First Principles Study of the Structure–Performance Relation of Pristine Wn+1Cn and Oxygen-Functionalized Wn+1CnO2 MXenes as Cathode Catalysts for Li-O2 Batteries
Abstract
:1. Introduction
2. Details of the Calculation
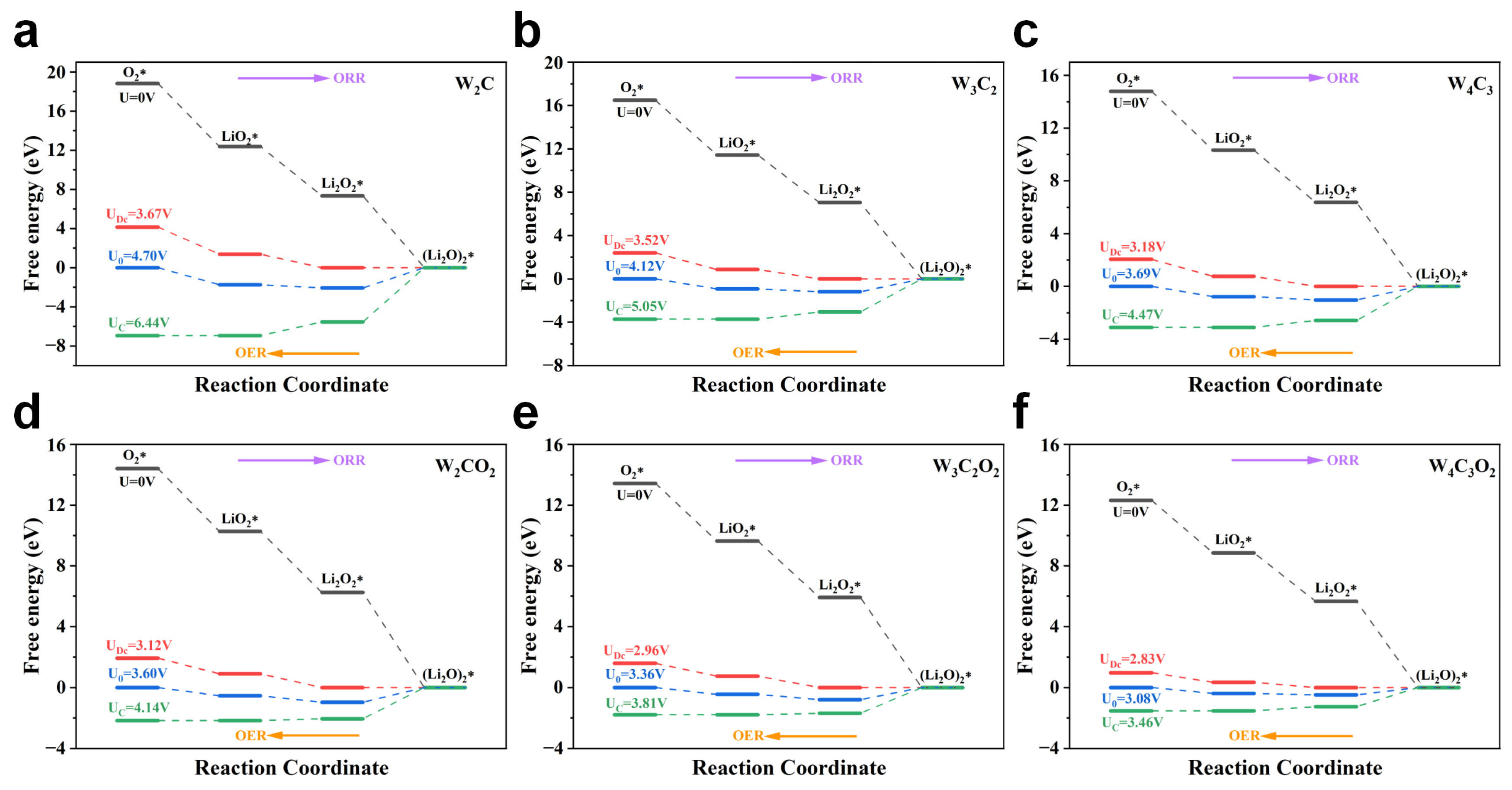
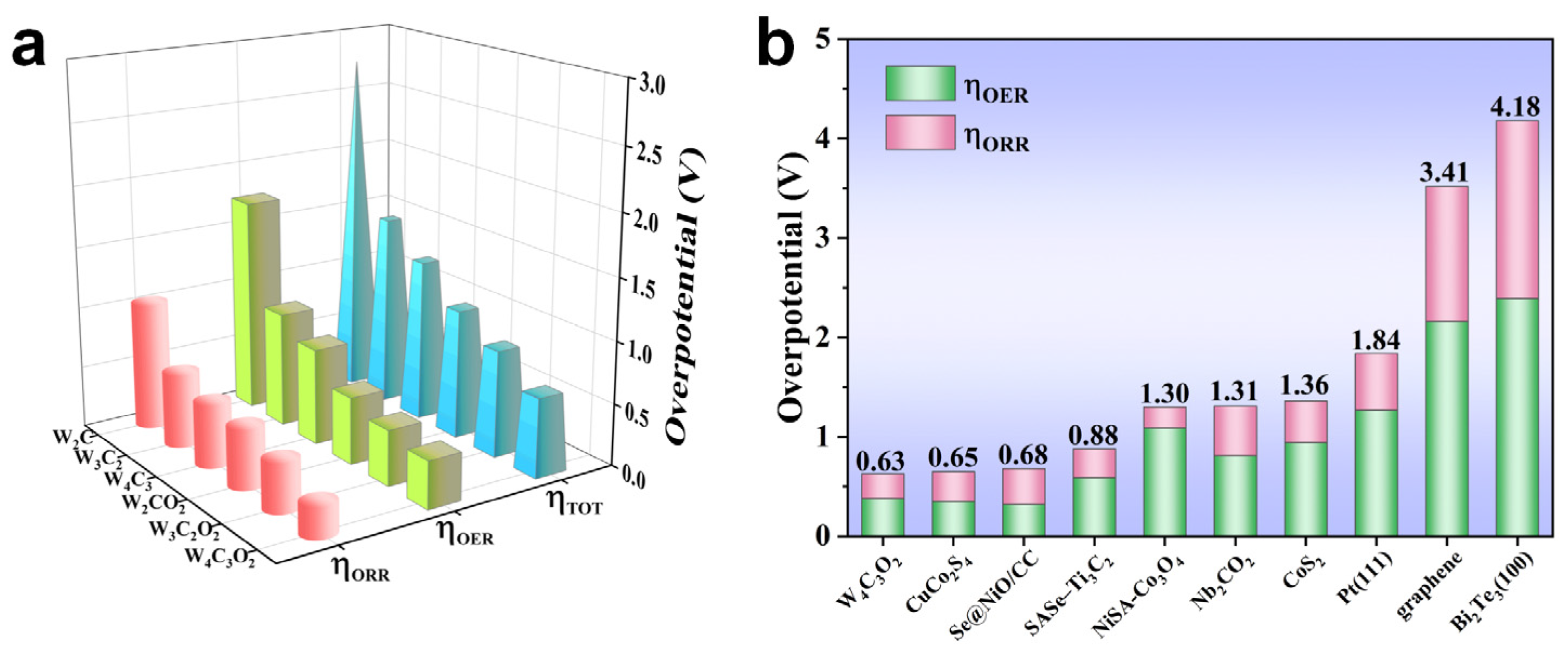
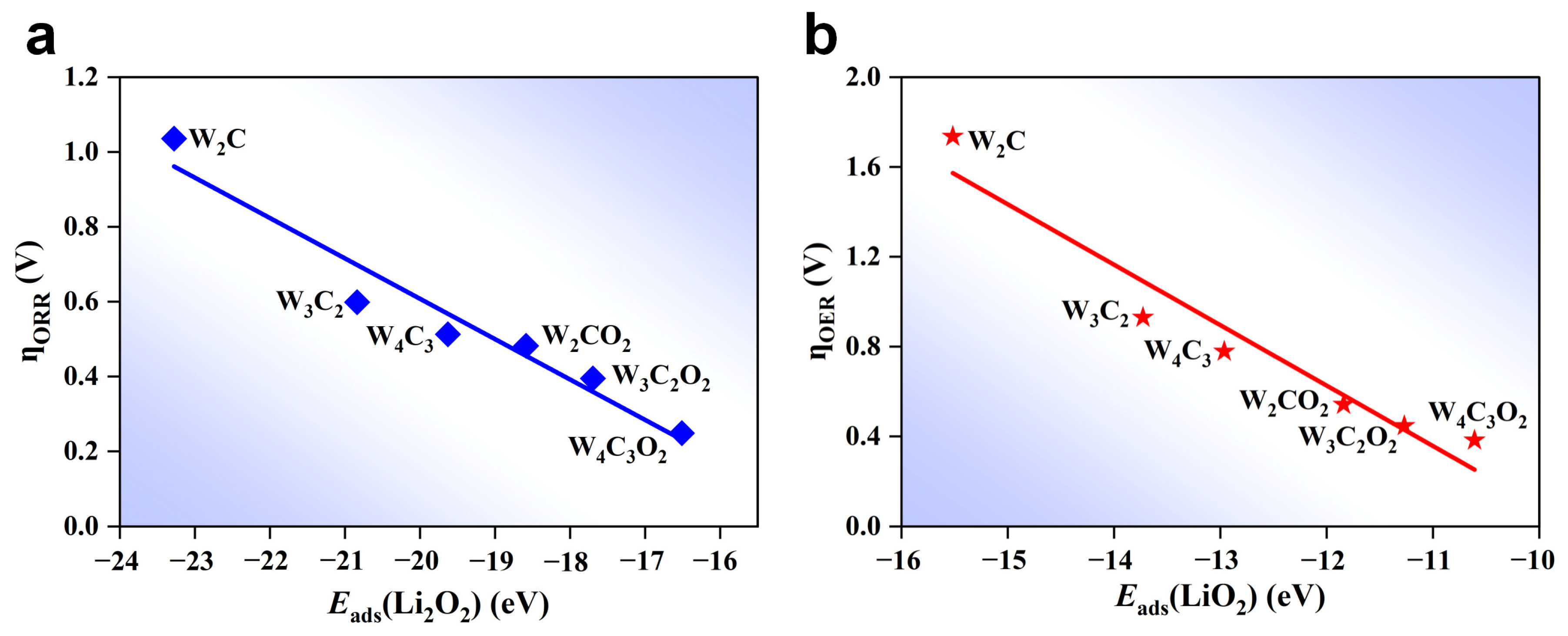
3. Results and Discussion
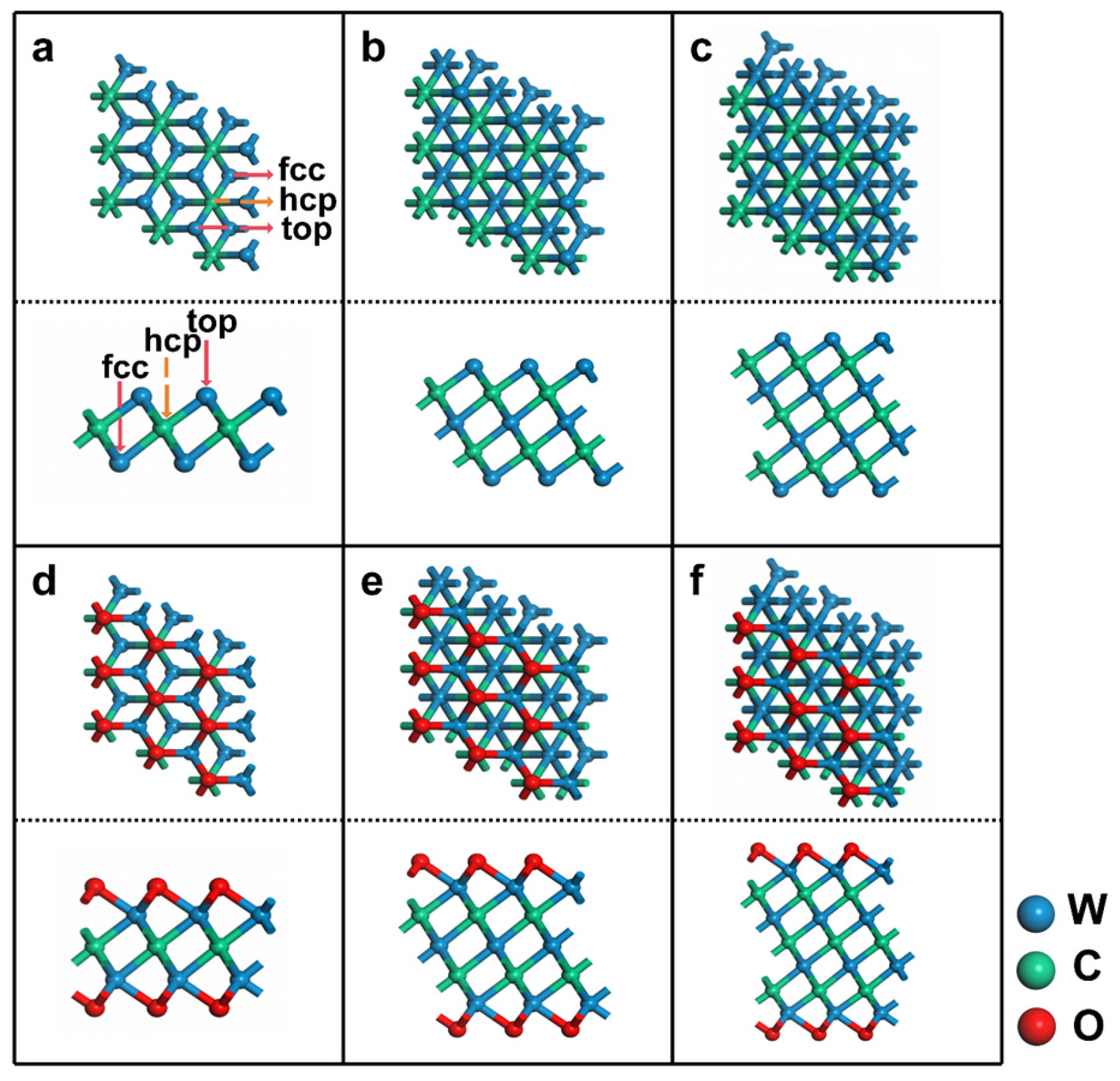
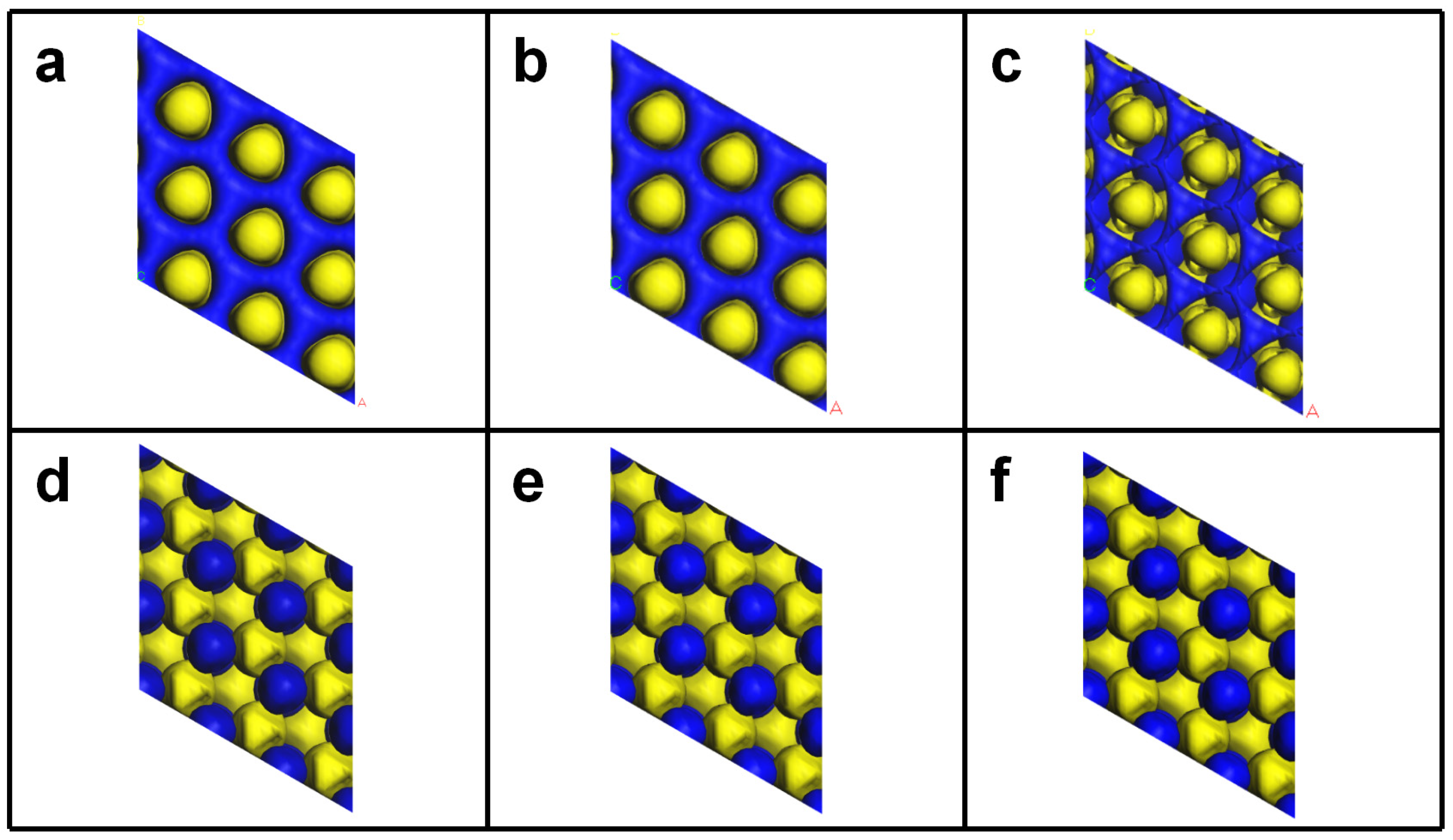
3.1. Structural Properties of WC MXenes
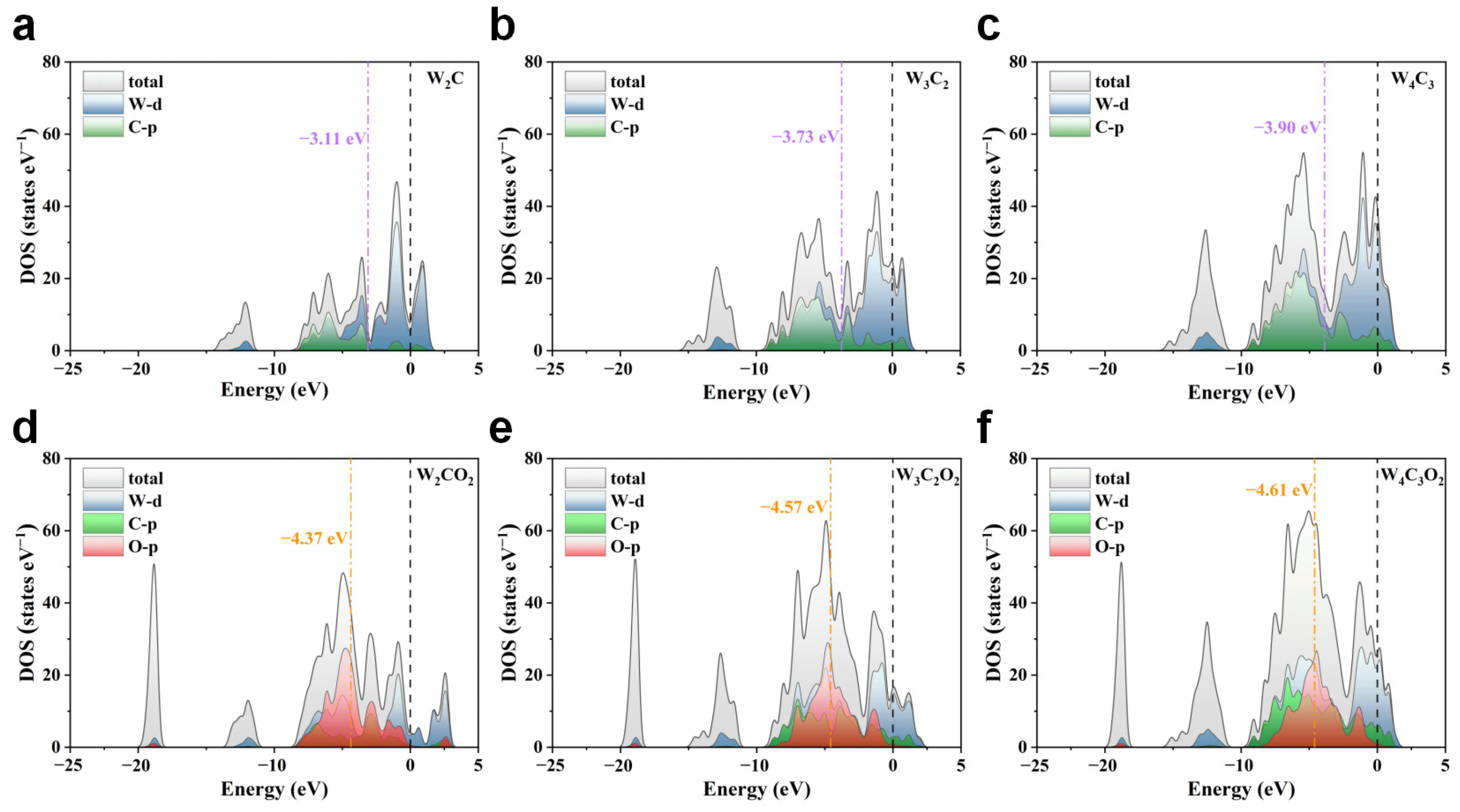
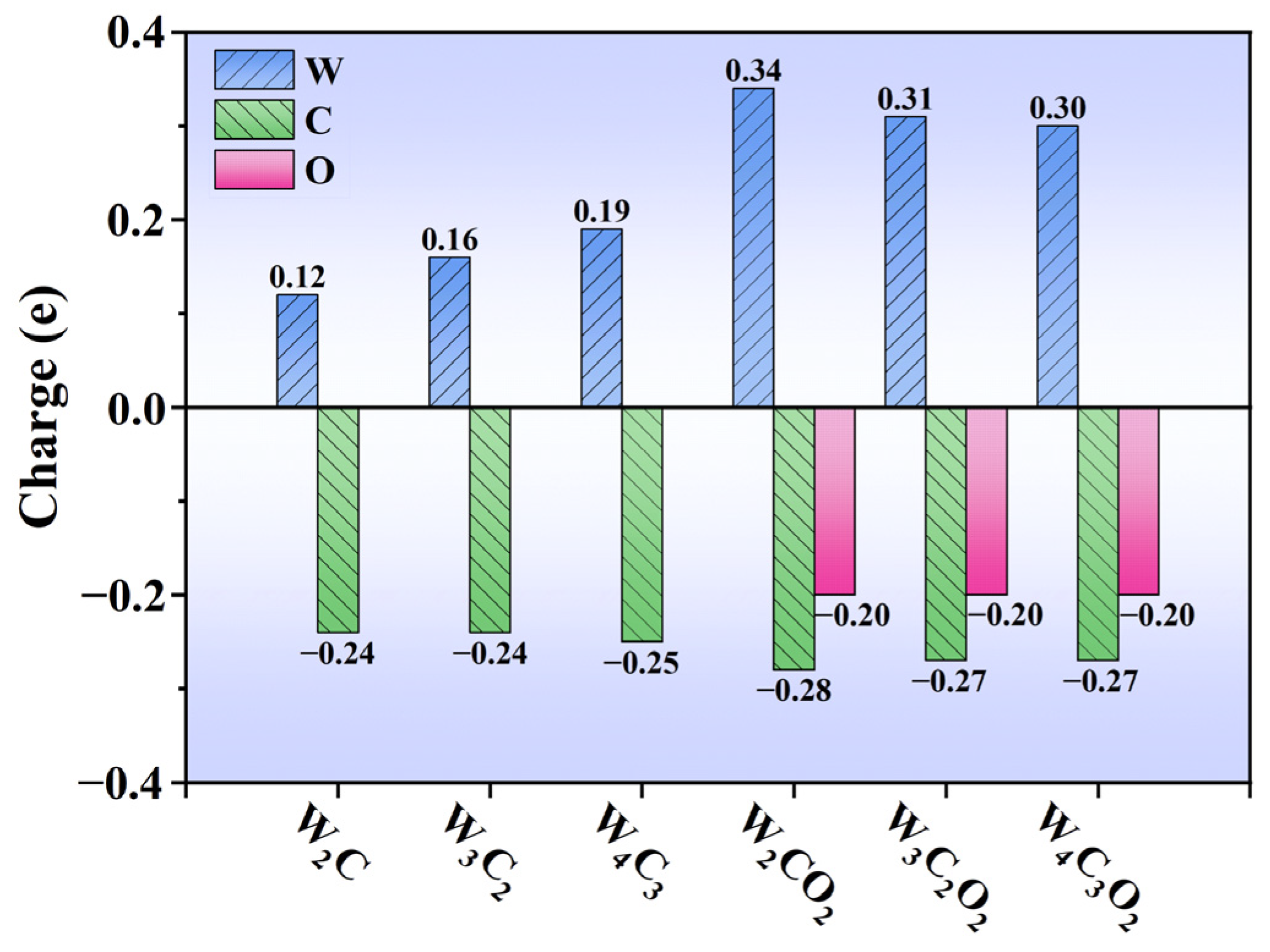
3.2. Evaluation of Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mu, X.; Xia, C.; Gao, B.; Guo, S.; Zhang, X.; He, J.; Wang, Y.; Dong, H.; He, P.; Zhou, H. Two-Dimensional Mo-Based Compounds for the Li-O2 Batteries: Catalytic Performance and Electronic Structure Studies. Energy Storage Mater. 2021, 41, 650–655. [Google Scholar] [CrossRef]
- Xia, Q.; Li, D.; Zhao, L.; Wang, J.; Long, Y.; Han, X.; Zhou, Z.; Liu, Y.; Zhang, Y.; Li, Y. Recent Advances in Heterostructured Cathodic Electrocatalysts for Non-Aqueous Li–O2 Batteries. Chem. Sci. 2022, 13, 2841–2856. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhu, Z.; Chan, K.-Y.; Li, F.; Chen, J. Photoelectrochemistry of Oxygen in Rechargeable Li–O2 Batteries. Chem. Soc. Rev. 2022, 51, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Li, H.; Lin, Y.; Yuan, M.; Nan, C.; Chen, C. Tunable Oxygen Vacancies of Cobalt Oxides in Lithium–Oxygen Batteries: Morphology Control of Discharge Product. Nano Lett. 2023, 23, 9119–9125. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Wang, C.; Hu, A.; Hou, Q.; Tan, Y.; Dou, W.; Yuan, R.; Zheng, M.; Dong, Q. High-Performance Lithium–Oxygen Batteries Using a Urea-Based Electrolyte with Kinetically Favorable One-Electron Li2O2 Oxidation Pathways. Angew. Chem. 2022, 134, E202207570. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, Z.; Dou, Y.; Li, M.; Wu, J.; Zhang, Z.; Zhou, Z. An Efficient Multifunctional Soluble Catalyst for Li-O2 Batteries. CCS Chem. 2023. