Structural, Optical and Dielectric Properties of Some Nanocomposites Derived from Copper Oxide Nanoparticles Embedded in Poly(vinylpyrrolidone) Matrix
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of CuO Nanoparticles
2.3. Preparation of Film Samples
2.4. Characterization
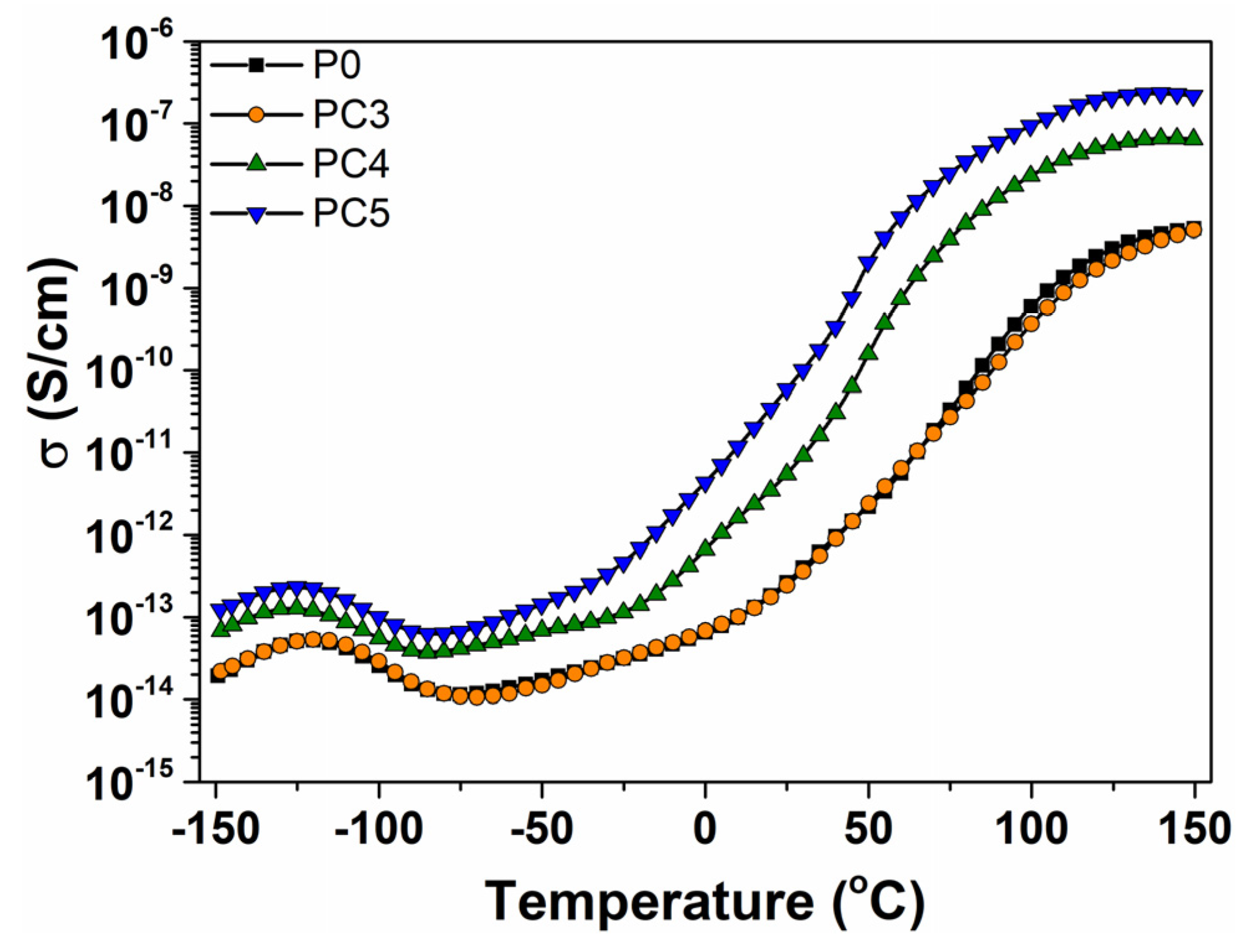
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Chakraborty, U.; Kaur, G.; Rubahn, H.G.; Kaushik, A.; Chauhary, G.R.; Mishra, Y.K. Advanced metal oxides nanostructures to recognize and eradicate water pollutants. Prog. Mater. Sci. 2023, 139, 101169. [Google Scholar]
- Soliman, T.S.; Vshivkov, S.A.; Elkalashy, S.I. Structural, linear and nonlinear optical properties of Ni nanoparticles—Polyvinyl alcohol nanocomposite films for optoelectronic applications. Opt. Mater. 2020, 107, 110037. [Google Scholar] [CrossRef]
- Barabaszova, K.C.; Holesova, S.; Bily, M.; Hundakova, M. CuO and CuO/vermiculite based nanoparticles in antibacterial PVAc nanocomposites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4218–4227. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Abd-Elkader, O.H.; Lakshininarayana, G. Effect of ZnO/Co or Mn) ratios on the structures and optical spectroscopy parameters of PVA/PVP/PEG blended polymer. Opt. Mater. 2022, 128, 112411. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Dhatarwal, P. Nanofiller concentration-dependent appreciably tailorable and multifunctional properties of (PVP/PVA)/SnO2 nanocomposites for advanced flexible device technologies. J. Mater. Sci. Mater. Electron. 2021, 32, 9661–9674. [Google Scholar] [CrossRef]
- Bulinski, M.; Kuncser, V.; Plapcianu, C.; Krautwald, S.; Franke, H.; Rotaru, P.; Filoti, G. Optical and electronic properties of polyvinyl alcohol doped with pairs of mixed valence metal ions. J. Phys. D Appl. Phys. 2004, 37, 2437–2444. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Zhu, Y.; Li, S.; Jiang, P. High-k polymer nanocomposites with 1D fillers for dielectric and energy storage applications. Progr. Mater. Sci. 2019, 100, 187–225. [Google Scholar] [CrossRef]
