A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal
Abstract
1. Introduction
2. Experimental
2.1. Reagents
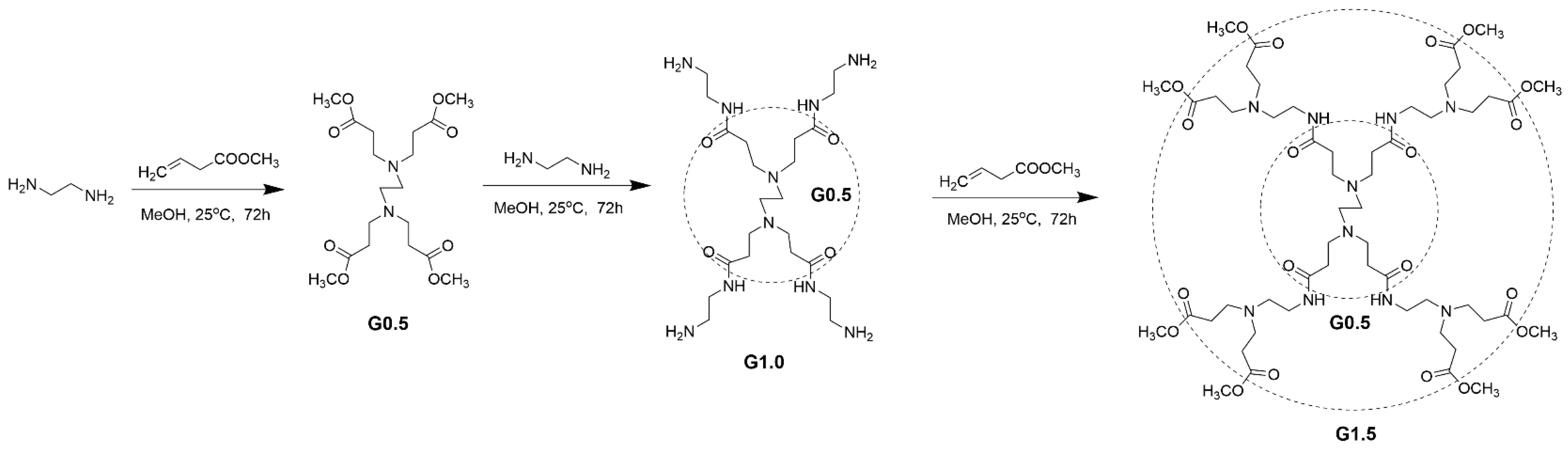
2.2. Preparation of PAMAM Dendrimer (G1.5)
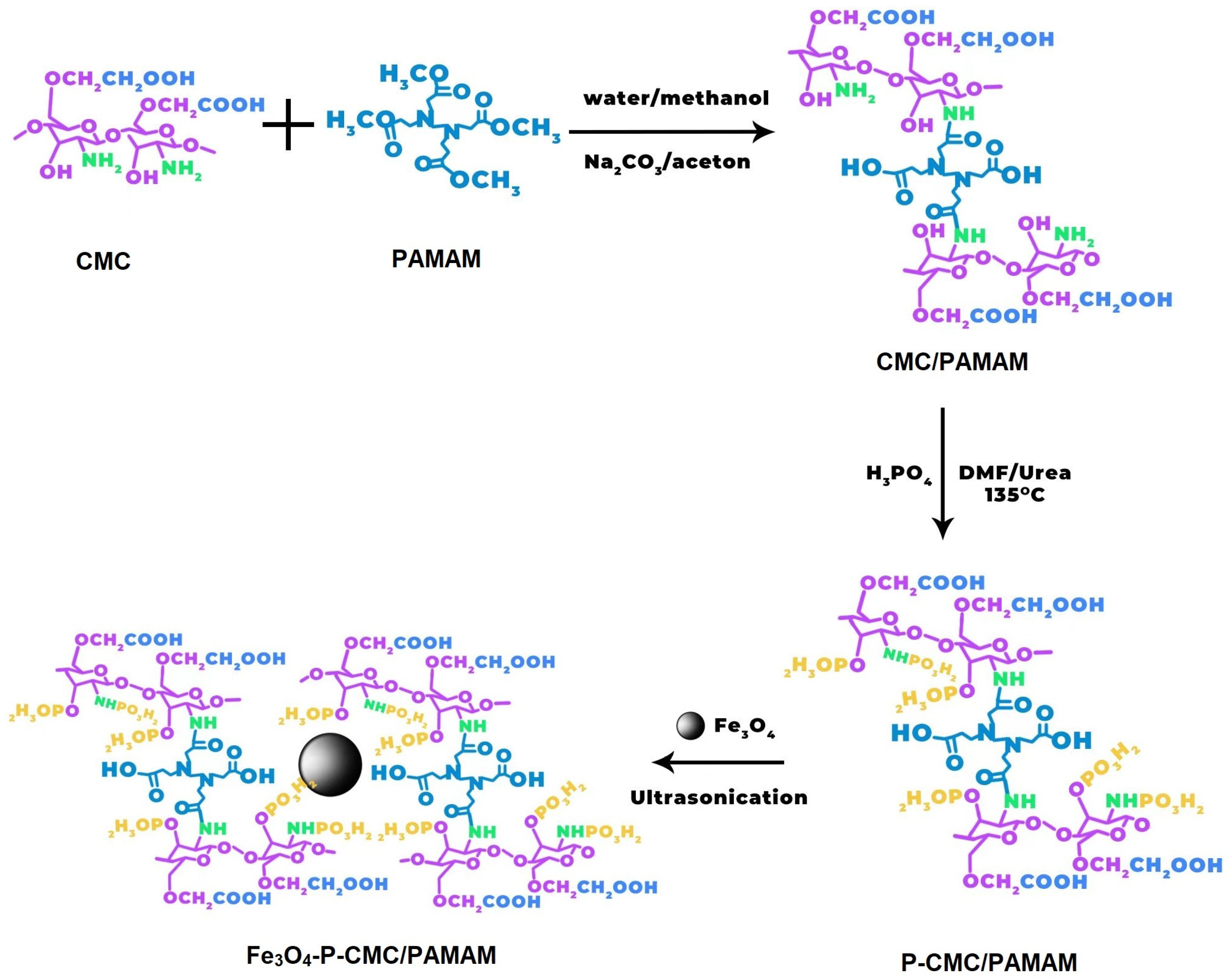
2.3. Preparation of CMC/G1.5 Nanoparticles
2.4. Preparation of P-CMC/G1.5 Nanoparticles
2.5. Preparation of Magnetic Fe3O4 Nanoparticles
2.6. Preparation of Fe3O4-P-CMC/G1.5 Nanoparticles
2.7. Effect of Different Factors on U(VI) Adsorption
2.8. Adsorption Kinetic
2.9. Adsorption Isotherms
2.10. Thermodynamic Studies
2.11. Desorption and Regeneration Experiments
3. Results and Discussion
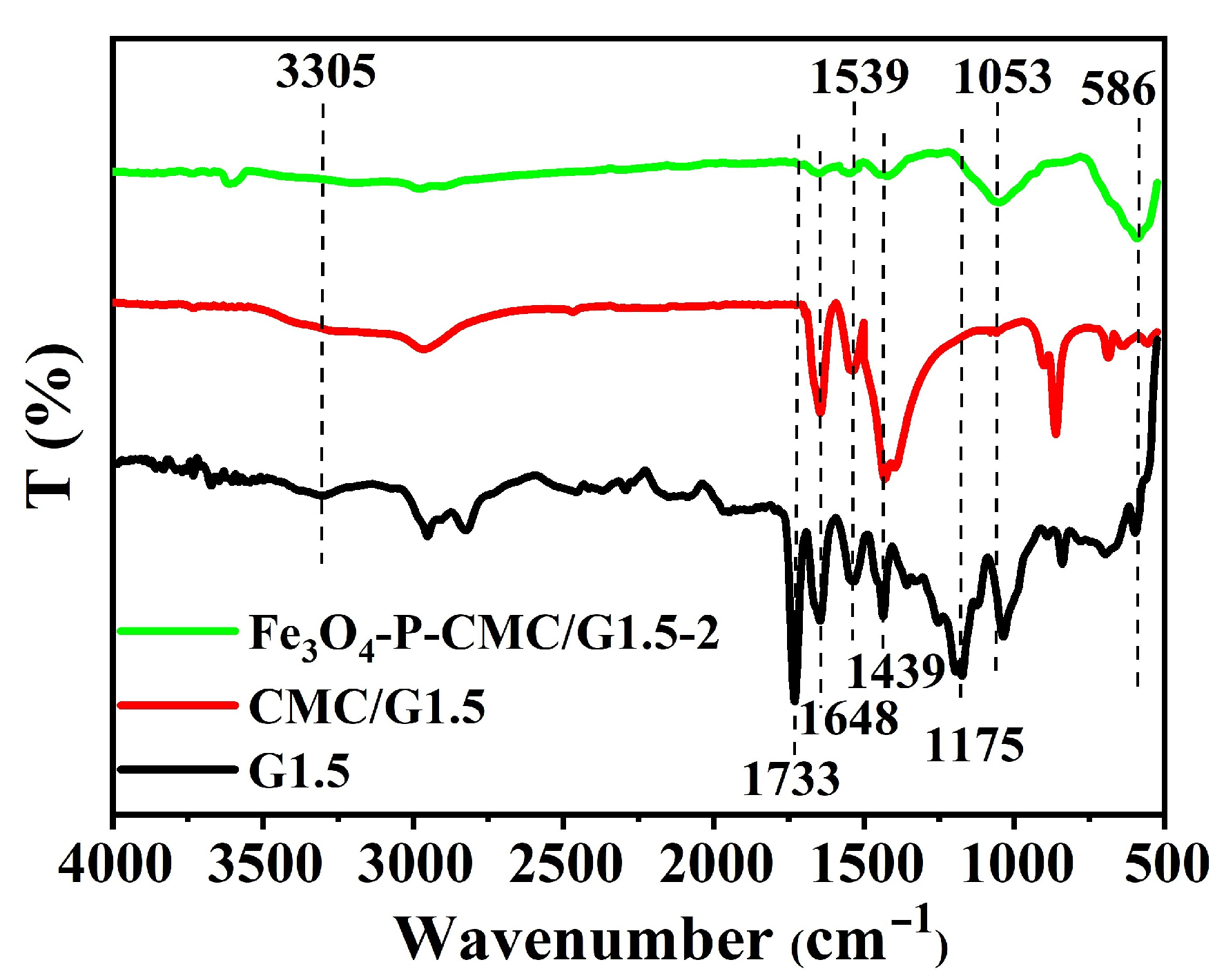
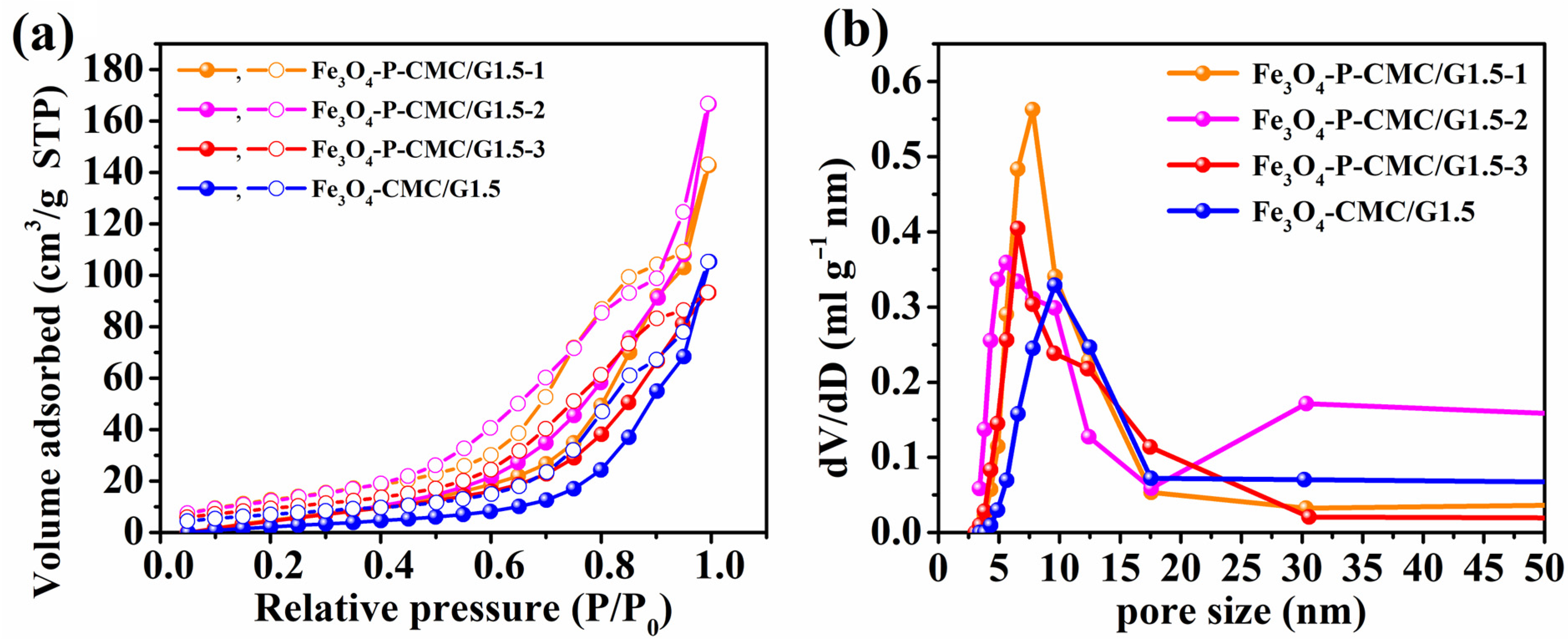
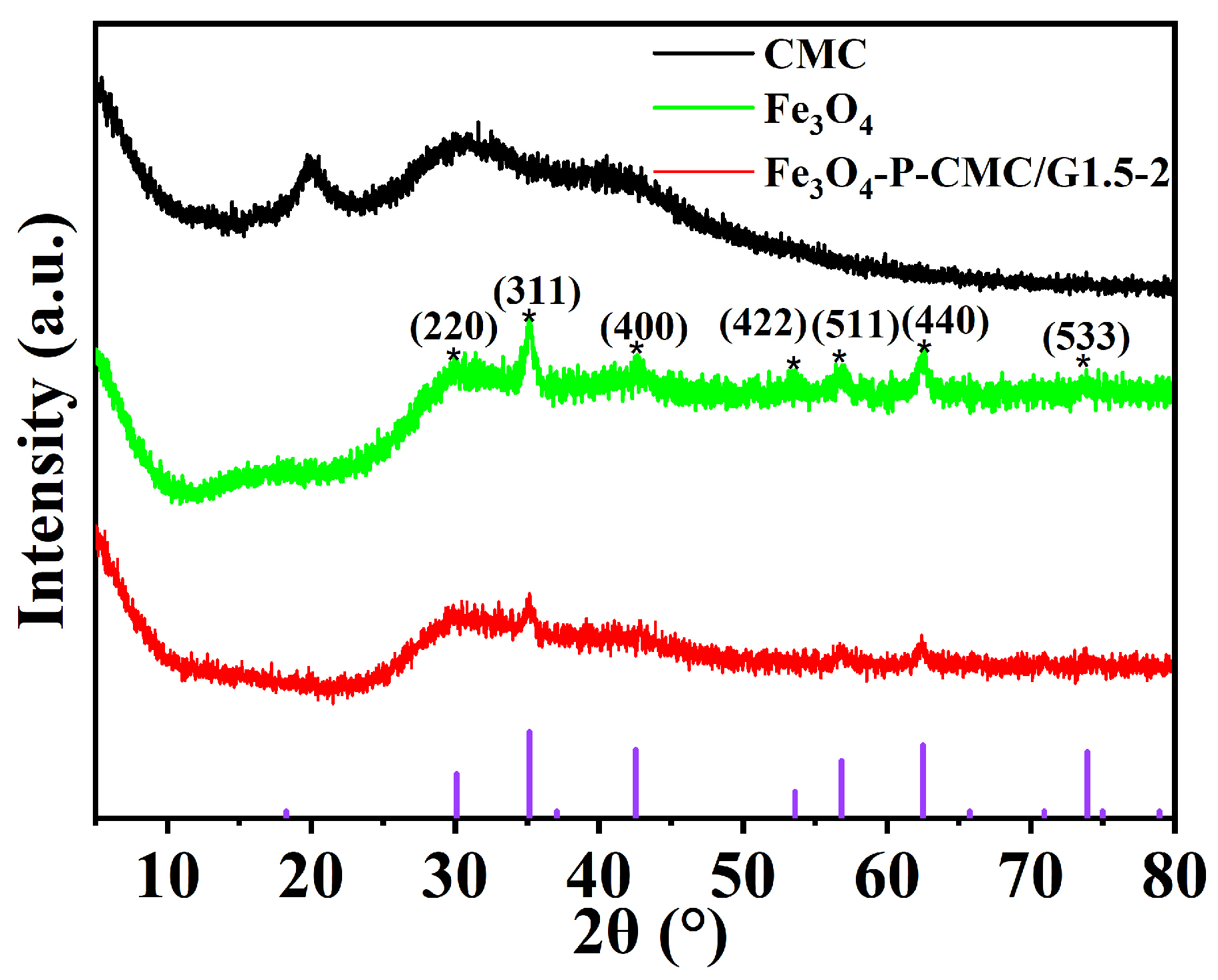
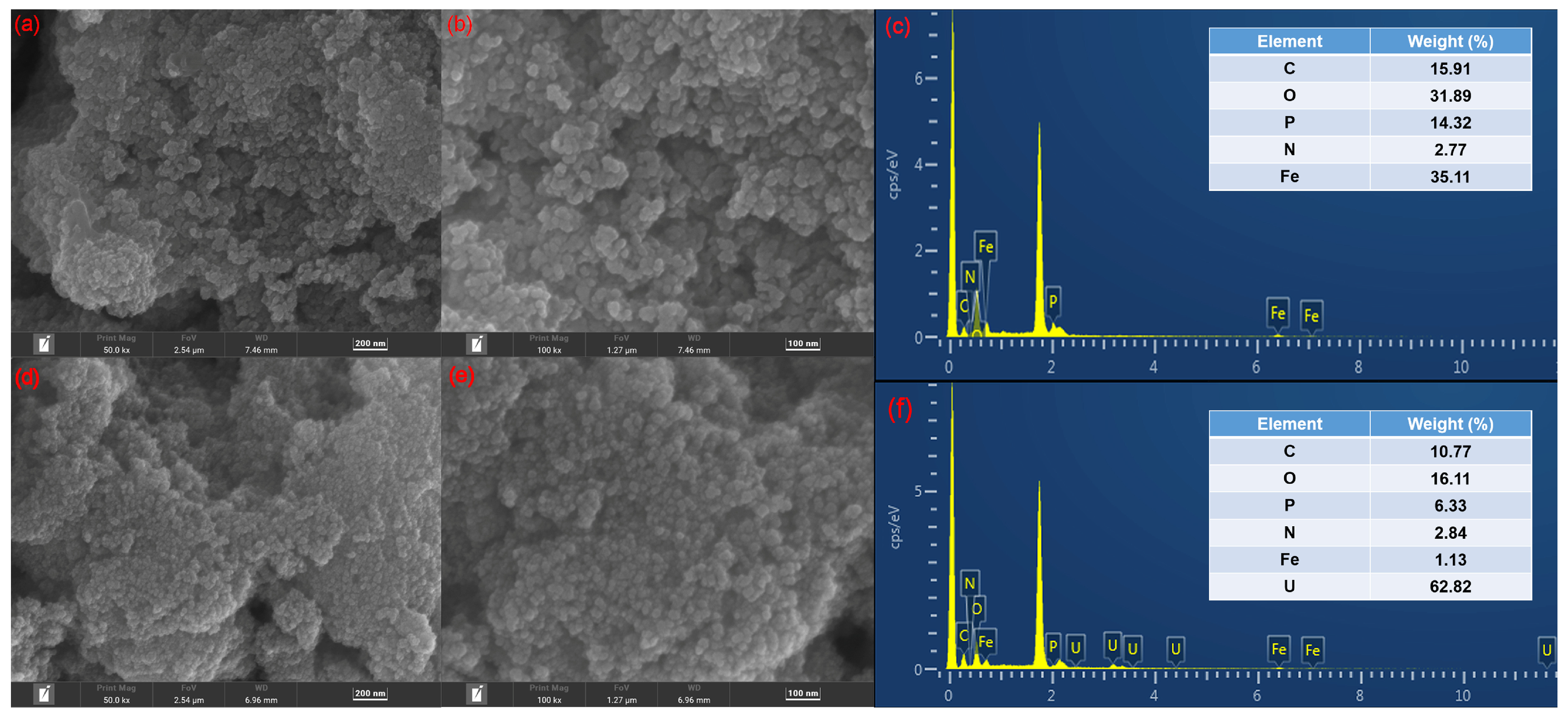
3.1. Characterization of Fe3O4-P-CMC/PAMAM
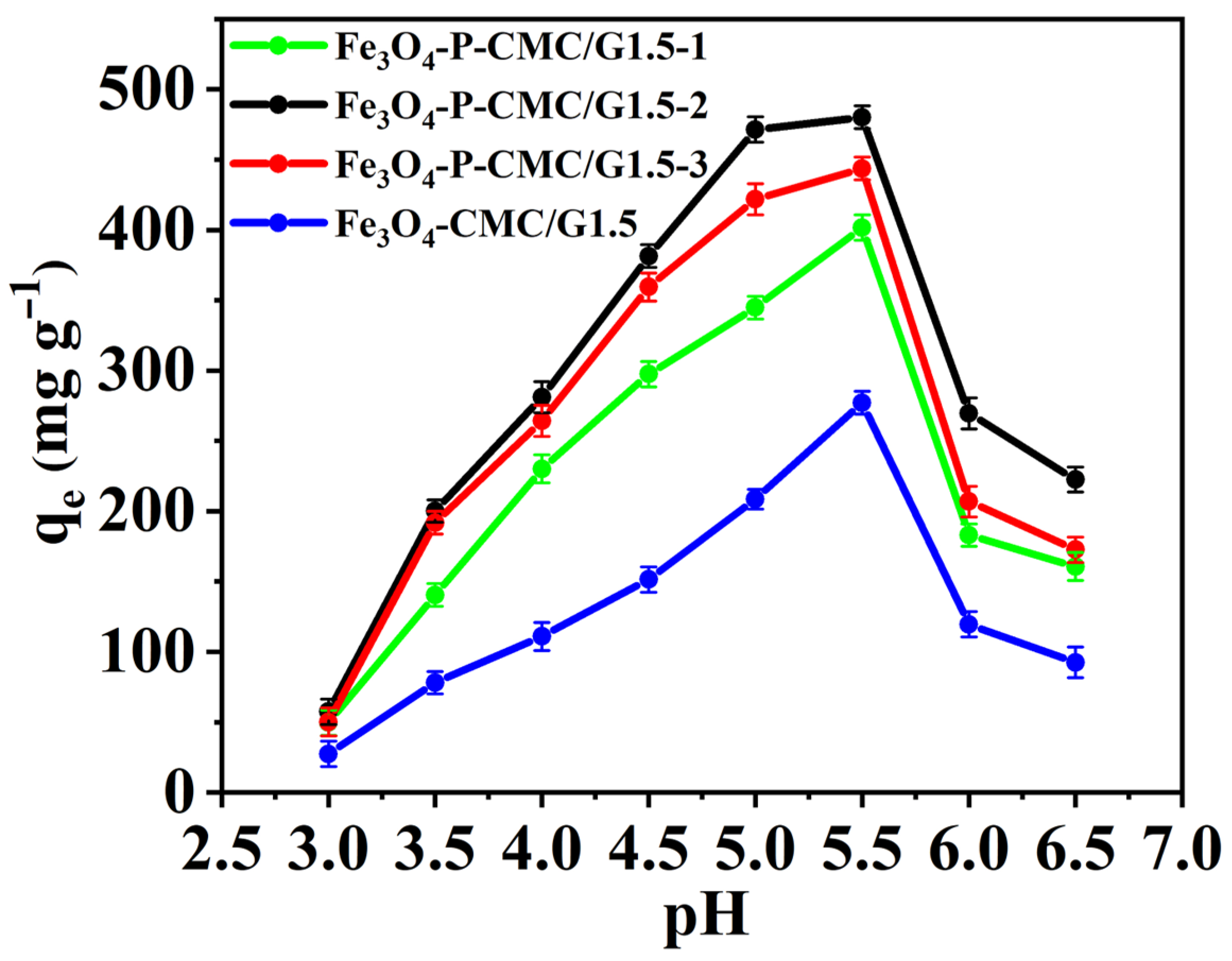
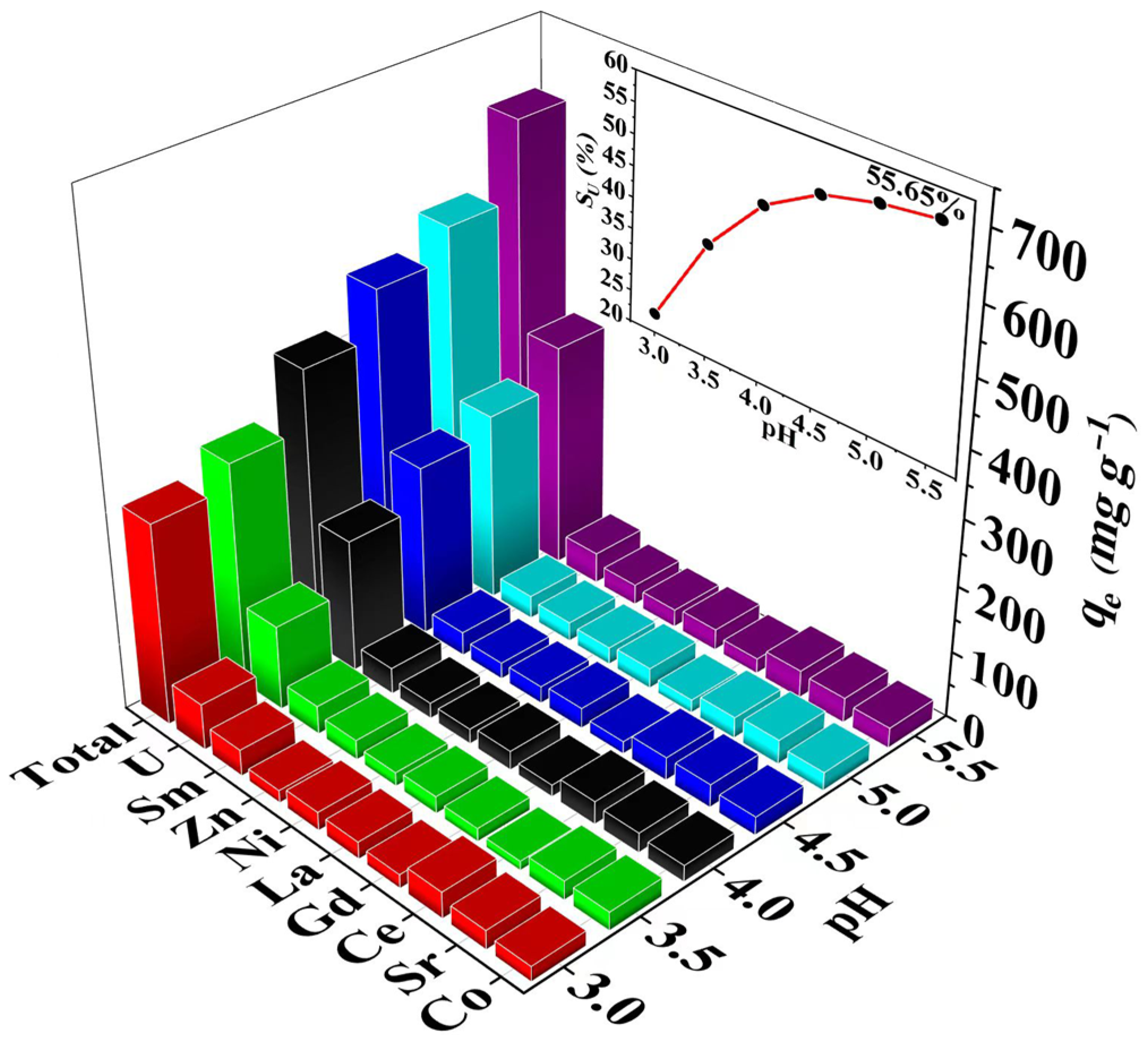
3.2. Effect of pH Values
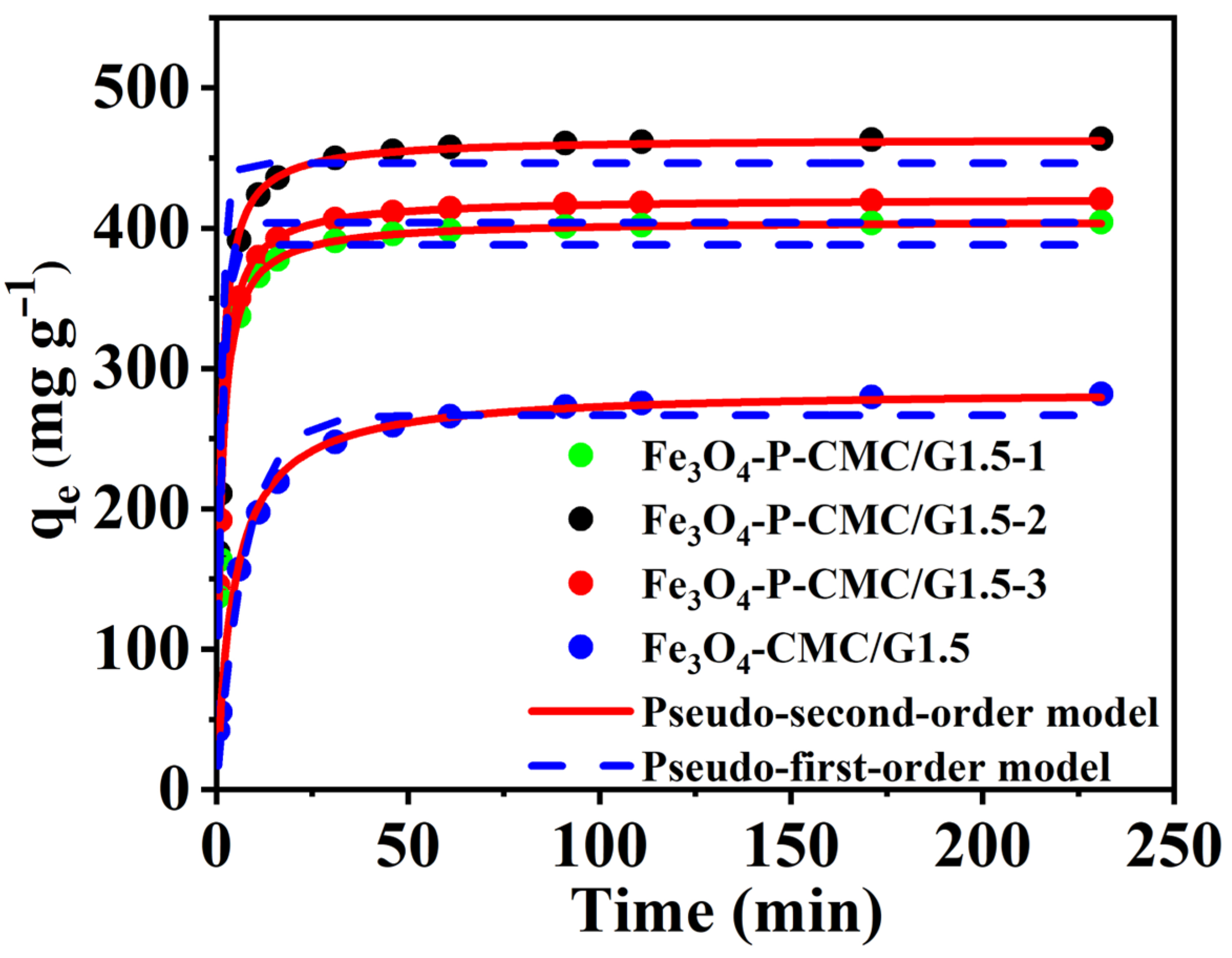
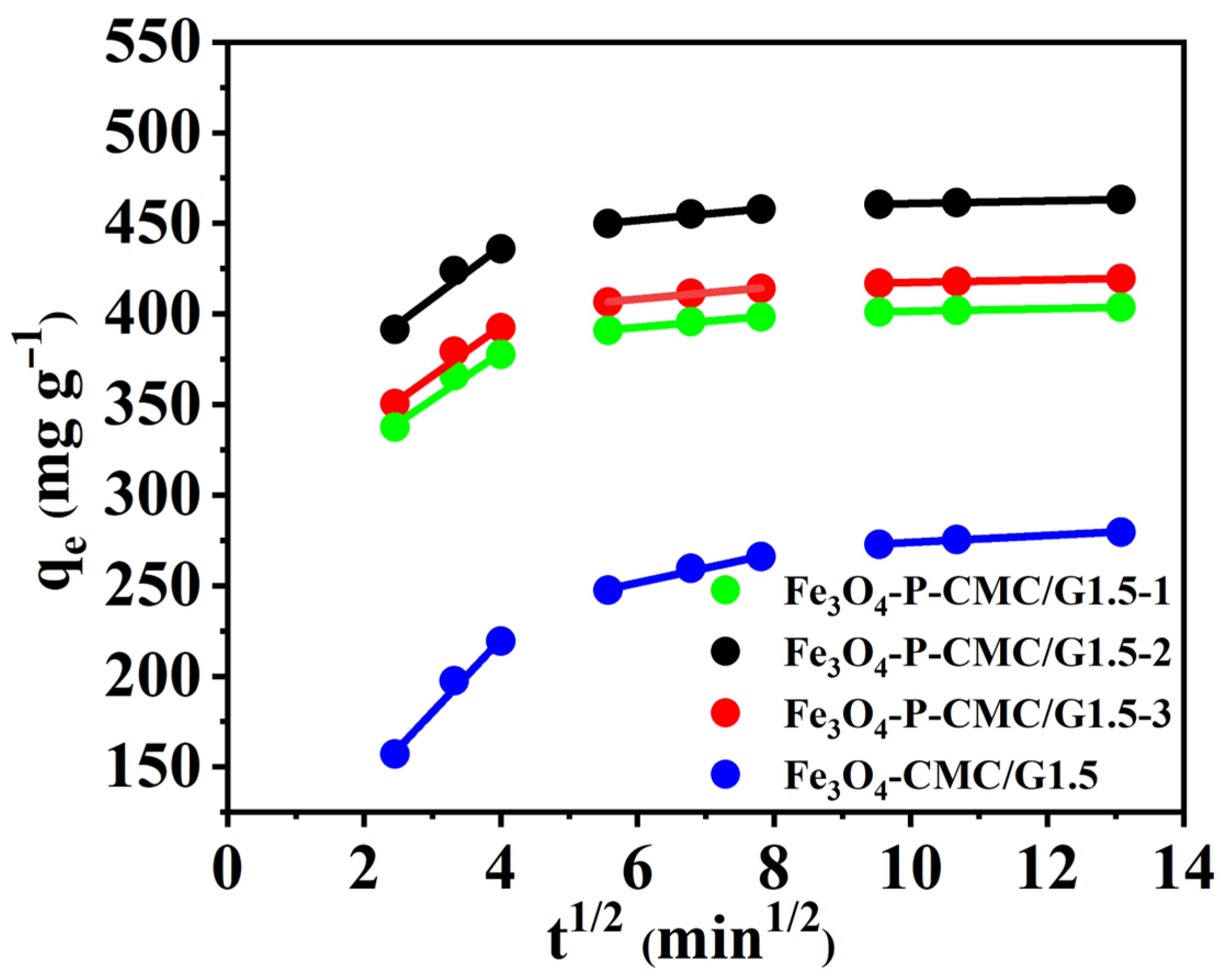
3.3. Adsorption Kinetics
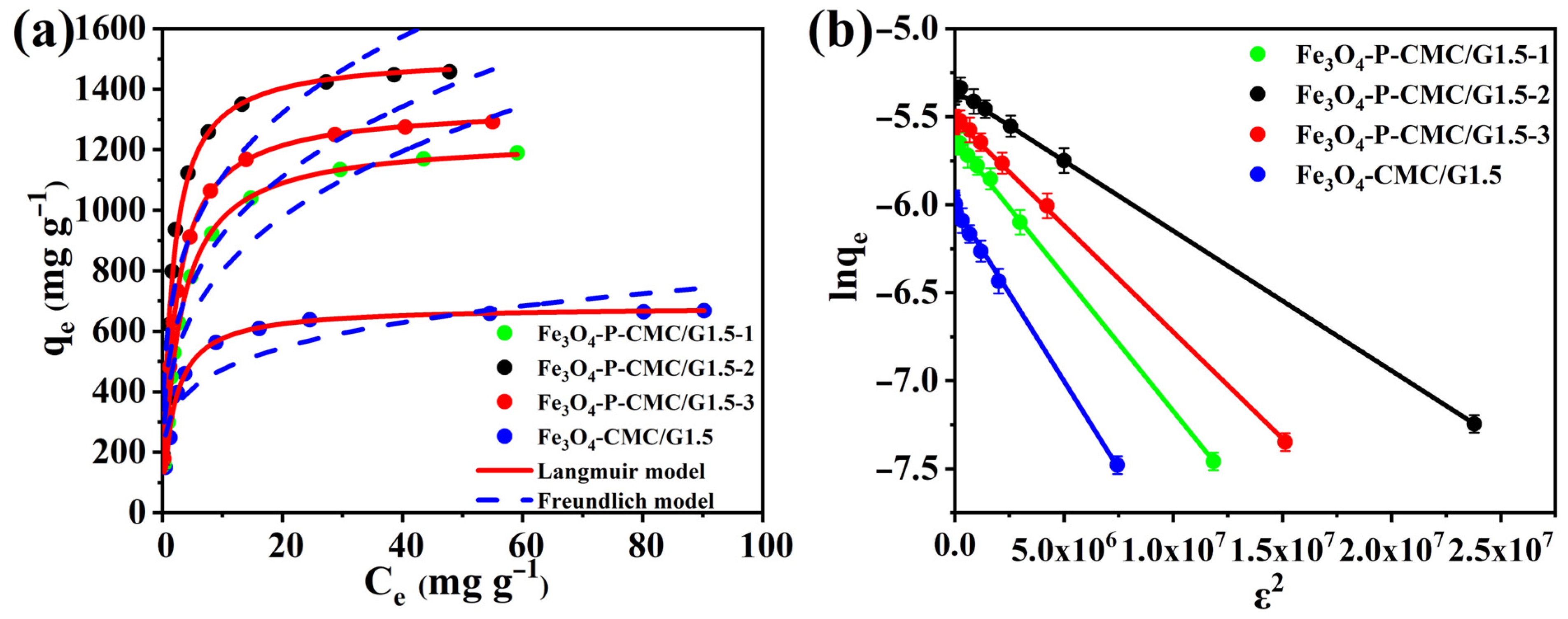
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamic
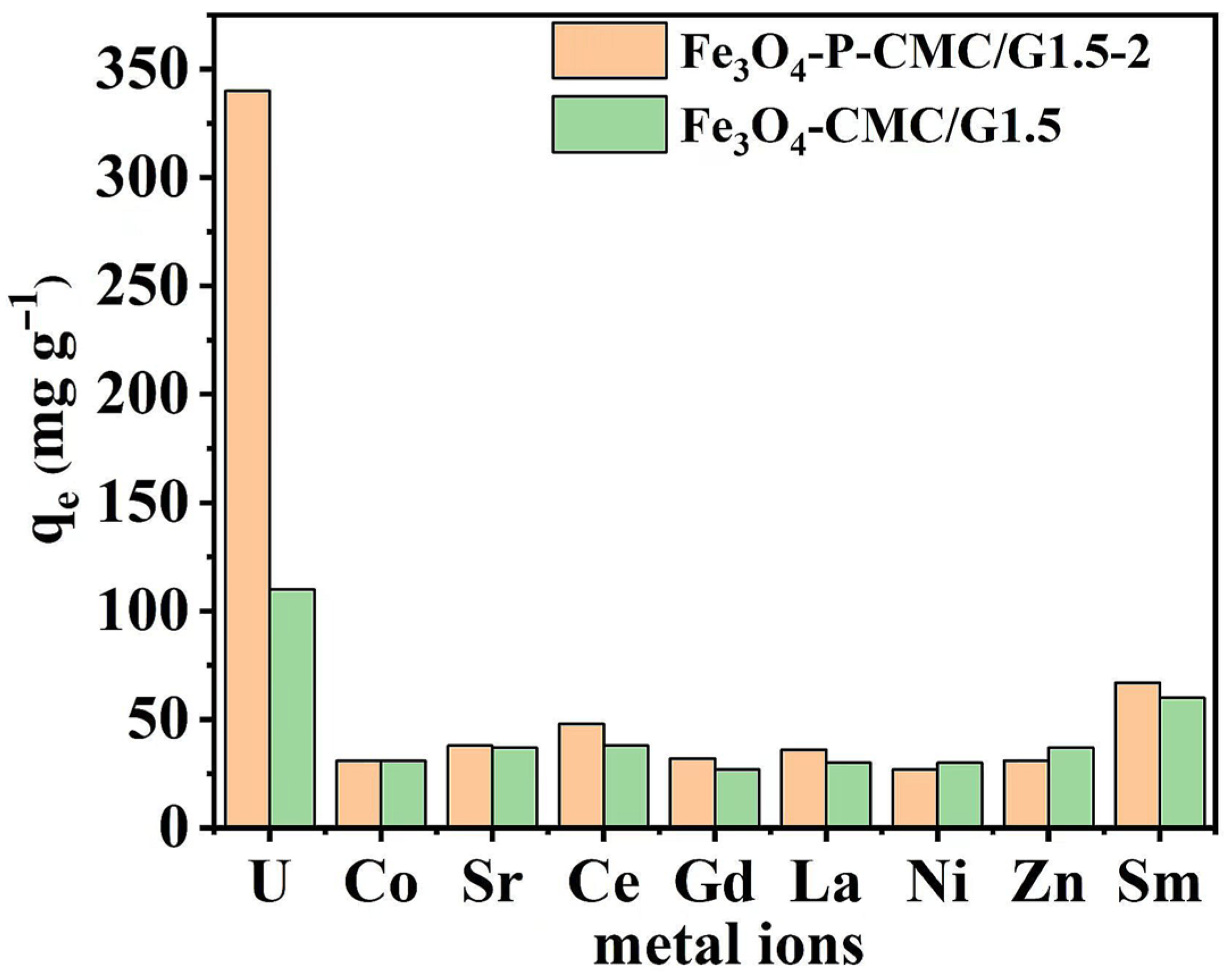
3.6. Effect of Competitive Ions
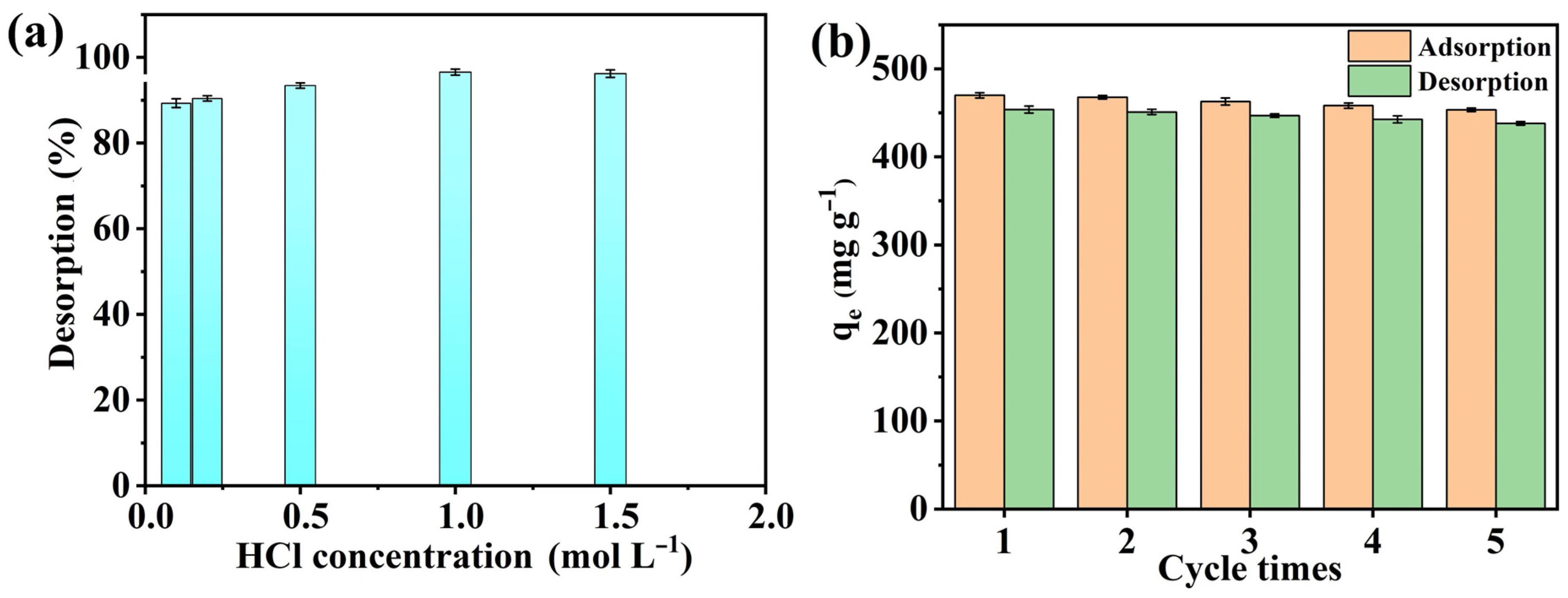
3.7. Desorption and Reusability
3.8. Possible Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMC | carboxymethyl chitosan | |
| PAMAM | poly(amidoamine) | |
| Nomenclature | ||
| C | the constant proportional to the extent of the boundary layer thickness (mg·g−1) | |
| C0 | the initial concentrations of the metal ion (mg∙L−1) | |
| Ce | the equilibrium concentrations of the metal ion (mg∙L−1), | |
| EDR | the mean free energy of adsorption | |
| k1 | the rate constants of pseudo-first-order model (min−1), | |
| k2 | the rate constants of pseudo-second-order model (g∙mg−1∙min−1) | |
| kint | the rate constants of intraparticle diffusion kinetics model (mg·g−1·min−0.5) | |
| Kd | the distribution coefficient (mL∙g−1) | |
| the distribution coefficients of uranyl ion | ||
| the distribution coefficients of other ions | ||
| KF | Freundlich constants, indicating adsorption intensity | |
| KL | the Langmuir adsorption equilibrium constant (L·mg−1) | |
