Microstructure, Mechanical Property and Thermal Conductivity of Porous TiCO Ceramic Fabricated by In Situ Carbothermal Reduction of Phenolic Resin and Titania
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Composition
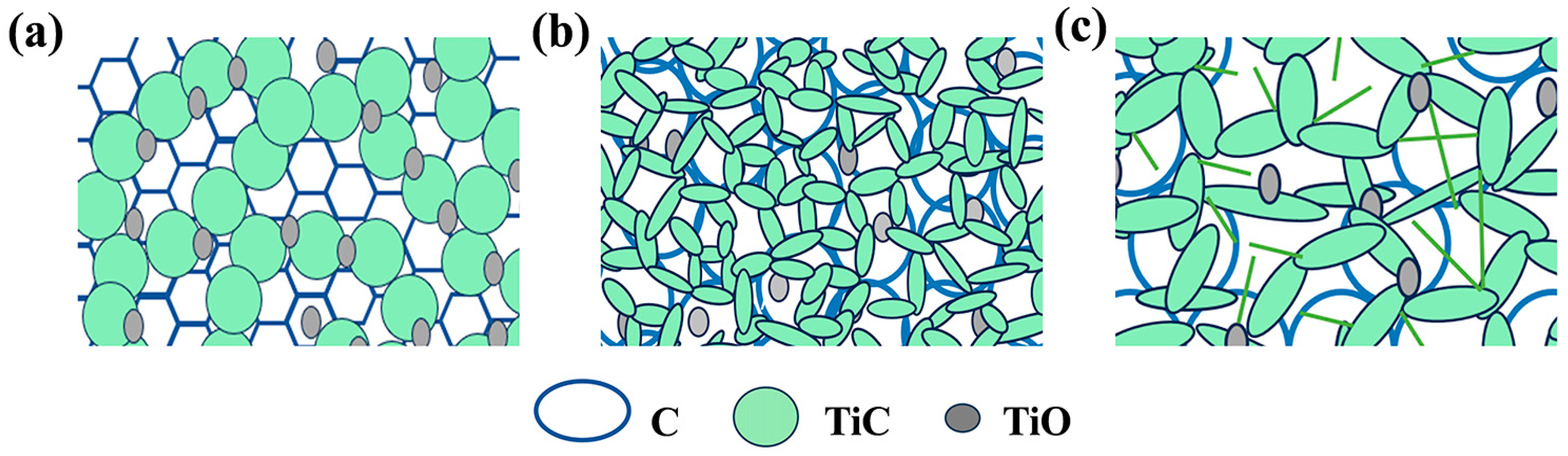
3.2. Microstructure
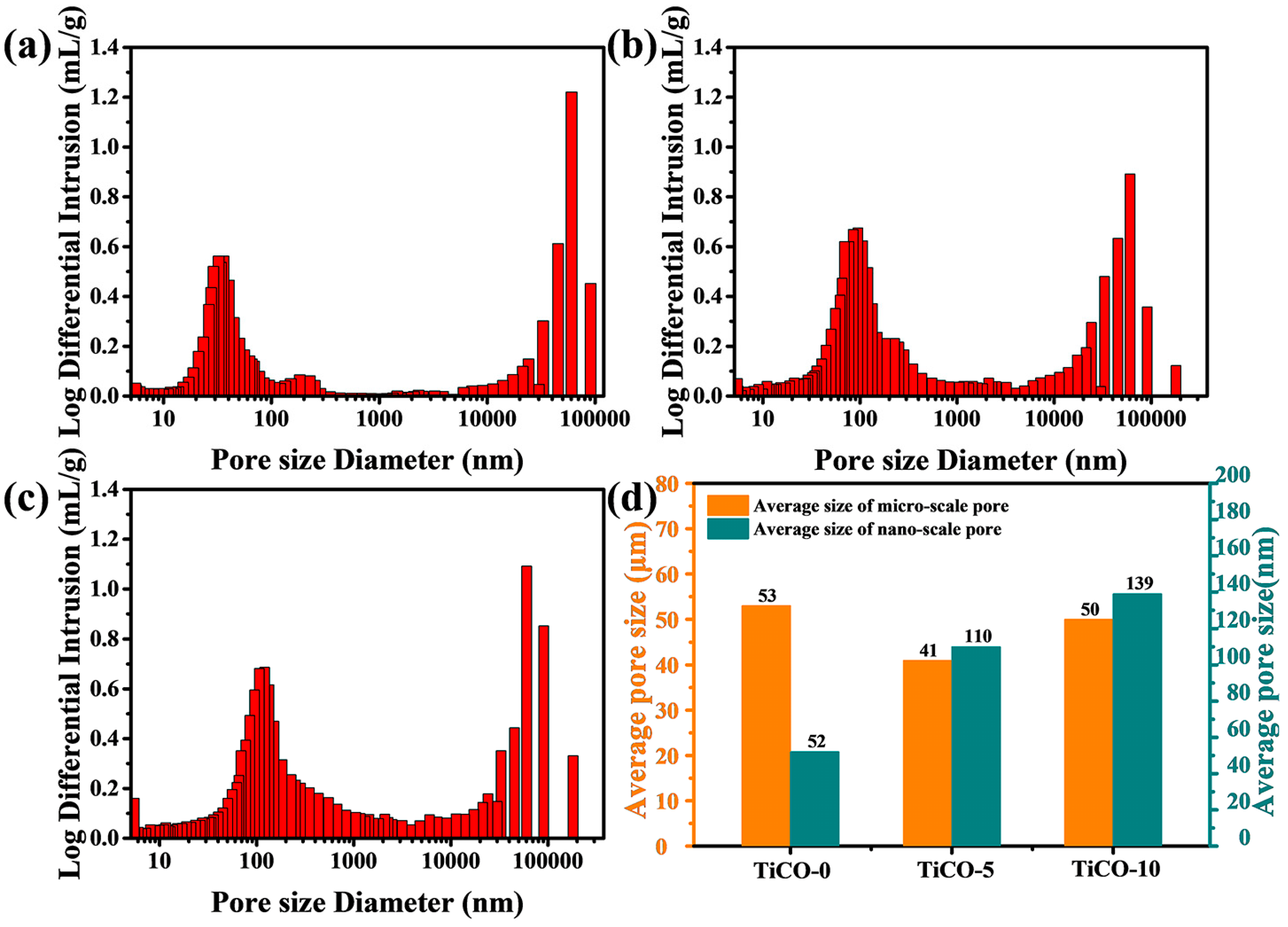
3.3. Pore Size Distribution
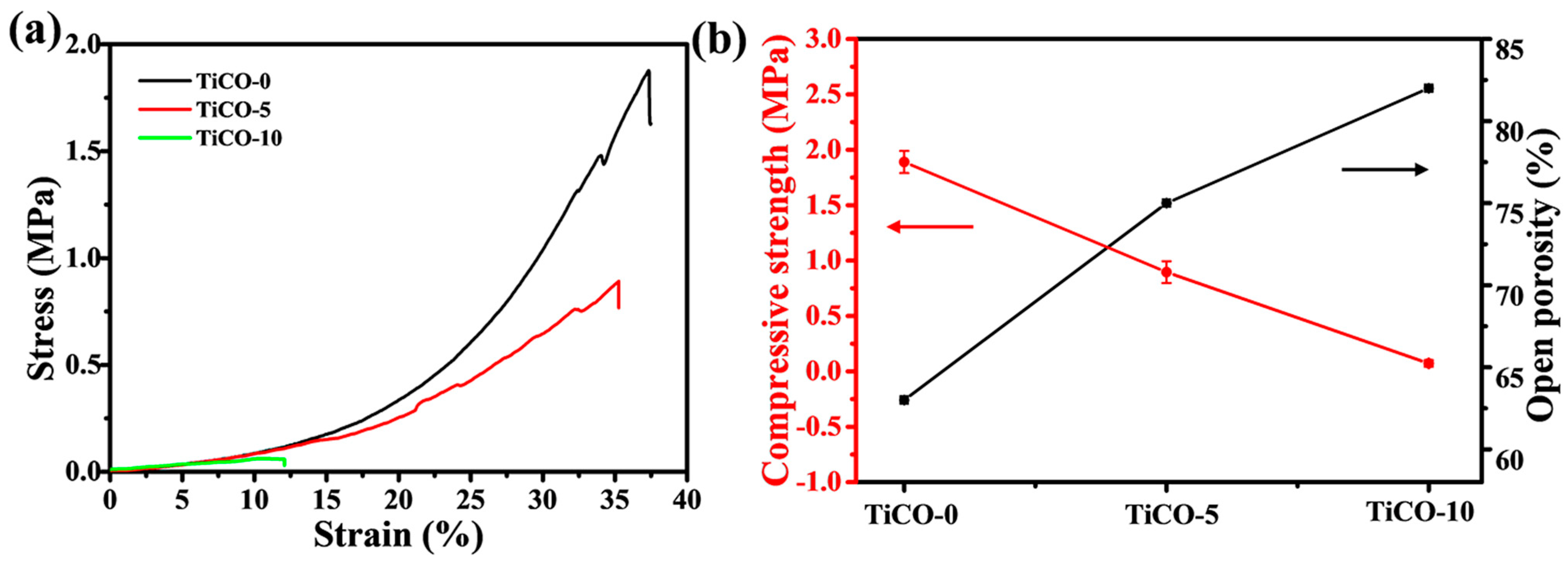
3.4. Compressive Strength
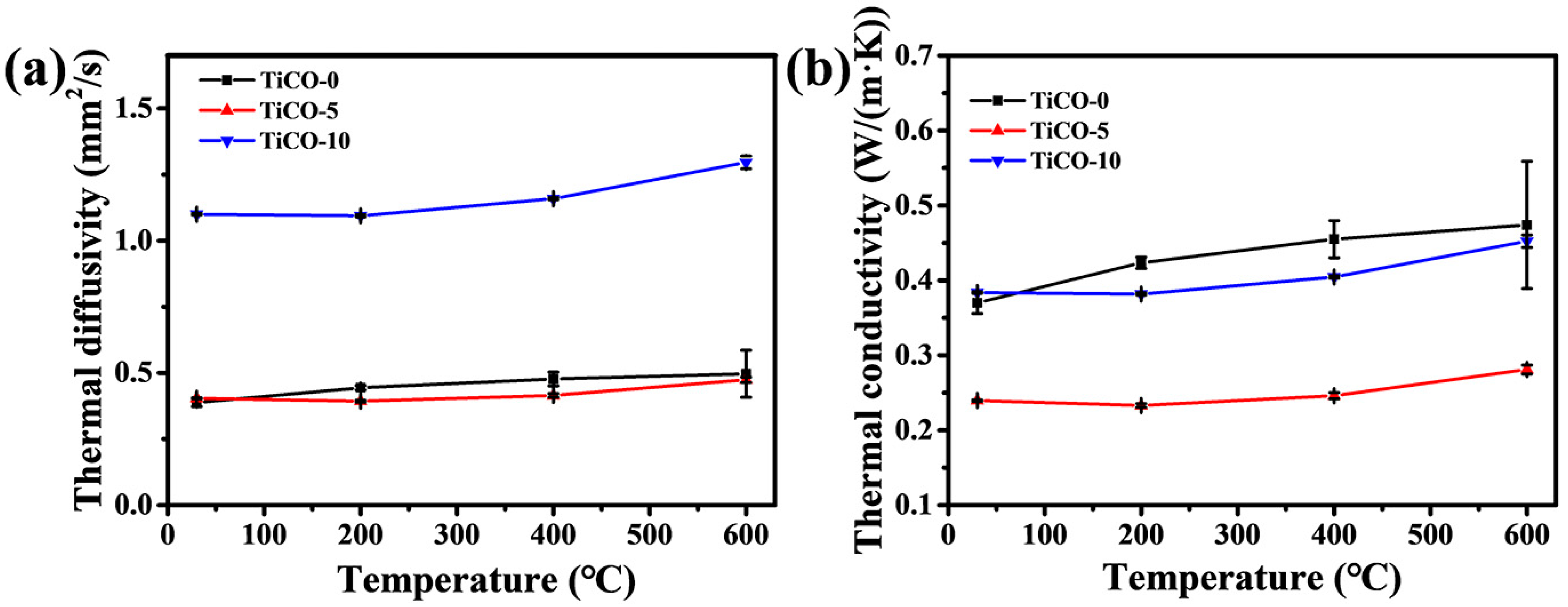
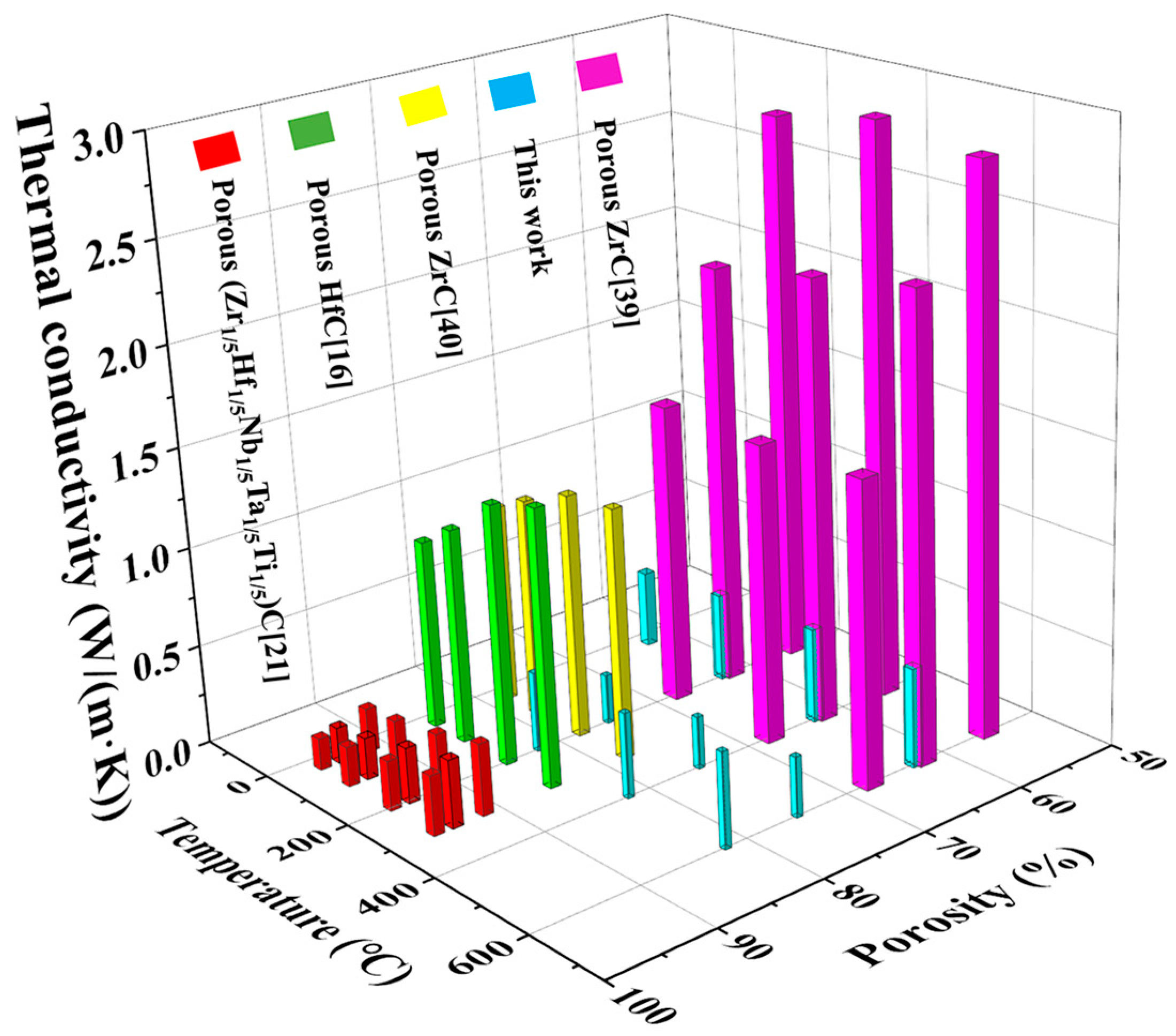
3.5. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Zhang, R.; Xie, G.; Manca, O. Thermal and thermomechanical performance of actively cooled pyramidal sandwich panels. Int. J. Therm. Sci. 2019, 139, 118–128. [Google Scholar] [CrossRef]
- Schlichting, K.W.; Padture, N.P.; Klemens, P.G. Thermal conductivity of dense and porous yttria-stabilized zirconia. J. Mater. Sci. 2001, 36, 3003–3010. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Tudorache, D.I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An updated overview of silica aerogel-based nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef]
- Chen, R.; Jia, W.; Hei, D.; Wang, Y. Toward excellent performance of Al2O3-ZrO2 reticulated porous ceramics: New insights based on residual stress. Ceram. Int. 2018, 44, 21478–21485. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Z.; Ma, S.; Zhang, C.; Guo, W. Theoretical model based on stress waves and experimental verification of residual stress in stereolithography printed ZrO2 porous ceramics. Ceram. Int. 2022, 48, 23983–23988. [Google Scholar] [CrossRef]