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Yin, R.; Li, B.; Huang, X.; Zhao, L.; Qian, L. Single-Atom Pt Supported on Holey Ultrathin G-C3N4 Nanosheets as Efficient Catalyst for Li-O2 Batteries. J. Colloid Interface Sci. 2020, 564, 28–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, Q.; Yin, K.; Li, Y.; Tao, L.; Tan, H.; Yang, Y.; Guo, S. Engineering Eg Orbital Occupancy of Pt with Au Alloying Enables Reversible Li− O2 Batteries. Angew. Chem. Int. Ed. 2022, 61, E202201416. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Luo, Y.; Zhou, Y.; Dong, W.; Chen, W. Application of Functionalized Graphene in Li–O2 Batteries. Nanotechnology 2021, 32, 132003. [Google Scholar] [CrossRef]
- Dang, C.; Feng, P.; He, S.; Zhao, L.; Shan, A.; Li, M.; Kong, L.; Gao, L. Nicop Based Carbon Nanotube Heterostructure for Improved Oxygen Redox Reaction Kinetics in Li-O2 Batteries. Electrochim. Acta 2023, 462, 142771. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Ding, Y.; Ma, J.; Das, P.; Liu, H.; Wu, Z.-S.; Bao, X. Two-Dimensional Mn3O4 Nanosheets with Dominant (101) Crystal Planes on Graphene as Efficient Oxygen Catalysts for Ultrahigh Capacity and Long-Life Li–O2 Batteries. ACS Catal. 2022, 12, 12765–12773. [Google Scholar] [CrossRef]
- Lian, Z.; Lu, Y.; Ma, S.; Li, Z.; Liu, Q. Metal Atom-Doped Co3O4 Nanosheets for Li-O2 Battery Catalyst: Study on the Difference of Catalytic Activity. Chem. Eng. J. 2022, 445, 136852. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Kong, X.; Yu, S.; Zhang, D.; Liu, W. Construction of Co-NC@ Mo2C Hetero-Interfaces for Improving the Performance of Li-O2 Batteries. Electrochim. Acta 2023, 446, 142096. [Google Scholar] [CrossRef]
- Meng, X.; Liao, K.; Dai, J.; Zou, X.; She, S.; Zhou, W.; Ye, F.; Shao, Z. Ultralong Cycle Life Li–O2 Battery Enabled by a MOF-Derived Ruthenium–Carbon Composite Catalyst with a Durable Regenerative Surface. ACS Appl. Mater. Interfaces 2019, 11, 20091–20097. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chang, Z.; Zhong, M.; Fu, Z.-X.; Luo, J.; Zhao, Y.