- Pascariu, P.; Airinei, A.; Grigoras, M.; Vacareanu, L.; Iacomi, F. Metal-polymer nanocomposites based on Ni nanoparticles and polythiophene obtained by electrochemical method. Appl. Surf. Sci. 2015, 352, 95–102. [Google Scholar] [CrossRef]
- El-Morsy, M.A.; Awwad, N.S.; Elhosiny Ali, H.; Menazea, A.A. Optical, thermal and dielectric properties of copper oxide (CuO)/chitosan (CS)/polyethylene oxide (PEO) blends. J. Polym. Res. 2022, 29, 177. [Google Scholar] [CrossRef]
- Mergen, O.B.; Arda, E. Determination of electrical and optical behaviors of carboxymethyl cellulose/graphene nanocomposites. J. Mater. Sci. Mater. Electron. 2023, 34, 179. [Google Scholar] [CrossRef]
- Mansour, S.A.; Elsad, R.A.; Izzularab, M.A. Dielectric properties enhancement of PVC nanodielectrics based on synthesized ZnO nanoparticles. J. Polym. Res. 2016, 23, 85. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Alzahrani, E.; Abo-Dief, H.M.; Saeed, A.; Alshammari, E.M.; Al-Harthi, A.M.; Tarabiah, A.E. Tuning the structural, optical, electrical and dielectric properties of PVA/PVP/CMC ternary polymer blend using ZnO nanoparticles for nanodielectric and optoelectronic devices. Opt. Mater. 2023, 140, 11390. [Google Scholar] [CrossRef]
- Agulto, V.C.; Empizo, M.J.F.; Kawano, K.; Minanci, Y.; Yamanoi, K.; Sarukura, N.; Yago, A.C.C.; Sarmago, R.V. Two-step fabrication of ZnO-PVP composites with tunable visible emissions. Opt. Mater. 2018, 76, 317–322. [Google Scholar] [CrossRef]
- Rajesh, K.; Crasta, V.; Kumar, N.B.R.; Shetty, G.; Rekha, P.D. Structural, optical, mechanical and dielectric properties of titanium oxide doped PVA/PVP nanocomposite. J. Polym. Res. 2019, 26, 99. [Google Scholar] [CrossRef]
- Mallakpoor, S. The use of poly(amide-imide)/CuO as a filler for the preparation of poly(vinyl pyrrolidone) nanocomposites: Thermal and morphological studies. J. Compos. Mater. 2016, 50, 1181–1188. [Google Scholar] [CrossRef]
