0.076 | 4.0 | −1315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Huang, W.; Komarneni, S. Polymer-Clay Nanocomposites for the Uptake of Hazardous Anions. Nanomaterials 2024, 14, 467. https://doi.org/10.3390/nano14050467
Zhang H, Huang W, Komarneni S. Polymer-Clay Nanocomposites for the Uptake of Hazardous Anions. Nanomaterials. 2024; 14(5):467. https://doi.org/10.3390/nano14050467
Chicago/Turabian StyleZhang, Huaibin, Wenyan Huang, and Sridhar Komarneni. 2024. "Polymer-Clay Nanocomposites for the Uptake of Hazardous Anions" Nanomaterials 14, no. 5: 467. https://doi.org/10.3390/nano14050467