-F.; Li, G.-B.; Bu, X.-H. Functionalizing MOF with Redox-Active Tetrazine Moiety for Improving the Performance as Cathode of Li–O2 Batteries. CCS Chem. 2021, 3, 1297–1305. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, M.; Huang, X.; Li, H.; Sun, G. In Situ Decoration of Cop/Ti3C2Tx Composite as Efficient Electrocatalyst for Li-Oxygen Battery. Chin. Chem. Lett. 2023, 34, 107152. [Google Scholar] [CrossRef]
- Teng, K.; Tang, W.; Qi, R.; Li, B.; Deng, Y.; Zhou, M.; Wu, M.; Zhang, J.; Liu, R.; Zhang, L. Nitrogen-Deficient G-C3N4 Compounded with NiCo2S4 (NiCo2S4@ ND-CN) as a Bifunctional Electrocatalyst for Boosting the Activity of Li-O2 Batteries. Catal. Today 2023, 409, 23–30. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, R.; Du, D.; Ren, L.; Li, R.; Wen, X.; Zhao, C.; Shu, C. V2C Mxene Enriched with-O Termination as High-Efficiency Electrocatalyst for Lithium-Oxygen Battery. Appl. Mater. Today 2022, 27, 101464. [Google Scholar] [CrossRef]
- Tariq, H.A.; Nisar, U.; Abraham, J.J.; Ahmad, Z.; Alqaradawi, S.; Kahraman, R.; Shakoor, R. Tio2 Encrusted Mxene as a High-Performance Anode Material for Li-Ion Batteries. Appl. Surf. Sci. 2022, 583, 152441. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Yang, D.; Soomro, R.A.; Xu, B. Flexible Carbon Dots-Intercalated Mxene Film Electrode with Outstanding Volumetric Performance for Supercapacitors. Adv. Funct. Mater. 2023, 33, 2209918. [Google Scholar] [CrossRef]
- Tu, W.; Chen, K.; Zhu, L.; Zai, H.E.B.; Ke, X.; Chen, C.; Sui, M.; Chen, Q.; Li, Y. Tungsten-Doping-Induced Surface Reconstruction of Porous Ternary Pt-Based Alloy Electrocatalyst for Oxygen Reduction. Adv. Funct. Mater. 2019, 29, 1807070. [Google Scholar] [CrossRef]
- Du, Y.; Chen, W.; Zhou, L.; Hu, R.; Wang, S.; Li, X.; Xie, Y.; Yang, L.; Liu, Y.; Liu, Z. One-Step, in Situ Formation of WN-W2C Heterojunctions Implanted on N Doped Carbon Nanorods as Efficient Oxygen Reduction Catalyst for Metal-Air Battery. Nano Res. 2023, 16, 8773–8781. [Google Scholar] [CrossRef]
