Enhanced Thermoelectric Properties of P-Type Sn-Substituted Higher Manganese Silicides
Abstract
:1. Introduction
2. Materials and Methods
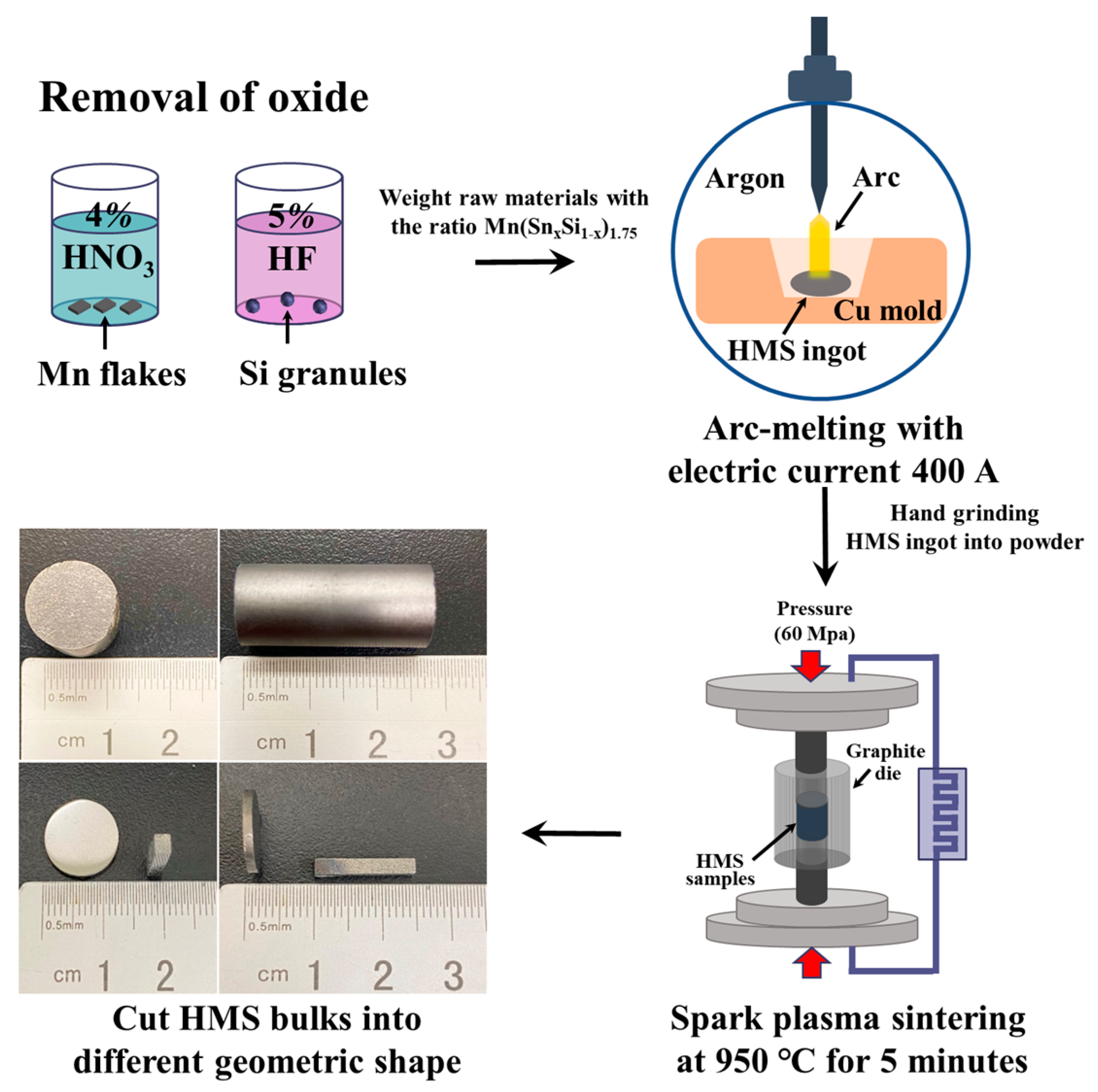
2.1. Synthesis of Bulk HMS
2.2. Material Characterizations
2.3. Measurements of Thermoelectric Properties
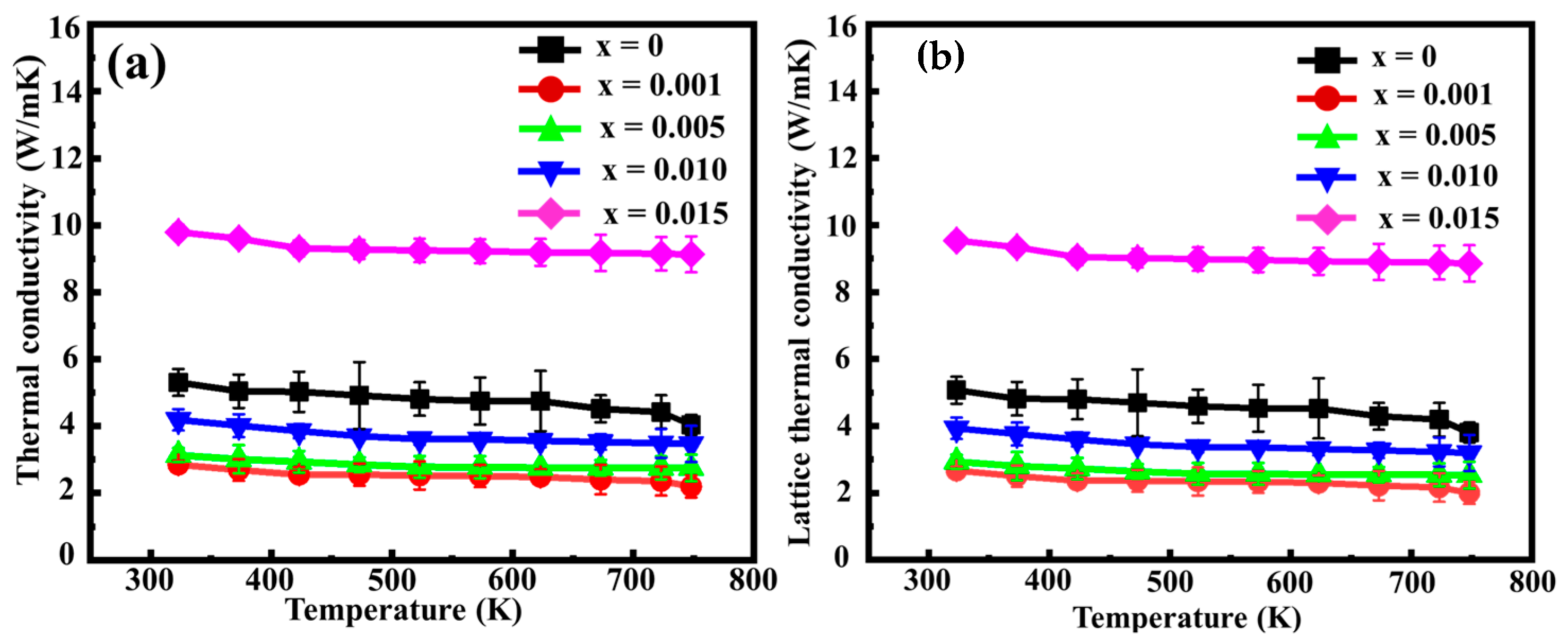
3. Results and Discussion
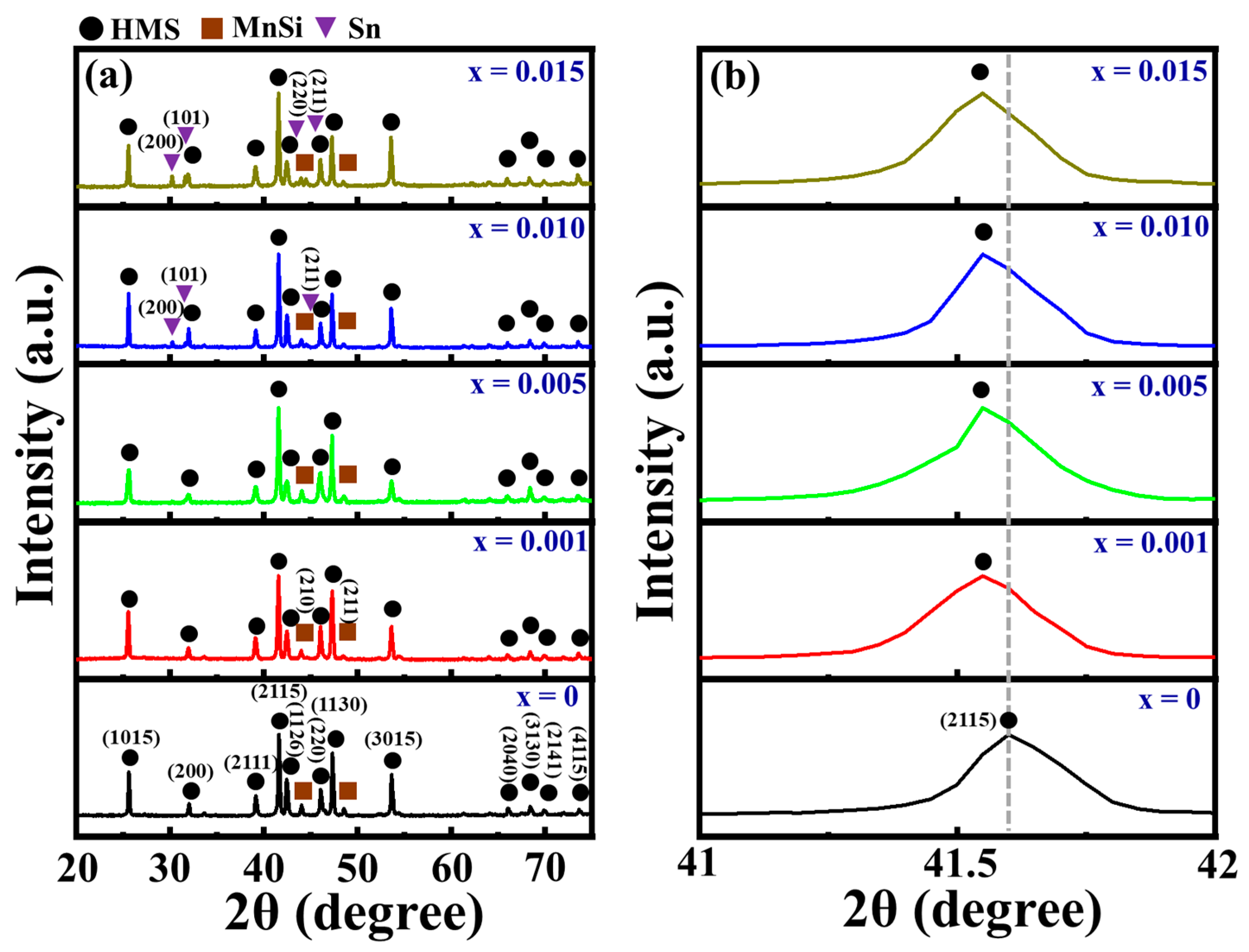
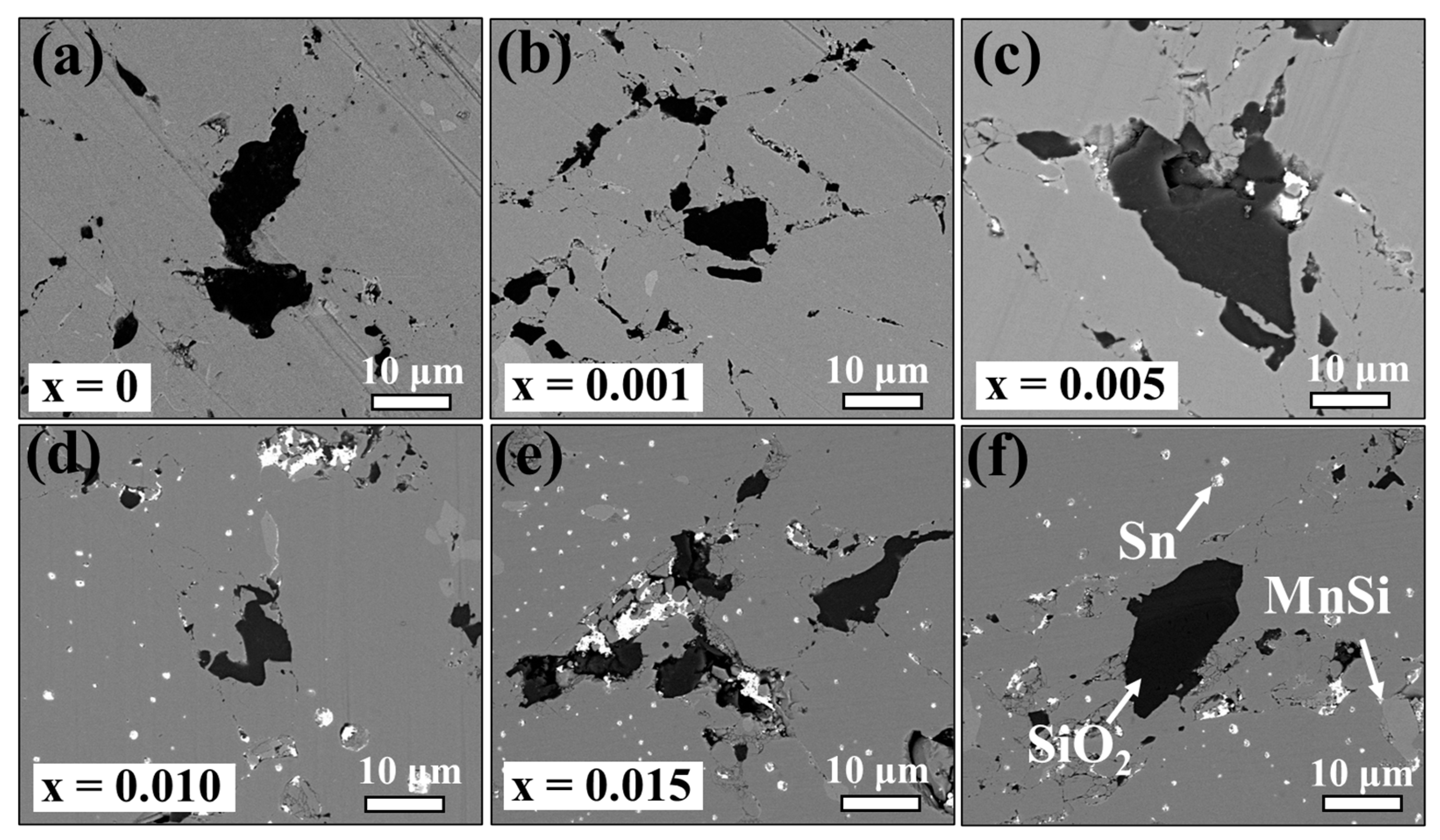
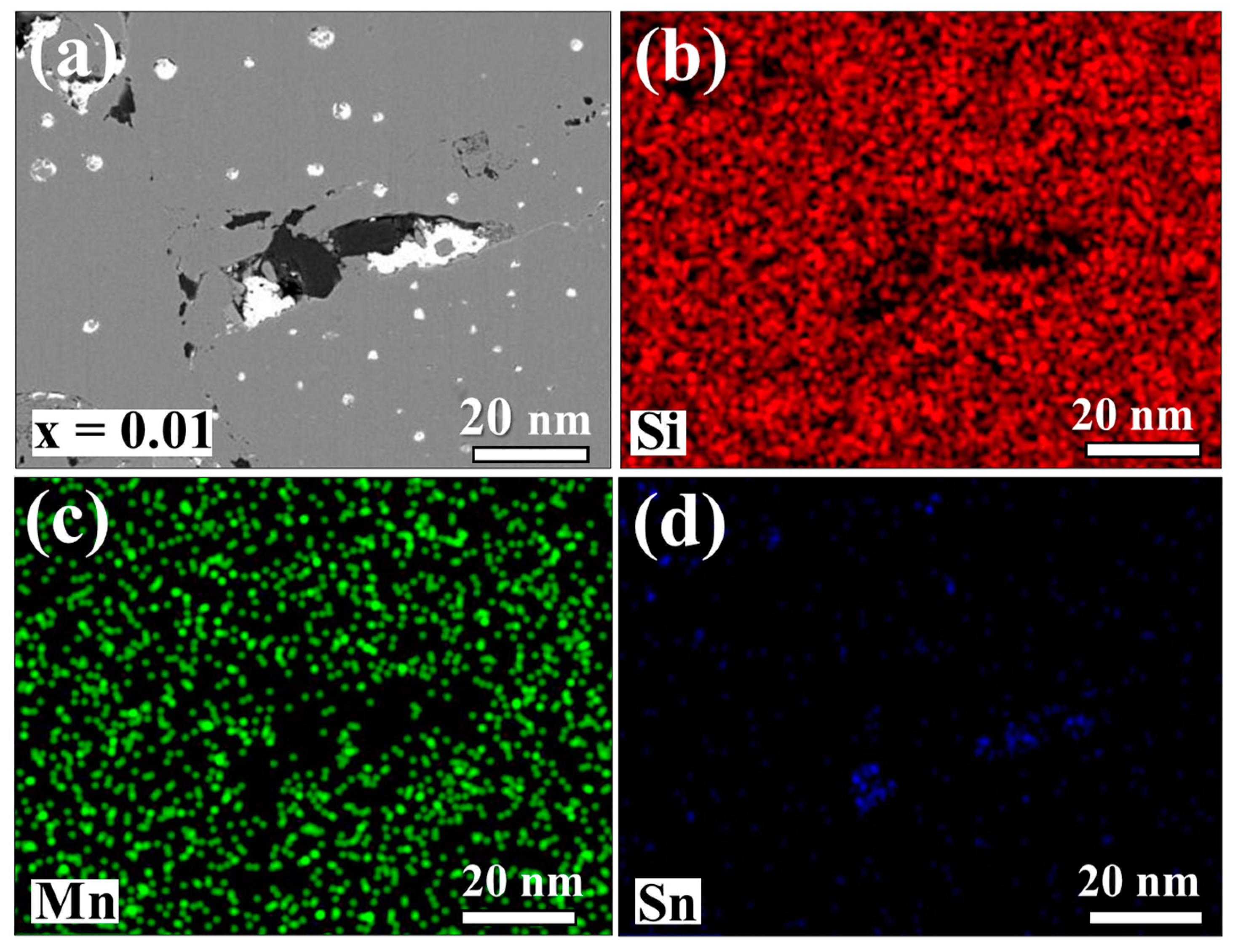
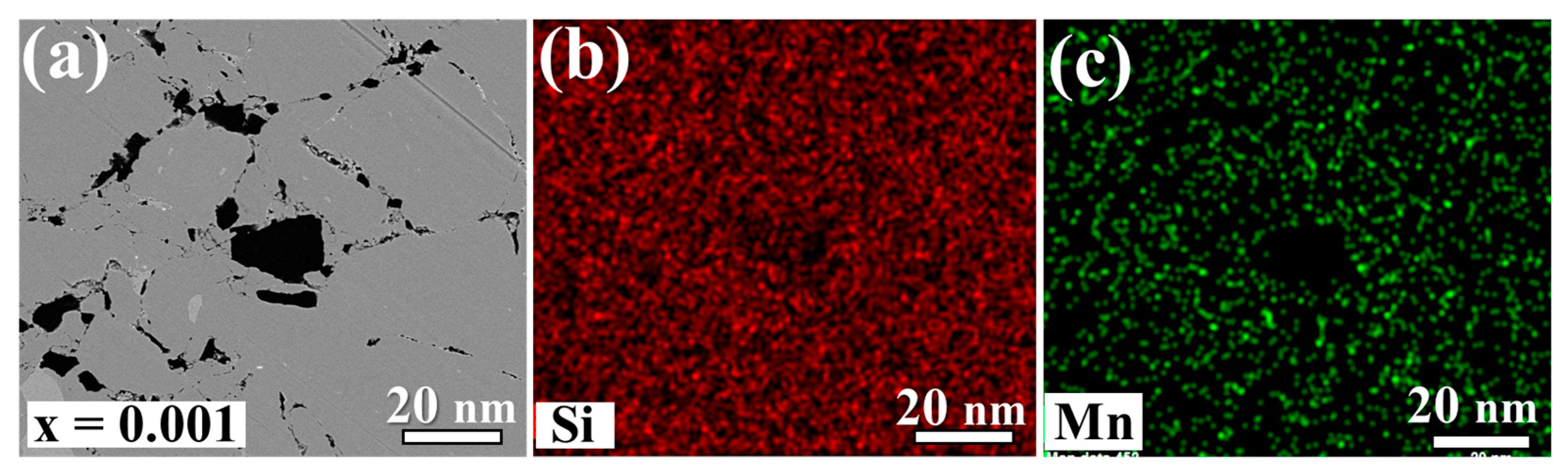
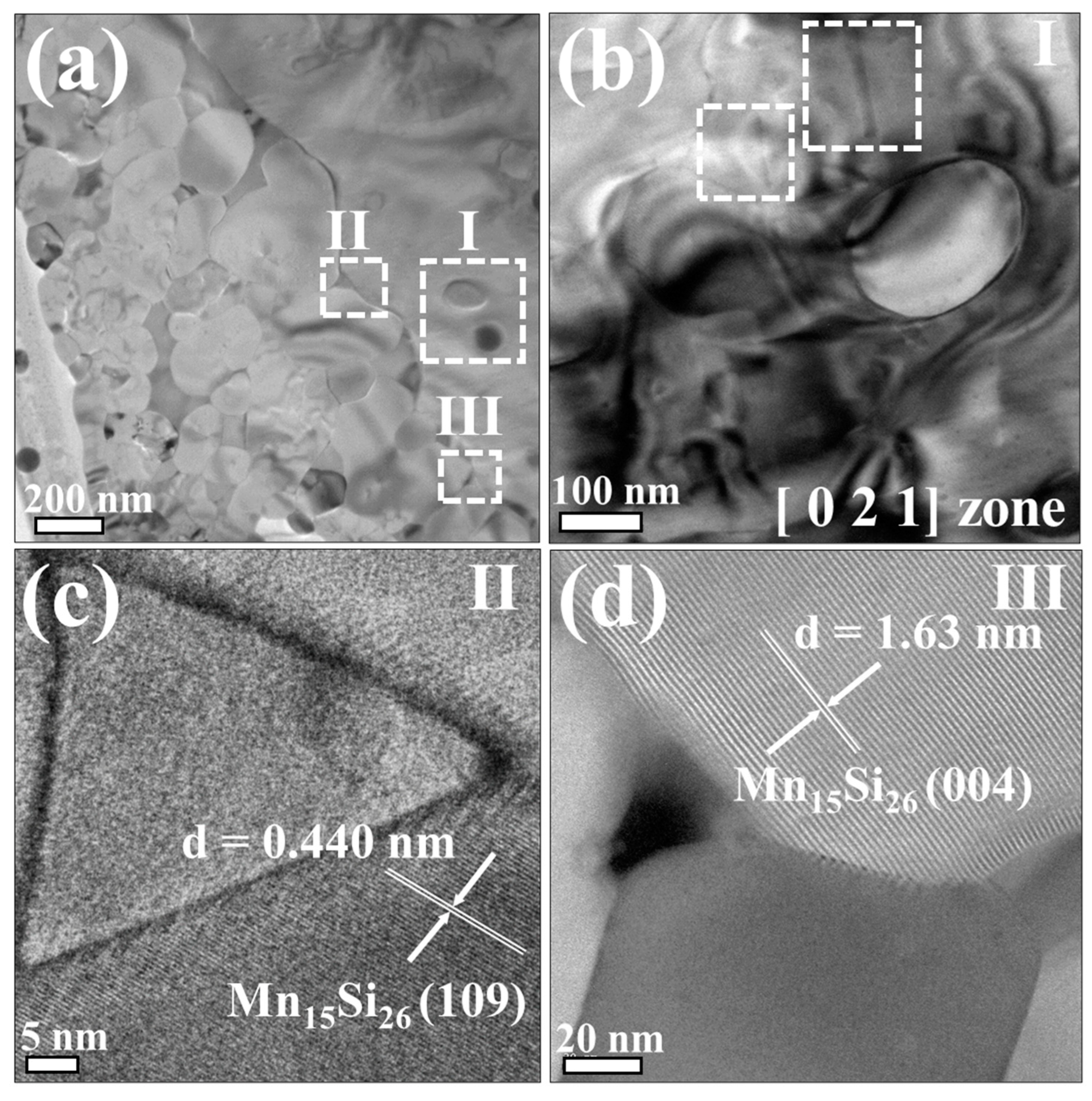
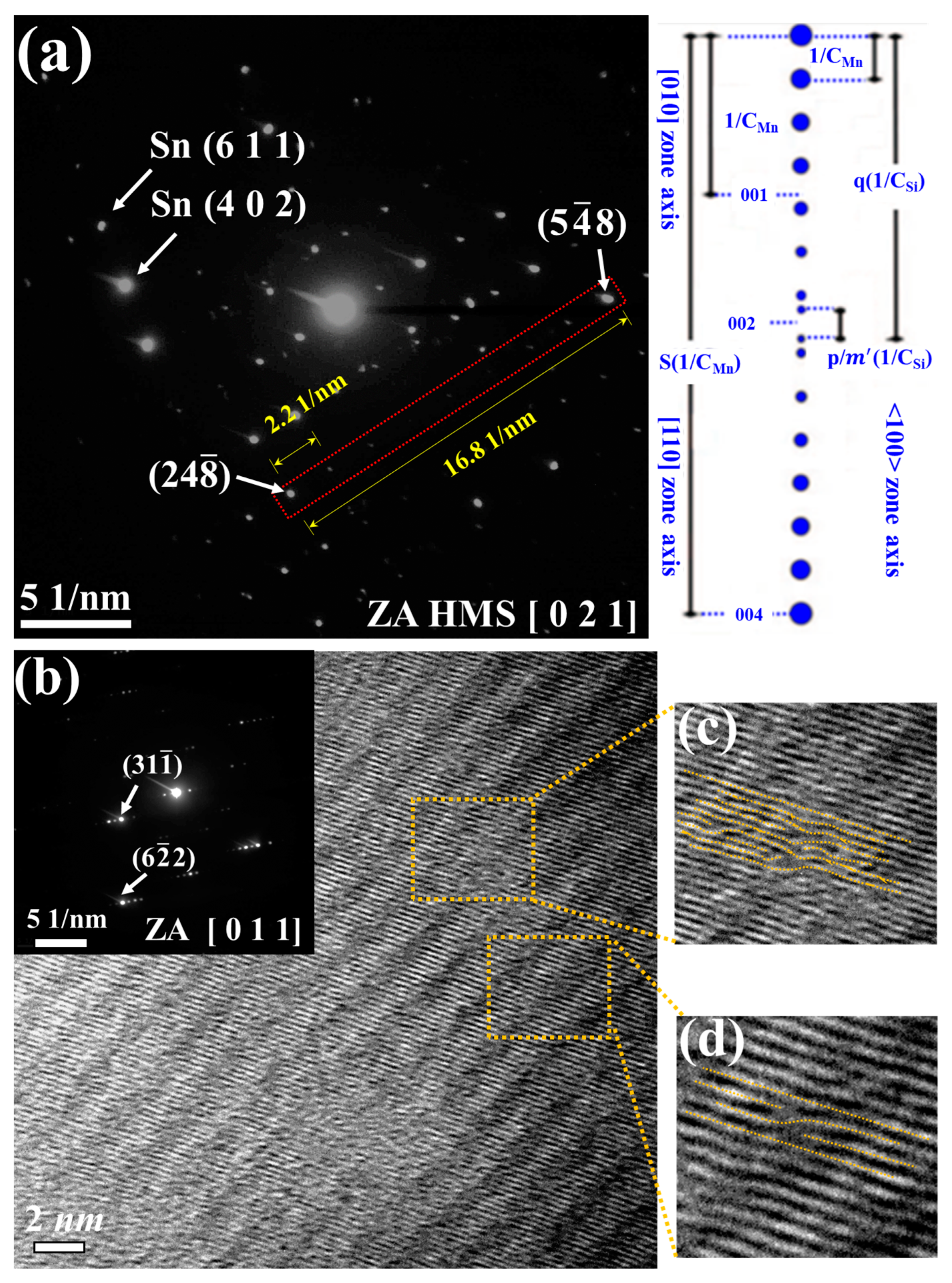
3.1. Material Characterizations of Sn-Substituted HMS
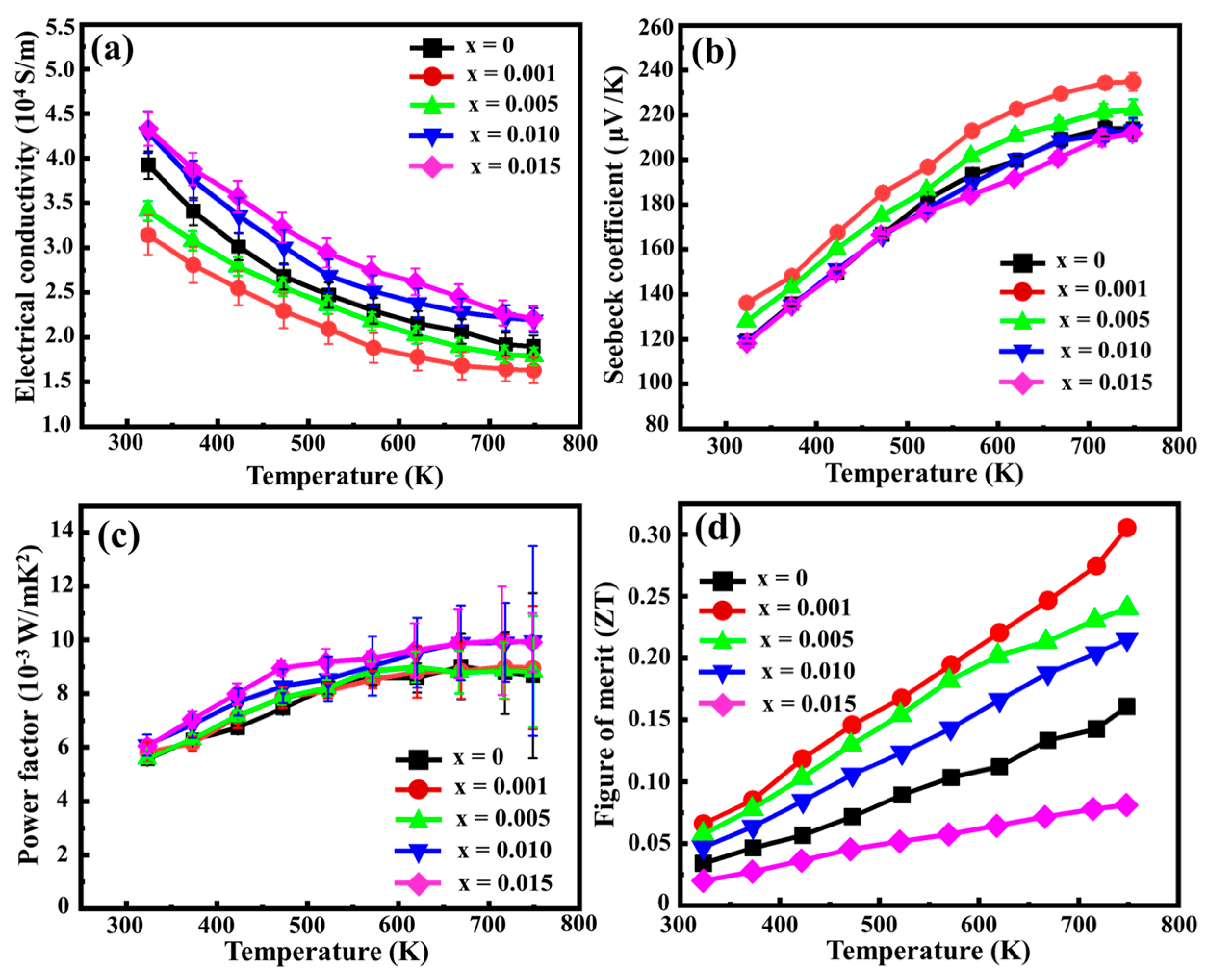
3.2. TE Properties of Sn-Substituted HMS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiSalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.C.; Minnich, A.; Yu, B.; Yan, X.A.; Wang, D.Z.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Agarwal, A.; Coutant, Z.A.; Hall, M.J.; Liu, J.; Liu, R.; Malhotra, A.; Norouzzadeh, P.; Öztürk, M.C.; Ramesh, V.P.; et al. Thermoelectric silicides: A review. Jpn. J. Appl. Phys. 2017, 56, 05DA04. [Google Scholar] [CrossRef]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl phases for thermoelectric devices. Dalton Trans. 2007, 21, 2099–2107. [Google Scholar] [CrossRef]
