Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Source and Growth Conditions
2.2. Pathogenicity Test
2.2.1. In Vitro Pathogenicity Test
2.2.2. Pathogenicity Test under Greenhouse Conditions
Inoculum Preparation and Soil Infestation
Virulence Assay in Bean Seedling
2.3. Biosynthesis of AgNPs Using Various Plant Extracts
2.3.1. Preparation of Plant Extract
2.3.2. Formation of AgNPs
2.4. AgNPs Efficacy on the Growth of the Root Rot Fungi
2.5. Characterization of the Biosynthesized AgNPs
2.6. Minimum Inhibitory Concentrations of AgNPs
2.7. Inhibition of Seed Infection by Root Rot Fungi Using the MIC of AgNPs
2.8. Antifungal Activity of AgNPs MIC under Greenhouse Conditions
2.9. Statistical Analysis
3. Results and Discussion
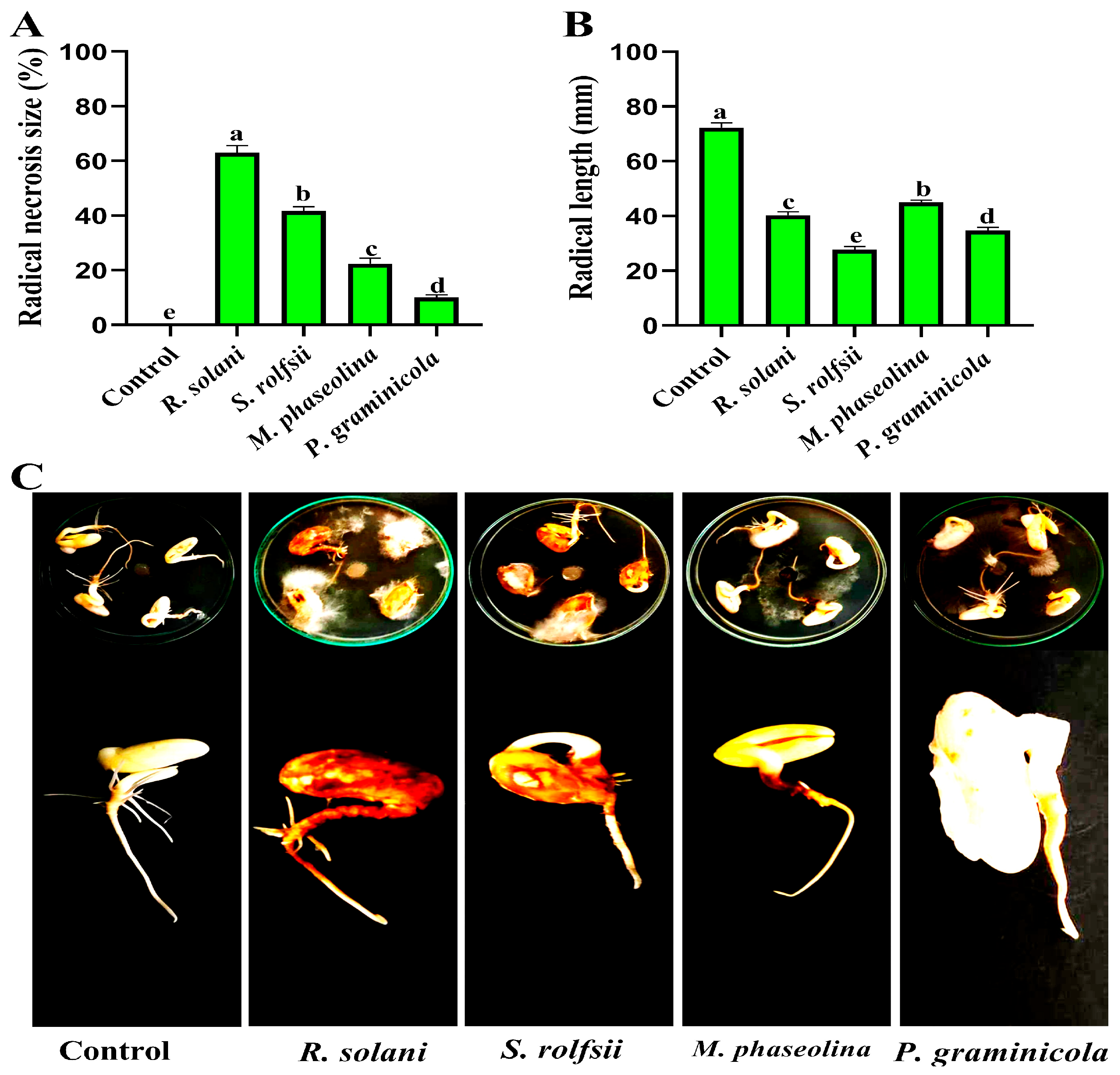
3.1. Pathogenicity Test
3.2. Biosynthesis of AgNPs
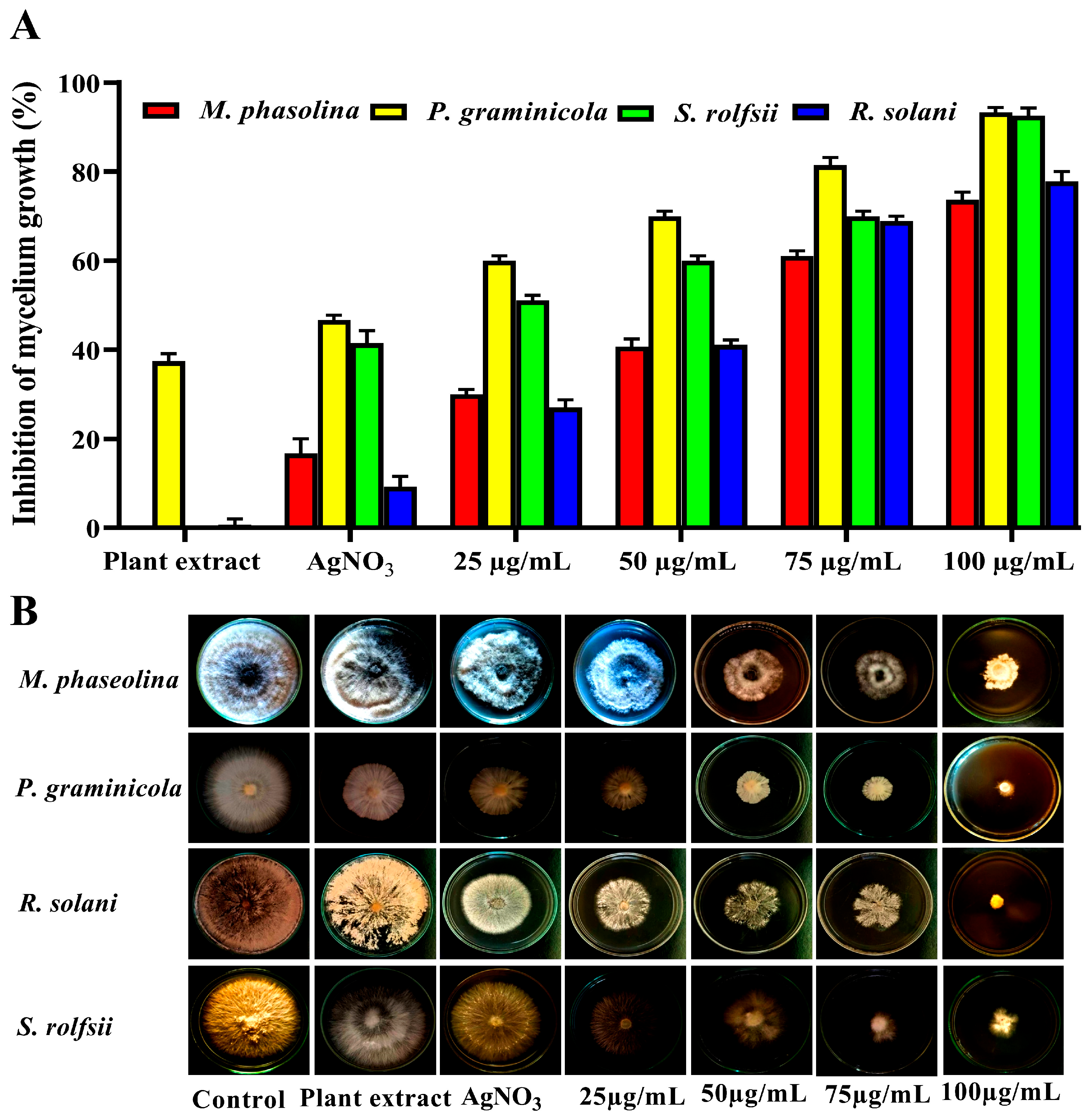
3.3. Efficacy of AgNP Treatment on Root Rot Fungi
3.4. Characterization of AgNPs Synthesized with A. graecorum
