Effects of Polyether Amine Canopy Structure on Heavy Metal Ions Adsorption of Magnetic Solvent-Free Nanofluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
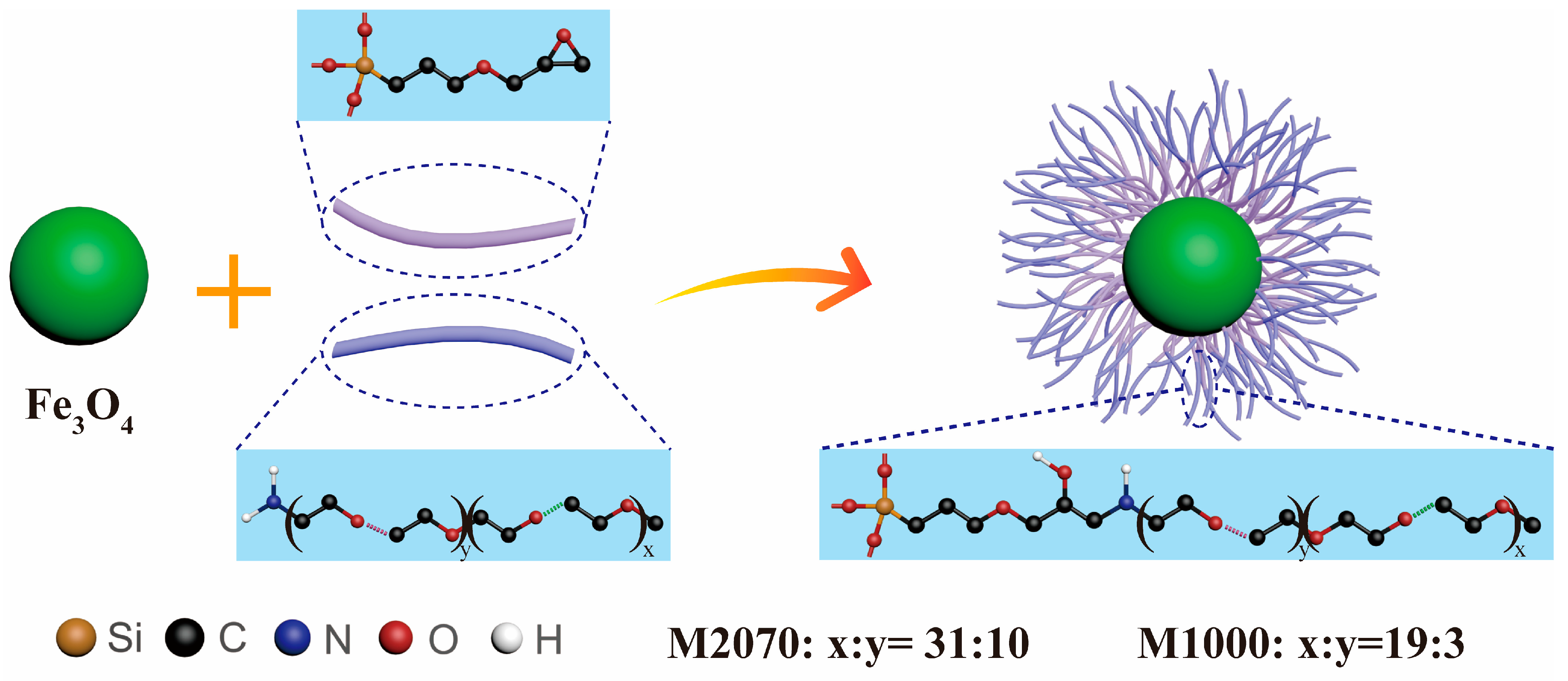
2.2. Preparation of Magnetic Solvent-Free Nanofluid
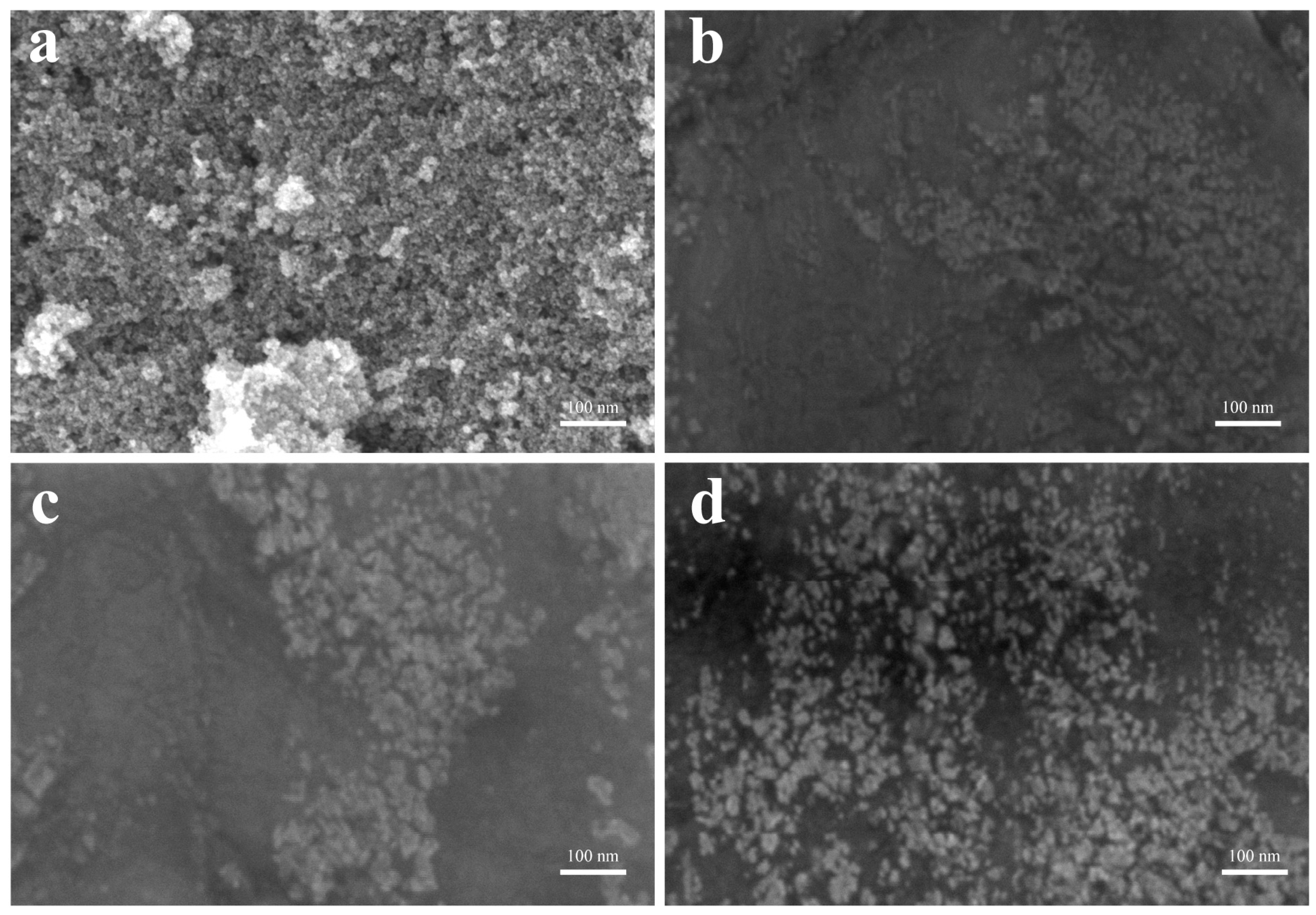
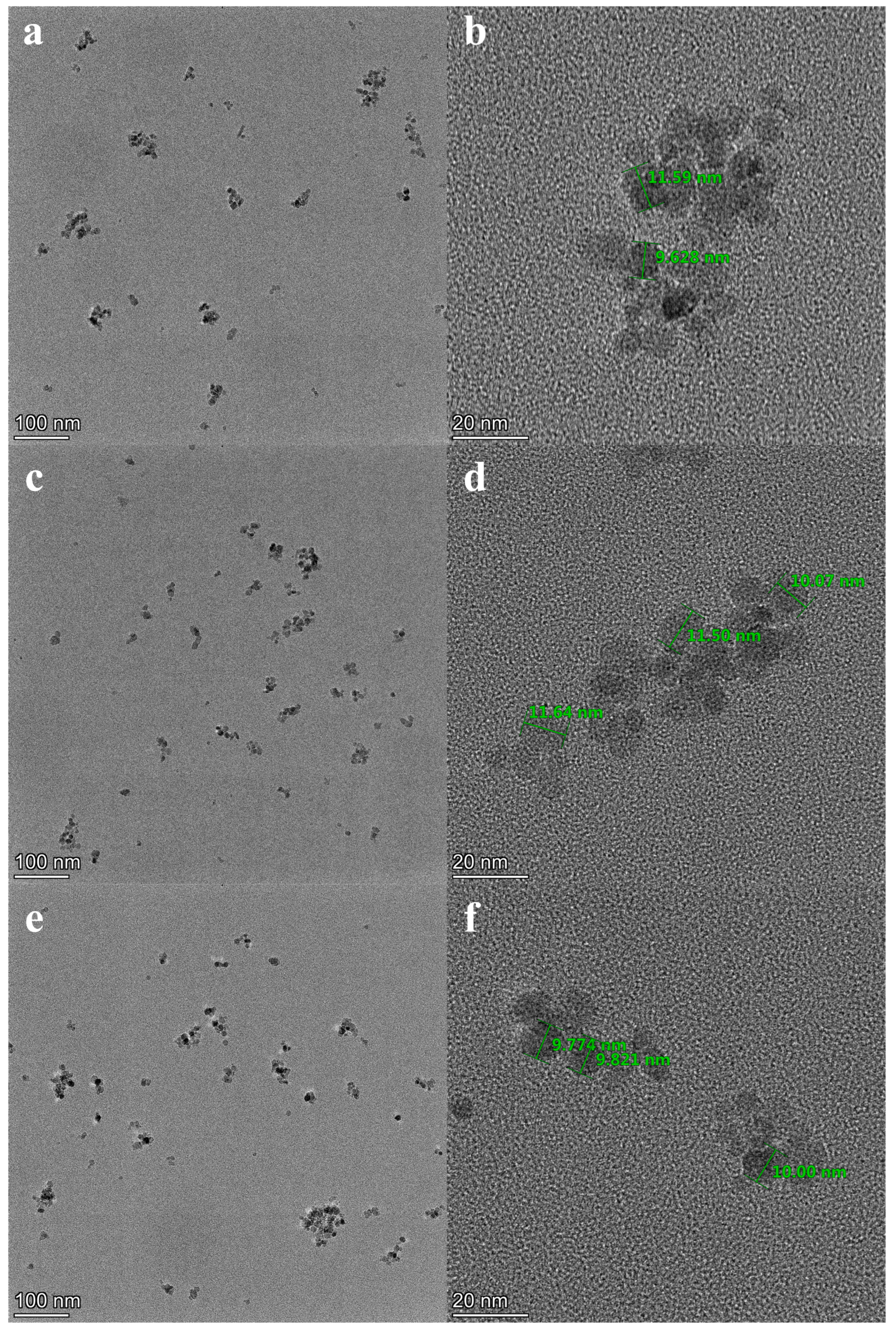
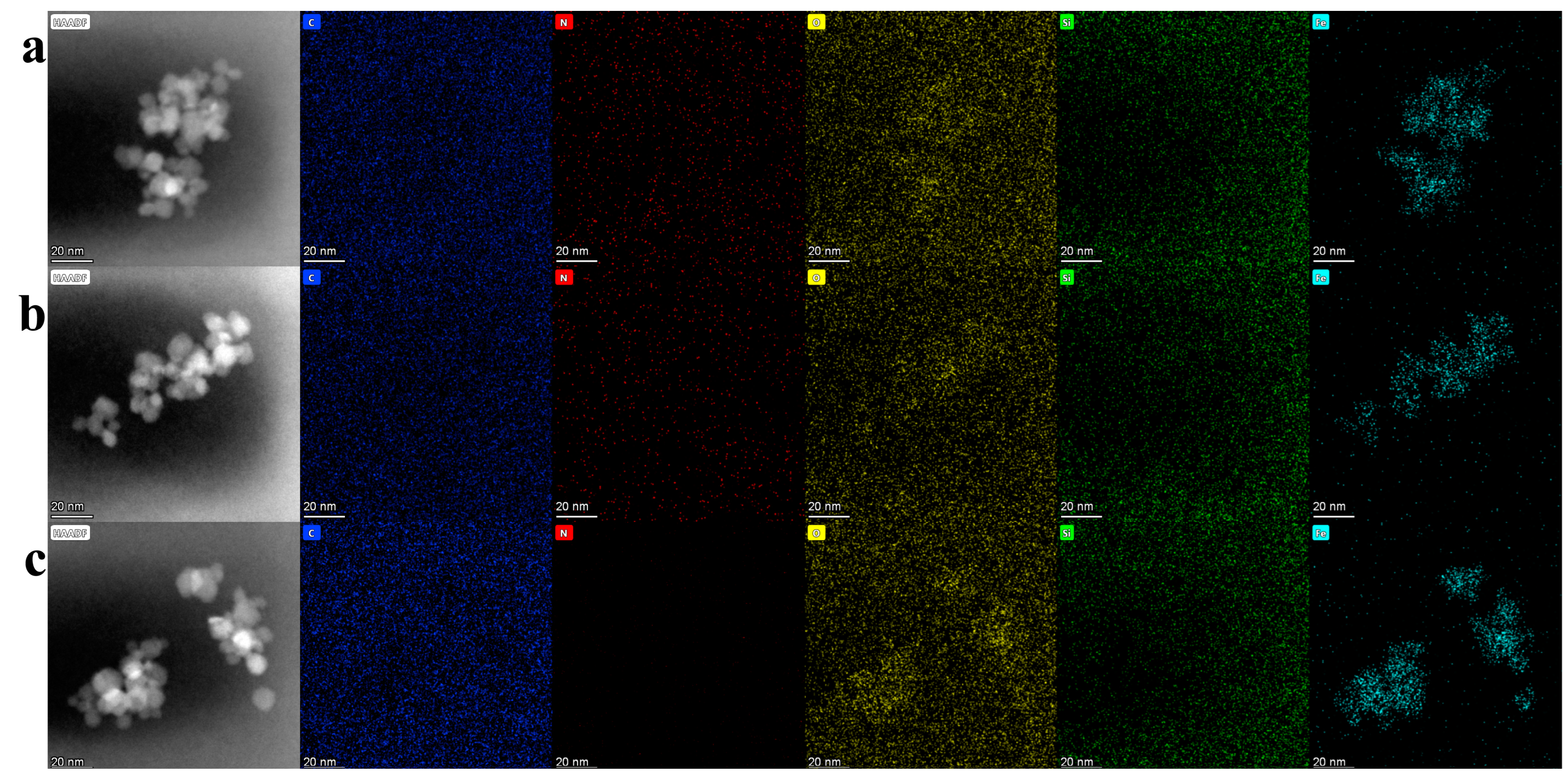
2.3. Characterization
2.4. Adsorption Equilibrium and Kinetics
2.5. Recyclability Test
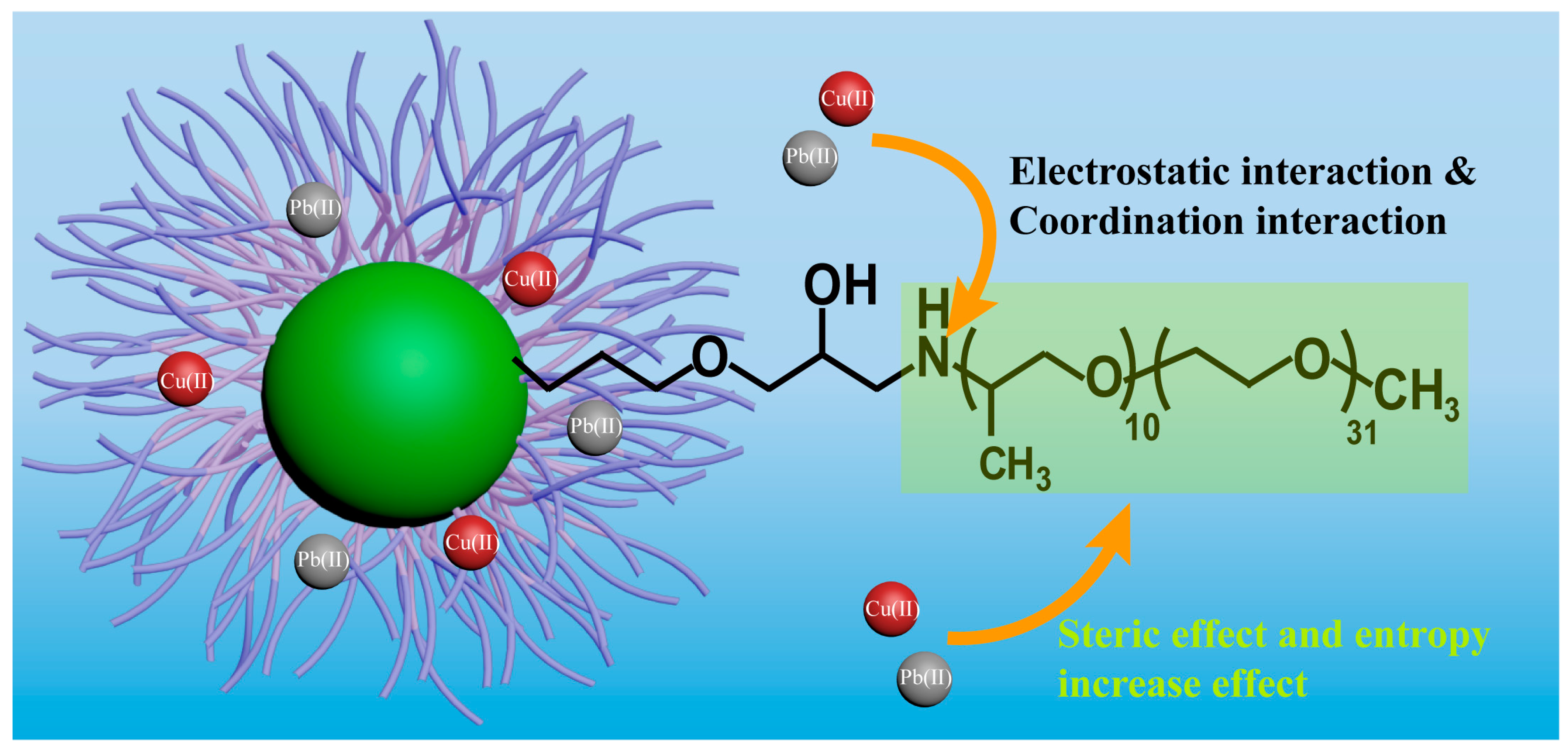
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Jia, S.; Yuan, X.; Ding, L.; Ai, T.; Yuan, K.; Wang, W.; Magagnin, L.; Jiang, Z. High-efficiency removal of Pb(II) and Cu(II) by amidoxime functionalized silica aerogels: Preparation, adsorption mechanisms and environmental impacts analysis. Sep. Purif. Technol. 2024, 335, 126079. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, C.; Cui, W.; He, F.; Zhang, J.; Zhang, T.C.; He, C. Adsorption of Pb2+ and Cu2+ ions on the CS2-modified alkaline lignin. Chem. Eng. J. 2020, 391, 123581. [Google Scholar] [CrossRef]
- Kim, J.G.; Ku, J.; Jung, J.; Park, Y.S.; Choi, G.H.; Hwang, S.S.; Lee, J.H.; Lee, A.S. Ion-exchangeable and sorptive reinforced membranes for efficient electrochemical removal of heavy metal ions in wastewater. J. Clean. Prod. 2024, 438, 140779. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Ruder, L.R.; Dawson, R.; Ogden, M.D. Ion exchange removal of Cu(II), Fe(II), Pb(II) and Zn(II) from acid extracted sewage sludge—Resin screening in weak acid media. Water Res. 2019, 158, 257–267. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Fagundez, J.L.; Netto, M.S.; Dotto, G.L.; Salau, N.P. A new method of developing ANN-isotherm hybrid models for the determination of thermodynamic parameters in the adsorption of ions Ag+, Co2+ and Cu2+ onto zeolites ZSM-5, HY, and 4A. J. Environ. Chem. Eng. 2021, 9, 106126. [Google Scholar] [CrossRef]
- Tang, W.; Zanli, B.L.; Chen, J. O/N/P-doped biochar induced to enhance adsorption of sulfonamide with coexisting Cu2+/Cr (VI) by air pre-oxidation. Bioresour. Technol. 2021, 341, 125794. [Google Scholar] [CrossRef]
- Phuengprasop, T.; Sittiwong, J.; Unob, F. Removal of heavy metal ions by iron oxide coated sewage sludge. J. Hazard. Mater. 2011, 186, 502–507. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef]
- Hai, B.; Wu, J.; Chen, X.; Protasiewicz, J.D.; Scherson, D.A. Metal-ion adsorption on carboxyl-bearing self-assembled monolayers covalently bound to magnetic nanoparticles. Langmuir 2005, 21, 3104–3105. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, S.H.; Qiao, Z.A.; Mahurin, S.M.; Chen, J.; Fang, Y.; Wan, S.; Nelson, K.; Zhang, P.; Dai, S. Porous liquids: A promising class of media for gas separation. Angew. Chem. Int. Ed. 2014, 54, 932–936. [Google Scholar] [CrossRef]
- Park, Y.; Shin, D.; Jang, Y.N.; Park, A.H. CO2 capture capacity and swelling measurements of liquid-like nanoparticle organic hybrid materials via attenuated total reflectance fourier transform infrared spectroscopy. J. Chem. Eng. Data 2011, 57, 40–45. [Google Scholar] [CrossRef]