- binti Najwa, H.; Calvin, Y.B.N.; Sadri, R.; Lee, M.K.; Chang, L.J.; Tripathi, M.; Dalton, A.; Goh, B.T. Controlled physical properties and growth mechanism of manganese silicide nanorods. J. Alloys Compd. 2021, 851, 156693. [Google Scholar]
- Levinson, L.M. Investigation of the defect manganese silicide MnnSi2n−m. J. Solid State Chem. 1973, 6, 126–135. [Google Scholar] [CrossRef]
- Amatya, R.; Ram, R.J. Trend for Thermoelectric materials and their earth abundance. J. Electron. Mater. 2012, 41, 1011–1019. [Google Scholar] [CrossRef]
- Liu, W.-D.; Chen, Z.G.; Zou, J. Eco-Friendly Higher Manganese Silicide Thermoelectric Materials: Progress and Future Challenges. Adv. Energy Mater. 2018, 8, 180056. [Google Scholar] [CrossRef]
- Fredrickson, D.C.; Lee, S.; Hoffmann, R. The Nowotny chimney ladder phases: Whence the 14 electron rule? Inorg. Chem. 2004, 43, 6159–6167. [Google Scholar] [CrossRef]
- Nishida, I.; Masumoto, K.; Kawasumi, I.; Sakata, M. Striations and crystal structure of the matrix in the MnSi-Si alloy system. J. Less Common Met. 1980, 71, 293–301. [Google Scholar] [CrossRef]