- Chen, J.; Ren, B.; Cui, H.; Wang, C. Constructing Pure Phase Tungsten-Based Bimetallic Carbide Nanosheet as An Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Small 2020, 16, 1907556. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Mao, Z.; Yan, X.; Su, R.; Guan, P.; Xu, B.; Zhang, X.; Qin, G.; Pennycook, S.J. Ultrasmall Tungsten Carbide Catalysts Stabilized in Graphitic Layers for High-Performance Oxygen Reduction Reaction. Nano Energy 2016, 28, 261–268. [Google Scholar] [CrossRef]
- GarcÍA-Romeral, N.; Morales-GarcÍA, Á.; Viñes, F.; De PR Moreira, I.; Illas, F. The Nature of The Electronic Ground State of M2C (M= Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, And W) Mxenes. Phys. Chem. Chem. Phys. 2023, 25, 31153–31164. [Google Scholar] [CrossRef]
- Qin, T.; Wang, Z.; Wang, Y.; Besenbacher, F.; Otyepka, M.; Dong, M. Recent Progress in Emerging Two-Dimensional Transition Metal Carbides. Nano-Micro Lett. 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, L.; Yao, C.; Niu, L. A DFT Computational Prediction of 2H Phase W2C Monolayer and the Effect of O Functional Groups. Phys. Lett. A 2022, 424, 127842. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Zhang, H.; Zhao, X. Exploring High-Efficiency Electrocatalysts of Metal-Doped Two-Dimensional C4N for Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions by First-Principles Screening. Phys. Chem. Chem. Phys. 2022, 24, 26061–26069. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, P.; Xiao, B.-B.; Mi, J.-L. Theoretical Study of P-Block Metal–Nitrogen–Carbon Single-Atom Catalysts for the Oxygen Reduction Reaction. Catal. Sci. Technol. 2022, 12, 6751–6760. [Google Scholar] [CrossRef]
- Jing, T.; Liang, D.; Deng, M.; Cai, S.; Qi, X. Density Functional Theory Studies of Heteroatom-Doped Graphene-Like Gan Monolayers as Electrocatalysts for Oxygen Evolution and Reduction. ACS Appl. Nano Mater. 2021, 4, 7125–7133. [Google Scholar] [CrossRef]
- Gouveia, J.D.; Morales-Garcia, A.; Vines, F.; Gomes, J.R.; Illas, F. Facile Heterogeneously Catalyzed Nitrogen Fixation by Mxenes. ACS Catal. 2020, 10, 5049–5056. [Google Scholar] [CrossRef]