| m | the amount of the sorbent (g) | |
| n | Freundlich constants, indicating adsorption capacity | |
| qe | the amount of adsorption at equilibrium (mg∙g−1) | |
| qe(U) | the adsorption capacity of uranium (mg·g−1) | |
| qe(Total) | the total amount of adsorption for all multi-ions (mg∙g−1). | |
| qDR | the theoretical adsorption capacity (mol·g−1) | |
| qm | the maximum or saturated amount of adsorption (mg∙g−1) | |
| qt | U(VI) adsorption capacity at t (mg·g−1) | |
| R | the ideal gas constant (8.314 kJ·mol−1 K−1) | |
| RL | the dimensionless constant separation factor | |
| SU | degree of selectivity of the adsorbent towards uranium | |
| SU(VI)/M(x) | the selectivity coefficient of uranyl ions relative to competing ions | |
| Sr | the relative selectivity coefficient | |
| t | the adsorption time (min) | |
| T | the absolute temperature (K). | |
| V | the volume of the solution (L) | |
| Greek letters | ||
| β | the constant associated with the adsorption energy (mol2·kJ−2) | |
| ε | the Polanyi potential | |
| ΔS° | standard entropy (J·mol−1·K−1) | |
| ΔH° | standard enthalpy (kJ·mol−1) | |
| ΔG° | standard Gibbs free energy (kJ·mol−1) | |
References
- Wang, Q.; Guo, J.; Li, R.; Jiang, X.-T. Exploring the role of nuclear energy in the energy transition: A comparative perspective of the effects of coal, oil, natural gas, renewable energy, and nuclear power on economic growth and carbon emissions. Environ. Res. 2023, 221, 115290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ye, Q.; Candel, S.; Vignon, D.; Guillaumont, R. A Chinese–French Study on Nuclear Energy and the Environment. Engineering 2023, 26, 159–172. [Google Scholar] [CrossRef]
- Grenthe, I.; Drożdżynński, J.; Fujino, T.; Buck, E.C.; Albrecht-Schmitt, T.E.; Wolf, S.F. The Chemistry of the Actinide and Transactinide Elements; Morrs, L.R., Edelstein, N.M., Fuger, J., Katz, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 253–689. [Google Scholar]