- Kawasumi, I.; Sakata, M.; Nishida, I.; Masumoto, K. Crystal growth of manganese silicide, MnSi∼1.73 and semiconducting properties of Mn15Si26. J. Mater. Sci. 1981, 16, 355–366. [Google Scholar] [CrossRef]
- Akselrud, L.; Cardoso, G.R.; Wagner, R.M.; Yu, G. Disorder in the composite crystal structure of the manganesedisilicide’MnSi1. 73 from powder X-ray diffraction data. J. Acta Crystallogr. B 2015, 71, 707–712. [Google Scholar] [CrossRef]
- Van Smaalen, S. Incommensurate crystal structures. Crystallogr. Rev. 1995, 4, 79–202. [Google Scholar] [CrossRef]
- Migas, D.B.; VShaposhnikov, L.; Filonov, A.B.; Borisenko, V.E.; Dorozhkin, N.N. Ab initio study of the band structures of different phases of higher manganese silicides. Phys. Rev. B 2008, 77, 9. [Google Scholar] [CrossRef]
- Girard, S.N.; Chen, X.; Meng, F.; Pokhrel, A.; Zhou, J.S.; Shi, L.; Jin, S. Thermoelectric Properties of undoped high purity higher manganese silicides grown by chemical vapor transport. Chem. Mater. 2014, 26, 5097–5104. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef]
- Majumdar, A. Thermoelectricity in semiconductor nanostructures. Science 2004, 303, 777–778. [Google Scholar] [CrossRef]
- Wang, Y.S.; Huang, L.L.; Zhu, C.; Zhang, J.; Li, D.; Xin, H.X.; Danish, M.H.; Qin, X.Y. Simultaneously enhanced power factor and phonon scattering in Bi0.4Sb1.6Te3 alloy doped with germanium. Scr. Mater. 2018, 154, 118–122. [Google Scholar] [CrossRef]