- Atta, M.R.; Algethami, N.; Farea, M.O.; Alsulami, Q.A.; Rajah, A. Enhancing the structural, thermal, and dielectric properties of the polymer nanocomposites based on polymer blend and barium titrate nanoparticles for application in energy storage. Int. J. Energy Res. 2022, 46, 8020–8029. [Google Scholar] [CrossRef]
- Tommalieh, M.J.; Awwad, N.S.; Ibrahim, H.A.; Menazea, A.A. Characterization and electrical enhancement of PVP/PVA matrix doped by gold nanoparticles prepared by laser ablation. Radiat. Phys. Chem. 2021, 179, 109195. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.M.; Ahmed, S.I.; Alhazime, A.A. Tailoring the optical properties of PVA/PVP blend by doping with Cu/MnS nanoparticles. J. Vinyl Addit. Technol. 2021, 27, 410–418. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N.K. Synthesis and biomedical applications of copper oxide nanoparticles: An expanding horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef]
- Bosigo, R.; Lepodise, L.M.; Kuvarega, A.; Muiva, C. Hydrothermal synthesis of CuO and CeO2/CuO nanostructures: Spectroscopy and temperature dependent electrical properties. J. Mater. Sci. Mater. Electron. 2021, 32, 7136–7152. [Google Scholar] [CrossRef]
- Mahdy, M.A.; El Zawawi, I.K.; Ahmad, M.M. Structure, optical and magnetic properties of PVA/CuO/CoFo2O4 nanocomposite films for flexible magneto-electronic applications. Mater. Sci. Eng. B 2024, 299, 117028. [Google Scholar] [CrossRef]
- Sone, B.T.; Diallo, A.; Fuku, X.G.; Gurib-Fakim, A.; Maaza, M. Biosynthesized CuO nano-platelets: Physical properties and enhanced thermal conductivities nanofluidics. Arab. J. Chem. 2020, 13, 160–170. [Google Scholar] [CrossRef]
- Gherasim, C.; Pascariu, P.; Asandulesa, M.; Dobromir, M.; Doroftei, F.; Fifere, N.; Dascalu, A.; Airinei, A. Copper oxide nanostructures: Preparation, structural, dielectric and catalytic properties. Ceram. Int. 2022, 48, 25556–25568. [Google Scholar] [CrossRef]