- Glechner, T.; Tomastik, C.; Badisch, E.; Polcik, P.; Riedl, H. Influence of WC/C Target Composition and Bias Potential on the Structure-Mechanical Properties of Non-Reactively Sputtered WC Coatings. Surf. Coat. Technol. 2022, 432, 128036. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.D.; Viñes, F.; Illas, F.; Gomes, J.R. Mxenes Atomic Layer Stacking Phase Transitions and their Chemical Activity Consequences. Phys. Rev. Mater. 2020, 4, 054003. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, L.; Liu, H.; Jiang, X.; Aleksandr, B.; Song, E.; Jiang, Q. Designing Fluorographene with Fen4 and Con4 Moieties for Oxygen Electrode Reaction: A Density Functional Theory Study. Appl. Surf. Sci. 2021, 537, 147846. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The Synchronous-Transit Method for Determining Reaction Pathways and Locating Molecular Transition States. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Lin, L.; Yang, X.; Shi, P.; Yan, L.; Xie, K.; Deng, C.; Chen, Z. Probing the Origin of Transition Metal Carbide VC for Oxygen Reduction Reaction: A DFT Study. Surf. Interfaces 2023, 40, 103100. [Google Scholar] [CrossRef]
- Radin, M.D.; Rodriguez, J.F.; Tian, F.; Siegel, D.J. Lithium Peroxide Surfaces are Metallic, while Lithium Oxide Surfaces are Not. J. Am. Chem. Soc. 2012, 134, 1093–1103. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yao, M.; Wang, X.; Huang, H. First-Principles Study of Rocksalt Early Transition-Metal Carbides as Potential Catalysts for Li–O2 Batteries. Phys. Chem. Chem. Phys. 2018, 20, 30231–30238. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, J.; Abbas, A.; Wang, C.; Wang, H. MOF-Derived Mn2O3 Nanocage with Oxygen Vacancies as Efficient Cathode Catalysts for Li–O2 Batteries. Small 2023, 19, 2302953. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, C.; Liu, C.; Chen, X.; Hu, A.; Long, J. Rationalizing the Effect of Oxygen Vacancy on Oxygen Electrocatalysis in Li–O2 Battery. Small 2020, 16, 2001812. [Google Scholar] [CrossRef] [PubMed]
