Interfacing Langmuir–Blodgett and Pickering Emulsions for the Synthesis of 2D Nanostructured Films: Applications in Copper Ion Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
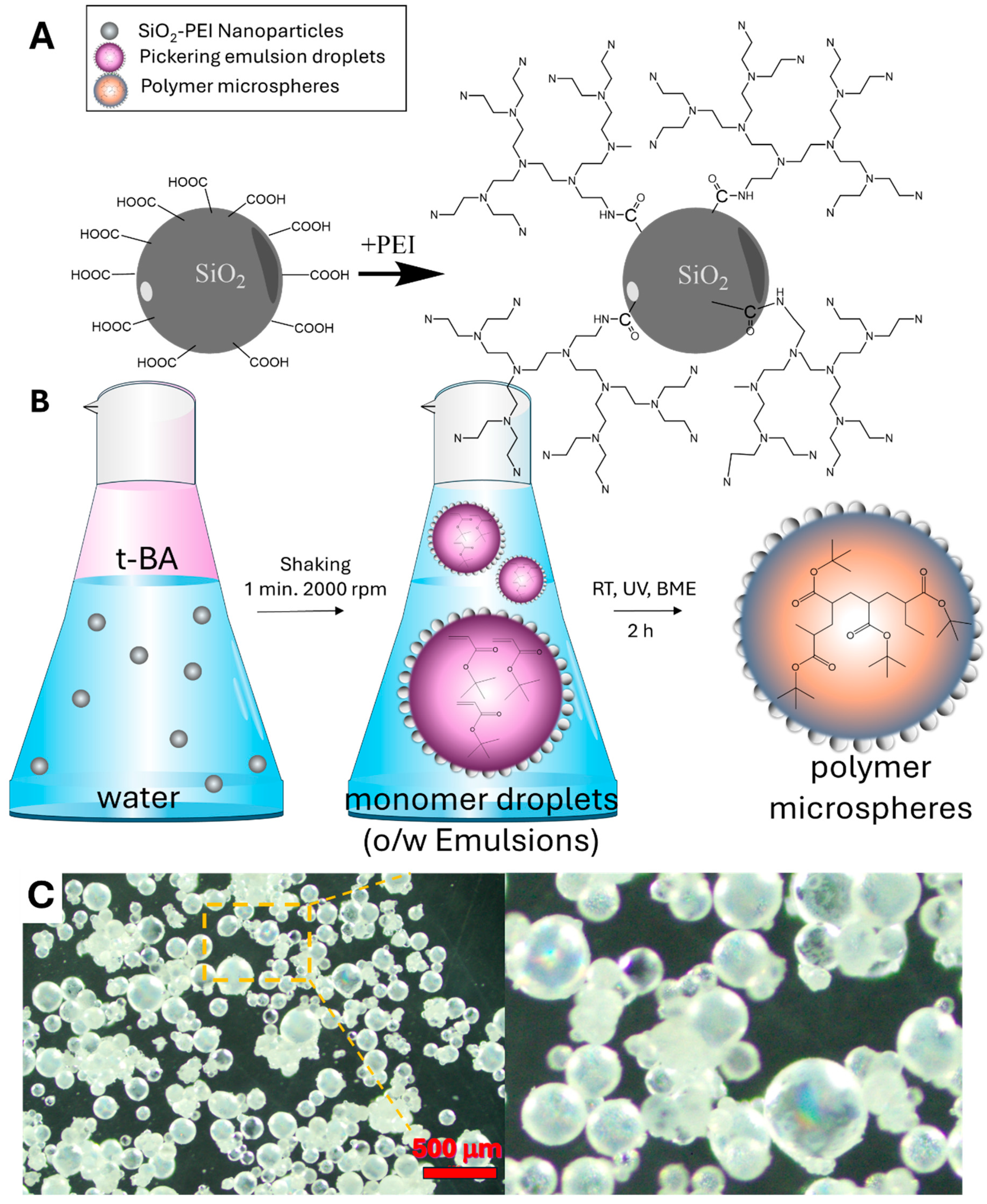
2.2.1. Synthesis and Functionalization of Silica Nanoparticles with PEI (SiO2-PEI NPs)
2.2.2. Microsphere Preparation via Pickering Emulsion Polymerization (P(t-BA)/SiO2-PEI NPs-Microsphere)
2.2.3. Preparation of 2D P(t-BA)/SiO2-PEI NPs and PVA/P(t-BA)/SiO2-PEI NP Films
2.2.4. Measurement of Ion Extraction and Recovery Capacity of Polymer Absorbents
2.3. Scanning Electron Microscopy (SEM)
2.4. Optical Microscopy
3. Results and Discussions
3.1. Synthesis SiO2-PEI NPs
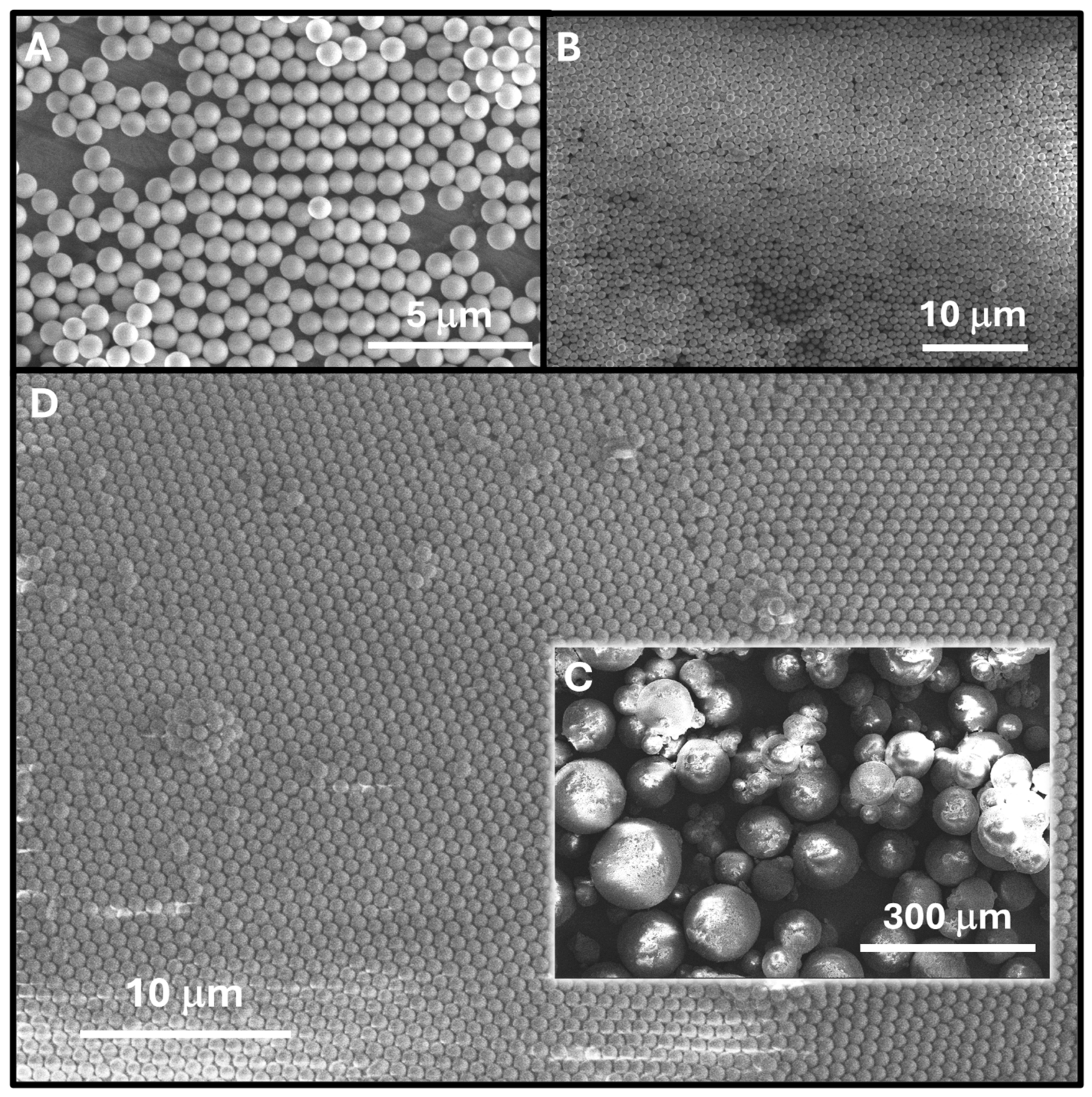
3.2. Synthesis of the P(t-BA)/SiO2-PEI NPs-Microspheres
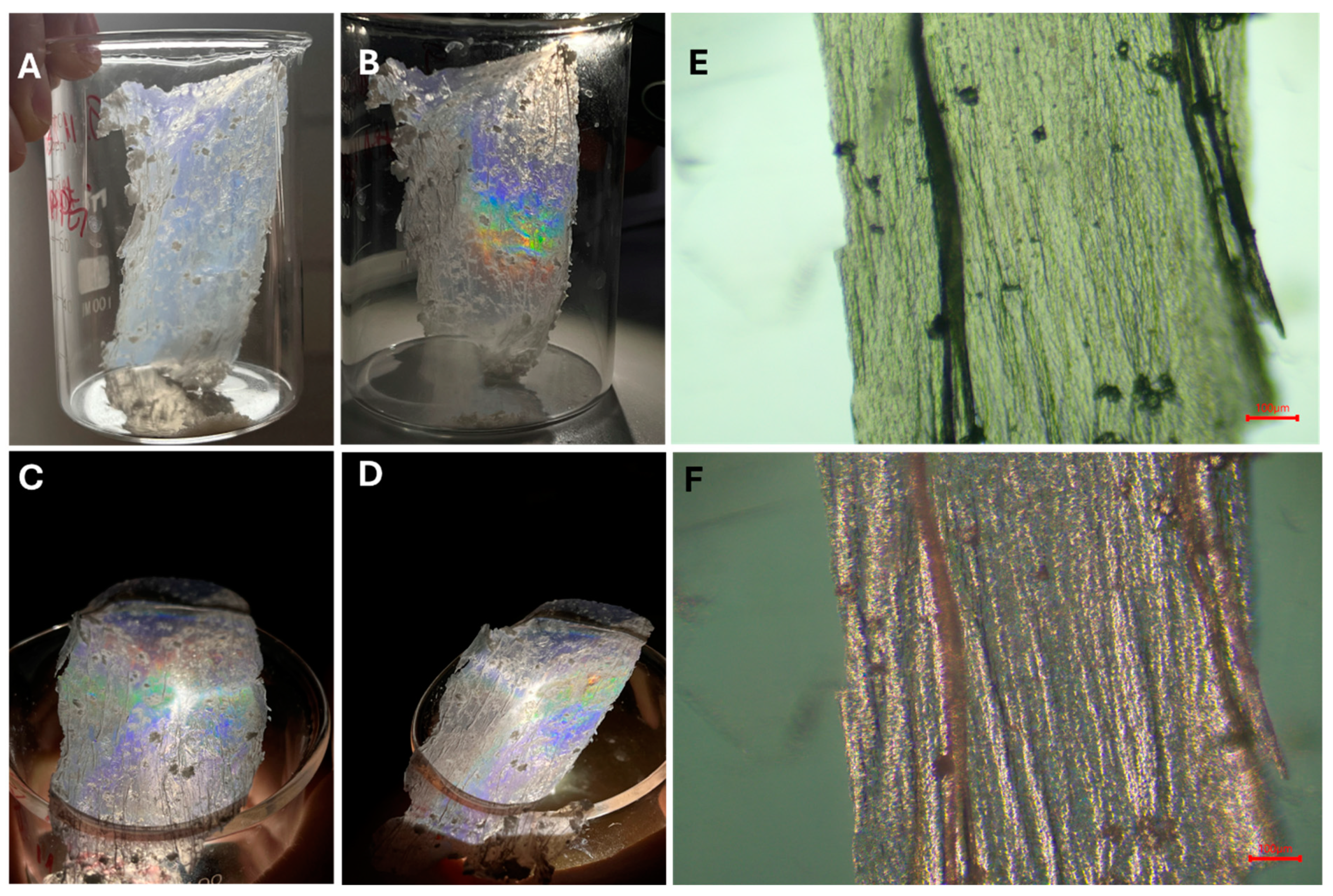
3.3. Synthesis of the Langmuir–Blodgett 2D P(t-BA)/SiO2-PEI NP Film
3.4. Application of Thin 2D P(t-BA)/SiO2-PEI NP Films for Ion Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parker, R.M.; Zhao, T.H.; Frka-Petesic, B.; Vignolini, S. Cellulose Photonic Pigments. Nat. Commun. 2022, 13, 3378. [Google Scholar] [CrossRef]
- Reculusa, S.; Ravaine, S. Colloidal Photonic Crystals Obtained by the Langmuir–Blodgett Technique. Appl. Surf. Sci. 2005, 246, 409–414. [Google Scholar] [CrossRef]
- Delléa, O.; Lebaigue, O. Boostream: A Dynamic Fluid Flow Process to Assemble Nanoparticles at Liquid Interface. Mech. Ind. 2017, 18, 602. [Google Scholar] [CrossRef]