- Molavi, R.; Sheikhi, M.H. Facile wet chemical synthesis of Al doped CuO nanoleaves for carbon oxide gas sensor applications. Mater. Sci. Semicond. Process. 2020, 106, 104767. [Google Scholar] [CrossRef]
- Alghunaim, N.S. Effect of CuO nanofiller on the spectroscopic properties, dielectric permittivity and dielectric modulus of CMC/PVP nanocomposites. J. Mater. Res. Technol. 2019, 8, 3596–3602. [Google Scholar] [CrossRef]
- Shetty, B.G.; Crasta, V.; Kumar, N.B.R.; Rajesh, K.; Bairy, R.; Patil, P.S. Promising PVA/TiO2, CuO filled nanocomposites for electrical and third nonlinear optical applications. Opt. Mater. 2019, 95, 109218. [Google Scholar] [CrossRef]
- Khan, H.U.; Tarig, M.; Shah, M.; Iqbal, M.; Jan, M.T. Inquest of highly sensitive, selective and stable ammonia (NH3) gas sensor: Structural, morphological and gas sensing properties of polyvinylpyrrolidone (PVP)/CuO nanocomposite. Synth. Met. 2020, 268, 116482. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Sen, S.; Gupta, P.K.; Saha, S. Negative photochemistry: Optical and structural characterization of PVP encapsulated CuO nanorods for the study of negative photoconductivity effect. Eur. Phys. J. Plus 2023, 138, 613. [Google Scholar] [CrossRef]
- Javed, R.; Ahmed, M.; Haq, I.; Nisa, S.; Zia, M. PVP and PEG doped CuO nanoparticles are more biologically active: Antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C 2017, 79, 108–115. [Google Scholar]
- Shahin, N.A.M.; Abd El Hamid, R.K.; Ezzat, H.A. Effect of CuO on the molecular, structural, optical, and electronic properties of polyvinyl pyrrolidone: Experimental and DFT approaches. Egypt. J. Chem. 2024, 67, 571–580. [Google Scholar]
- Dhatarwal, P.; Choudhary, S.; Segwa, R.J. Effectively nanofiller concentration tunable dielectric properties of PVP/SnO2 nanodielectrics. Mater. Lett. 2020, 273, 127913. [Google Scholar] [CrossRef]
- Ali, F.M. Structural and optical characterization of [(PVA:PVP)-Cu2+] composite films for promising semiconducting polymer devices. J. Mol. Struct. 2019, 1189, 352–359. [Google Scholar] [CrossRef]