- Rawat, P.K.; Paul, B.; Banerji, P. An alternative approach for optimal carrier concentration towards ideal thermoelectric performance. Phys. Status Solidi-Rapid Res. Lett. 2012, 6, 481–483. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554–557. [Google Scholar] [CrossRef]
- Zebarjadi, M.; Esfarjani, K.; Shakouri, A.; Bian, Z.X.; Bahk, J.H.; Zeng, G.H.; Bowers, J.; Lu, H.; Zide, J.; Gossard, A. Effect of nanoparticles on electron and thermoelectric transport. J. Electron. Mater. 2009, 38, 954–959. [Google Scholar] [CrossRef]
- Mikami, M.; Ozaki, K.; Takazawa, H.; Yamamoto, A.; Terazawa, Y.; Takeuchi, T. Effect of Ti substitution on thermoelectric properties of W-doped Heusler Fe2VAl Alloy. J. Electron. Mater. 2013, 42, 1801–1806. [Google Scholar] [CrossRef]
- Takagiwa, Y.; Maeda, R.; Ohhashi, S.; Tsai, A.P. Reduction of thermal conductivity for icosahedral Al-Cu-Fe quasicrystal through heavy element substitution. Materials 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, I.; Fedorov, M.I.; Zaitsev, V.K.; Solomkin, F.Y.; Eremin, I.S.; Samunin, A.Y.; Mukoujima, M.; Sano, S.; Tsuji, T. Effects of Ge doping on micromorphology of MnSi in MnSi similar to 1.7 and on their thermoelectric transport properties. Jpn. J. Appl. Phys. Part 1-Regul. Pap. Brief Commun. Rev. Pap. 2005, 44, 8562–8570. [Google Scholar] [CrossRef]
