Automated Monitoring System for Suspended Photocatalytic Batch Reactions Based on Online Circulatory Spectrophotometry
Abstract
:1. Introduction
- The development of the debubbler to address the SNB problem;
- The development of the ASD to minimize the influence of the PDP and PDW;
- The construction of the DAS-OCS-AMS to provide a generic framework of online automated monitoring using various spectroscopy techniques for the SPBRs.
2. Materials and Methods
2.1. Materials
2.2. Method
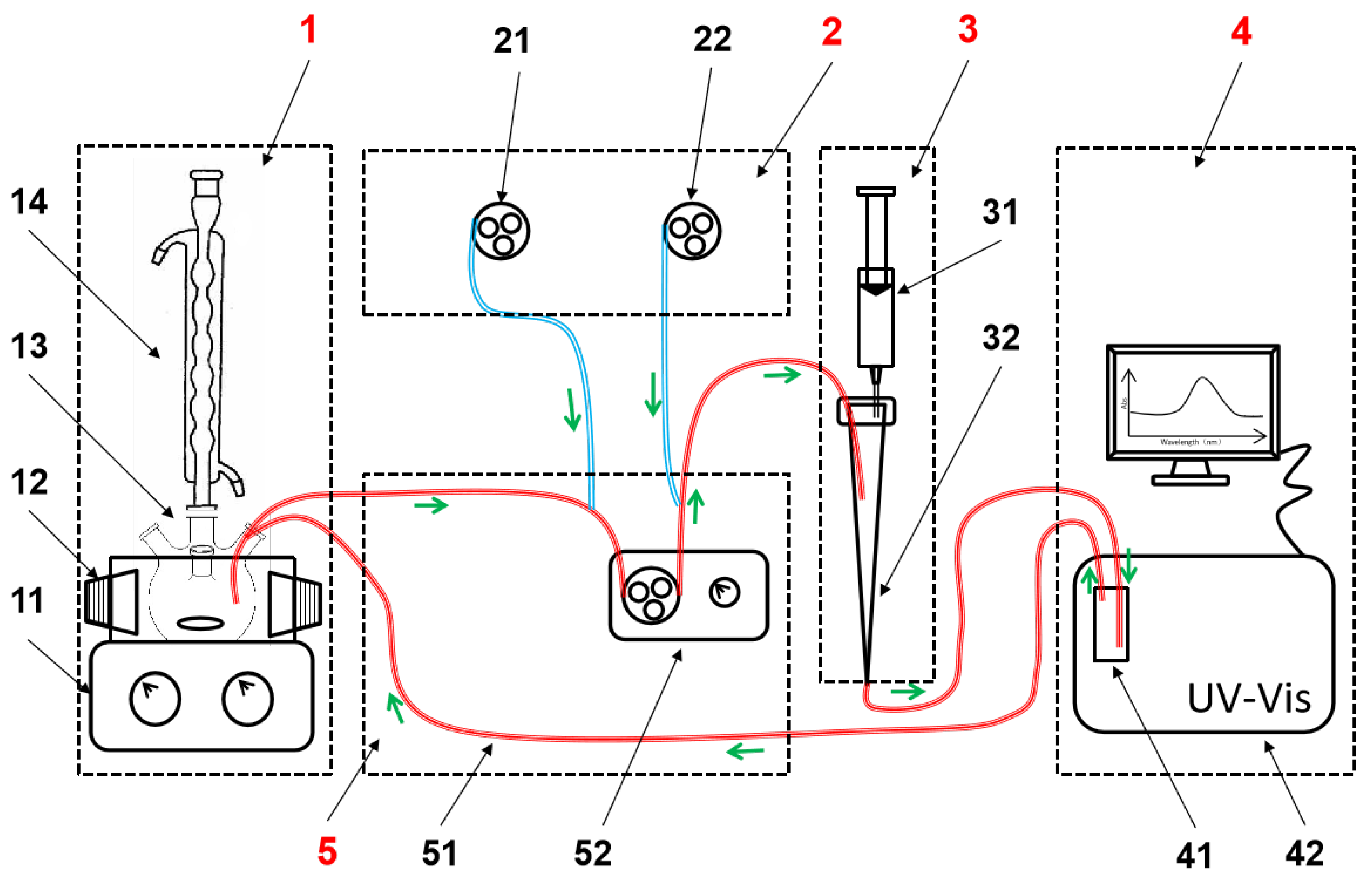
2.2.1. The Proposed DAS-OCS-AMS
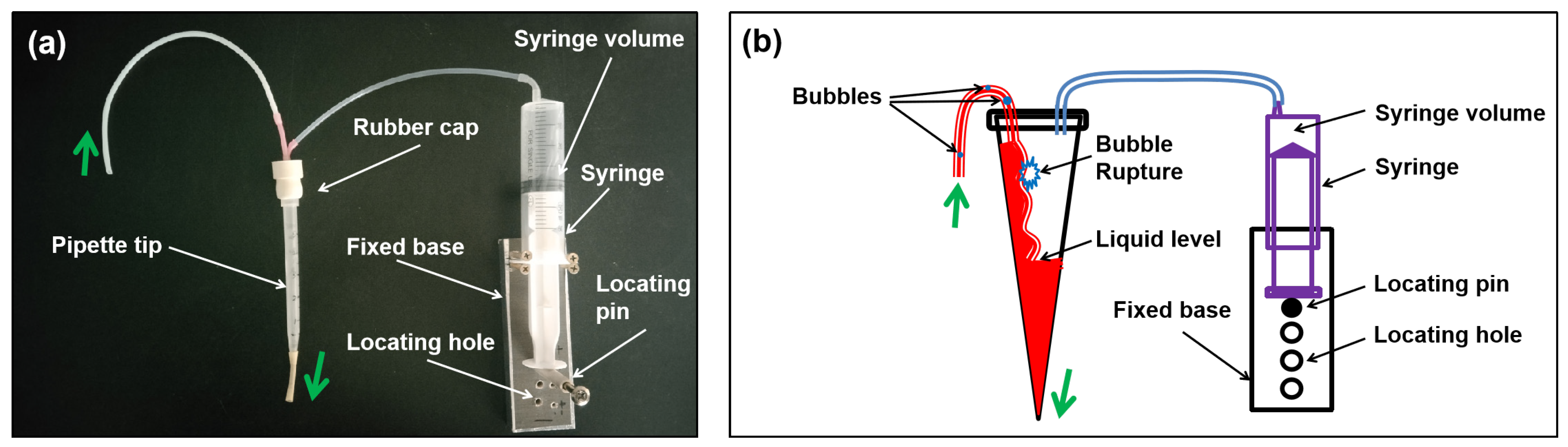
2.2.2. The Proposed Debubbler
2.2.3. The Proposed ASD
2.2.4. The Traditional OCS-AMS
2.3. Case Studies
3. Results and Discussion
3.1. Results for the Water
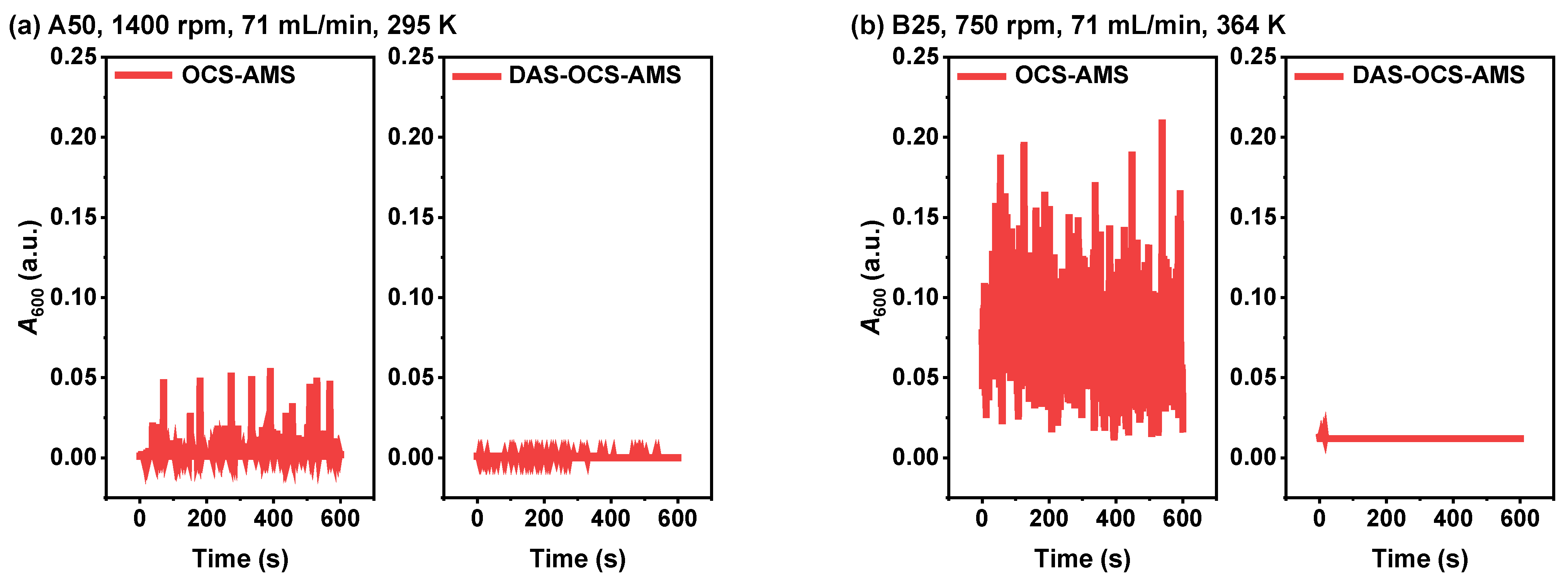
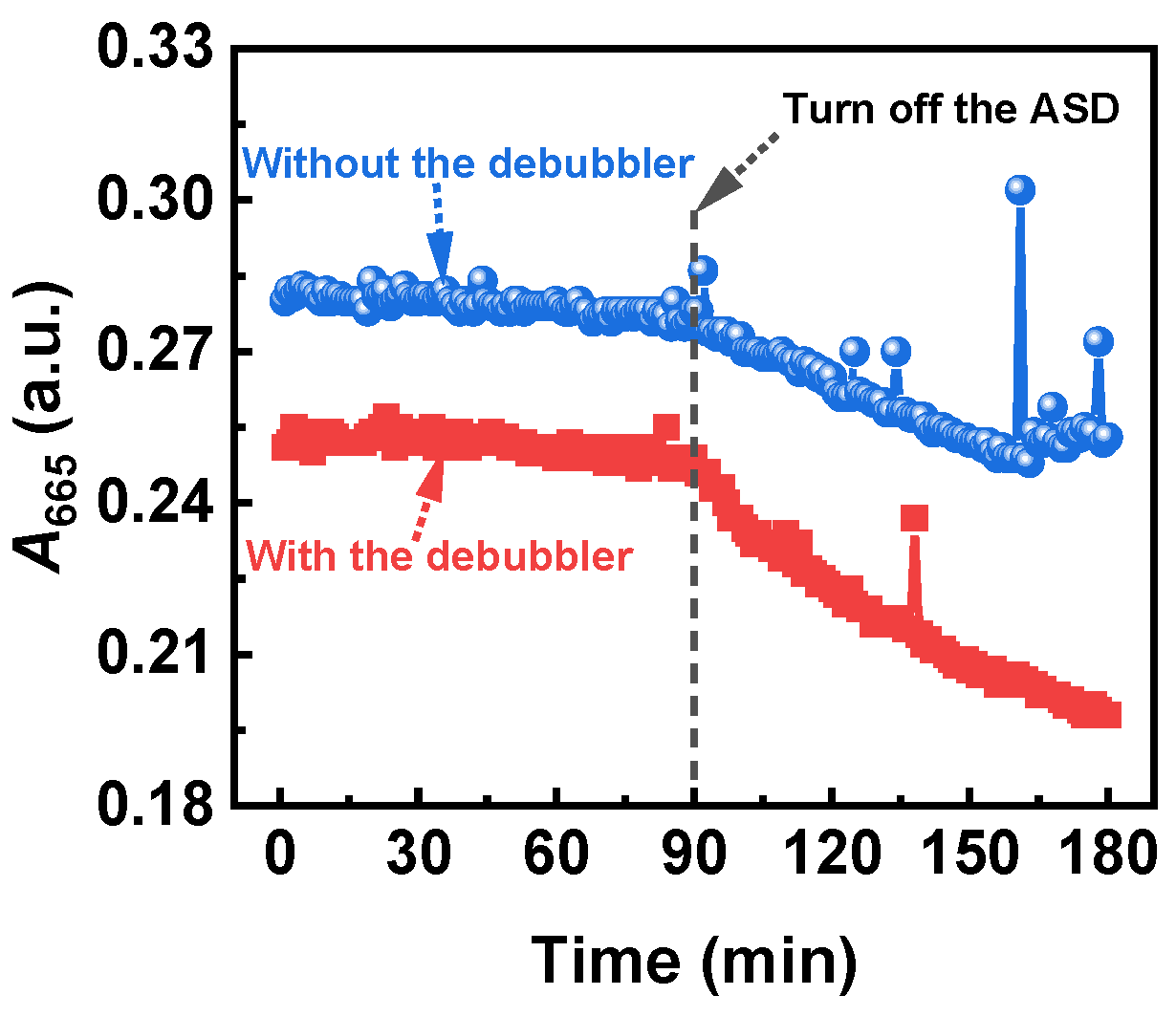
3.1.1. Comparison of Signal Stability under Two Severe Experimental Conditions
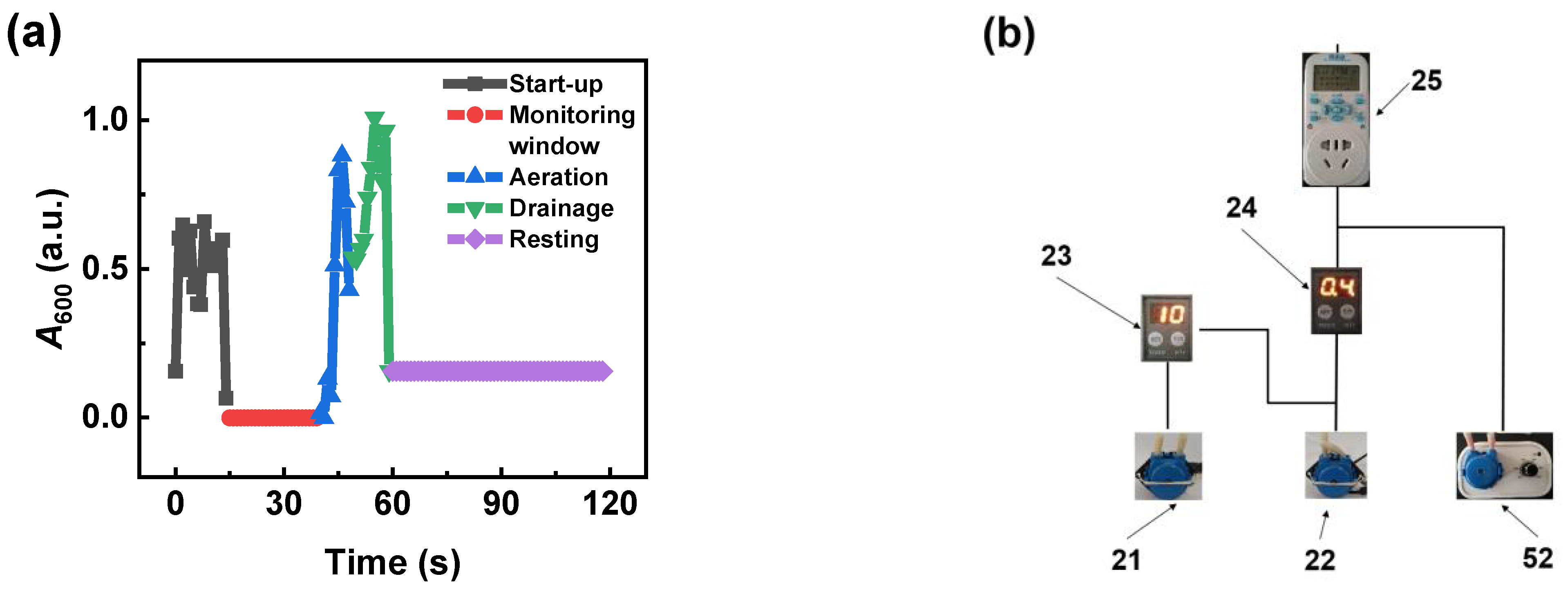
3.1.2. Analysis of the Mechanism of SNB Generation in the OCS-AMS
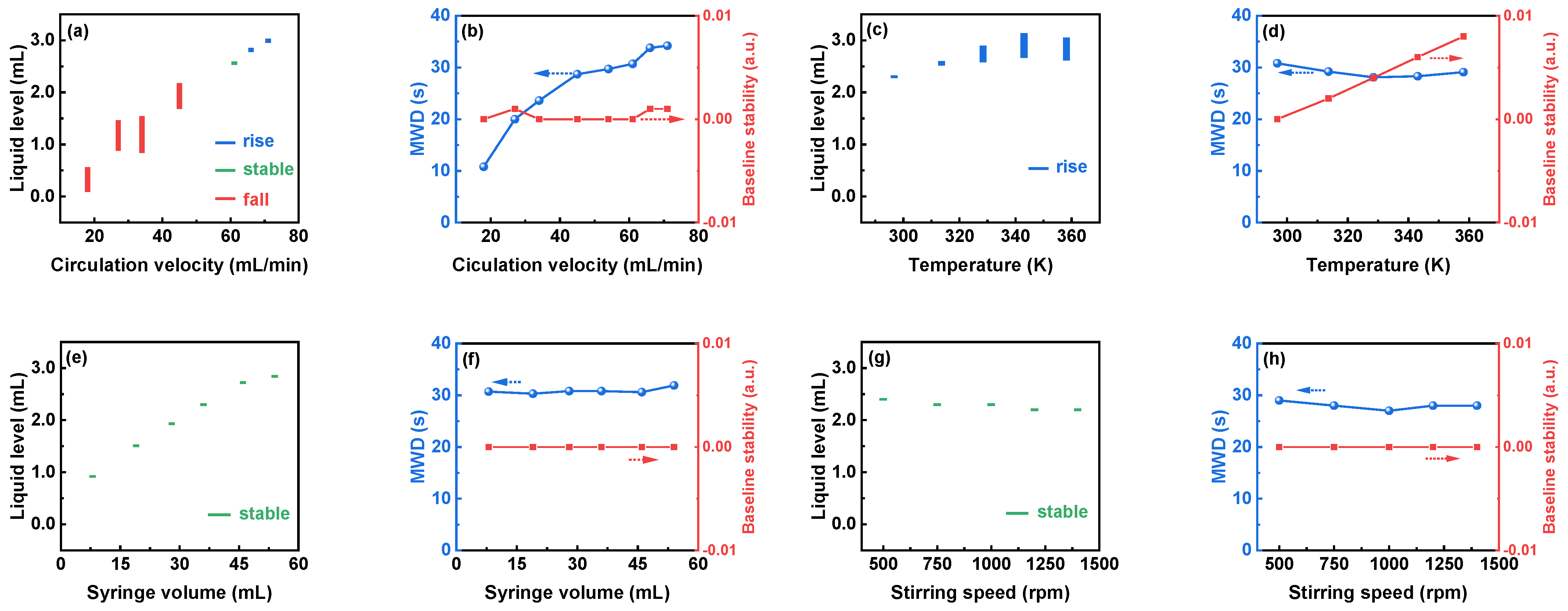
3.1.3. Influence of Parameters on the Operational Stability of the Debubbler
3.2. Results of Suspensions
3.2.1. Comparison with the OCS-AMS for AC Suspension with Relatively Stable Absorbance
3.2.2. Comparison with the OCS-AMS for TiO2 Suspension
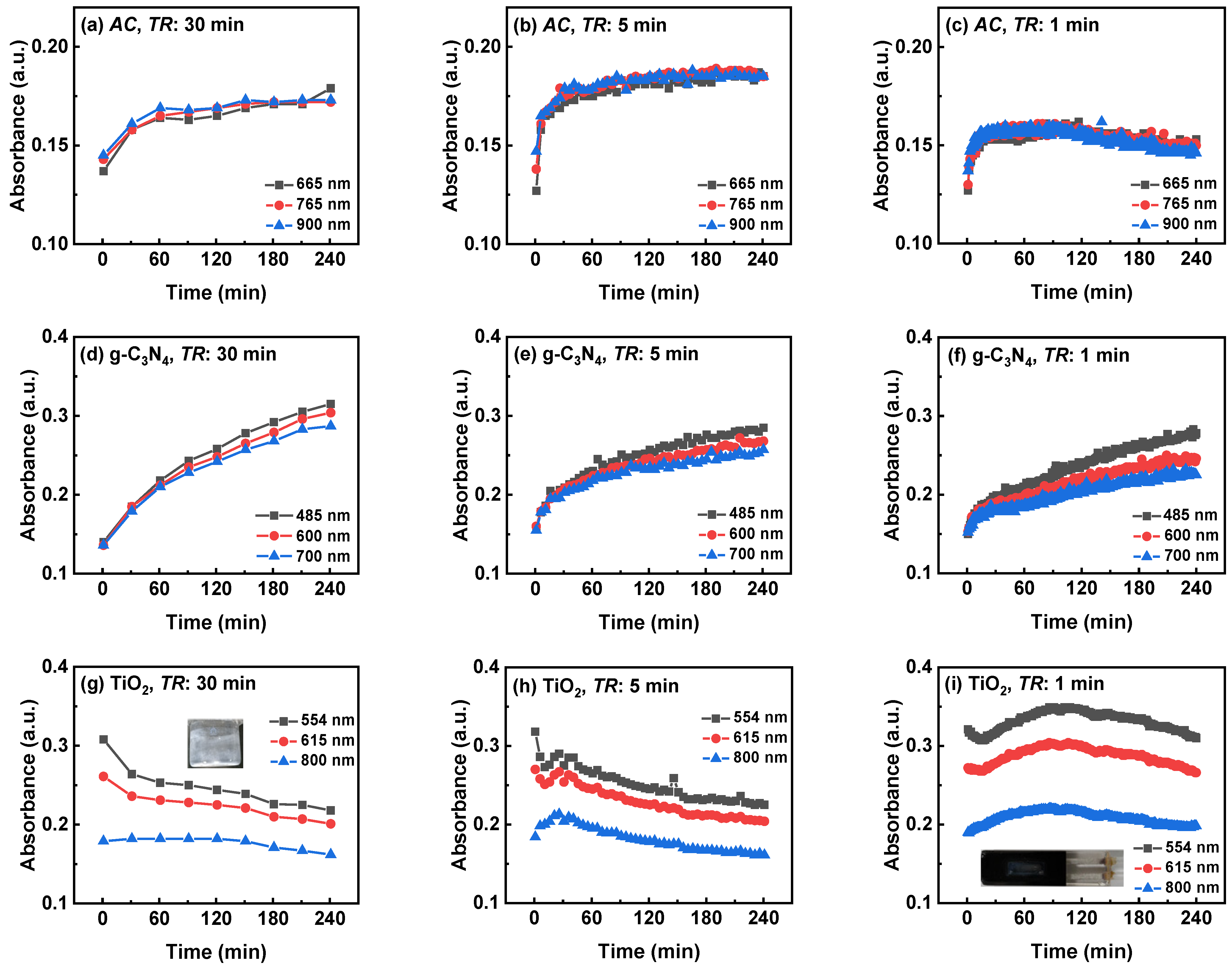
3.2.3. Three-Wavelength Monitoring Results of Three Suspensions with Different TR