- Akter, J.; Sapkota, K.P.; Hasuf, M.A.; Islam, M.A.; Abbas, H.G.; Hahn, J.R. Kinetically controlled selective synthesis of Cu2O and CuO nanoparticles toward enhanced degradation of methylene blue using ultraviolet and sun light. Mater. Sci. Semicond. Process. 2021, 123, 105570. [Google Scholar] [CrossRef]
- Lanford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Kumar, V.; Rawal, I.; Kumar, V.; Goyal, P.K. Efficient UV photodetectors based on Ni-doped ZnS nanoparticles prepared by facial chemical reduction method. Phys. B Cond. Mater. 2019, 575, 411690. [Google Scholar] [CrossRef]
- Ningaraju, S.; Pracash, A.P.G.; Ravikumar, H.B. Studies on the free volume controlled electrical properties of PVA/NiO and PVA/TiO2 polymer nanocomposites. Solid State Ion. 2018, 320, 132–147. [Google Scholar] [CrossRef]
- Atisme, T.B.; Yu, C.Y.; Tseng, E.N.; Chen, Y.C.; Shu, P.K.; Chen, S.Y. Interface interactions in conjugated polymer composite with metal oxide nanoparticles. Nanomaterials 2019, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J. Optical properties of amorphous semiconductors. In Amorphous and Liquid Semiconductors; Tauc, J., Ed.; Plenum Publishing Co.: London, UK, 1974; pp. 159–200. [Google Scholar]
- Tauc, J.; Menth, A. States in the gap. J. Non Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Ahmad, A.A.; Qaltan, I.A.; El-Ali, A.R.; Al Fawares, S.A.; Al-Bataineh, Q.M. Synthesis of optically tunable and thermally stable PMMA-PVA/CuO NPs hybrid nanocomposite thin films. Polymers 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, I.; Saib, F.; Laachachi, A.; Ozocar, M.; Bessekhouad, Y. Optoelectronic characteristics of PVC/SrxZnO nanocomposite films. Opt. Mater. 2024, 147, 114693. [Google Scholar] [CrossRef]
- Alzahrani, H.A.H. CuO and MWCNTs nanoparticles filled PVA/PVP nanocomposites: Morphological, optical, thermal, dielectric, and electrical characteristics. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1913–1923. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity of the electronic absorption of solids. Phys. Rev. B 1953, 92, 1324–1333. [Google Scholar] [CrossRef]