- Peterson, I.R. Langmuir-Blodgett Films. J. Phys. Appl. Phys. 1990, 23, 379. [Google Scholar] [CrossRef]
- Metzger, R.M. Unimolecular Electronics. Chem. Rev. 2015, 115, 5056–5115. [Google Scholar] [CrossRef]
- Bardosova, M.; Pemble, M.E.; Povey, I.M.; Tredgold, R.H. The Langmuir-Blodgett Approach to Making Colloidal Photonic Crystals from Silica Spheres. Adv. Mater. 2010, 22, 3104–3124. [Google Scholar] [CrossRef] [PubMed]
- Parchine, M.; McGrath, J.; Bardosova, M.; Pemble, M.E. Large Area 2D and 3D Colloidal Photonic Crystals Fabricated by a Roll-to-Roll Langmuir–Blodgett Method. Langmuir 2016, 32, 5862–5869. [Google Scholar] [CrossRef]
- Huang, S.; Minami, K.; Sakaue, H.; Shingubara, S.; Takahagi, T. Effects of the Surface Pressure on the Formation of Langmuir−Blodgett Monolayer of Nanoparticles. Langmuir 2004, 20, 2274–2276. [Google Scholar] [CrossRef] [PubMed]
- Chitu, L.; Siffalovic, P.; Majkova, E.; Jergel, M.; Vegso, K.; Luby, S.; Capek, I.; Satka, A.; Perlich, J.; Timmann, A.; et al. Modified Langmuir-Blodgett Deposition of Nanoparticles—Measurement of 2D to 3D Ordered Arrays. Meas. Sci. Rev. 2010, 10, 162. [Google Scholar] [CrossRef]
- Shin, Y.; Song, J.; Kim, D.; Kang, T. Facile Preparation of Ultrasmall Void Metallic Nanogap from Self-Assembled Gold–Silica Core–Shell Nanoparticles Monolayer via Kinetic Control. Adv. Mater. 2015, 27, 4344–4350. [Google Scholar] [CrossRef] [PubMed]