- Zhou, A.J.; Zhu, T.J.; Zhao, X.B.; Yang, S.H.; Dasgupta, T.; Stiewe, C.; Hassdorf, R.; Mueller, E. Improved thermoelectric performance of higher manganese silicides with Ge additions. J. Electron. Mater. 2010, 39, 2002–2007. [Google Scholar] [CrossRef]
- Luo, W.H.; Li, H.; Fu, F.; Hao, W.; Tang, X.F. Improved thermoelectric properties of Al-doped higher manganese silicide prepared by a rapid solidification method. J. Electron. Mater. 2011, 40, 1233–1237. [Google Scholar] [CrossRef]
- Chen, X.; Girard, S.N.; Meng, F.; Lara-Curzio, E.; Jin, S.; Goodenough, J.B.; Zhou, J.S.; Shi, L. Approaching the minimum thermal conductivity in rhenium-substituted higher manganese silicides. Adv. Energy Mater. 2014, 4, 10. [Google Scholar] [CrossRef]
- Homma, T.; Kamata, T.; Saito, N.; Ghodke, S.; Takeuchi, T. Effects of Re substitution for Mn on microstructures and properties in Re-substituted higher manganese silicide thermoelectric material. J. Alloys Compd. 2019, 776, 8–15. [Google Scholar] [CrossRef]