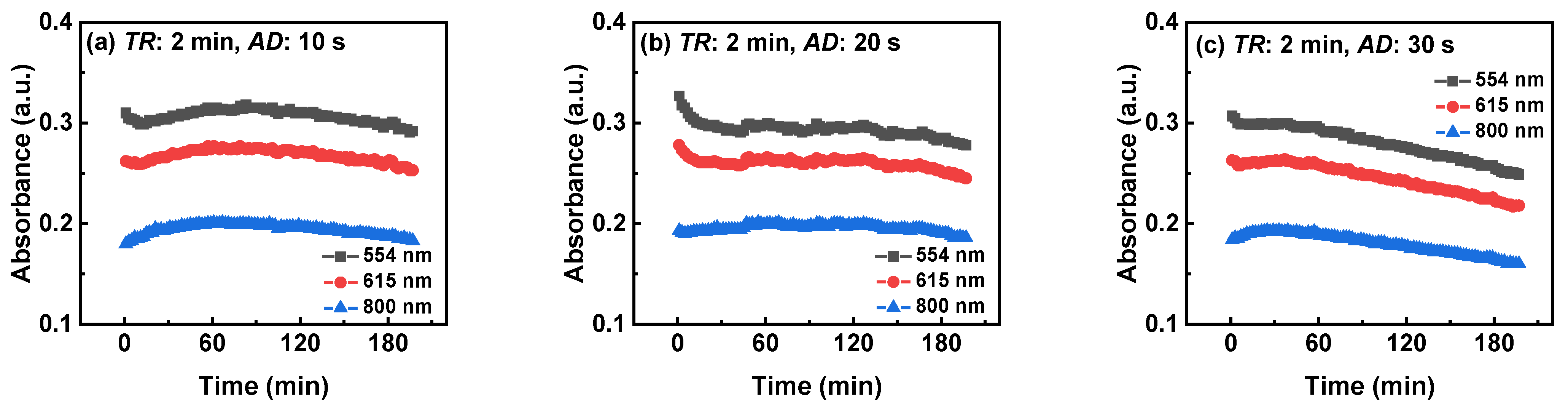
3.2.4. Three-Wavelength Monitoring Results of TiO2 Suspension with Different AD
3.3. Comparison with the OCS-AMS for the Photocatalytic Degradation of AOII by TiO2
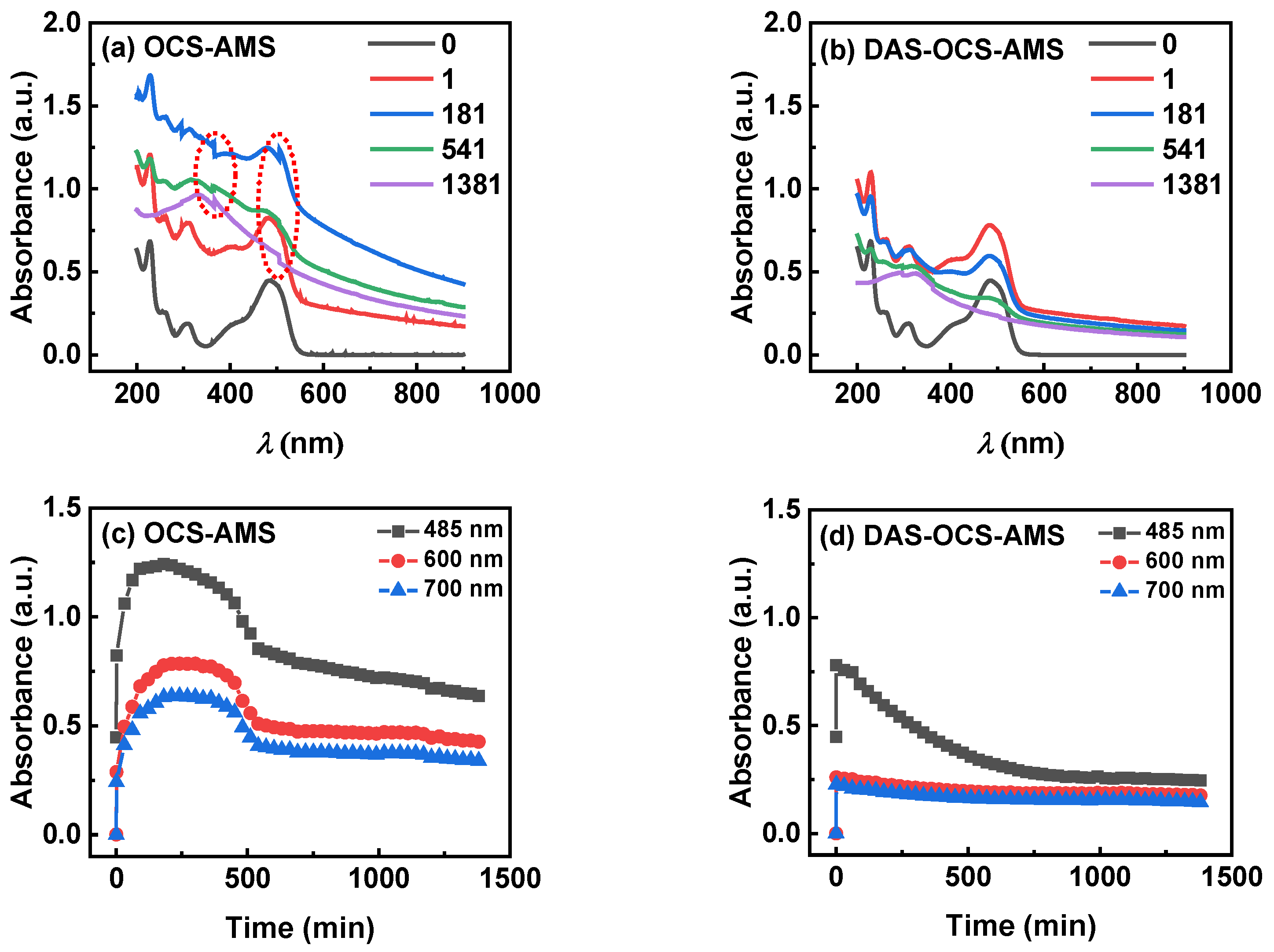
3.3.1. Results under Visible Illumination
3.3.2. Results under UV Illumination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoque, M.A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Zhang, Y.; Lv, X.X.; Cui, M.S.; Cui, K.P.; Dai, Z.L.; Wang, B.; Weerasooriya, R.; Chen, X. Efficient Degradation of Sulfamethoxazole by Diatomite-Supported Hydroxyl-Modified UIO-66 Photocatalyst after Calcination. Nanomaterials 2023, 13, 3116. [Google Scholar] [CrossRef] [PubMed]
- El-Ghobashy, M.A.; Hashim, H.; Darwish, M.A.; Khandaker, M.U.; Sulieman, A.; Tamam, N.; Trukhanov, S.V.; Trukhanov, A.V.; Salem, M.A. Eco-Friendly NiO/Polydopamine Nanocomposite for Efficient Removal of Dyes from Wastewater. Nanomaterials 2022, 12, 1103. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.; Rivero, M.; Zúñiga, V.; Rituerto, J. Analysis of a Hybrid Suspended-Supported Photocatalytic Reactor for the Treatment of Wastewater Containing Benzothiazole and Aniline. Water 2019, 11, 337. [Google Scholar] [CrossRef]