- Montelongo, Y.; Sikdar, D.; Ma, Y.; McIntosh, A.J.S.; Velleman, L.; Kucernak, A.R.; Edel, J.B.; Kornyshev, A.A. Electrotunable Nanoplasmonic Liquid Mirror. Nat. Mater. 2017, 16, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, X.; Ji, F.; Luo, B.; Ice, N.F.; Liu, Q.; Zhang, Q.; Chen, Q. Polymorphic Assembly from Beveled Gold Triangular Nanoprisms. Nano Lett. 2017, 17, 3270–3275. [Google Scholar] [CrossRef]
- Tian, H.; Li, H.; Fang, Y. Binary Thiol-Capped Gold Nanoparticle Monolayer Films for Quantitative Surface-Enhanced Raman Scattering Analysis. ACS Appl. Mater. Interfaces 2019, 11, 16207–16213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Tao, P.; Shang, W.; Song, C.; Deng, T. Vertical Segregation in the Self-Assembly of Nanoparticles at the Liquid/Air Interface. Nanoscale 2014, 6, 14662–14666. [Google Scholar] [CrossRef]
- Han, G.; Liu, S.; Yang, Q.; Zeng, F.; Li, W.; Mao, X.; Xu, J.; Zhu, J. Polymer-Grafted Nanoparticle Superlattice Monolayers over 100 Cm2 through a Modified Langmuir-Blodgett Method. Polymer 2022, 259, 125308. [Google Scholar] [CrossRef]
- Song, L.; Xu, B.B.; Cheng, Q.; Wang, X.; Luo, X.; Chen, X.; Chen, T.; Huang, Y. Instant Interfacial Self-Assembly for Homogeneous Nanoparticle Monolayer Enabled Conformal “Lift-on” Thin Film Technology. Sci. Adv. 2021, 7, eabk2852. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef]
- Honciuc, A.; Negru, O.-I. NanoTraPPED—A New Method for Determining the Surface Energy of Nanoparticles via Pickering Emulsion Polymerization. Nanomaterials 2021, 11, 3200. [Google Scholar] [CrossRef]