3.5. Minimum Inhibition Concentration (MIC) of AgNPs
3.6. In Vitro MIC Inhibited Bean Root Rot Diseases
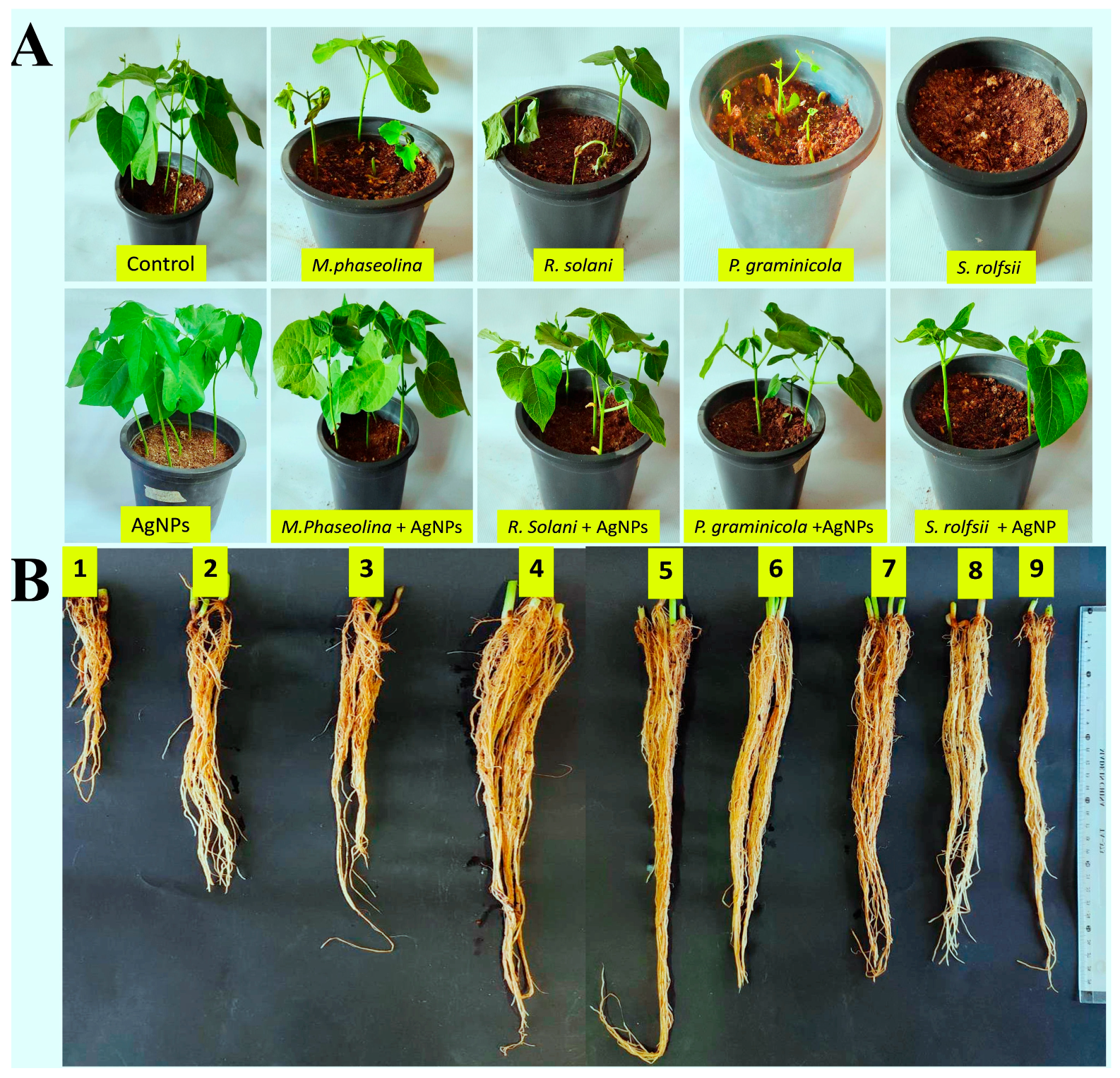
3.7. Under Greenhouse MIC Inhibited Bean Root Rot Diseases
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, G. Feeding the World in 2050 and Beyond—Part 1: Productivity Challenges. Michigan State University Extension. 3 December 2018. Available online: https://www.canr.msu.edu/news/feeding-the-world-in-2050-and-beyond-part-1 (accessed on 11 January 2024).