- Lei, Y.; Xiong, C.; Dong, L.; Guo, H.; Su, X.; Yao, J.; You, Y.; Tian, D.; Shang, X. Ionic liquid of ultralong carbon nanotubes. Small 2007, 3, 1889–1893. [Google Scholar] [CrossRef]
- Moganty, S.S.; Srivastava, S.; Lu, Y.; Schaefer, J.L.; Rizvi, S.A.; Archer, L.A. Ionic liquid-tethered nanoparticle suspensions: A novel class of ionogels. Chem. Mater. 2012, 24, 1386–1392. [Google Scholar] [CrossRef]
- Gu, S.Y.; Gao, X.F.; Zhang, Y.H. Synthesis and characterization of solvent-free ionic molybdenum disulphide (MoS2) nanofluids. Mater. Chem. Phys. 2015, 149–150, 587–593. [Google Scholar] [CrossRef]
- Shirazi, N.A.; Rahimpour, M.R. Chapter 20—Environmental and industrialization challenges of nanofluids. In Nanofluids and Mass Transfer; Rahimpour, M.R., Makarem, M.A., Kiani, M.R., Sedghamiz, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 467–481. [Google Scholar]
- Mehrali, M.; Sadeghinezhad, E.; Akhiani, A.R.; Tahan Latibari, S.; Metselaar, H.S.; Kherbeet, A.S.; Mehrali, M. Heat transfer and entropy generation analysis of hybrid graphene/Fe3O4 ferro-nanofluid flow under the influence of a magnetic field. Powder Technol. 2017, 308, 149–157. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, R.; Wu, F.; Li, D.; Wang, N.; Zhang, A. A functional liquid-like multiwalled carbon nanotube derivative in the absence of solvent and its application in nanocomposites. RSC Adv. 2014, 4, 30004–30012. [Google Scholar] [CrossRef]