- Masuda, K.; Kobayashi, S. Direct and quantitative monitoring of catalytic organic reactions under heterogeneous conditions using direct analysis in real time mass spectrometry. Chem. Sci. 2020, 11, 5105–5112. [Google Scholar] [CrossRef]
- Visan, A.; van Ommen, J.R.; Kreutzer, M.T.; Lammertink, R.G.H. Photocatalytic Reactor Design: Guidelines for Kinetic Investigation. Ind. Eng. Chem. Res. 2019, 58, 5349–5357. [Google Scholar] [CrossRef]
- Matos, R.S.; Attah-Baah, J.M.; Monteiro, M.D.S.; Costa, B.F.O.; Macedo, M.A.; Da Paz, S.P.A.; Angelica, R.S.; de Souza, T.M.; Talu, S.; Oliveira, R.; et al. Evaluation of the Photocatalytic Activity of Distinctive-Shaped ZnO Nanocrystals Synthesized Using Latex of Different Plants Native to the Amazon Rainforest. Nanomaterials 2022, 12, 2889. [Google Scholar] [CrossRef]
- Gilja, V.; Novakovic, K.; Travas-Sejdic, J.; Hrnjak-Murgic, Z.; Rokovic, M.K.; Zic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef]
- Salazar-Beltran, D.; Hinojosa-Reyes, L.; Maya-Alejandro, F.; Turnes-Palomino, G.; Palomino-Cabello, C.; Hernandez-Ramirez, A.; Guzman-Mar, J.L. Automated on-line monitoring of the TiO2-based photocatalytic degradation of dimethyl phthalate and diethyl phthalate. Photochem. Photobiol. Sci. 2019, 18, 863–870. [Google Scholar] [CrossRef]
- Guevara-Almaraz, E.; Hinojosa-Reyes, L.; Caballero-Quintero, A.; Ruiz-Ruiz, E.; Hernandez-Ramirez, A.; Guzman-Mar, J.L. Potential of multisyringe chromatography for the on-line monitoring of the photocatalytic degradation of antituberculosis drugs in aqueous solution. Chemosphere 2015, 121, 68–75. [Google Scholar] [CrossRef]