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef]
- Castro-Guerrero, N.A.; Isidra-Arellano, M.C.; Mendoza-Cozatl, D.G.; Valdés-López, O. Common bean: A legume model on the rise for unraveling responses and adaptations to iron, zinc, and phosphate deficiencies. Front. Plant Sci. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)–model food legumes. Plant Soil. 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Porteous-Álvarez, A.J.; Mayo-Prieto, S.; Álvarez-García, S.; Reinoso, B.; Casquero, P.A. Genetic Response of Common Bean to the Inoculation with Indigenous Fusarium Isolates. J. Fungi 2020, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Mulas, D.; García-Fraile, P.; Carro, L.; Ramírez-Bahena, M.-H.; Casquero, P.; Velázquez, E.; González-Andrés, F. Distribution and efficiency of Rhizobium leguminosarum strains nodulating Phaseolus vulgaris in Northern Spanish soils: Selection of native strains that replace conventional N fertilization. Soil Biol. Biochem. 2011, 43, 2283–2293. [Google Scholar] [CrossRef]
- Bramhanwade, K.; Shende, S.; Bonde, S.; Gade, A.; Rai, M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016, 14, 229–235. [Google Scholar] [CrossRef]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef]
- Valenciano, J.; Casquero, P.; Boto, J.; Guerra, M. Effect of sowing techniques and seed pesticide application on dry bean yield and harvest components. Field Crop. Res. 2006, 96, 2–12. [Google Scholar] [CrossRef]
- Schwartz, H.F.; Steadman, J.R.; Hall, R.; Forster, R.L. Compendium of Bean Diseases; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2005. [Google Scholar]
- Pathania, A.; Sharma, S.K.; Sharma, P.N. Common bean. In Broadening the Genetic Base of Grain Legumes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 11–50. [Google Scholar]
- El-Mohamedy, R.; Abd Alla, M. Bio-priming seed treatment for biological control of soil borne fungi causing root rot of green bean (Phaseolus vulgaris L.). J. Agric. Technol. 2013, 9, 589–599. [Google Scholar]
- Naseri, B. Root rot of common bean in Zanjan, Iran: Major pathogens and yield loss estimate Austral. Plant Pathol. 2008, 37, 546–551. [Google Scholar] [CrossRef]
- Leon, M.C.C.; Stone, A.; Dick, R.P. Organic soil amendments: Impacts on snap bean common root rot (Aphanomyes euteiches) and soil quality. Appl. Soil Ecol. 2006, 31, 199–210. [Google Scholar] [CrossRef]
- Pieczarka, D.; Abawi, G. Effect of interaction between Fusarium, Pythium, and Rhizoctonia on severity of bean root rot. Phytopathology 1978, 68, 403–408. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Sallam, N.M.; Mohamed, A.A.; Hassan, M.H. Effect of mycorrhiza and biofertilisers on reducing the incidence of Fusarium root and pod rot diseases of peanut. Arch. Phytopathol. Plant Protect. 2013, 46, 868–881. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Upadhyaya, C.P.; Singh, A.; Abd-Elsalam, K.A.; Prasad, R. Applications of silver nanoparticles in plant protection. In Nanobiotechnology Applications in Plant Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 247–265. [Google Scholar]