- Dong, S.; Ding, Y.; Zhang, X.; Hu, J.; Wang, M.; You, X.; Yan, J.; Yang, J.; Ruan, J. Porous SiC ceramic matrix composite reinforced by SiC nanowires with high strength and low thermal conductivity. J. Inorg. Mater. 2022, 37, 459–466. [Google Scholar]
- Wang, S.; Yin, Y.; Chen, L.; Liu, X.; Jia, Q.; Zhang, S. Controllable preparation of porous ZrB2-SiC ceramics via using KCl space holders. Ceram. Int. 2021, 47, 33978–33987. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, K.; Zhou, C.; Han, W.; Du, Z. Preparation and properties of high-porosity ZrB2-SiC ceramics by water-based freeze casting. J. Eur. Ceram. Soc. 2021, 41, 2239–2246. [Google Scholar] [CrossRef]
- Lan, X.L.; Li, Y.B.; Wang, Z.J. High-temperature electromagnetic wave absorption, mechanical and thermal insulation properties of in-situ grown SiC on porous SiC skeleton. Chem. Eng. J. 2020, 9, 397. [Google Scholar] [CrossRef]
- Chabi, S.; Rocha, V.G.; García-Tuñón, E.; Ferraro, C.; Saiz, E.; Xia, Y.; Zhu, Y. Ultralight, strong, three-dimensional SiC structures. ACS Nano 2015, 10, 1871–1876. [Google Scholar] [CrossRef]