- Bai, H.; Zheng, Y.; Wang, T.; Peng, N. Magnetic solvent-free nanofluid based on Fe3O4/polyaniline nanoparticles and its adjustable electric conductivity. J. Mater. Chem. A 2016, 4, 14392–14399. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, M.; Hao, Y. High efficient removal of Pb(II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2012, 191, 104–111. [Google Scholar] [CrossRef]
- Xin, X.; Wei, Q.; Yang, J.; Yan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Tan, Z.; Peng, H.; Liu, H.; Wang, L.; Chen, J.; Lu, X. Facile preparation of EDTA-functionalized chitosan magnetic adsorbent for removal of Pb(II). J. Appl. Polym. Sci. 2015, 132, 42384. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Gharayebi, Y.; Shameli, K.; Nadi, B. Fabrication and characterization of SiO2/(3-aminopropyl)triethoxysilane-coated magnetite nanoparticles for lead(II) removal from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2013, 23, 599–607. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Q.; Shi, J.; Zheng, Y.; Wang, D.; Zhang, J.; Liu, S.; Fu, Z. A novel magnetic loading porous liquid absorbent for removal of Cu(II) and Pb(II) from the aqueous solution. Sep. Purif. Technol. 2023, 314, 123605. [Google Scholar] [CrossRef]
- Cuenca, J.A.; Bugler, K.; Taylor, S.; Morgan, D.; Williams, P.; Bauer, J.; Porch, A. Study of the magnetite to maghemite transition using microwave permittivity and permeability measurements. J. Phys. Condens. Matter 2016, 28, 106002. [Google Scholar] [CrossRef]