- Ahmed, T.; Luo, J.; Noman, M.; Ijaz, M.; Wang, X.; Masood, H.A.; Manzoor, N.; Wang, Y.; Li, B. Microbe-mediated nanoparticle intervention for the management of plant diseases. Crop Health 2023, 1, 3. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, M.J.; Gorczyca, A.; Szymocha, A. Physiological effects of nanosilver on vegetative mycelium, conidia and the development of the entomopathogenic fungus, Isaria fumosorosea. Biocontrol Sci. Technol. 2015, 25, 873–887. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Xiong, Q.; Xie, Z.; Cheng, J.; Yu, B.; Zhang, H.; Su, Y.; Zhao, J. Functional, eco-friendly, and starch-based nanocarriers with sustained release of carvacrol for persistent control of tomato gray mold. Crop Health 2023, 1, 13. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Wang, J.; Ijaz, M.; Shahid, M.; Islam, M.S.; Azizullah; Manzoor, I.; Li, D.; Song, F. Nano-enabled crop resilience against pathogens: Potential, mechanisms and strategies. Crop Health 2023, 1, 15. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological synthesis of silver nanoparticles and prospects in plant disease management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, R.; Guo, J.; Ahmed, T.; Ibrahim, E.; Ali, M.A.; Rizwan, M.; Ijaz, M.; An, Q.; Wang, Y.; Wang, J. Integrative transcriptomic and metabolomic analyses reveals the toxicity and mechanistic insights of bioformulated chitosan nanoparticles against Magnaporthe oryzae. Chemosphere 2024, 356, 141904. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, R.; Guo, J.; Ahmed, T.; Jiang, H.; Raza, M.; Shahid, M.; Ibrahim, E.; Wang, Y.; Wang, J.; Yan, C. Bio-formulated chitosan nanoparticles enhance disease resistance against rice blast by physiomorphic, transcriptional, and microbiome modulation of rice (Oryza sativa L.). Carbohydr. Polym. 2024, 334, 122023. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Huo, P.; Chandran, K.; Dakshinamoorthi, B.M.; Yun, S.-I.; Liu, B. A review on the toxicity of silver nanoparticles against different biosystems. Chemosphere 2022, 292, 133397. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Duarte, A.C.; Pereira, E.; Ahmad, I. Silver nanoparticles in soil–plant systems. J. Nanopart. Res. 2013, 15, 1896. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Socol, Y.; Abramson, O.; Gedanken, A.; Meshorer, Y.; Berenstein, L.; Zaban, A. Suspensive electrode formation in pulsed sonoelectrochemical synthesis of silver nanoparticles. Langmuir 2002, 18, 4736–4740. [Google Scholar] [CrossRef]
- Solanki, J.N.; Murthy, Z. Highly monodisperse and sub-nano silver particles synthesis via microemulsion technique. Colloid Surf. A-Physicochem. Eng. Asp. 2010, 359, 31–38. [Google Scholar] [CrossRef]