- Boubaker, K. A physical explanation to the controversial Urbach tailing universality. Eur. Phys. J. Plus 2011, 126, 10. [Google Scholar] [CrossRef]
- Morsi, M.A.; Abdelghany, A.M. UV-irradiation assisted control of the structural, optical and thermal properties of PEO/PVP blended gold nanoparticles. Mater. Chem. Phys. 2017, 201, 100–112. [Google Scholar] [CrossRef]
- Boranna, M.P.; Gummagol, N.B.; Patil, P.S.; Ravikumar, H.B. Effect of free volume on nonlinear optical characteristics of P(St-co-MMA)/CuO and PSAN/CuO polymer nanocomposites. Mater. Sci. Eng. B 2024, 302, 117239. [Google Scholar] [CrossRef]
- Fifere, N.; Ardeleanu, R.; Doroftei, F.; Dobromir, M.; Airinei, A. Tailoring the structural and optical properties of cerium oxide nanoparticles prepared by an ecofriendly green route using plant extracts. Int. J. Mol. Sci. 2024, 25, 681. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Sengwa, R.J. ZnO nanoparticles dispersed PVA-PVP blend matrix based high performance flexible multifunctional microelectronic devices. Curr. Appl. Phys. 2018, 18, 1041–1058. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Linear and nonlinear optical properties of simple oxides. J. Appl. Phys. 1966, 79, 1741–1745. [Google Scholar] [CrossRef]
- Spitzer, W.G.; Fan, H.Y. Determination of optical constants and carrier effective mass of semiconductors. Phys. Rev. 1957, 106, 882–890. [Google Scholar] [CrossRef]
- Zemel, J.N.; Jensen, J.D.; Schoolar, R.B. Electrical and optical properties of epitaxial films of PbS, PbSe, PbTe, and SnTe. Phys. Rev. B 1965, 140, A330–A342. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Enhanced dielectric properties of the ZnO and TiO2 nanoparticles dispersed poly(vinyl pyrrolidone) matrix based nanocomposites. J. Macromol. Sci. Part B 2020, 59, 853–866. [Google Scholar] [CrossRef]