- Hawn, D.D.; DeKoven, B.M. Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surf. Interface Anal. 2004, 10, 63–74. [Google Scholar] [CrossRef]
- Muhler, M.; Schlögl, R.; Ertl, G. The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene 2. Surface chemistry of the active phase. J. Catal. 1992, 138, 413–444. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Buchwald, T.; Siwińska-Ciesielczyk, K.; Strzemiecka, B.; Jesionowski, T.; Voelkel, A. Novel highly efficient ionic liquid-functionalized silica for toxic metals removal. Sep. Purif. Technol. 2021, 265, 118483. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Esmailzadeh, F.; Asgharnasl, S.; Ganjali, F.; Taheri-Ledari, R.; Maleki, A. Efficient removal of Pb(II)/Cu(II) from aqueous samples by a guanidine-functionalized SBA-15/Fe3O4. Sep. Purif. Technol. 2022, 291, 120956. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, G.; Zhu, J.; Wu, Z. Adsorption of heavy metal ions in water by surface functionalized magnetic composites: A review. Environ. Sci. Water Res. Technol. 2022, 8, 907–925. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, S.; Li, S.; Xiao, Z.; Song, Y.; An, Q.; Tian, G. Pb(II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem. Eng. J. 2013, 215–216, 461–471. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.; Liu, P.; Fang, W.; Geng, J. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr(VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef]