- Yang, J.; Wu, K.; Li, X.; Wang, X.; Pi, G.; Fang, W.; Yan, Q. A porous TiC supported nanostructured complex metal oxide ceramic electrode for oxygen evolution reaction. Ceram. Int. 2023, 49, 27662–27667. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, Y.; Feng, Y.; Teng, X.; Yan, S.; Li, R.; Yu, W.; Huang, Z.; Li, S.; Li, Z. Synthesis and mechanical properties of TiC-Fe interpenetrating phase composites fabricated by infiltration process. Ceram. Int. 2018, 44, 21742–21749. [Google Scholar] [CrossRef]
- Yan, N.; Fu, Q.; Wang, R.; Zhang, J.; Liu, N.; Li, B.; Shen, Q.; Sun, J. Quasi-static and dynamic compressive behaviors of porous ZrC ceramic reinforced pyrocarbon composites. Compos. Part A Appl. Sci. Manuf. 2022, 153, 106749. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, F.; Cheng, L.; Yang, J. ZrC-SiC closed-cell ceramics with low thermal conductivity: Exploiting unique spherical closed-cell structure through tape casting and CVI techniques. J. Mater. Sci. Technol. 2023, 184, 101–110. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Liu, X.; Jia, Q.; Zhang, S. Carbothermal reduction synthesis of high porosity and low thermal conductivity ZrC-SiC ceramics via an one-step sintering technique. J. Eur. Ceram. Soc. 2022, 42, 4465–4471. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Zhou, Y. Low thermal conductivity and high porosity ZrC and HfC ceramics prepared by in-situ reduction reaction/partial sintering method for ultrahigh temperature applications. J. Mater. Sci. Technol. 2019, 35, 2778–2784. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, Y.; Liu, M.; Li, Q.; Huang, Z. Oxidation mechanism and kinetics of SiBCN/HfC ceramic composites at high temperatures. J. Mater. Res. Technol. 2020, 9, 2289–2298. [Google Scholar] [CrossRef]
- Fei, Y.; Song, X.; Du, L.; Wang, Y.; Du, Z. Study on the sintering mechanism and properties of porous ceramics prepared by silicon carbide abrasive particles with multi-mineral sintering additives and silica sols. Ceram. Int. 2022, 48, 27324–27333. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, G.; Li, H.; Liu, L.; Liu, Y.; Shen, Z. Fabrication and microstructure of porous SiC ceramics with Al2O3 and CeO2 as sintering additives. Ceram. Int. 2016, 42, 12613–12616. [Google Scholar] [CrossRef]
- Obada, D.O.; Dodoo, A.D.; Dauda, M.; Anafi, F.O.; Ahmed, A.S.; Ajayi, O.A. Pressureless sintering and gas flux properties of porous ceramic membranes for gas applications. Results Phys. 2017, 7, 3838–3846. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, Z.; Sun, L.; Liang, X.; Luo, Z.; Chen, H.; Li, J.; Wang, J. High entropy ultra-high temperature ceramic thermal insulator (Zr1/5Hf1/5Nb1/5Ta1/5Ti1/5)C with controlled microstructure and outstanding properties. J. Mater. Sci. Technol. 2022, 119, 190–199. [Google Scholar] [CrossRef]
- Persembe, E.; Parra, C.C.; Ameloot, R. Ceramic foams with controlled geometries through 3D printed sacrificial templates. Adv. Eng. Mater. 2023, 25, 2300076. [Google Scholar] [CrossRef]