- Singh, P.K.; Gaur, M.S.; Chauhan, R.S. Dielectric properties of sol-gel synthesized polysulfone-ZnO nanocomposites. J. Therm. Anal. Calorim. 2015, 122, 725–740. [Google Scholar] [CrossRef]
- Samet, M.; Levchenko, V.; Boiteux, G.; Seytre, G.; Kallel, A.; Serghei, A. Electrode polarization vs. Maxwell-Wagner-Silars interfacial polarization in dielectric spectra of materials: Characteristic frequencies and scaling laws. J. Chem. Phys. 2015, 142, 194703. [Google Scholar] [CrossRef]
- Igbal, T.; Irfan, M.; Ramay, S.M.; Mahmood, A.; Saleen, M.; Siddigi, S.A. ZnO-PVA polymer matrix with transition metals oxide nano-fillers for high dielectric medium. J. Polym. Environ. 2020, 28, 2422–2432. [Google Scholar] [CrossRef]
- Varsudevan, P.; Thomas, S.; Arunkumar, K.V.; Karthika, S.; Unnikrishnan, N.V. Synthesis and dielectric studies of poly(vinyl pyrrolidone)/titanium dioxide nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012015. [Google Scholar] [CrossRef]
- Salim, E.; Hany, W.; Elshahawy, A.G.; Oraly, A.H. Investigation of optical, structural and electrical properties of solid-state polymer nanocomposites electrolyte incorporated with Ag nanoparticles. Sci. Rep. 2022, 12, 21201. [Google Scholar] [CrossRef] [PubMed]
- Shoubak, W.M.; Hasan, A.; Mahrous, S.; Hassen, A. Controlling the physical properties of polyacrylonitrile by strontium hexaferrite nanoparticles. Polym. Bull. 2024, 81, 697–718. [Google Scholar] [CrossRef]
- Bu, Q.; Yin, S.; Wan, W.; Zheng, S.; Zhou, S.; Yu, L.; Quyang, Y. Effect of silane coupling agent KH550 on the dielectric properties of CaCu3Ti4O12/polyurethane composite films. Ceram. Int. 2024, 50, 12389–12396. [Google Scholar] [CrossRef]
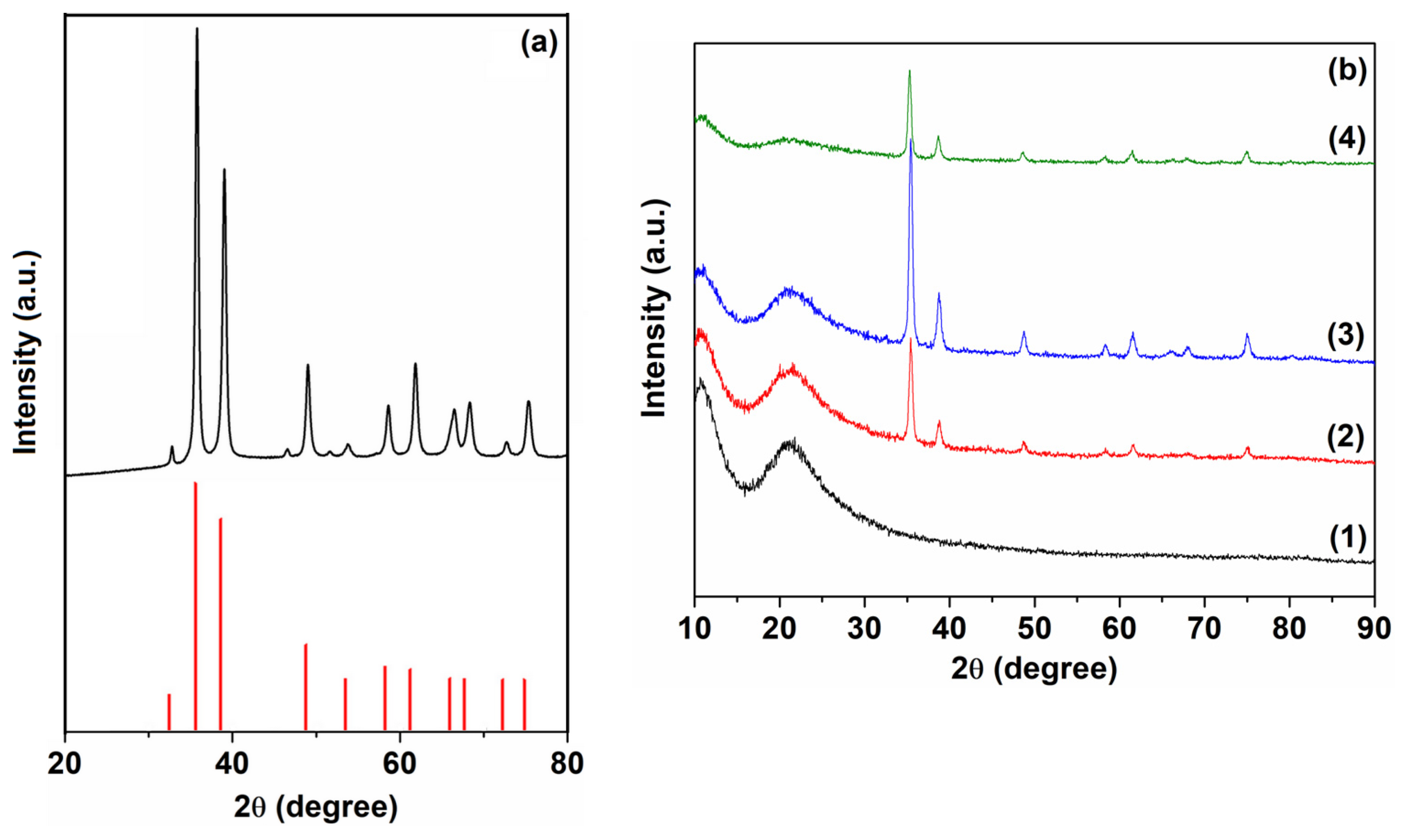
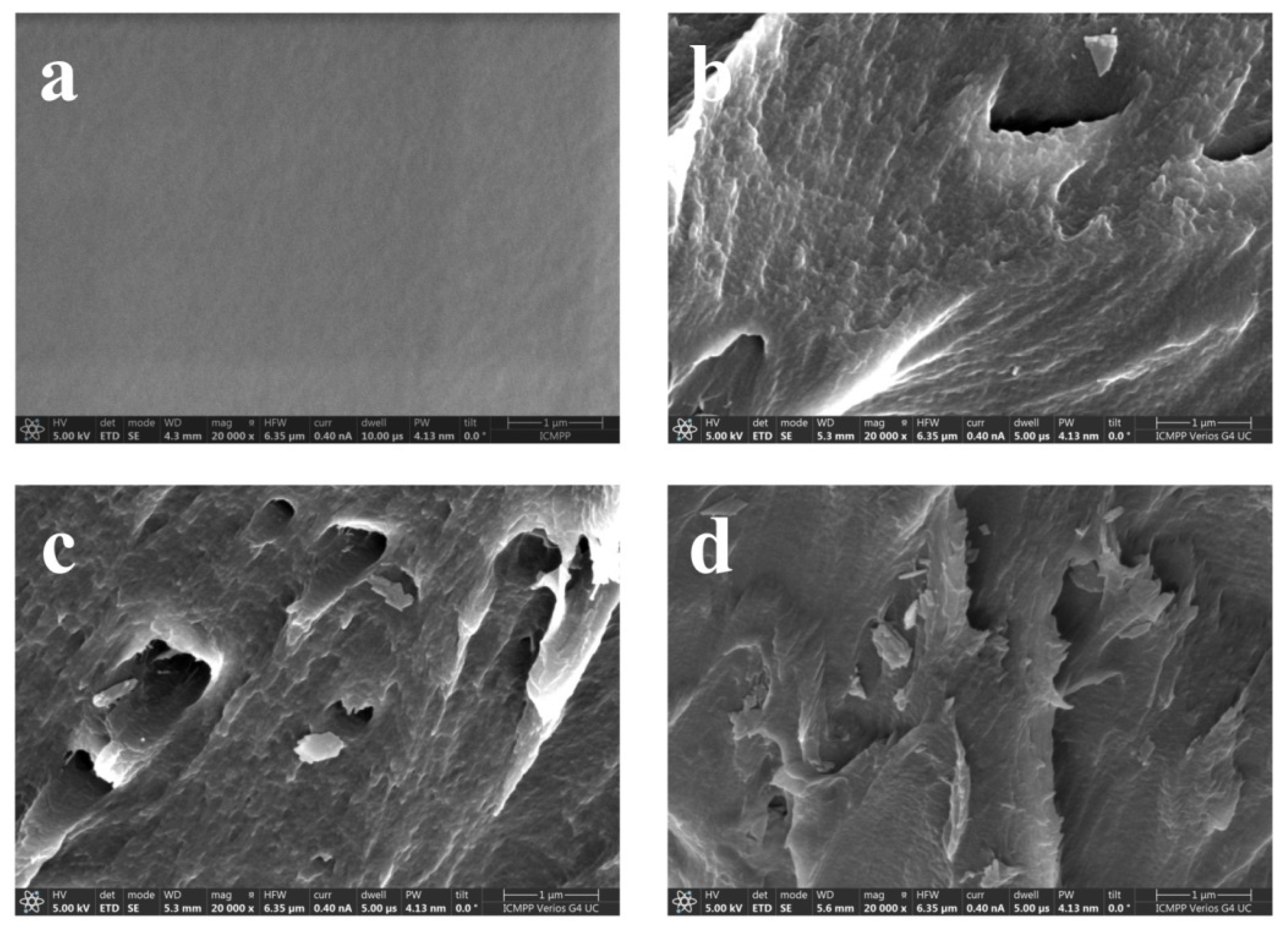
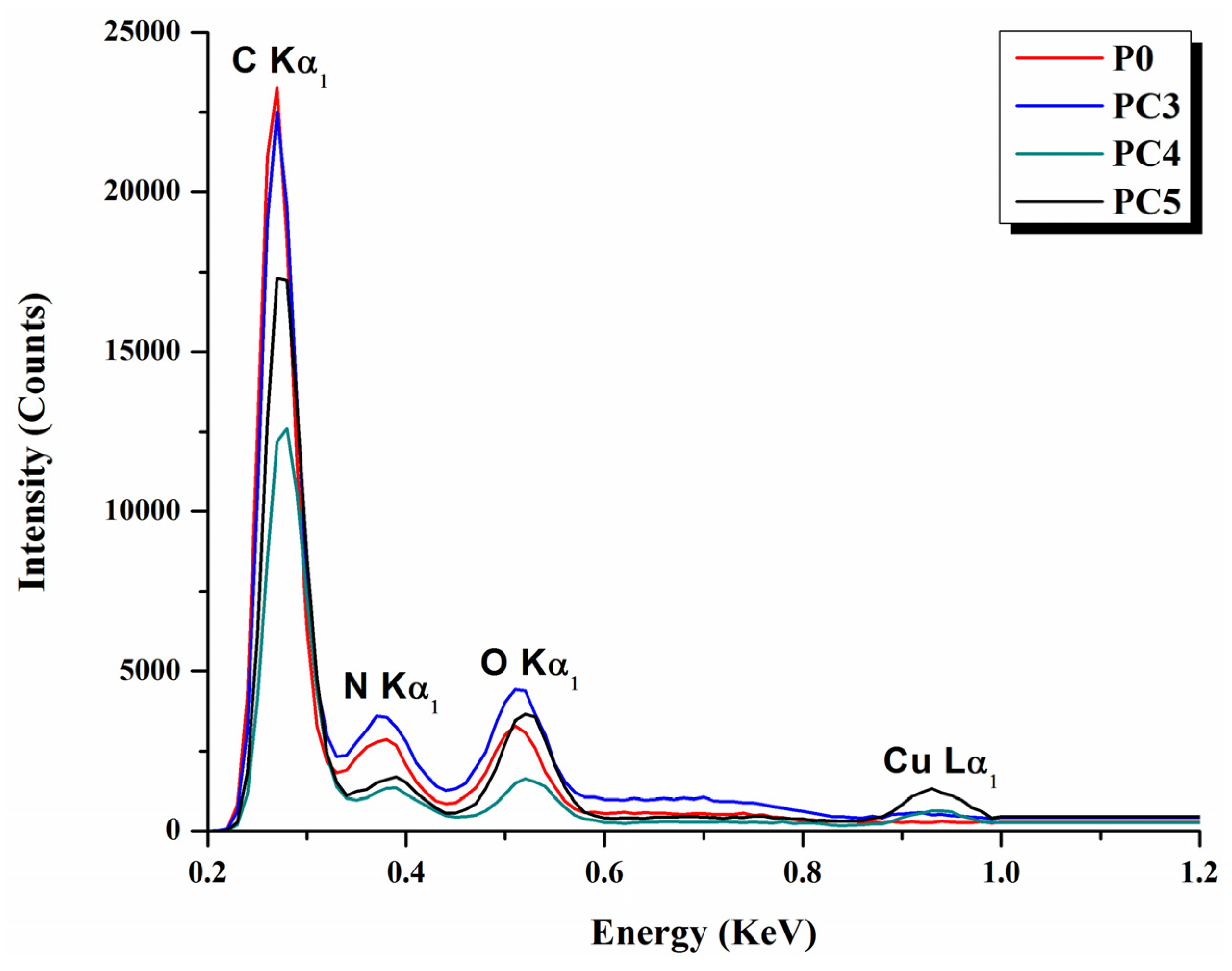
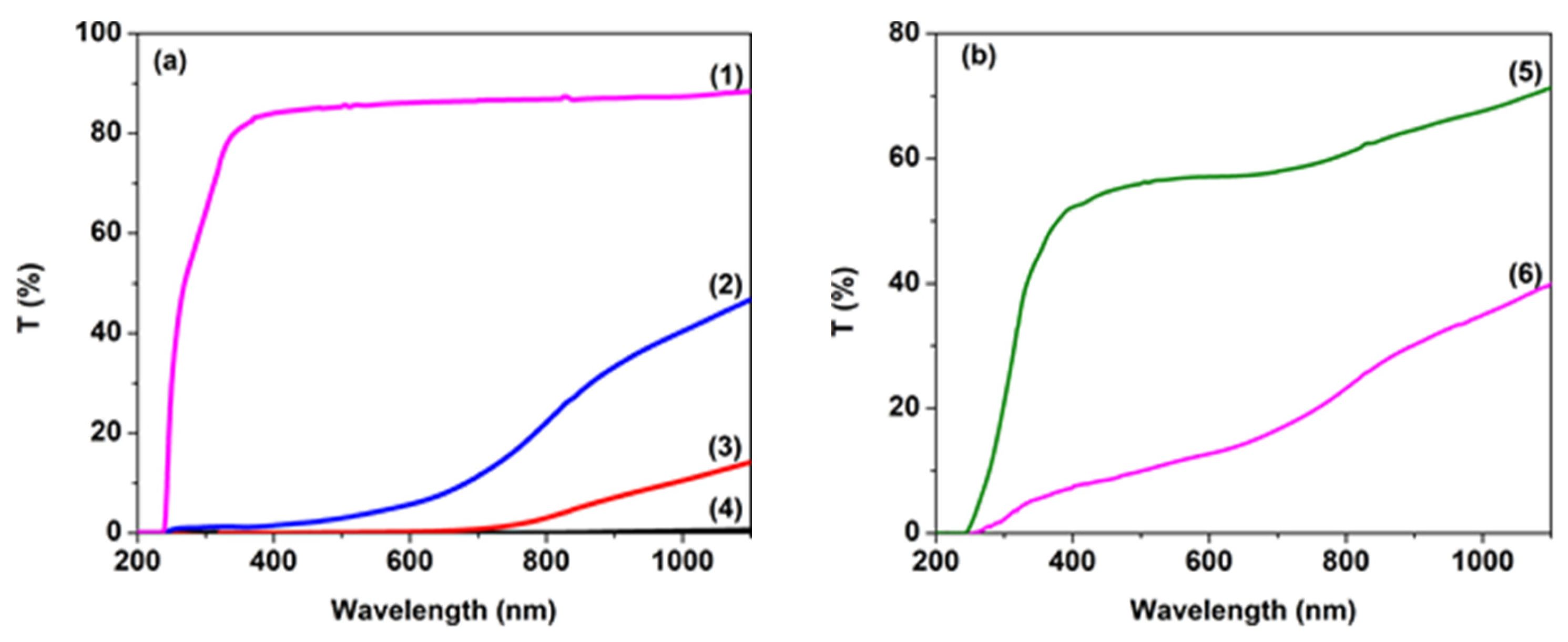
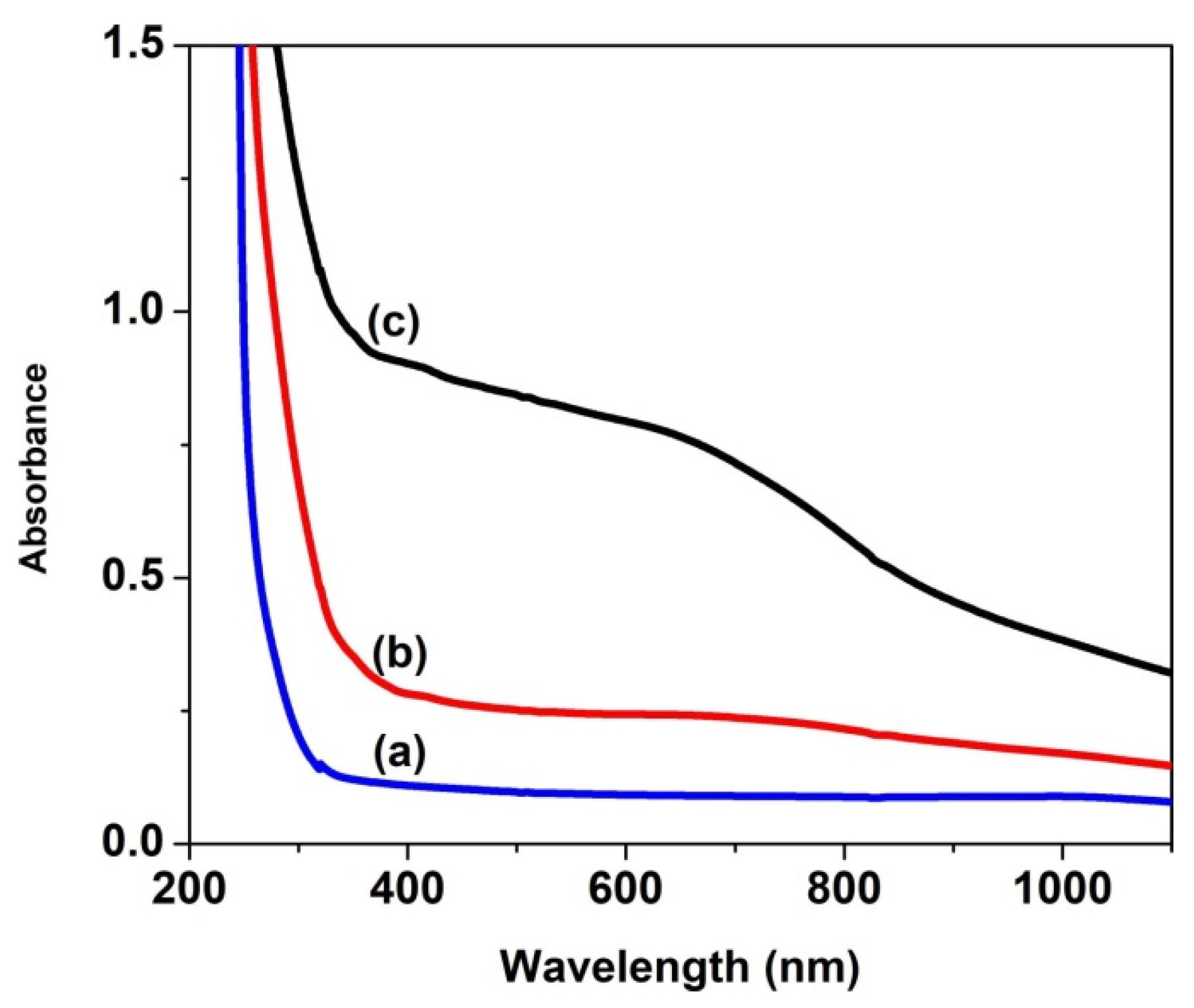
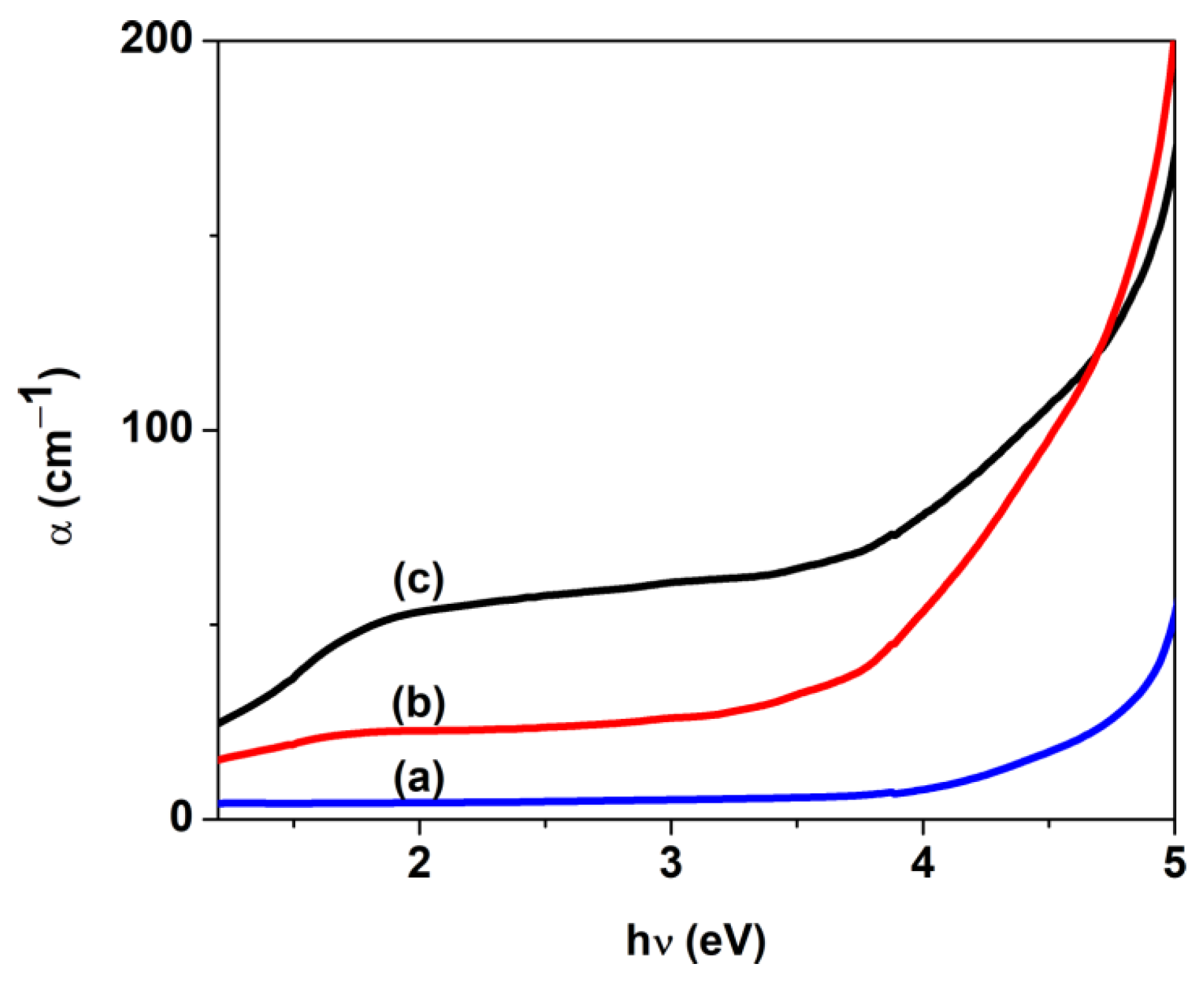
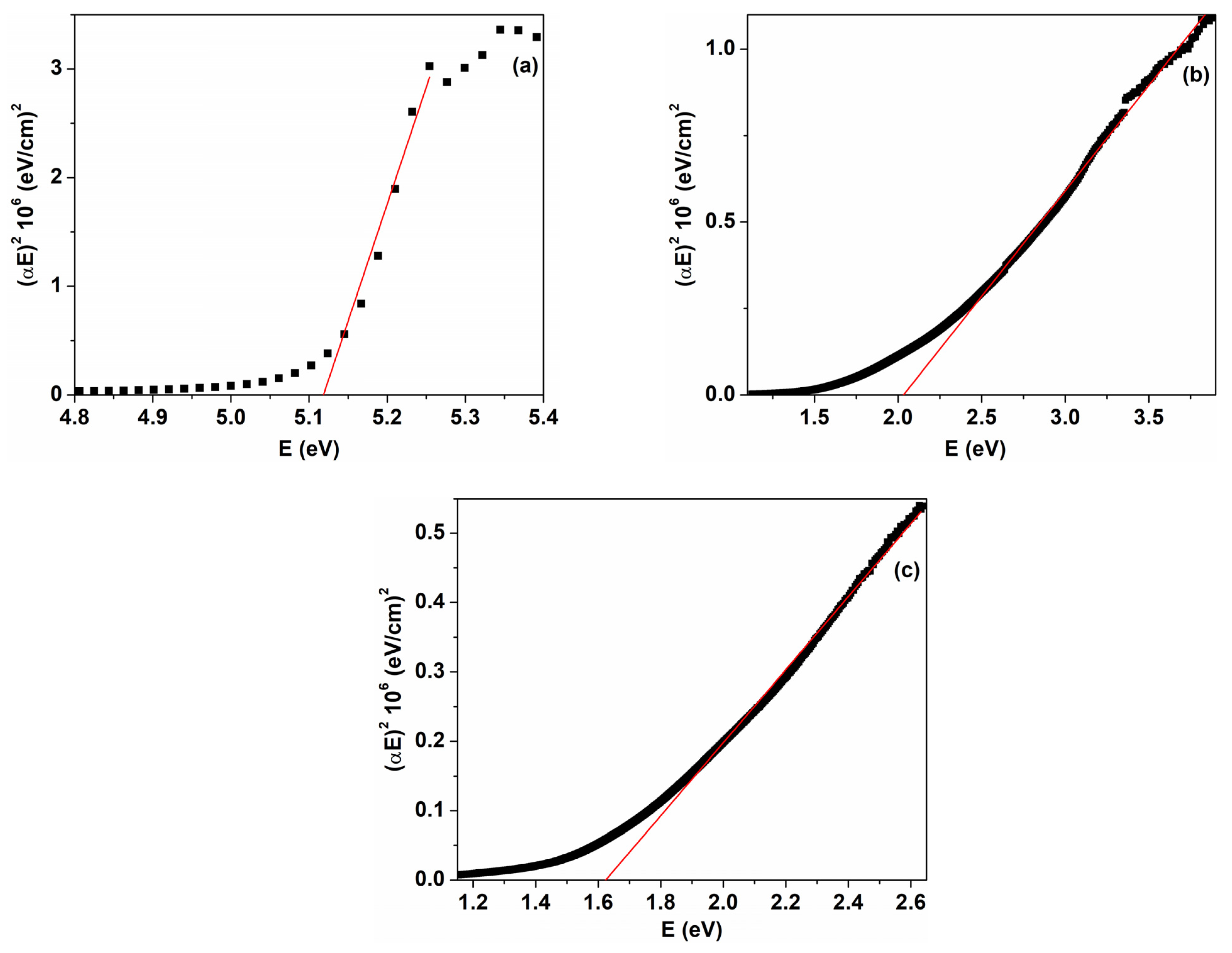
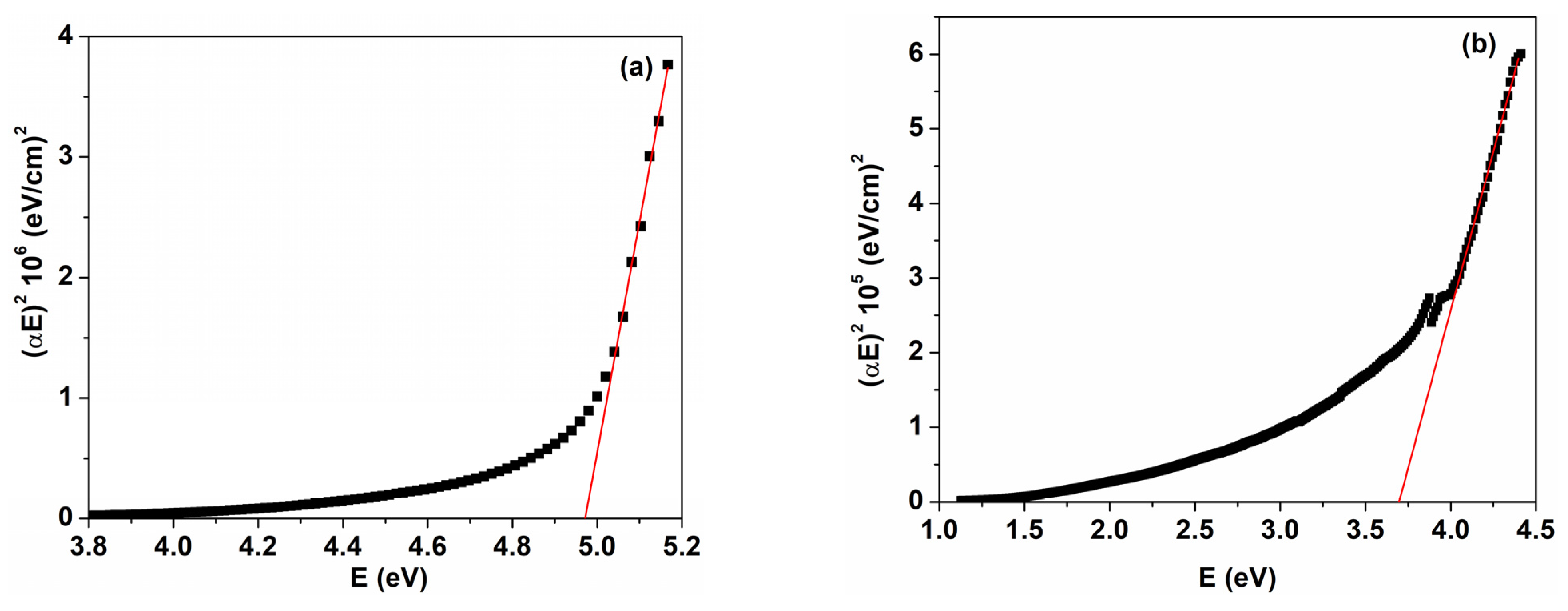
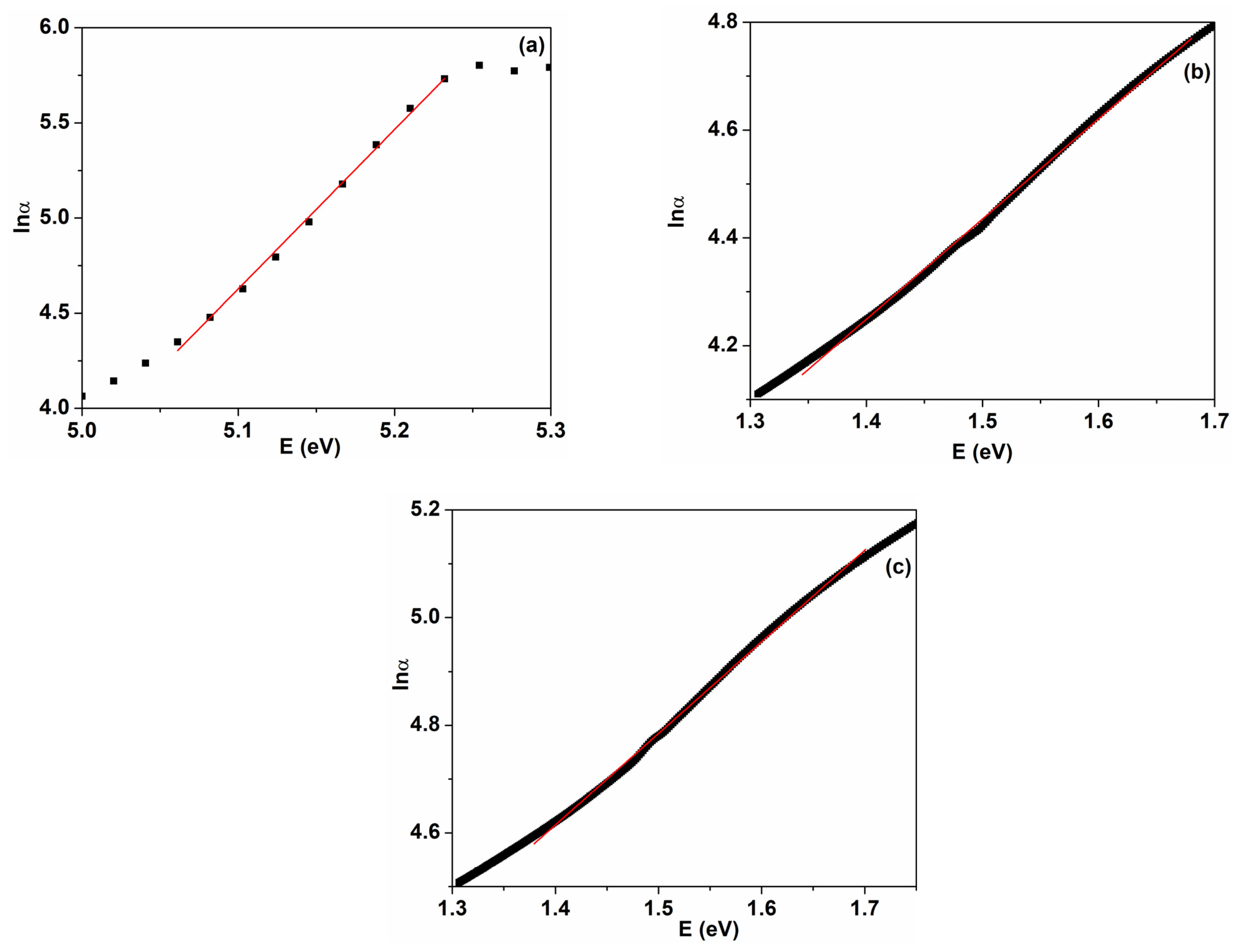
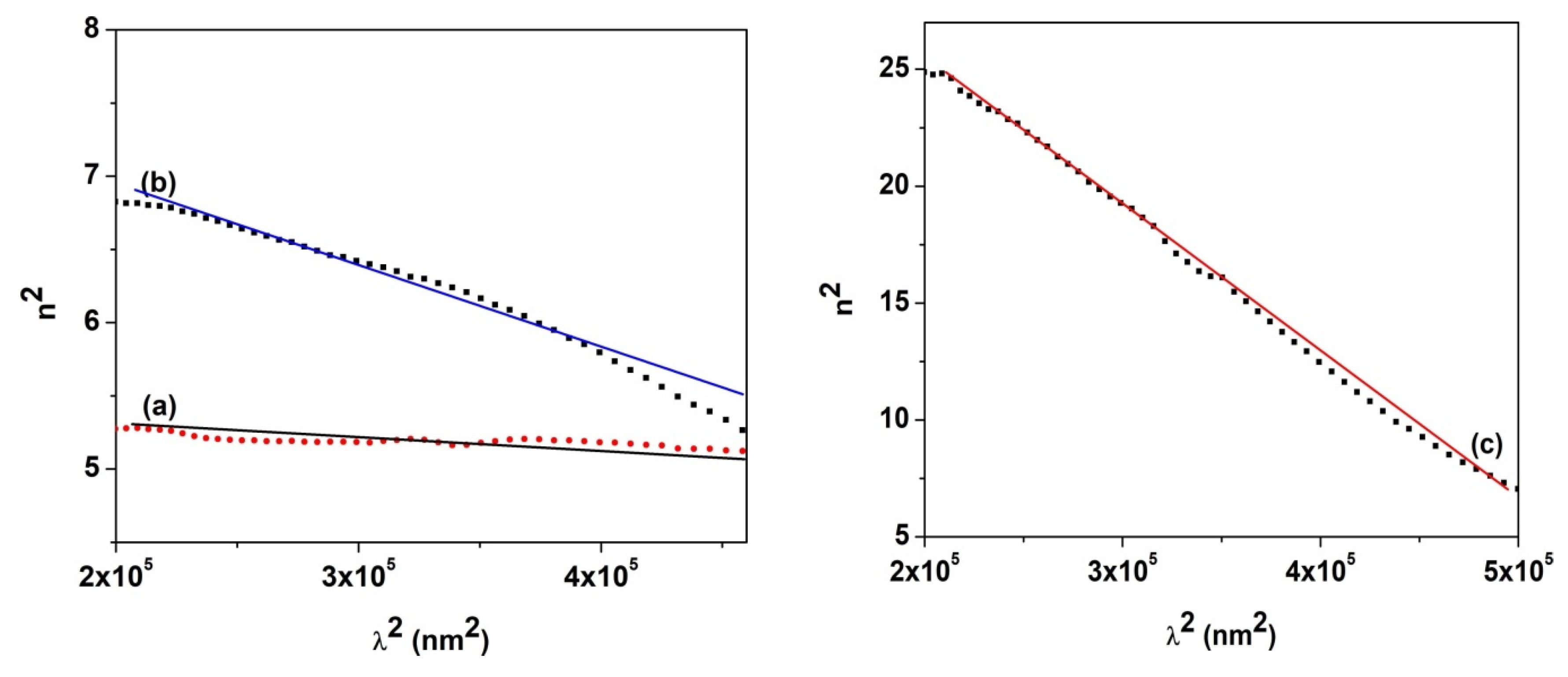