- Jeong, S.; Hu, L.; Lee, H.R.; Garnett, E.; Choi, J.W.; Cui, Y. Fast and Scalable Printing of Large Area Monolayer Nanoparticles for Nanotexturing Applications. Nano Lett. 2010, 10, 2989–2994. [Google Scholar] [CrossRef]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A Large-Area, Flexible Pressure Sensor Matrix with Organic Field-Effect Transistors for Artificial Skin Applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef]
- Song, L.; Huang, Y.; Nie, Z.; Chen, T. Macroscopic Two-Dimensional Monolayer Films of Gold Nanoparticles: Fabrication Strategies, Surface Engineering and Functional Applications. Nanoscale 2020, 12, 7433–7460. [Google Scholar] [CrossRef] [PubMed]
- Daqiqeh Rezaei, S.; Dong, Z.; You En Chan, J.; Trisno, J.; Ng, R.J.H.; Ruan, Q.; Qiu, C.-W.; Mortensen, N.A.; Yang, J.K.W. Nanophotonic Structural Colors. ACS Photonics 2021, 8, 18–33. [Google Scholar] [CrossRef]
- Fu, Y.; Tippets, C.A.; Donev, E.U.; Lopez, R. Structural Colors: From Natural to Artificial Systems. WIREs Nanomed. Nanobiotechnology 2016, 8, 758–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nero, M.; Jansson, K.; Willhammar, T.; Sipponen, M.H. Photonic Crystals with Rainbow Colors by Centrifugation-Assisted Assembly of Colloidal Lignin Nanoparticles. Nat. Commun. 2023, 14, 3099. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds, Tables of Spectral Data; Fourth, Revised and Enlarged Edition; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93810-1. [Google Scholar]