- Rajahmundry, G.K.; Garlapati, C.; Kumar, P.S.; Alwi, R.S.; Vo, D.-V.-N. Statistical analysis of adsorption isotherm models and its appropriate selection. Chemosphere 2021, 276, 130176. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, W.; Guo, M.; Wang, Y.; Jia, J.; Hu, Z. Preparation of cotton-based fibrous adsorbents for the removal of heavy metal ions. Carbohydr. Polym. 2019, 225, 115218. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, D.; Wang, X.; Yu, S.; Wang, H.; Wen, T.; Song, G.; Yu, Z.; Wang, X. Highly efficient Pb(II) and Cu(II) removal using hollow Fe3O4@PDA nanoparticles with excellent application capability and reusability. Inorg. Chem. Front. 2018, 5, 2174–2182. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Sun, J.; Wu, G.; Wang, J.; Zhang, D. Synthesis of pyridyl Schiff base functionalized SBA-15 mesoporous silica for the removal of Cu(II) and Pb(II) from aqueous solution. J. Solgel Sci. Technol. 2019, 94, 658–670. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, Y.; Zhang, T.A. Preparation of polyamidoamine dendrimer-functionalized chitosan beads for the removal of Ag(I), Cu(II), and Pb(II). Int. J. Biol. Macromol. 2023, 242, 124543. [Google Scholar] [CrossRef]
- Güçoğlu, M.; Şatıroğlu, N. Adsorption of Pb(II), Cu(II), Cd(II), Ni(II), and Co(II) ions by newly synthesized 2-(2′-Hydroxyphenyl)Benzothiazole-functionalized silica. J. Mol. Liq. 2022, 348, 118388. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Shen, M.; Tan, X.; Ding, Y.; Jiang, Z.; Wang, C. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal. Chem. Eng. J. 2015, 268, 290–299. [Google Scholar] [CrossRef]