- Hedayat, N.; Du, Y.; Ilkhani, H. Review on fabrication techniques for porous electrodes of solid oxide fuel cells by sacrificial template methods. Renew. Sustain. Energy Rev. 2017, 77, 1221–1239. [Google Scholar] [CrossRef]
- Lukacs, V.A.; Stanculescu, R.; Curecheriu, L.; Ciomaga, C.E.; Horchidan, N.; Cioclea, C.; Mitoseriu, L. Structural and functional properties of BaTiO3 porous ceramics produced by using pollen as sacrificial template. Ceram. Int. 2020, 46, 523–530. [Google Scholar] [CrossRef]
- Biggemann, J.; Stumpf, M.; Fey, T. Porous alumina ceramics with multimodal pore size distributions. Materials 2021, 14, 3294. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, L.; Yang, F.; Shao, Z.; Zhang, H.; Zhao, Z.; Wang, K. (La0.2Y0.2Nd0.2Gd0.2Sr0.2)CrO3: A novel conductive porous high-entropy ceramic synthesized by the sol-gel method. J. Alloys Compd. 2021, 863, 158763. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Mayorov, V.Y.; Modin, E.B.; Portnyagin, A.S.; Gridasova, E.A.; Agafonova, I.G.; Zakirova, A.E.; Tananaev, I.G.; Avramenko, V.A. Sol-gel and SPS combined synthesis of highly porous wollastonite ceramic materials with immobilized Au-NPs. Ceram. Int. 2017, 43, 8509–8516. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Li, X.; Su, D.; Ji, H.; Yu, H.; Hu, Z. Large-scale and ultra-low thermal conductivity of ZrO2 fibrofelt/ZrO2-SiO2 aerogels composites for thermal insulation. Ceram. Int. 2018, 44, 8742–8748. [Google Scholar] [CrossRef]