- Gomaa, H.; Shenashen, M.A.; Cheira, M.F.; Sueki, K.; El-Nasr, T.A.S.; Selim, M.M.; El-Safty, S.A. Green extraction of uranium (238U) from natural radioactive resources. Chem. Eng. J. 2023, 461, 142014. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. Life cycle sustainability assessment of advanced treatment techniques for urban wastewater reuse and sewage sludge resource recovery. Sci. Total. Environ. 2023, 869, 161771. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.O.; Chen, Z.; Ohoro, C.R.; Oniye, M.; Igwegbe, C.A.; Elimhingbovo, I.; Khongthaw, B.; Dulta, K.; Yap, P.-S.; Anastopoulos, I. A review of remediation technologies for uranium-contaminated water. Chemosphere 2024, 352, 141322. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, L.; Liu, L.; Zhao, B.; Hu, B.; Yu, S.; Wang, X. Uranium extraction from seawater by novel materials: A review. Sep. Purif. Technol. 2023, 320, 124204. [Google Scholar] [CrossRef]
- Korak, J.A.; Mungan, A.L.; Watts, L.T. Critical review of waste brine management strategies for drinking water treatment using strong base ion exchange. J. Hazard. Mater. 2023, 441, 129473. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, H.-H.; Rho, H.; Seo, J.; Jeon, J.-W.; Nam, S.-N.; Park, C.M.; Yoon, Y. Recovery of rare-earth and radioactive elements from contaminated water through precipitation: A review. Chem. Eng. J. 2023, 475, 146222. [Google Scholar] [CrossRef]
- Yu, K.; Pan, H.; Jiang, Y.; Zhang, T.; Zhang, H.; Ma, F.; Song, H.; Yuan, Y.; Pan, J. Anti-biological contamination strategies for enhanced uranium extraction from seawater. Desalination 2023, 566, 116893. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Sharif, H.M.A.; Zhang, J.; Ali, J.; Zhao, X. Sustainable and easy recoverable magnetic TiO2-Lignocellulosic Biomass@Fe3O4 for solar photocatalytic water remediation. J. Clean. Prod. 2019, 233, 841–847. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Khalifa, M.A.; El Wakeel, Y.M.; Header, M.S.; Abdel-Fattah, T.M. Engineered nano-magnetic iron oxide-urea-activated carbon nanolayer sorbent for potential removal of uranium (VI) from aqueous solution. J. Nucl. Mater. 2017, 487, 13–22. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Q.; Liu, J.; Zhang, H.; Yu, J.; Chen, R.; Song, D.; Li, R.; Wang, J. Magnetic metal-organic frameworks/carbon dots as a multifunctional platform for detection and removal of uranium. Appl. Surf. Sci. 2019, 491, 640–649. [Google Scholar] [CrossRef]