- Yang, J.; Meisner, G.P.; Chen, L. Strain field fluctuation effects on lattice thermal conductivity of ZrNiSn-based thermoelectric compounds. Appl. Phys. Lett. 2004, 85, 1140–1142. [Google Scholar] [CrossRef]
- Liu, W.D.; Shi, X.L.; Moshwan, R.; Sun, Q.; Yang, L.; Chen, Z.G.; Zou, J. Effectively restricting MnSi precipitates for simultaneously enhancing the Seebeck coefficient and electrical conductivity in higher manganese silicide. J. Mater. Chem. C 2019, 7, 7212–7218. [Google Scholar] [CrossRef]
- Chakraborti, N.; Lukas, H. Calculation and optimization of the Mn-Si phase diagram. Calphad 1989, 13, 293–300. [Google Scholar] [CrossRef]
- Higgins, J.M.; Schmitt, A.L.; Guzei, I.A.; Jin, S. Higher manganese silicide nanowires of nowotny chimney ladder phase. J. Am. Chem. Soc. 2008, 130, 16086–16094. [Google Scholar] [CrossRef]
- Ye, H.; Amelinckx, S. High-resolution electron microscopic study of manganese silicides MnSi2−x. J. Solid State Chem. 1986, 61, 8–39. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Fredrickson, D.C.; Lee, S.; Hoffmann, R.; Lin, J.H. The Nowotny chimney ladder phases: Following the c(pseudo) clue toward an explanation of the 14 electron rule. Inorg. Chem. 2004, 43, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, Z.M.; Kim, H.S.; Wang, H.; Snyder, G.J. Band gap estimation from temperature dependent Seebeck measurement-deviations from the 2e vertical bar S vertical bar T-max(max) relation. Appl. Phys. Lett. 2015, 106, 5. [Google Scholar] [CrossRef]