- Honciuc, A.; Negru, O.-I. Asymmetrically Nanostructured 2D Janus Films Obtained from Pickering Emulsions Polymerized in a Langmuir–Blodgett Trough. Micromachines 2023, 14, 1459. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Asher, S.A.; Meng, Z.; Wang, F.; Lu, W.; Xue, M.; Qi, F. Two-Dimensional Colloidal Crystal Heterostructures. RSC Adv. 2015, 5, 18939–18944. [Google Scholar] [CrossRef]
- Honciuc, A.; Negru, O.-I. Monitoring the Surface Energy Change of Nanoparticles in Functionalization Reactions with the NanoTraPPED Method. Nanomaterials 2023, 13, 1246. [Google Scholar] [CrossRef] [PubMed]
- Pauli, O.; Honciuc, A. Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions. Nanomaterials 2022, 12, 3738. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Z.; Wu, D.; Lee, H.T.; Wang, H.; Honciuc, A.; Chew, J.W. Synthesis of Ligand-Carrying Polymeric Nanoparticles for Use in Extraction and Recovery of Metal Ions. Colloids Surf. Physicochem. Eng. Asp. 2017, 533, 179–186. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic Amorphous Silica Nanoparticles: Toxicity, Biomedical and Environmental Implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Nnaji, C.O.; Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Pan, S.; Barhoum, A. Chapter 6—Engineered Nanomaterials for Wastewater Treatment: Current and Future Trends. In Fundamentals of Nanoparticles; Barhoum, A., Hamdy Makhlouf, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–168. ISBN 978-0-323-51255-8. [Google Scholar]