- Nasser, R.; Ibrahim, E.; Fouad, H.; Ahmad, F.; Li, W.; Zhou, Q.; Yu, T.; Chidwala, N.; Mo, J. Termiticidal, biochemical, and morpho-histological effects of botanical based nanoemulsion against a subterranean termite, Odontotermes formosanus Shiraki. Front. Plant Sci. 2024, 14, 1292272. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically synthesized metal nanoparticles: Recent advancement and future perspectives in cancer theranostics. Future Sci. OA 2017, 3, FSO203. [Google Scholar]
- Basbagci, G.; Unal, F.; Uysal, A.; Dolar, F.S. Identification and pathogenicity of Rhizoctonia solani AG-4 causing root rot on chickpea in Turkey. Span. J. Agric. Res. 2019, 17, 1007. [Google Scholar] [CrossRef]
- Ichielevich-Auster, M.; Sneh, B.; Koltin, Y.; Barash, I. Suppression of damping-off caused by Rhizoctonia species by a nonpathogenic isolate of R. solani. Phytopathology 1985, 75, 1080–1084. [Google Scholar] [CrossRef]
- Prajapati, S.; Godika, S.; Kumar, N.; Lakhran, L.; Maurya, S.; Sharma, J. Isolation, identification and pathogenicity of Sclerotinia sclerotiorum causing Sclerotinia rot of chilli. J Pharmacogn. Phytochem. 2020, 9, 20–23. [Google Scholar]
- Güney, İ.G.; Güldür, E. Inoculation techniques for assessing pathogenicity of Rhizoctonia solani, Macrophomina phaseolina, Fusarium oxysporum and Fusarium solani on pepper seedlings. Türkiye Tarımsal Araştırmalar Derg. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cell. Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Masum, M.; Islam, M.; Siddiqa, M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Luo, J.; Ahmed, T.; Wu, W.; Yan, C.; Li, B. Biosynthesis of Silver Nanoparticles Using Onion Endophytic Bacterium and Its Antifungal Activity against Rice Pathogen Magnaporthe oryzae. J. Fungi 2020, 6, 294. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-mediated ZnO nanoparticles: A green tool for controlling soil-borne pathogens in cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef]
- García, R.; Robinson, R.A.; Aguilar, J.A.; Sandoval, S.; Guzman, R. Recurrent selection for quantitative resistance to soil-borne diseases in beans in the Mixteca region, Mexico. Euphytica 2003, 130, 241–247. [Google Scholar] [CrossRef]
- Liton, M.J.A.; Bhuiyan, M.; Jannat, R.; Ahmed, J.; Rahman, M.; Rubayet, M.T. Efficacy of Trichoderma-fortified compost in controlling soil-borne diseases of bush bean (Phaseolus vulgaris L.) and sustainable crop production. Adv. Agric. 2019, 7, 123–136. [Google Scholar]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Palanisamy, S.; Goutham, G.; Koodalingam, A.; Prabhu, N.; Kannapiran, E.; Basu, M.J.; Arulvasu, C. Biosynthesis of silver nanoparticles using aqueous extract of Phyllanthus acidus L. fruits and characterization of its anti-inflammatory effect against H2O2 exposed rat peritoneal macrophages. Process Biochem. 2017, 55, 172–181. [Google Scholar] [CrossRef]
- Murugan, K.; Senthilkumar, B.; Senbagam, D.; Al-Sohaibani, S. Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int. J. Nanomed. 2014, 9, 2431. [Google Scholar]