- Ramos-Huerta, L.A.; Valadés-Pelayo, P.J.; Llanos, A.G.; Ruiz, R.S.; Cabello, J.J.; Castillo-Araiza, C.O. Development of a new methodology to determine suspended photocatalyst optical properties. Chem. Eng. J. 2021, 413, 127458. [Google Scholar] [CrossRef]
- Zhou, R.; Guzman, M.I. Photocatalytic Reduction of Fumarate to Succinate on ZnS Mineral Surfaces. J. Phys. Chem. C 2016, 120, 7349–7357. [Google Scholar] [CrossRef]
- Tapia-Tlatelpa, T.; Trull, J.; Romeral, L. In situ Decolorization Monitoring of Textile Dyes for an Optimized UV-LED/TiO2 Reactor. Catalysts 2019, 9, 669. [Google Scholar] [CrossRef]
- Rößler, M.; Huth, P.U.; Liauw, M.A. Process analytical technology (PAT) as a versatile tool for real-time monitoring and kinetic evaluation of photocatalytic reactions. React. Chem. Eng. 2020, 5, 1992–2002. [Google Scholar] [CrossRef]
- Keller, M.H.; Moreira, R.; Souza, B.S. Automated monitoring the kinetics of homogeneous and heterogeneous chemical processes using a smartphone. Sci. Rep. 2022, 12, 15774. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tatarchuk, T.; Kannan, K.; Shyichuk, A. Optimization of TiO2-P25 photocatalyst dose and H2O2 concentration for advanced photo-oxidation using smartphone-based colorimetry. Water Sci. Technol. 