- Hummelshøj, J.; Luntz, A.C.; Nørskov, J. Theoretical Evidence for Low Kinetic Overpotentials in Li-O2 Electrochemistry. J. Chem. Phys. 2013, 138, 034703. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, X.; Qin, Y.; Wang, X.; Yao, M.; Qin, Z.; Huang, H. Oxygen Evolution Reaction on Pristine and Oxidized Tic (100) Surface in Li–O2 Battery. J. Phys. Chem. C 2018, 122, 12665–12672. [Google Scholar] [CrossRef]
- Ding, S.; Wu, L.; Yuan, X. Regulating D-Orbital Electronic Configuration of Ni-Based Chalcogenides to Enhance the Oxygen Electrode Reactions in Li-O2 Batteries. Chem. Eng. J. 2023, 478, 147473. [Google Scholar] [CrossRef]
- Lian, Z.; Lu, Y.; Zhao, S.; Li, Z.; Liu, Q. Engineering the Electronic Interaction Between Atomically Dispersed Fe and Ruo2 Attaining High Catalytic Activity and Durability Catalyst for Li-O2 Battery. Adv. Sci. 2023, 10, 2205975. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, M.; Zhao, Y.; Li, Z.; Shi, K.; Li, H.; Sun, G. Status and Prospects of Mxene-Based Lithium–Oxygen Batteries: Theoretical Prediction and Experimental Modulation. Adv. Energy Mater. 2023, 13, 2204019. [Google Scholar] [CrossRef]
- Bashir, T.; Ismail, S.A.; Wang, J.; Zhu, W.; Zhao, J.; Gao, L. Mxene Terminating Groups O,–F Or–OH,–F Or O,–OH,–F, or O,–OH,–Cl? J. Energy Chem. 2023, 76, 90–104. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Li, B.; Lin, M.; Wang, T.; Zhen, Y.; Huang, Z.; Xing, W.; Zhao, L. Theoretical Study of Catalytic Performance of WN Mxenes as Cathodes for Li-O2 Batteries: Effects of Surface Functionalization and Atomic Layers. Appl. Surf. Sci. 2023, 638, 158027. [Google Scholar] [CrossRef]
- Bai, X.; Guan, J. Applications of Mxene-Based Single-Atom Catalysts. Small Struct. 2023, 4, 2200354. [Google Scholar] [CrossRef]
- Tian, G.; Xu, H.; Wang, X.; Wen, X.; Zeng, T.; Liu, S.; Fan, F.; Xiang, W.; Shu, C. Optimizing D-Orbital Occupation on Interfacial Transition Metal Sites via Heterogeneous Interface Engineering to Accelerate Oxygen Electrode Reaction Kinetics in Lithium-Oxygen Batteries. Nano Energy 2023, 117, 108863. [Google Scholar] [CrossRef]