- Liu, D.B.; Zhang, C.; Xue, Y.H. Synthesis and characterization of sol-gel derived lanthanum zirconate ceramic aerogels toward ultralow thermal conductivity. Mater. Sci. Eng. B 2023, 87, 116127. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, X.; Pei, X.L.; He, L.; Huang, Z.R.; Huang, Q. Preparation of highly porous SiC via ceramic precursor conversion and evaluation of its thermal insulation performance. Adv. Appl. Ceram. 2020, 119, 398–406. [Google Scholar] [CrossRef]
- Yu, Z.; Mao, K.; Feng, Y. Single-source-precursor synthesis of porous W-containing SiC-based nanocomposites as hydrogen evolution reaction electrocatalysts. J. Adv. Ceram. 2021, 10, 1338–1349. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Zhang, S.; Luo, X.; Liu, F.; Li, Y. In situ synthesis of Al2O3-SiC powders via molten-salt-assisted aluminum/carbothermal reduction method. Ceram. Int. 2022, 48, 24471–24475. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Zhang, Y.L.; Ren, J.C.; Li, T.; Wang, H.H. Large-scale synthesis of hafnium carbide nanowires via a Ni-assisted polymer infiltration and pyrolysis. J. Am. Ceram. Soc. 2019, 102, 2924–2931. [Google Scholar] [CrossRef]