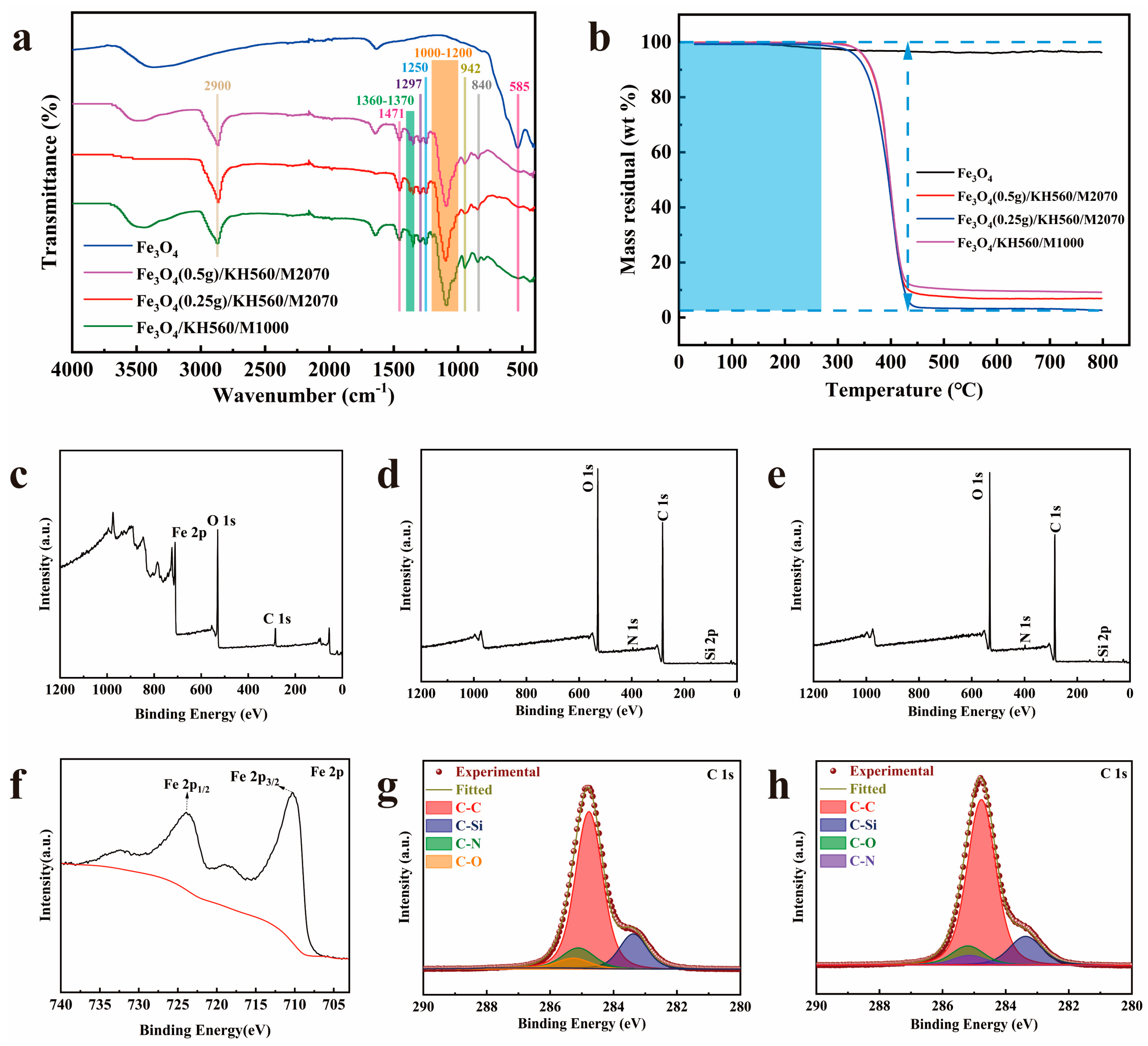
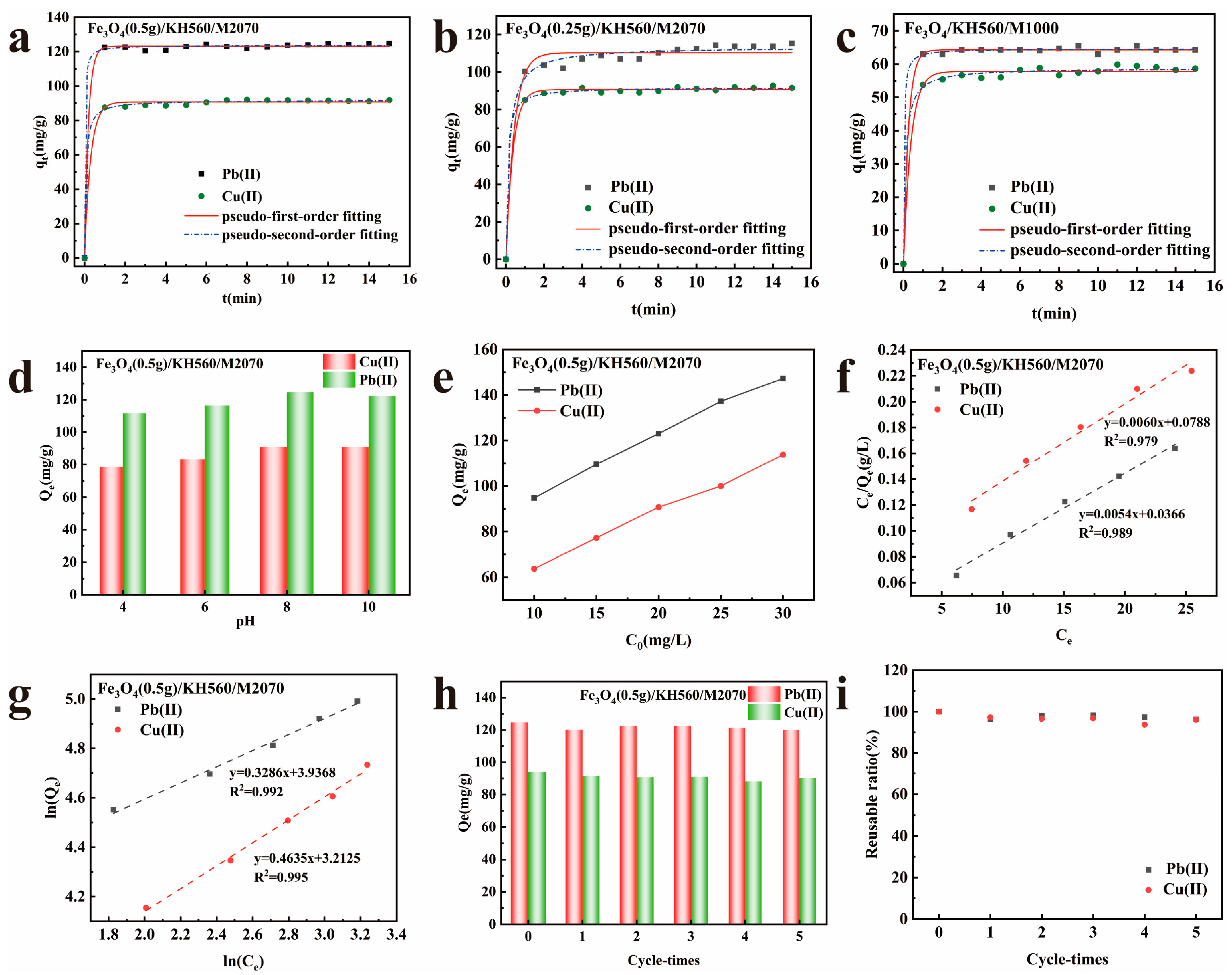
| Samples | ωc (%) | ω0 (%) | Mc | M0 | n |
|---|---|---|---|---|---|
| Fe3O4(0.5 g)/KH560/M2070 | 6.9 | 93.1 | 232 | 2236 | 1.40 |
| Fe3O4(0.25 g)/KH560/M2070 | 3.2 | 96.8 | 232 | 2236 | 3.14 |
| Fe3O4/KH560/M1000 | 9.7 | 90.3 | 232 | 1236 | 1.75 |
| Samples | Cu(II) | Pb(II) |
|---|---|---|
| Fe3O4(0.5 g)/KH560/M2070 | 3.45 | 1.45 |
| Fe3O4(0.25 g)/KH560/M2070 | 3.35 | 1.29 |
| Fe3O4/KH560/M1000 | 1.28 | 0.43 |
| Adsorbents | Models | Parameters | Cu(II) | Pb(II) |
|---|---|---|---|---|
| Fe3O4(0.5 g)/KH560/M2070 | PFO | Qe (mg g−1) | 90.70 | 123.11 |
| K1 (min−1) | 3.297 | 5.191 | ||
| R2 | 0.997 | 0.998 | ||
| PSO | Qe (mg g−1) | 91.70 | 123.56 | |
| K2 (g mg−1 min−1) | 0.175 | 0.438 | ||
| R2 | 0.998 | 0.999 | ||
| Fe3O4(0.25 g)/KH560/M2070 | PFO | Qe (mg g−1) | 90.66 | 110.24 |
| K1 (min−1) | 2.748 | 2.279 | ||
| R2 | 0.997 | 0.981 | ||
| PSO | Qe (mg g−1) | 91.80 | 113.39 | |
| K2 (g mg−1 min−1) | 0.137 | 0.052 | ||
| R2 | 0.998 | 0.992 | ||
| Fe3O4/KH560/M1000 | PFO | Qe (mg g−1) | 57.81 | 64.25 |
| K1 (min−1) | 2.606 | 3.898 | ||
| R2 | 0.992 | 0.998 | ||
| PSO | Qe (mg g−1) | 58.83 | 64.56 | |
| K2 (g mg−1 min−1) | 0.158 | 0.553 | ||
| R2 | 0.996 | 0.999 |