- Liu, F.; Hua, S.; Xia, F.; Hu, B. Efficient extraction of radionuclides with MXenes/persimmon tannin functionalized cellulose nanofibers: Performance and mechanism. Appl. Surf. Sci. 2023, 609, 155254. [Google Scholar] [CrossRef]
- Guo, H.; Qin, Q.; Chang, J.-S.; Lee, D.-J. Modified alginate materials for wastewater treatment: Application prospects. Bioresour. Technol. 2023, 387, 129639. [Google Scholar] [CrossRef]
- Castro-Riquelme, C.L.; López-Maldonado, E.A.; Ochoa-Terán, A.; Alcántar-Zavala, E.; Trujillo-Navarrete, B.; Pérez-Sicairos, S.; Miranda-Soto, V.; Zizumbo-López, A. Chitosan-carbamoylcarboxylic acid grafted polymers for removal of metal ions in wastewater. Chem. Eng. J. 2023, 456, 141034. [Google Scholar] [CrossRef]
- Michailidou, G.; Koumentakou, I.; Liakos, E.V.; Lazaridou, M.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Adsorption of Uranium, Mercury, and Rare Earth Elements from Aqueous Solutions onto Magnetic Chitosan Adsorbents: A Review. Polymers 2021, 13, 3137. [Google Scholar] [CrossRef]
- Patidar, A.; Thakur, D.S. Dendrimers: Potential carriers for drug delivery. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1383–1389. [Google Scholar]
- Kannan, R.M.; Pitha, I.; Parikh, K.S. A new era in posterior segment ocular drug delivery: Translation of systemic, cell-targeted, dendrimer-based therapies. Adv. Drug Deliv. Rev. 2023, 200, 115005. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnology 2023, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Yasir, A.T.; Benamor, A.; Hawari, A.H.; Mahmoudi, E. Poly (amido amine) dendrimer based membranes for wastewater treatment—A critical review. Chem. Eng. Sci. 2023, 273, 118665. [Google Scholar] [CrossRef]
- Hussain, I.; Muhammad, N.; Subhani, Q.; Shou, D.; Jin, M.; Yu, L.; Lu, G.; Wen, X.; Intisar, A.; Yan, Z. A review on structural aspects and applications of PAMAM dendrimers in analytical chemistry: Frontiers from separation sciences to chemical sensor technologies. TrAC Trends Anal. Chem. 2022, 157, 116810. [Google Scholar] [CrossRef]
- Patel, V.; Rajani, C.; Paul, D.; Borisa, P.; Rajpoot, K.; Youngren-Ortiz, S.R.; Tekade, R.K. Dendrimers as novel drug-delivery system and its applications. In Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 333–392. [Google Scholar]