- Sharma, Y.C.; Srivastava, V.; Singh, V.K.; Kaul, S.N.; Weng, C.H. Nano-adsorbents for the Removal of Metallic Pollutants from Water and Wastewater. Environ. Technol. 2009, 30, 583–609. [Google Scholar] [CrossRef] [PubMed]
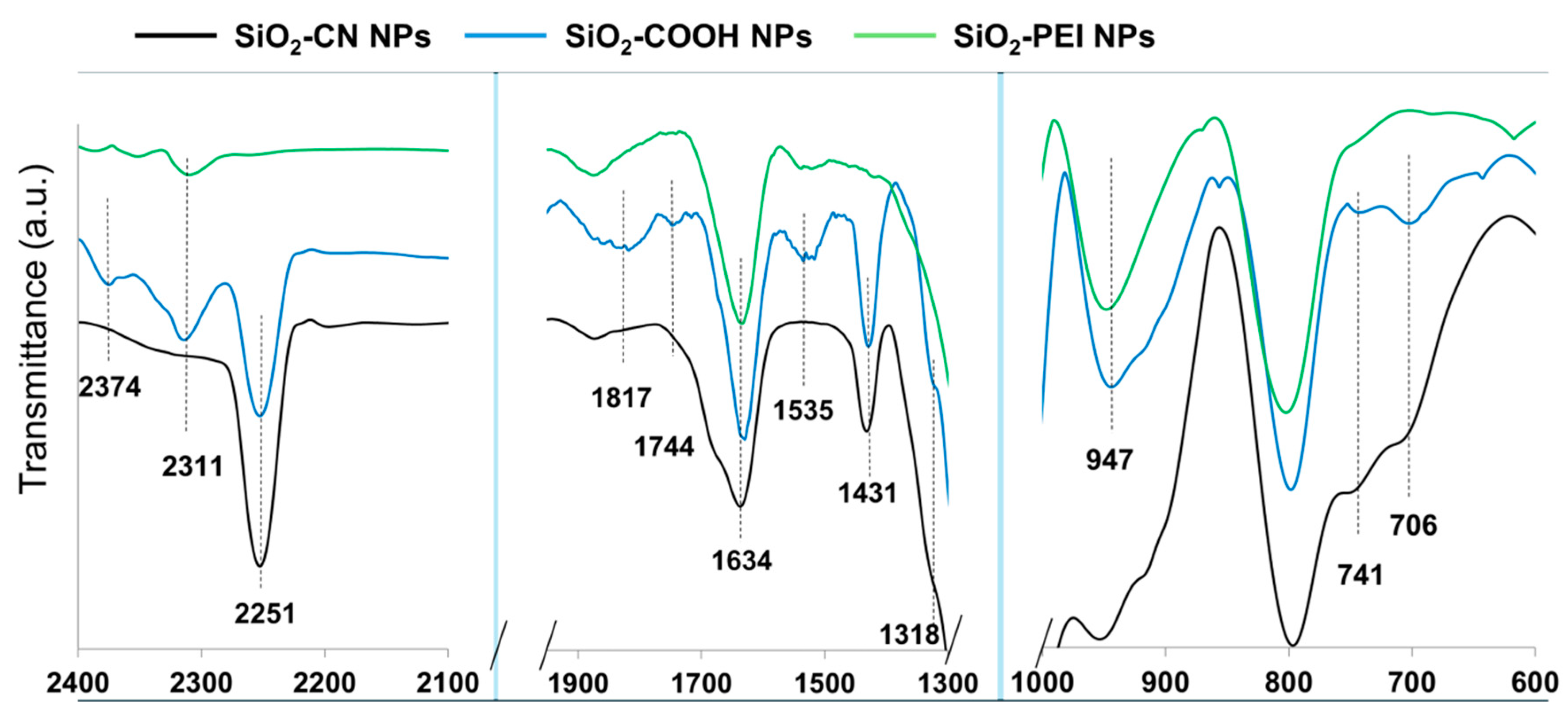



| Functional Group at the Surface | ζ-Potential [mV] |
|---|---|
| SiO2 NPs | −48.7 ± 1.4 |
| SiO2-CN NPs | −51.3 ± 0.5 |
| SiO2-COOH NPs | −37.5 ± 0.8 |
| SiO2-PEI NPs | +48.9 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honciuc, A.; Negru, O.-I.; Honciuc, M. Interfacing Langmuir–Blodgett and Pickering Emulsions for the Synthesis of 2D Nanostructured Films: Applications in Copper Ion Adsorption. Nanomaterials 2024, 14, 809. https://doi.org/10.3390/nano14090809
Honciuc A, Negru O-I, Honciuc M. Interfacing Langmuir–Blodgett and Pickering Emulsions for the Synthesis of 2D Nanostructured Films: Applications in Copper Ion Adsorption. Nanomaterials. 2024; 14(9):809. https://doi.org/10.3390/nano14090809
Chicago/Turabian StyleHonciuc, Andrei, Oana-Iuliana Negru, and Mirela Honciuc. 2024. "Interfacing Langmuir–Blodgett and Pickering Emulsions for the Synthesis of 2D Nanostructured Films: Applications in Copper Ion Adsorption" Nanomaterials 14, no. 9: 809. https://doi.org/10.3390/nano14090809
APA StyleHonciuc, A., Negru, O.-I., & Honciuc, M. (2024). Interfacing Langmuir–Blodgett and Pickering Emulsions for the Synthesis of 2D Nanostructured Films: Applications in Copper Ion Adsorption. Nanomaterials, 14(9), 809. https://doi.org/10.3390/nano14090809