- Tian, S.; Li, H.J.; Zhang, Y.; Zhang, S.; Wang, Y.J.; Ren, J.C. Single-crystalline hafnium carbide nanowire growth below the eutectic temperature by CVD. J. Cryst. Growth 2013, 384, 44–49. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Q.; Deng, D.; Liu, Z. Modeling compressive strength of cement asphalt composite based on pore size distribution. Constr. Build. Mater. 2017, 150, 714–722. [Google Scholar] [CrossRef]
- Yoon, B.H.; Choi, W.Y.; Kim, H.E.; Kim, J.H.; Koh, Y.H. Aligned porous alumina ceramics with high compressive strengths for bone tissue engineering. Scr. Mater. 2008, 58, 537–540. [Google Scholar] [CrossRef]
- Fan, J.; Hui, S.; Bailey, T.P.; Page, A.; Uher, C.; Yuan, F. Ultralow thermal conductivity in grapheme-silica porous ceramics with a special saucer structure of graphene aerogels. J. Mater. Chem. A 2019, 7, 1574–1584. [Google Scholar] [CrossRef]
- Bourret, J.; Michot, A.; Tessier, D.N.; Naït, A.B.; Pennec, F.; Alzina, A.; Vicente, J.; Peyratout, C.S.; Smith, D.S. Thermal conductivity of very porous kaolin-based ceramics. J. Am. Ceram. Soc. 2014, 97, 938–944. [Google Scholar] [CrossRef]
- Yan, N.; Fu, Q.G.; Zhang, Y.; Li, K.; Xie, W.; Zhang, J.; Zhuang, L.; Shi, X.H. Preparation of pore-controllable zirconium carbide ceramics with tunable mechanical strength, thermal conductivity and sound absorption coefficient. Ceram. Int. 2020, 46, 19609–19616. [Google Scholar] [CrossRef]
- Li, F.; Kang, Z.; Huang, X.; Wang, X.G.; Zhang, G.J. Preparation of zirconium carbide foam by direct foaming method. J. Eur. Ceram. Soc. 2014, 34, 3513–3520. [Google Scholar] [CrossRef]