- Liang, W.-X.; Wei, Y.; Qiao, M.; Fu, J.-W.; Wang, J.-X. High-gravity-assisted controlled synthesis of lanthanum carbonate for highly-efficient adsorption of phosphate. Sep. Purif. Technol. 2023, 307, 122696. [Google Scholar] [CrossRef]
- Ahmed, W.; Núñez-Delgado, A.; Mehmood, S.; Ali, S.; Qaswar, M.; Shakoor, A.; Chen, D.-Y. Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: Adsorption behavior and mechanism. Environ. Res. 2021, 201, 111518. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.V.; Pinto, D.; Salau, N.P.; Pinto, L.A.; Cadaval, T.R., Jr.; Silva, L.F.; Lopes, T.J.; Dotto, G.L. Modeling of anthocyanins adsorption onto chitosan films: An approach using the pore volume and surface diffusion model. Sep. Purif. Technol. 2022, 292, 121062. [Google Scholar] [CrossRef]
- Su, M.; Li, H.; Liu, Z.; Peng, H.; Huang, S.; Zhou, Y.; Liao, C.; Song, G.; Chen, D. Highly-efficient and easy separation of γ-Fe2O3 selectively adsorbs U (VI) in waters. Environ. Res. 2022, 210, 112917. [Google Scholar] [CrossRef]
- Wen, D.; Dong, Z.; Ao, Y.; Xie, K.; Zhai, M.; Zhao, L. Aminotriazole isomers modified cellulose microspheres for selective adsorption of U(VI): Performance and mechanism investigation. Carbohydr. Polym. 2021, 257, 117666. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; El-Sheshtawy, H.S.; El-Magied, M.O.A.; Dhmees, A.S. Mesoporous Al2O3 derived from blast furnace slag as a cost-effective adsorbent for U(VI) removal from aqueous solutions. Int. J. Environ. Anal. Chem. 2021, 103, 2948–2964. [Google Scholar] [CrossRef]
- Abin-Bazaine, A.; Trujillo, A.C.; Olmos-Marquez, M. Adsorption Isotherms: Enlightenment of the Phenomenon of Adsorption. In Wastewater Treatment; Ince, M., Ince, O.K., Eds.; IntechOpen Publishing: London, UK, 2022; pp. 1–15. [Google Scholar]
- Jin, X.; Li, H.; Zhu, X.; Li, N.; Owens, G.; Chen, Z. Enhanced removal of oxytetracycline from wastewater using bimetallic Fe/Ni nanoparticles combined with ZIF-8 nanocomposites. J. Environ. Manag. 2022, 318, 115526. [Google Scholar] [CrossRef]