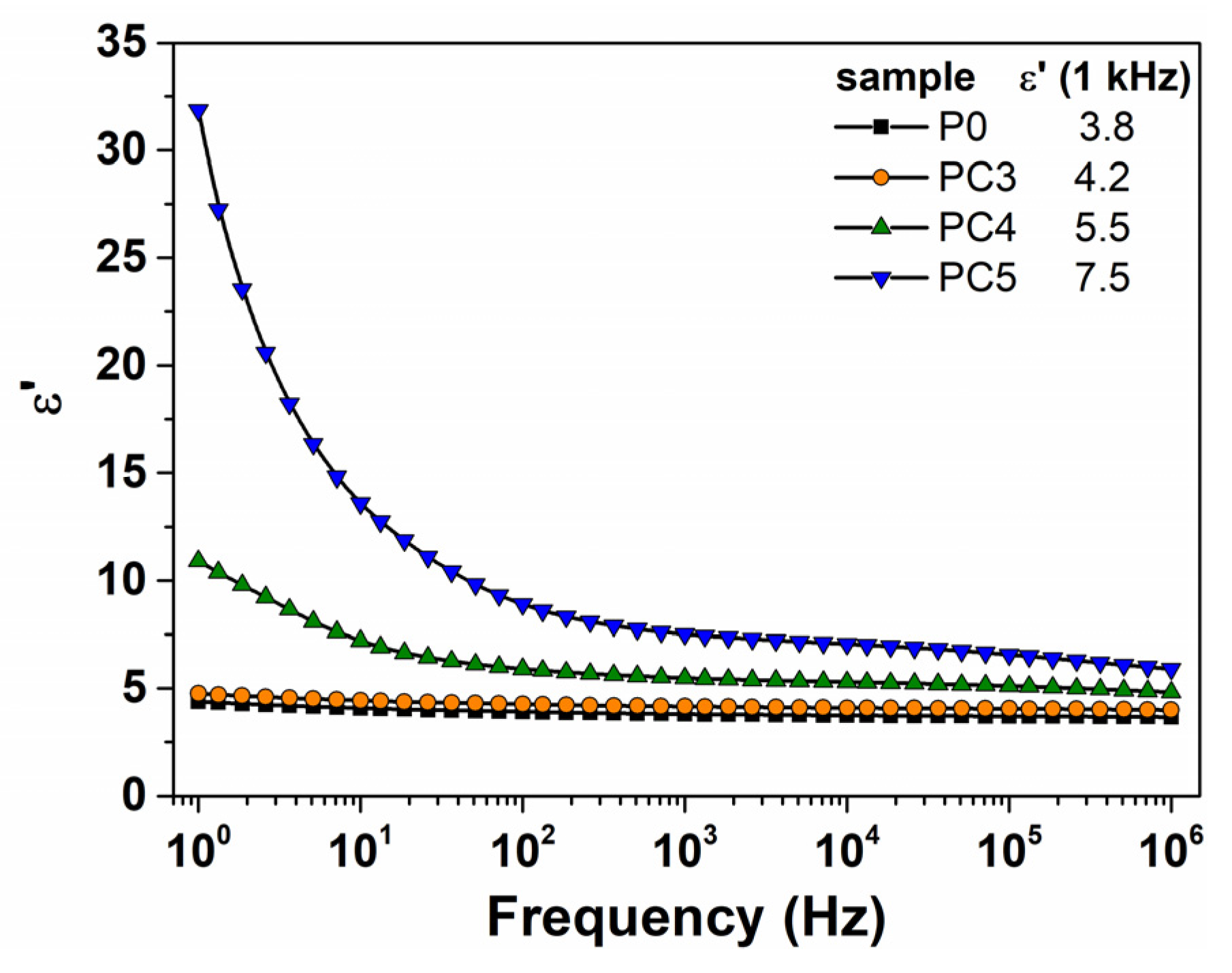
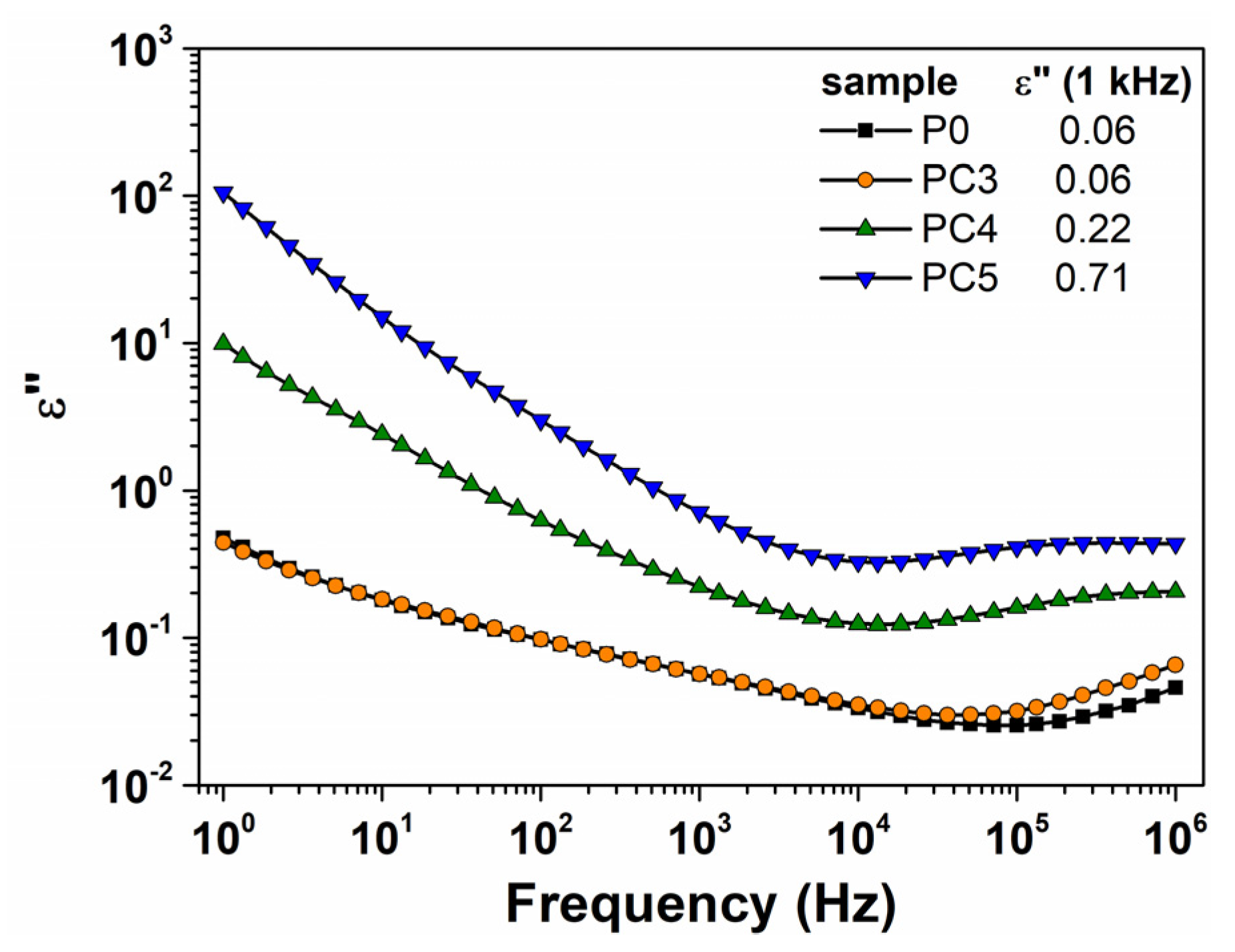
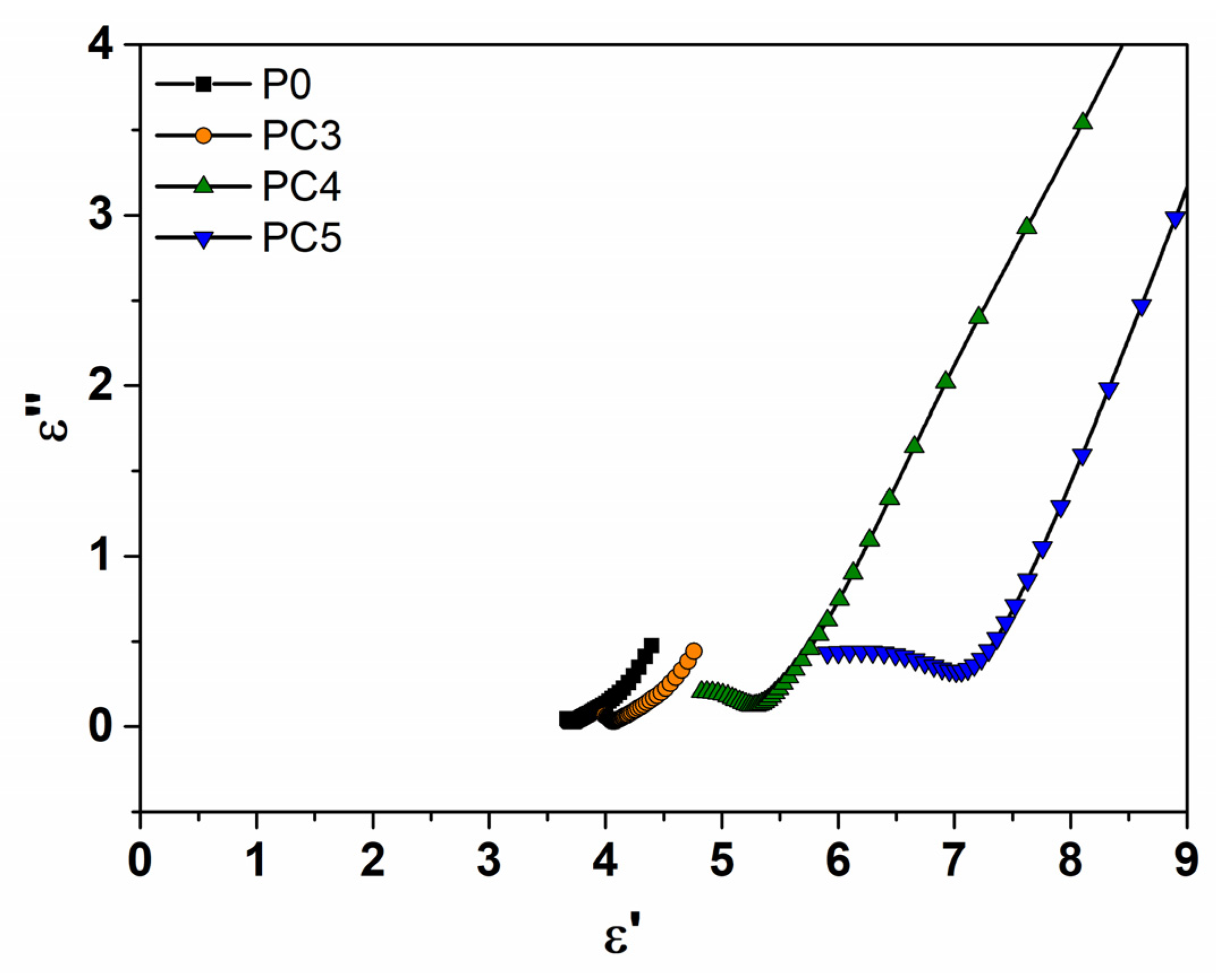
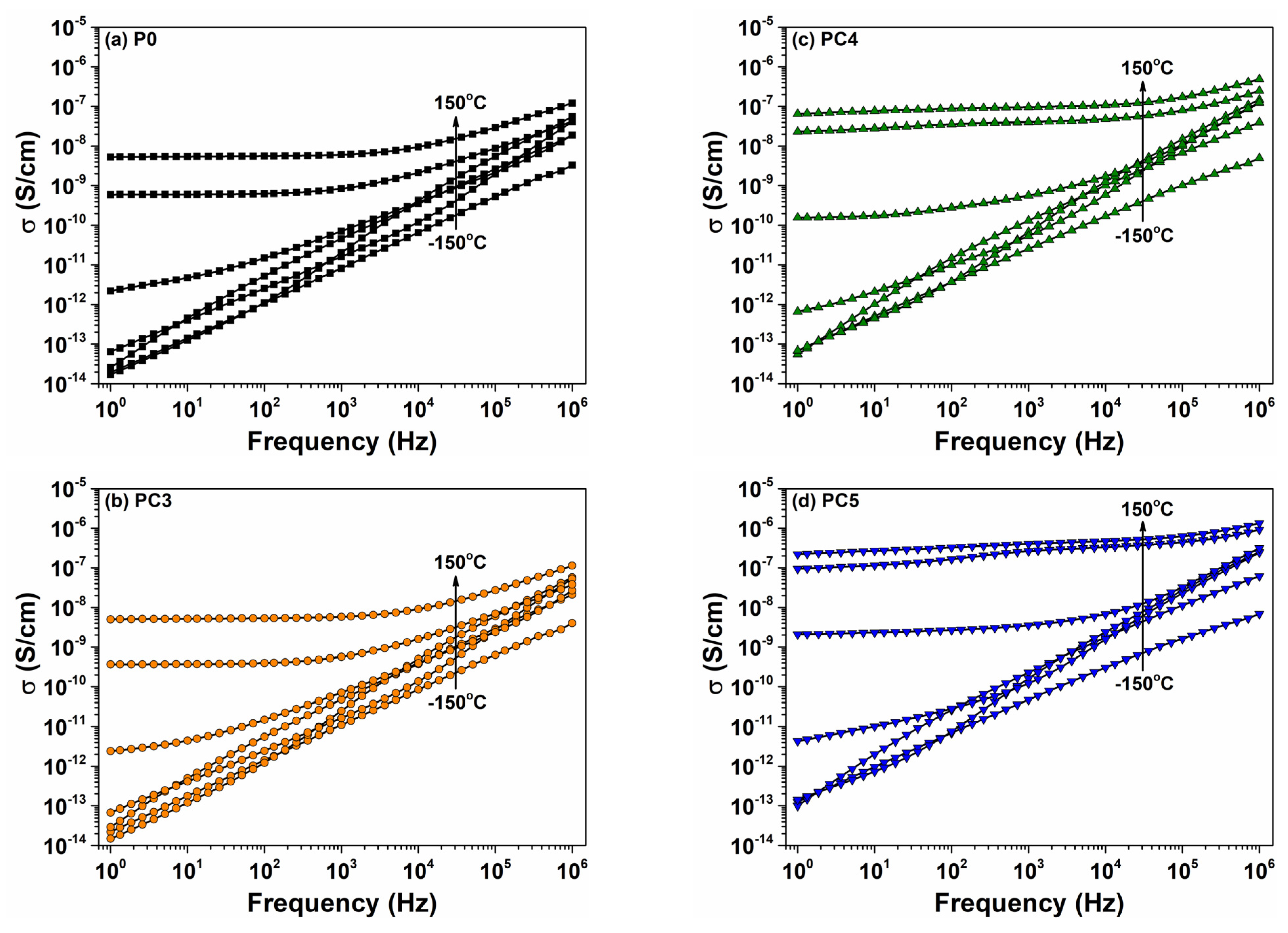

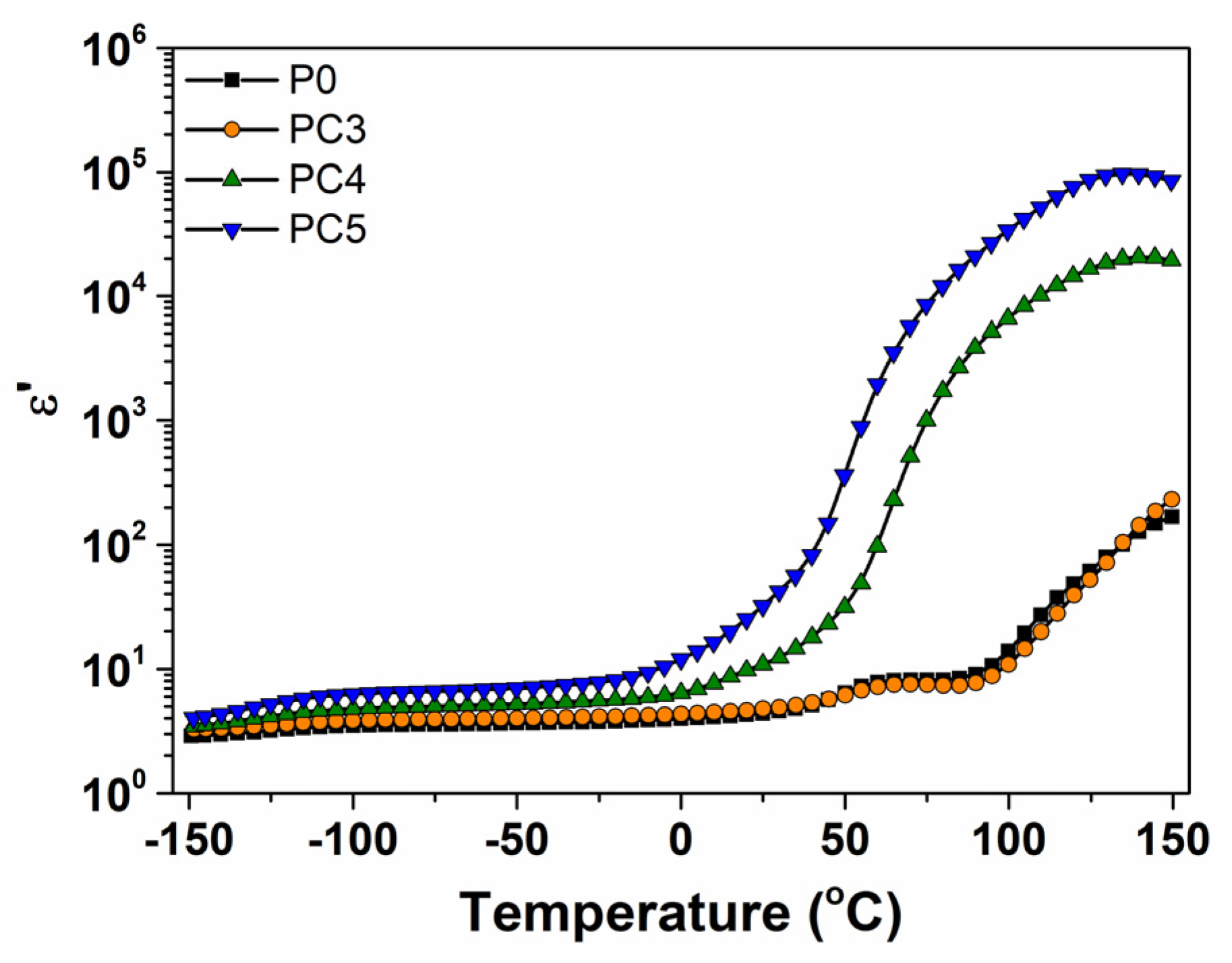
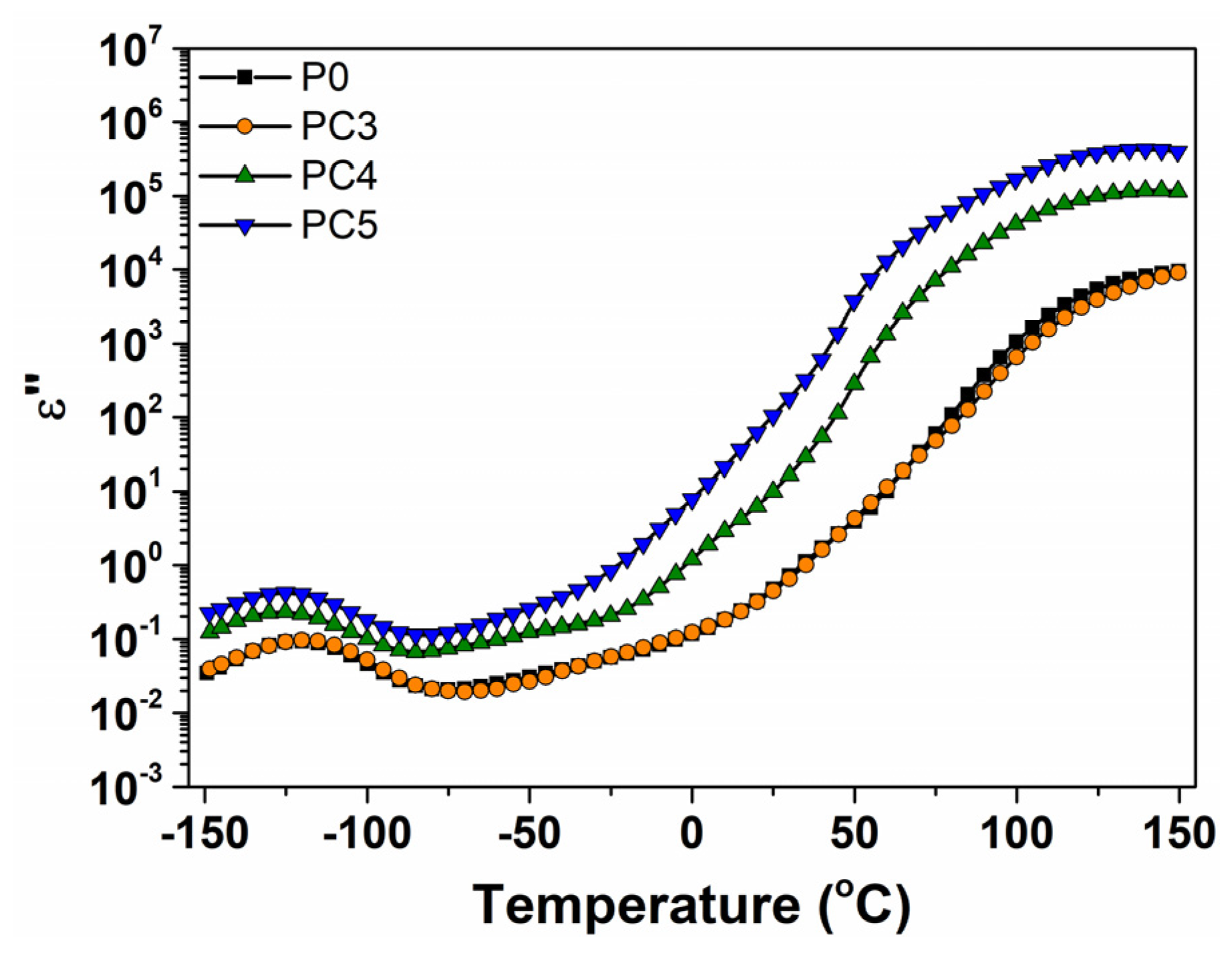
| Sample | D (nm) | ε × 103 | d (Å) | Eg (eV) | EU (meV) | n | N/m |
|---|---|---|---|---|---|---|---|
| CuO | 17.24 | 1.69 | 2.525 | ||||
| PC3 | 17.87 | 6.38 | 2.533 | 2.03 | 537.7 | 2.0045 | 0.52 × 10−6 |
| PC4 | 18.55 | 6.15 | 2.531 | 1.62 | 588.2 | 2.9223 | 1.39 × 10−6 |
| PC5 | 17.82 | 6.42 | 2.542 | 1.08 | 3.3030 | 3.79 × 10−6 | |
| P0 | 5.12 | 119.4 |
| Sample | P0 | PC3 | PC4 | PC5 | ||||
|---|---|---|---|---|---|---|---|---|
| At% | Wt% | At% | Wt% | At% | Wt% | At% | Wt% | |
| C K | 57.9 | 50.3 | 49.0 | 40.6 | 59.1 | 47.6 | 54.9 | 44.5 |
| O K | 25.8 | 26.2 | 32.4 | 31.4 | 26.1 | 24.5 | 20.0 | 18.9 |
| N K | 16 | 18.5 | 17.7 | 19.6 | 12.8 | 13.7 | 22.4 | 24.2 |
| Cu K | – | – | 0.3 | 1.3 | 1.3 | 5.7 | 2.6 | 11.0 |
| PtM | 0.4 | 5.0 | 0.5 | 7.1 | 0.7 | 8.6 | 0.1 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasim, C.; Asandulesa, M.; Fifere, N.; Doroftei, F.; Tîmpu, D.; Airinei, A. Structural, Optical and Dielectric Properties of Some Nanocomposites Derived from Copper Oxide Nanoparticles Embedded in Poly(vinylpyrrolidone) Matrix. Nanomaterials 2024, 14, 759. https://doi.org/10.3390/nano14090759
Gherasim C, Asandulesa M, Fifere N, Doroftei F, Tîmpu D, Airinei A. Structural, Optical and Dielectric Properties of Some Nanocomposites Derived from Copper Oxide Nanoparticles Embedded in Poly(vinylpyrrolidone) Matrix. Nanomaterials. 2024; 14(9):759. https://doi.org/10.3390/nano14090759
Chicago/Turabian StyleGherasim, Carmen, Mihai Asandulesa, Nicusor Fifere, Florica Doroftei, Daniel Tîmpu, and Anton Airinei. 2024. "Structural, Optical and Dielectric Properties of Some Nanocomposites Derived from Copper Oxide Nanoparticles Embedded in Poly(vinylpyrrolidone) Matrix" Nanomaterials 14, no. 9: 759. https://doi.org/10.3390/nano14090759