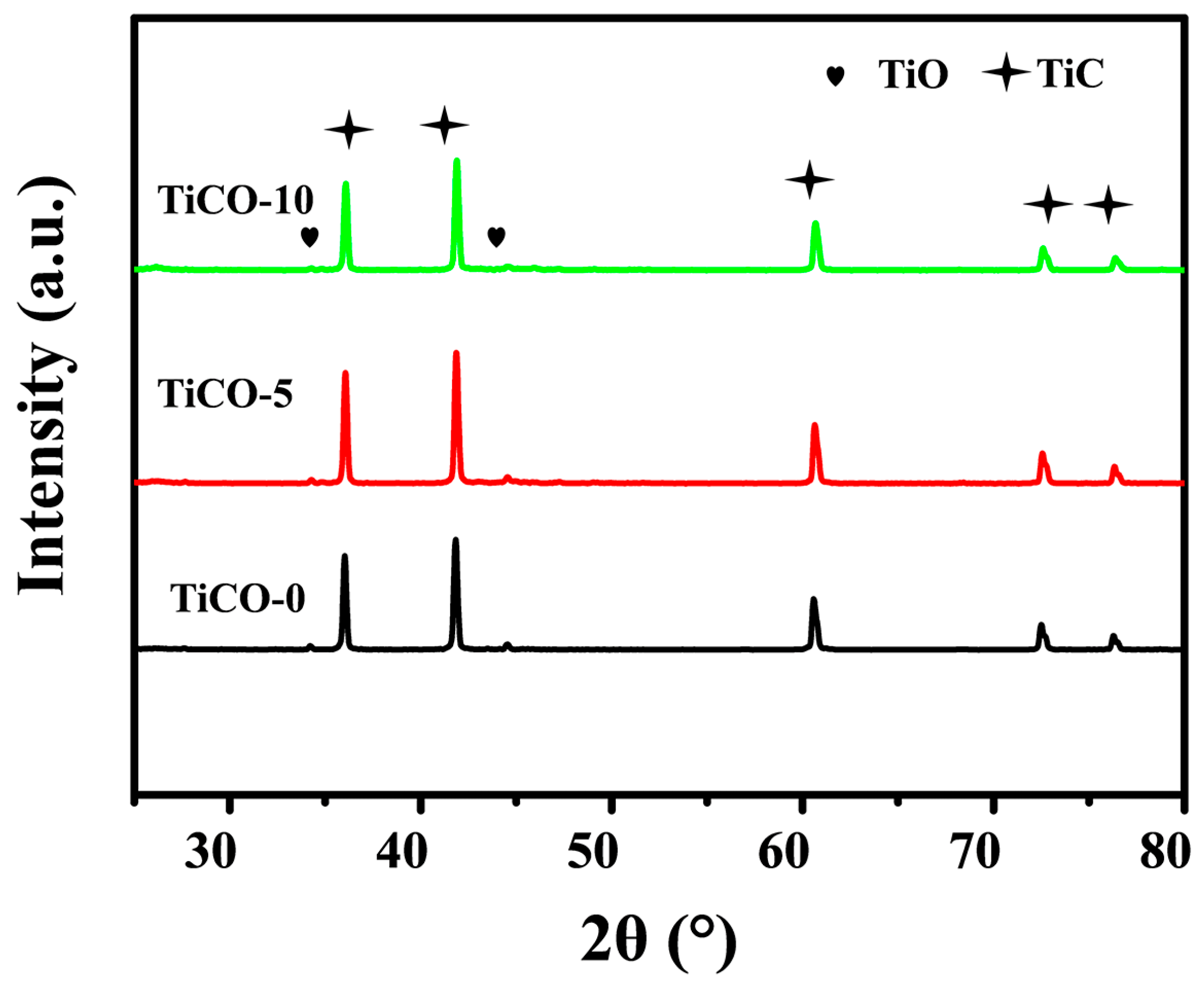
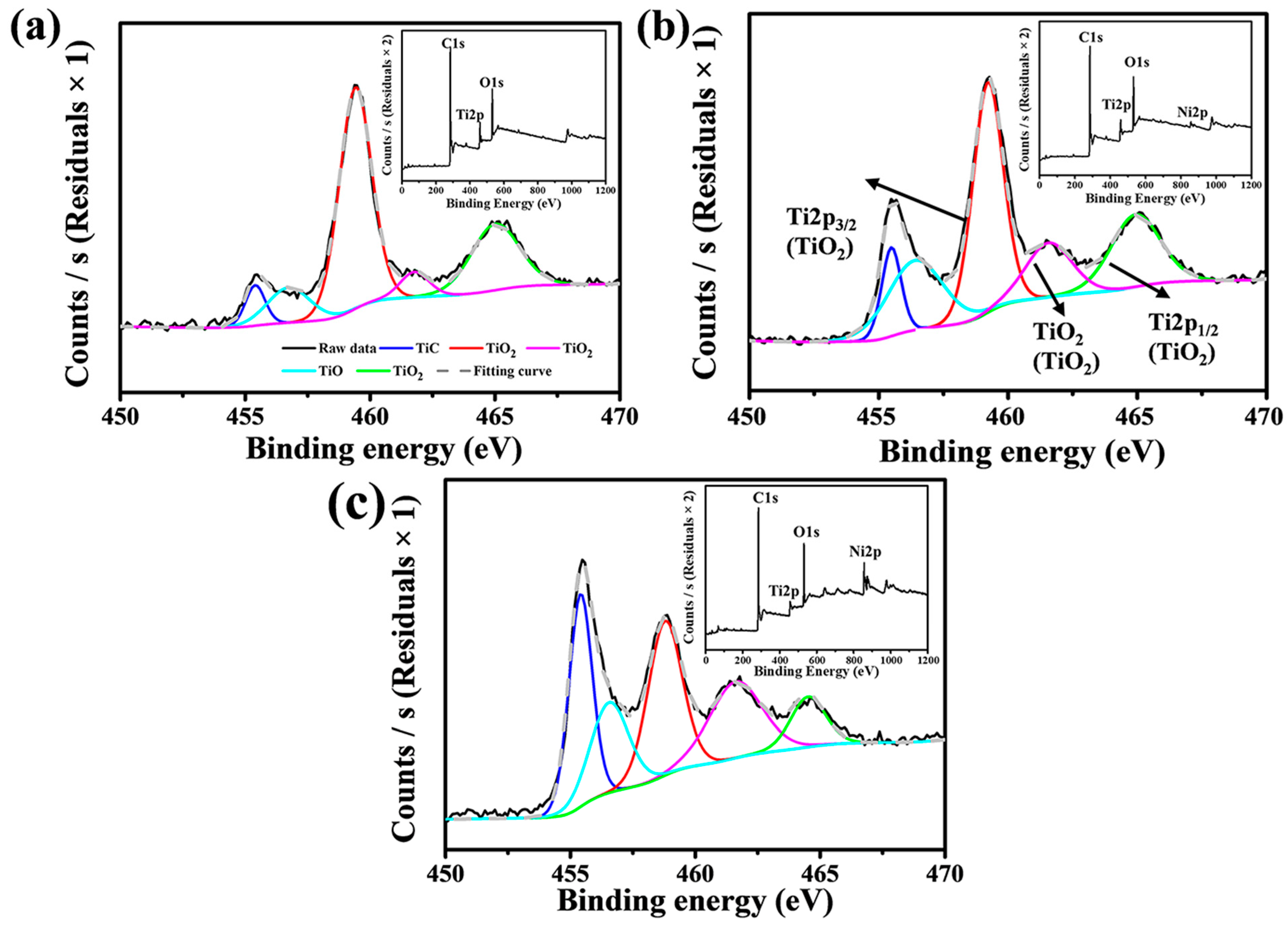
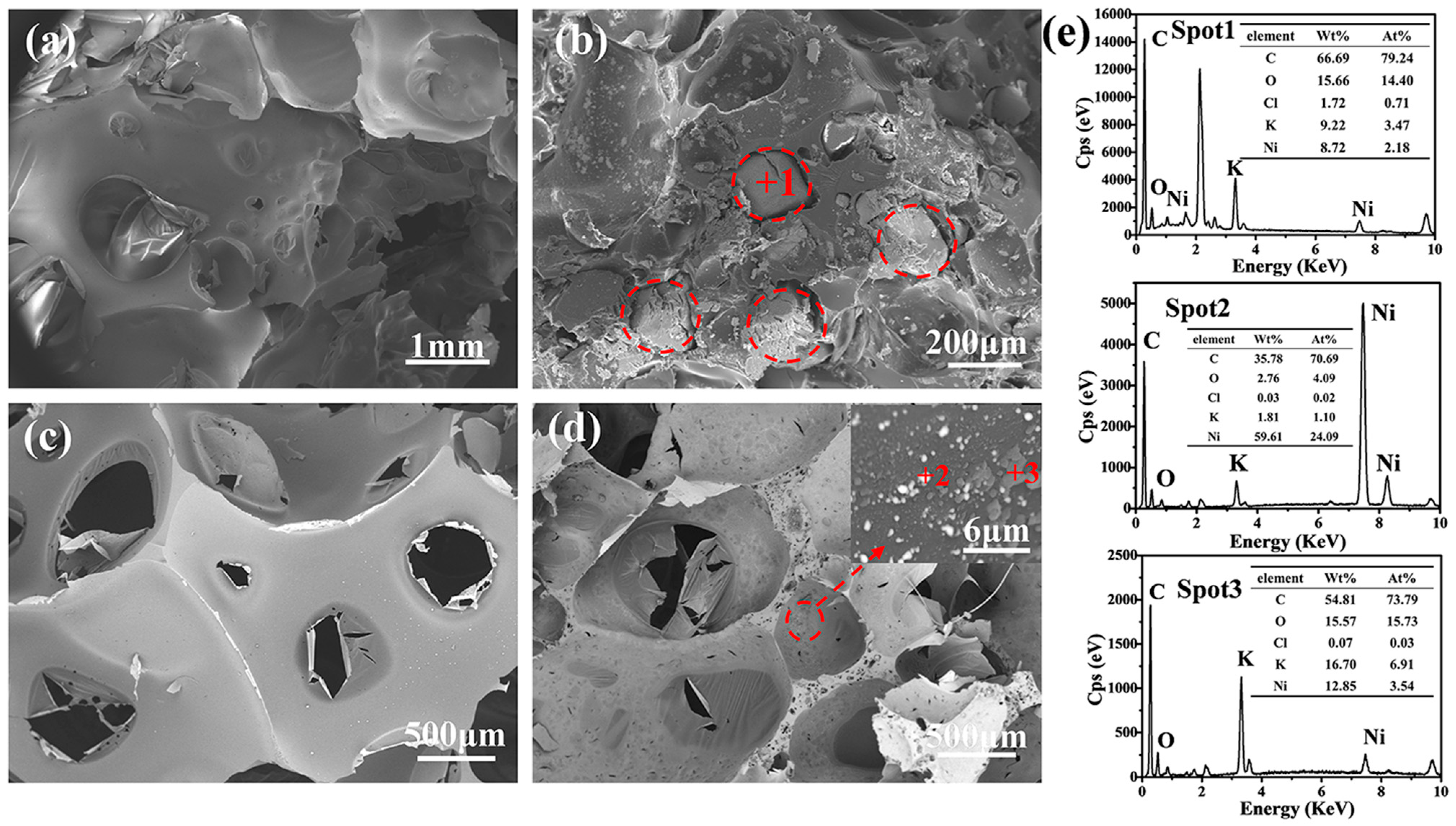
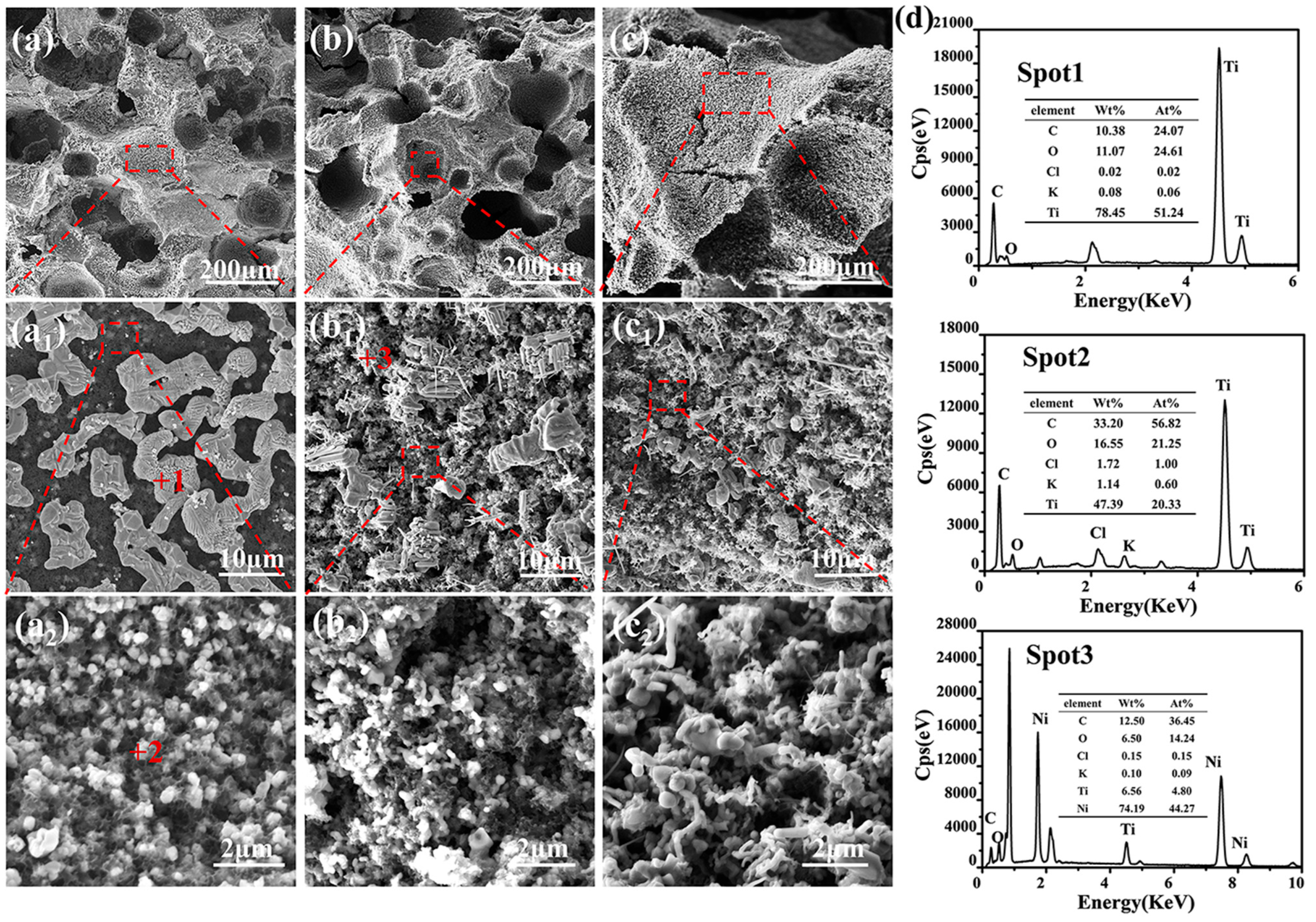
| Sample | Open Porosity (%) | Bulk Density (g·cm−3) | Compressive Strength (MPa) | Thermal Conductivity W/(m·K) |
|---|---|---|---|---|
| TiCO-0 | 63 | 1.05 | 1.89 ± 0.100 | 0.38 ± 0.014 |
| TiCO-5 | 75 | 0.87 | 0.89 ± 0.098 | 0.25 ± 0.001 |
| TiCO-10 | 82 | 0.72 | 0.07 ± 0.030 | 0.40 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Wang, C.; Li, Y.; Zhang, Z.; Feng, L. Microstructure, Mechanical Property and Thermal Conductivity of Porous TiCO Ceramic Fabricated by In Situ Carbothermal Reduction of Phenolic Resin and Titania. Nanomaterials 2024, 14, 515. https://doi.org/10.3390/nano14060515
Cao X, Wang C, Li Y, Zhang Z, Feng L. Microstructure, Mechanical Property and Thermal Conductivity of Porous TiCO Ceramic Fabricated by In Situ Carbothermal Reduction of Phenolic Resin and Titania. Nanomaterials. 2024; 14(6):515. https://doi.org/10.3390/nano14060515
Chicago/Turabian StyleCao, Xiaoyu, Chenhuan Wang, Yisheng Li, Zehua Zhang, and Lei Feng. 2024. "Microstructure, Mechanical Property and Thermal Conductivity of Porous TiCO Ceramic Fabricated by In Situ Carbothermal Reduction of Phenolic Resin and Titania" Nanomaterials 14, no. 6: 515. https://doi.org/10.3390/nano14060515