- Matei, P.M.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.T.; Martín-Ramos, P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Baka, Z.A. Green Synthesis of Silver Nanoparticles Using Pomegranate and Orange Peel Extracts and Their Antifungal Activity against Alternaria solani, the Causal Agent of Early Blight Disease of Tomato. Plants 2021, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, A.; Patade, V.; Balakrishna, G.; Pandey, H.; Singh, A. Synthesis of silver nanoparticles using extract of Ocimum kilimandscharicum and its antimicrobial activity against plant pathogens. SN Appl. Sci. 2019, 1, 1652. [Google Scholar] [CrossRef]
- Ajlouni, A.-W.; Hamdan, E.H.; Alshalawi, R.A.E.; Shaik, M.R.; Khan, M.; Kuniyil, M.; Alwarthan, A.; Ansari, M.A.; Khan, M.; Alkhathlan, H.Z. Green synthesis of silver nanoparticles using aerial part extract of the Anthemis pseudocotula boiss. plant and their biological activity. Molecules 2022, 28, 246. [Google Scholar] [CrossRef]
- Umadevi, M.; Shalini, S.; Bindhu, M. Synthesis of silver nanoparticle using D. carota extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025008. [Google Scholar] [CrossRef]
- Hossain, A.; Hong, X.; Ibrahim, E.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeya dadantii. Molecules 2019, 24, 2303. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.J.P.; Murugan, M.; Gurunathan, S. Biosynthesis of silver nanoparticles from the culture supernatant of Bacillus marisflavi and their potential antibacterial activity. J. Ind. Eng. Chem. 2014, 20, 1505–1510. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Wani, A.; Shah, M. A unique and profound effect of MgO and ZnO nanoparticles on some plant pathogenic fungi. J. Appl. Pharm. Sci. 2012, 2, 4. [Google Scholar]
- Abdel-Aziz, M.M.; Emam, T.M.; Elsherbiny, E.A. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater. Sci. Eng. C 2020, 109, 110617. [Google Scholar] [CrossRef] [PubMed]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef] [PubMed]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2013, 112, 384–387. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
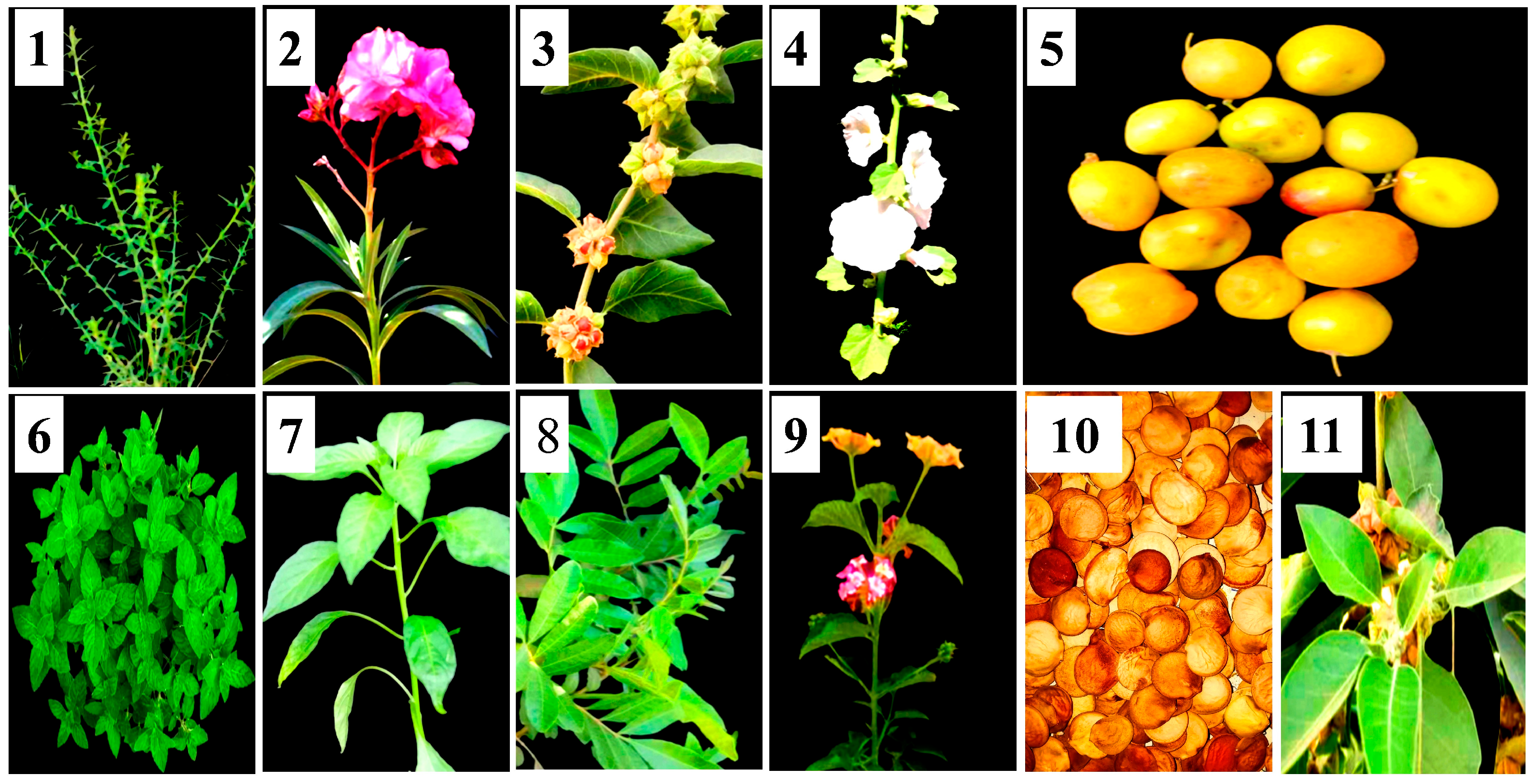








| English Name | Latin Name | Used Part |
|---|---|---|
| Alhagi graecorum | Leaves |
| Nerium oleander | Flowers |
| Withania somnifera | Fruits |
| Althoea officinalis | Flowers |
| Ziziphus spina shristi | Leaves |
| Mentha arvensis | Leaves |
| Capsicum annuum | Fruits |
| Schinus terebinthifolius | Leaves |
| Lantana camara | Flowers |
| Bauhinia variegata | Seeds |
| Withania smnifera | Leaves |
| Treatments | Disease Severity (%) | Growth Rates | ||||
|---|---|---|---|---|---|---|
| Foliar Symptom | Root Rot Symptom | Shoot Length (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) | |
| S. rolfsii | 100.00 | 100.00 | 11.00 | 0.00 | 0.40 | 0.20 |
| P. graminicola | 83.33 | 77.00 | 10.00 | 4.00 | 0.75 | 0.42 |
| R. solani | 55.00 | 38.00 | 26.20 | 7.00 | 1.44 | 0.61 |
| M. phaseolina | 44.14 | 32.00 | 28.00 | 8.00 | 2.10 | 0.80 |
| Control | 0.00 | 0.00 | 33.00 | 15.00 | 3.50 | 1.30 |
| Treatments | Inhibition of Mycelium Growth (%) | |||
|---|---|---|---|---|
| R. solani | P. graminicola | S. rolfsii | M. phaseolina | |