- Musah, M.; Azeh, Y.; Mathew, J.; Umar, M.; Abdulhamid, Z.; Muhammad, A. Adsorption Kinetics and Isotherm Models: A Review. Caliphate J. Sci. Technol. 2022, 4, 20–26. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Xiao, S.; Xue, W.; Zhao, X.; Yang, Q. Efficient Capture of Th(IV) and U(VI) by Radiation-Resistant Oxygen-Rich Ion Traps Based on a Metal–Organic Framework. ACS Appl. Mater. Interfaces 2023, 15, 25029–25040. [Google Scholar] [CrossRef]
- Xin, Q.; Wang, Q.; Luo, K.; Lei, Z.; Hu, E.; Wang, H. Mechanism for the seleikctive adsorption of uranium from seawater using carboxymethyl-enhanced polysaccharide-based amidoxime adsorbent. Carbohydr. Polym. 2023, 324, 121576. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K.; Çaǧın, T.; Wang, G.; Goddard, W.A. Structure of PAMAM Dendrimers: Generations 1 through 11. Macromolecules 2004, 37, 6236–6254. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, Z.; Zheng, D.; Qiu, X. pH-responsive magnetic Fe3O4/carboxymethyl chitosan/aminated lignosulfonate nanoparticles with uniform size for targeted drug loading. Int. J. Biol. Macromol. 2023, 225, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.K.; Aal, M.M.A.; El-Rahem, K.A.A.; Allam, E.M.; Dayem, S.M.A.; Elshehy, E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Cheira, M.F. Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials 2022, 12, 3866. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ding, C.; Nie, X.; Duan, T.; Ding, R. Fabrication of 3D Macroscopic Graphene Oxide Composites Supported by Montmorillonite for Efficient U(VI) Wastewater Purification. ACS Sustain. Chem. Eng. 2017, 5, 5503–5511. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Wu, F.; Yu, S.; Hu, Y.; Song, W.; Liu, Y.; Wang, H.; Hayat, T.; Wang, X. Controllable Synthesis of Ca-Mg-Al Layered Double Hydroxides and Calcined Layered Double Oxides for the Efficient Removal of U(VI) from Wastewater Solutions. ACS Sustain. Chem. Eng. 2016, 5, 1173–1185. [Google Scholar] [CrossRef]
- Xin, Q.; Wang, Q.; Gan, J.; Lei, Z.; Hu, E.; Wang, H. Enhanced performance in uranium extraction by the synergistic effect of functional groups on chitosan-based adsorbent. Carbohydr. Polym. 2023, 300, 120270. [Google Scholar] [CrossRef] [PubMed]
- Yang, G. Sorption and reduction of hexavalent uranium by natural and modified silicate minerals: A review. Environ. Chem. Lett. 2023, 21, 2441–2470. [Google Scholar] [CrossRef]