2021, 84, 469–483. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tatarchuk, T.; Mironyuk, I.; Kotsyubynsky, V.; Mandzyuk, V. Performance of commercial titanium dioxide samples in terms of dye photodegradation assessed using smartphone-based measurements. Phys. Chem. Solid State 2022, 23, 582–589. [Google Scholar] [CrossRef]
- Fu, Z.; Onishi, H. Photocatalytic Reaction in Aqueous Suspension: FTIR Spectroscopy with Attenuated Total Reflection in Diamonds. ACS Omega 2023, 8, 33825–33830. [Google Scholar] [CrossRef]
- Ruiz-Flores, A.; Garcia, A.; Pineda, A.; Brox, M.; Gersnoviez, A.; Canete-Carmona, E. Low-Cost Photoreactor to Monitor Wastewater Pollutant Decomposition. Sensors 2023, 23, 775. [Google Scholar] [CrossRef]
- Ditroi, T.; Kalmar, J.; Pino-Chamorro, J.A.; Erdei, Z.; Lente, G.; Fabian, I. Construction of a multipurpose photochemical reactor with on-line spectrophotometric detection. Photochem. Photobiol. Sci. 2016, 15, 589–594. [Google Scholar] [CrossRef]
- Bukman, L.; de Freitas, C.F.; Caetano, W.; Fernandes, N.R.C.; Hioka, N.; Batistela, V.R. Kinetic spectrophotometric method for real-time monitoring of ultraviolet photoreactions: A mini-photoreactor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 330–335. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Danyliuk, N.; Shyichuk, A.; Macyk, W.; Naushad, M. Photocatalytic degradation of dyes using rutile TiO2 synthesized by reverse micelle and low temperature methods: Real-time monitoring of the degradation kinetics. J. Mol. Liq. 2021, 342, 117407. [Google Scholar] [CrossRef]