- Thesberg, M.; Kosina, H.; Neophytou, N. On the Lorenz number of multiband materials. Phys. Rev. B 2017, 95, 14. [Google Scholar] [CrossRef]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.L.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 5. [Google Scholar] [CrossRef]
- Chen, X.; Weathers, A.; Salta, D.; Zhang, L.B.; Zhou, J.S.; Goodenough, J.B.; Shi, L. Effects of (Al,Ge) double doping on the thermoelectric properties of higher manganese silicides. J. Appl. Phys. 2013, 114, 10. [Google Scholar] [CrossRef]
| x | a (Å) | c (Å) | Density (g/cm3) |
|---|---|---|---|
| 0 | 5.55 | 66.9 | 5.07 |
| 0.001 | 5.56 | 67.1 | 4.96 |
| 0.005 | 5.56 | 67.1 | 4.72 |
| 0.01 | 5.56 | 67.1 | 4.79 |
| 0.015 | 5.56 | 67.1 | 4.84 |
| x | Compositions of phase | Hole concentration (1021 cm−3) | Mobility (cm2 V−1 s−1) | |||
|---|---|---|---|---|---|---|
| HMS | MnSi | Sn | SiO2 | |||
| 0 | 92.8% | 7.2% | N.D. | N.D. | 1.55 | 1.47 |
| 0.001 | 94.7% | 5.3% | N.D. | N.D. | 1.60 | 1.37 |
| 0.005 | 94.9% | 5.1% | N.D. | N.D. | 1.65 | 1.35 |
| 0.01 | 94.2% | 5.1% | 0.7% | N.D. | 1.72 | 1.31 |