- Kenney, J.P.; Lezama-Pacheco, J.; Fendorf, S.; Alessi, D.S.; Weiss, D.J. Uranium surface processes with sandstone and volcanic rocks in acidic and alkaline solutions. J. Colloid Interface Sci. 2023, 645, 715–723. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Cao, X.; Feng, Y.; Zhang, Z.; Wang, Y.; Liu, Y. Effects of different phosphorus sources on the adsorption of U (VI) by Zr (IV) organophosphate hybrids. J. Solid State Chem. 2021, 302, 122434. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Y.; Li, X.; He, X.; Ma, J.; Yang, L. Zirconium phosphonate doped PVA/Chitosan hybrid gel beads for enhanced selective extraction of Pb2+ from water. J. Taiwan Inst. Chem. Eng. 2015, 56, 103–112. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Wang, X.; Liu, D.; Liu, D. Preparation of amidoxime functionalized titanate nanosheets for efficient extraction of uranium from aqueous solution. J. Solid State Chem. 2020, 290, 121562. [Google Scholar] [CrossRef]
- BaiZQ, Y. Introduction of amino groups into acid-resistant MOFs for enhanced U (VI) sorption. J. Mater. Chem. A 2015, 3, 525. [Google Scholar]
- Xin, W.-B.; Xu, G.-C.; Li, M. Synthesis and characterization of a new organic–inorganic hybrid ferroelectric: (C4H10N)6[InBr6][InBr4]3·H2O. Dalton Trans. 2019, 48, 17402–17407. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; An, X.; Wan, G.; Zhu, W.; Luo, Y. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism. Sci. Total. Environ. 2019, 688, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Zhou, W.; Chen, C.; Xiong, Z. Investigation of U(VI) adsorption properties of poly(trimesoyl chloride-co-polyethyleneimine). J. Solid State Chem. 2021, 296, 121966. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, C.; Ren, X.; Wang, X.; Wang, H.; Wang, X. Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Prog. Mater. Sci. 2019, 103, 180–234. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Z.; Chen, S.; Yao, J.; Mu, Q.; Peng, D.; Zhao, H. Synthesis and facile functionalization of siloxane based hyper-cross-linked porous polymers and their applications in water treatment. Eur. Polym. J. 2019, 119, 94–101. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Xu, X.; Alsaedi, A.; Hayat, T.; Li, J. Adsorption and desorption of U(VI) on different-size graphene oxide. Chem. Eng. J. 2018, 360, 941–950. [Google Scholar] [CrossRef]