- Xu, H.; Yu, T.; Wang, J.; Li, M.; Liu, Y. Online monitoring of Fenton-mediated reactive red 6B oxidation kinetics. Environ. Prog. Sustain. Energy 2015, 34, 1019–1027. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Xu, W. Monitoring of decolorization kinetics of Reactive Brilliant Blue X-BR by online spectrophotometric method in Fenton oxidation process. J. Hazard Mater. 2008, 158, 445–453. [Google Scholar] [CrossRef]
- Lin, K.; Ma, J.; Pai, S.-C.; Huang, Y.; Feng, S.; Yuan, D. Determination of Nitrite, Phosphate, and Silicate by Valveless Continuous Analysis with a Bubble-Free Flow Cell and Spectrophotometric Detection. Anal. Lett. 2016, 50, 510–529. [Google Scholar] [CrossRef]
- Sakib, M.N.; Shuvo, M.S.; Rahman, R.; Saha, S. Particle deposition and fluid flow characteristics in turbulent corrugated pipe flow using Eulerian-Lagrangian approach. Heliyon 2023, 9, e14603. [Google Scholar] [CrossRef]
- Talebizadehsardari, P.; Rahimzadeh, H.; Ahmadi, G.; Inthavong, K.; Keshtkar, M.M.; Moghimi, M.A. Nano-particle deposition in laminar annular pipe flows. Adv. Powder Technol. 2020, 31, 3134–3143. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zou, H.; Li, M.; Wang, G.; Peng, M.; Zhang, J.; Tang, Z. Tuning the gas-liquid-solid segmented flow for enhanced heterogeneous photosynthesis of Azo-compounds. Chem. Eng. J. 2021, 423, 130226. [Google Scholar] [CrossRef]