| 0.015 | 93.9% | 4.4% | 1.7% | N.D. | 1.75 | 1.29 |
| x | σ (104 S/m) | S (μV/K) | ktotal (W/mK) | kL (W/mK) | ZT |
|---|---|---|---|---|---|
| 0 | 1.896 | 213.9 | 4.0 | 3.8 | 0.16 |
| 0.001 | 1.627 | 234.8 | 2.2 | 2.0 | 0.31 |
| 0.005 | 1.786 | 222.3 | 2.7 | 2.5 | 0.24 |
| 0.01 | 2.189 | 213.4 | 3.5 | 3.2 | 0.22 |
| 0.015 | 2.211 | 211.7 | 9.1 | 8.9 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.-X.; Yang, S.-R.; Tsao, I.-Y.; Wardhana, B.S.; Hsueh, S.-F.; Jang, J.S.-C.; Hsin, C.-L.; Lee, S.-W. Enhanced Thermoelectric Properties of P-Type Sn-Substituted Higher Manganese Silicides. Nanomaterials 2024, 14, 494. https://doi.org/10.3390/nano14060494
Jiang M-X, Yang S-R, Tsao I-Y, Wardhana BS, Hsueh S-F, Jang JS-C, Hsin C-L, Lee S-W. Enhanced Thermoelectric Properties of P-Type Sn-Substituted Higher Manganese Silicides. Nanomaterials. 2024; 14(6):494. https://doi.org/10.3390/nano14060494
Chicago/Turabian StyleJiang, Ming-Xun, Sang-Ren Yang, I-Yu Tsao, Bayu Satriya Wardhana, Shih-Feng Hsueh, Jason Shian-Ching Jang, Cheng-Lun Hsin, and Sheng-Wei Lee. 2024. "Enhanced Thermoelectric Properties of P-Type Sn-Substituted Higher Manganese Silicides" Nanomaterials 14, no. 6: 494. https://doi.org/10.3390/nano14060494