- Negm, S.H.; Abd El-Magied, M.O.; El Maadawy, W.M.; Abdel Aal, M.M.; Abd El Dayem, S.M.; Taher, M.A.; Abd El-Rahem, K.A.; Rashed, M.N.; Cheira, M.F. Appreciatively Efficient Sorption Achievement to U (VI) from the El Sela Area by ZrO2/Chitosan. Separations 2022, 9, 311. [Google Scholar] [CrossRef]
- Dai, Z.; Zhao, S.; Lian, J.; Li, L.; Ding, D. Efficient visible-light-driven photoreduction of U(VI) by carbon dots modified porous g-C3N4. Sep. Purif. Technol. 2022, 298, 121590. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, C.; Liu, Z.; Zhang, L.; Chen, L.; Wang, J.; Wang, X.; Yang, S.; Wang, S. Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U(vi). Environ. Sci. Nano 2017, 4, 1876–1886. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, L.; Wang, Q.; Xin, Q.; Xiong, Y.; Hu, E.; Lei, Z.; Wang, H. Phosphorylation improved the competitive U/V adsorption on chitosan-based adsorbent containing amidoxime for rapid uranium extraction from seawater. Int. J. Biol. Macromol. 2023, 238, 124074. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Sang, H.; Chen, Y.; Wei, Y.; Wu, Y. Layer-by-layer grafting synthesis of silicon-based zirconium organophosphate for the enhanced and selective adsorption of lanthanides. J. Environ. Chem. Eng. 2022, 10, 108608. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Luo, Q.; Han, R.; Wang, H.; Yang, J.; Liu, C. A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal. Nanomaterials 2024, 14, 810. https://doi.org/10.3390/nano14090810
Ma M, Luo Q, Han R, Wang H, Yang J, Liu C. A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal. Nanomaterials. 2024; 14(9):810. https://doi.org/10.3390/nano14090810
Chicago/Turabian StyleMa, Mingyang, Qunyin Luo, Ruidong Han, Hongyi Wang, Junjie Yang, and Chunyuan Liu. 2024. "A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal" Nanomaterials 14, no. 9: 810. https://doi.org/10.3390/nano14090810
APA StyleMa, M., Luo, Q., Han, R., Wang, H., Yang, J., & Liu, C. (2024). A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal. Nanomaterials, 14(9), 810. https://doi.org/10.3390/nano14090810