- Yue, J.; Falke, F.H.; Schouten, J.C.; Nijhuis, T.A. Microreactors with integrated UV/Vis spectroscopic detection for online process analysis under segmented flow. Lab. Chip 2013, 13, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Skeggs, L.T. An Automatic Method for Colorimetric Analysis. Am. J. Clin. Pathol. 1957, 28, 311–322. [Google Scholar] [CrossRef]
- Koscielniak, P. Modern Flow Analysis. Molecules 2020, 25, 2897. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.J.; Chocholous, P. Next generation of flow analysis is based on flow programming. Talanta 2024, 269, 125410. [Google Scholar] [CrossRef]
- Chidhambaram, N.; Ravichandran, K. Single step transformation of urea into metal-free g-C3N4 nanoflakes for visible light photocatalytic applications. Mater. Lett. 2017, 207, 44–48. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Han, G.; Li, C.; He, L.; Xiong, X.; Wang, S. Experimental investigation on particle transport of coal fines in unsteady terrain slug flow. J. Pet. Sci. Eng. 2018, 166, 747–758. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2011, 6, 27. [Google Scholar] [CrossRef]
- Yurdakal, S.; Loddo, V.; Bayarri Ferrer, B.; Palmisano, G.; Augugliaro, V.; Giménez Farreras, J.; Palmisano, L. Optical Properties of TiO2 Suspensions: Influence of pH and Powder Concentration on Mean Particle Size. Ind. Eng. Chem. Res. 2007, 46, 7620–7626. [Google Scholar] [CrossRef]
- Esser, F.; Masselter, T.; Speck, T. Silent Pumpers: A Comparative Topical Overview of the Peristaltic Pumping Principle in Living Nature, Engineering, and Biomimetics. Adv. Intell. Syst. 2019, 1, 1900009. [Google Scholar] [CrossRef]
- Beale, A.M.; van der Eerden, A.M.; Kervinen, K.; Newton, M.A.; Weckhuysen, B.M. Adding a third dimension to operando spectroscopy: A combined UV-Vis, Raman and XAFS setup to study heterogeneous catalysts under working conditions. Chem. Commun. 2005, 24, 3015–3017. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, D.-P.; Huang, J.-H. Automated Monitoring System for Suspended Photocatalytic Batch Reactions Based on Online Circulatory Spectrophotometry. Nanomaterials 2024, 14, 508. https://doi.org/10.3390/nano14060508
Lei D-P, Huang J-H. Automated Monitoring System for Suspended Photocatalytic Batch Reactions Based on Online Circulatory Spectrophotometry. Nanomaterials. 2024; 14(6):508. https://doi.org/10.3390/nano14060508
Chicago/Turabian StyleLei, Da-Peng, and Jian-Hua Huang. 2024. "Automated Monitoring System for Suspended Photocatalytic Batch Reactions Based on Online Circulatory Spectrophotometry" Nanomaterials 14, no. 6: 508. https://doi.org/10.3390/nano14060508