- Tereshchuk, P.; Golodnitsky, D.; Natan, A. Trends in the Adsorption of Oxygen and Li2O2 on Transition-Metal Carbide Surfaces: A Theoretical Study. J. Phys. Chem. C 2020, 124, 7716–7724. [Google Scholar] [CrossRef]
- Jiang, Z.; Wen, B.; Huang, Y.; Guo, Y.; Wang, Y.; Li, F. New Reaction Pathway of Superoxide Disproportionation Induced by a Soluble Catalyst in Li-O2 Batteries. Angew. Chem. 2024, 136, E202315314. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Wang, R.; Wang, J.; Zhai, Y.; Liu, X. Single-Atom Pd-N4 Catalysis for Stable Low-Overpotential Lithium-Oxygen Battery. Small 2023, 19, 2204559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.J.; Song, L.N.; Wang, X.X.; Liang, S.; Wang, H.F.; Du, X.Y.; Xu, J.J. Intrinsic Stress-Strain in Barium Titanate Piezocatalysts Enabling Lithium− Oxygen Batteries with Low Overpotential and Long Life. Angew. Chem. Int. Ed. 2023, 62, E202311739. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, Y.; Li, Y.; Zhang, T.; Wu, Z.S. Regulating Surface Electron Structure of PtNi Nanoalloy Via Boron Doping for High-Current-Density Li-O2 Batteries with Low Overpotential and Long-Life Cyclability. Smartmat 2024, 5, E1150. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Peng, S.; He, B.; Wang, J.; Du, Y.; Zhang, W.; Han, K.; Dang, F. Highly Efficient Nb2C Mxene Cathode Catalyst with Uniform O-Terminated Surface for Lithium–Oxygen Batteries. Adv. Energy Mater. 2021, 11, 2002721. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Xie, M.; Zhao, S.; Li, Z.; Liu, Q. Interfacially Engineered Induced Nickel-Based Heterostructures as Efficient Catalysts for Li-O2 Batteries. Electrochim. Acta 2023, 437, 141476. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Di, H.; Zhang, P.; Hui, X.; Yin, L. Single Semi-Metallic Selenium Atoms on Ti3C2 Mxene Nanosheets as Excellent Cathode for Lithium–Oxygen Batteries. Adv. Funct. Mater. 2021, 31, 2010544. [Google Scholar] [CrossRef]