| 1 | 92.60 | 94.44 | 75.93 | 79.63 |
| 2 | 89.61 | 85.56 | 62.96 | 63.33 |
| 3 | 62.52 | 90.00 | 70.74 | 66.67 |
| 4 | 51.21 | 31.48 | 61.11 | 60.00 |
| 5 | 15.70 | 93.33 | 55.19 | 3.33 |
| 6 | 60.08 | 91.85 | 8.89 | 68.52 |
| 7 | 55.65 | 76.67 | 68.89 | 35.19 |
| 8 | 14.19 | 87.78 | 26.30 | 2.22 |
| 9 | 53.68 | 90.00 | 56.67 | 30.00 |
| 10 | 87.24 | 53.70 | 72.22 | 75.19 |
| 11 | 83.71 | 92.22 | 52.22 | 37.41 |
| Treatments | Disease Assessment | |||
|---|---|---|---|---|
| Pre (%) | Post (%) | FS (%) | RS (%) | |
| M. phasolina | 33.30 | 49.67 | 51.00 | 36.00 |
| R. solani | 66.70 | 83.33 | 59.33 | 43.67 |
| P. graminicola | 66.70 | 83.33 | 79.33 | 78.33 |
| S. rolfsii | 100.00 | 100.00 | 100.00 | 100.00 |
| M. phasolina + AgNPs | 0.00 | 8.33 | 12.67 | 6.67 |
| R. solani + AgNPs | 8.30 | 16.67 | 34.67 | 24.67 |
| P. graminicola + AgNPs | 16.70 | 19.33 | 54.00 | 43.00 |
| S. rolfsii + AgNPs | 58.30 | 50.00 | 70.00 | 63.00 |
| AgNPs | 0.00 | 0.00 | 0.00 | 0.00 |
| Control | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatments | Growth Parameters | |||||
|---|---|---|---|---|---|---|
| Sg (%) | Rl (cm) | Sl (cm) | Vi | Fw (g) | Dw (g) | |
| M. phasolina | 66.67 | 16.00 | 13.33 | 1955.56 | 14.53 | 1.03 |
| R. solani | 33.33 | 15.00 | 16.00 | 1033.33 | 13.53 | 0.70 |
| P. graminicola | 33.33 | 12.33 | 8.00 | 677.78 | 6.17 | 0.40 |
| S. rolfsii | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| M. phasolina + AgNPs | 100.00 | 28.33 | 20.33 | 4866.67 | 40.30 | 2.87 |
| R. solani + AgNPs | 91.67 | 31.33 | 24.00 | 5072.22 | 33.77 | 2.40 |
| P. graminicola + AgNPs | 83.33 | 28.67 | 22.00 | 4222.22 | 27.37 | 1.90 |
| S. rolfsii + AgNPs | 41.67 | 21.00 | 18.33 | 1638.89 | 20.83 | 1.27 |
| AgNPs | 100.00 | 55.00 | 35.33 | 9033.33 | 61.33 | 4.83 |
| Control | 100.00 | 45.00 | 26.00 | 7100.00 | 50.00 | 3.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, E.; Ahmad, A.A.; Abdo, E.-S.; Bakr, M.A.; Khalil, M.A.; Abdallah, Y.; Ogunyemi, S.O.; Mohany, M.; Al-Rejaie, S.S.; Shou, L.; et al. Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles. Nanomaterials 2024, 14, 710. https://doi.org/10.3390/nano14080710
Ibrahim E, Ahmad AA, Abdo E-S, Bakr MA, Khalil MA, Abdallah Y, Ogunyemi SO, Mohany M, Al-Rejaie SS, Shou L, et al. Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles. Nanomaterials. 2024; 14(8):710. https://doi.org/10.3390/nano14080710
Chicago/Turabian StyleIbrahim, Ezzeldin, Abdelmonim Ali Ahmad, El-Sayed Abdo, Mohamed Ahmed Bakr, Mohamed Ali Khalil, Yasmine Abdallah, Solabomi Olaitan Ogunyemi, Mohamed Mohany, Salim S. Al-Rejaie, Linfei Shou, and et al. 2024. "Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles" Nanomaterials 14, no. 8: 710. https://doi.org/10.3390/nano14080710
APA StyleIbrahim, E., Ahmad, A. A., Abdo, E.-S., Bakr, M. A., Khalil, M. A., Abdallah, Y., Ogunyemi, S. O., Mohany, M., Al-Rejaie, S. S., Shou, L., Li, B., & Galal, A. A. (2024). Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles. Nanomaterials, 14(8), 710. https://doi.org/10.3390/nano14080710