| Adsorbate | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | KF (L·mg−1) | n | R2 | |
| Cu(II) | 166.67 | 0.076 | 0.979 | 24.8 | 2.157 | 0.995 |
| Pb(II) | 185.19 | 0.148 | 0.989 | 51.3 | 3.043 | 0.992 |
| Adsorbents | Qm (mg·g−1)/Cu(II) | Qm (mg·g−1)/Pb(II) | Ref. |
|---|---|---|---|
| CTPC | 81.97 | 123.46 | [38] |
| Fe3O4@PDA | 86.35 | 57.25 | [39] |
| SBA-NPA | 48.26 | 106.62 | [40] |
| CB-G3 | 88.82 | 97.87 | [41] |
| SiO2-NH-HPBT | 20.5 | 68.6 | [42] |
| P-PAN fibers | 131.41 | 176.99 | [43] |
| Fe3O4(0.5 g)/KH560/M2070 | 166.67 | 185.19 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, J.; Shi, J.; Yang, R. Effects of Polyether Amine Canopy Structure on Heavy Metal Ions Adsorption of Magnetic Solvent-Free Nanofluids. Nanomaterials 2024, 14, 505. https://doi.org/10.3390/nano14060505
Zhang Q, Zhang J, Shi J, Yang R. Effects of Polyether Amine Canopy Structure on Heavy Metal Ions Adsorption of Magnetic Solvent-Free Nanofluids. Nanomaterials. 2024; 14(6):505. https://doi.org/10.3390/nano14060505
Chicago/Turabian StyleZhang, Qi, Jian Zhang, Jian Shi, and Ruilu Yang. 2024. "Effects of Polyether Amine Canopy Structure on Heavy Metal Ions Adsorption of Magnetic Solvent-Free Nanofluids" Nanomaterials 14, no. 6: 505. https://doi.org/10.3390/nano14060505
APA StyleZhang, Q., Zhang, J., Shi, J., & Yang, R. (2024). Effects of Polyether Amine Canopy Structure on Heavy Metal Ions Adsorption of Magnetic Solvent-Free Nanofluids. Nanomaterials, 14(6), 505. https://doi.org/10.3390/nano14060505