- Tian, G.; Ren, L.; Xu, H.; Zeng, T.; Wang, X.; Wen, X.; Du, D.; Yan, Y.; Liu, S.; Shu, C. Metal Sulfide Heterojunction with Tunable Interfacial Electronic Structure as An Efficient Catalyst for Lithium-Oxygen Batteries. Sci. China Mater. 2023, 66, 1341–1351. [Google Scholar] [CrossRef]
- Ding, S.; Wu, L.; Zhang, F.; Yuan, X. Modulating Electronic Structure with Copper Doping to Promote the Electrocatalytic Performance of Cobalt Disulfide in Li–O2 Batteries. Small 2023, 19, 2300602. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Guo, L.; Su, W.; Zhao, L.; Li, G.; Chen, T.; Wang, C.; Dang, F. Stacking Surface Derived Catalytic Capability and By-Product Prevention for High Efficient Two Dimensional Bi2Te3 Cathode Catalyst in Li-Oxygen Batteries. Appl. Catal. B Environ. 2022, 318, 121844. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, T.; Shi, L.; Tan, P.; An, L. First-Principles Study of Nitrogen-, Boron-Doped Graphene and Co-Doped Graphene as the Potential Catalysts in Nonaqueous Li–O2 Batteries. J. Phys. Chem. C 2016, 120, 6612–6618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Wang, J.; Liu, J.; Wang, R.; Lin, M.; Wang, T.; Zhen, Y.; Xu, J.; Zhao, L. First Principles Study of the Structure–Performance Relation of Pristine Wn+1Cn and Oxygen-Functionalized Wn+1CnO2 MXenes as Cathode Catalysts for Li-O2 Batteries. Nanomaterials 2024, 14, 666. https://doi.org/10.3390/nano14080666
Zhu L, Wang J, Liu J, Wang R, Lin M, Wang T, Zhen Y, Xu J, Zhao L. First Principles Study of the Structure–Performance Relation of Pristine Wn+1Cn and Oxygen-Functionalized Wn+1CnO2 MXenes as Cathode Catalysts for Li-O2 Batteries. Nanomaterials. 2024; 14(8):666. https://doi.org/10.3390/nano14080666
Chicago/Turabian StyleZhu, Liwei, Jiajun Wang, Jie Liu, Ruxin Wang, Meixin Lin, Tao Wang, Yuchao Zhen, Jing Xu, and Lianming Zhao. 2024. "First Principles Study of the Structure–Performance Relation of Pristine Wn+1Cn and Oxygen-Functionalized Wn+1CnO2 MXenes as Cathode Catalysts for Li-O2 Batteries" Nanomaterials 14, no. 8: 666. https://doi.org/10.3390/nano14080666






