Recent Advances in Ruddlesden–Popper Phase-Layered Perovskite Sr2TiO4 Photocatalysts
Abstract
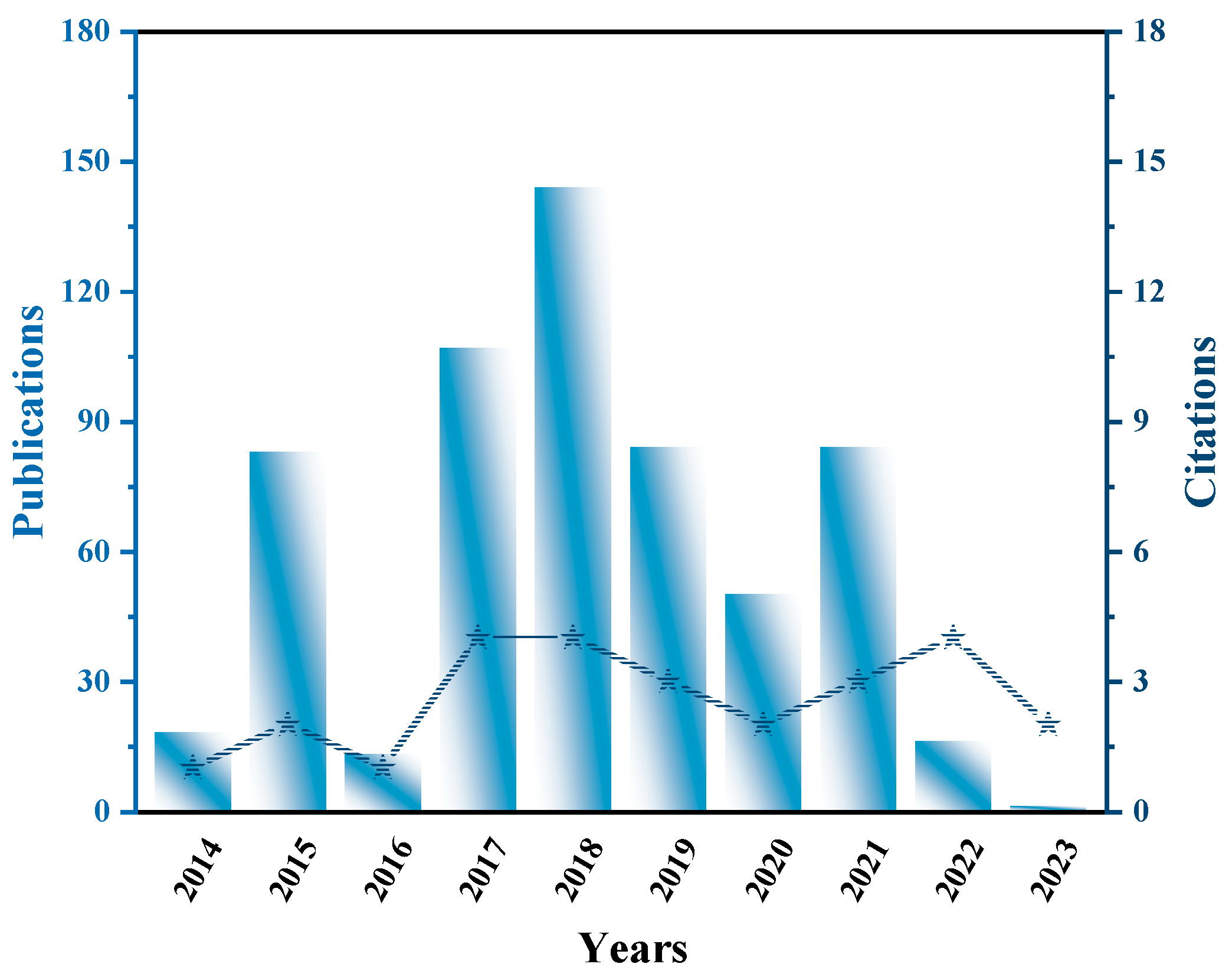
:1. Introduction
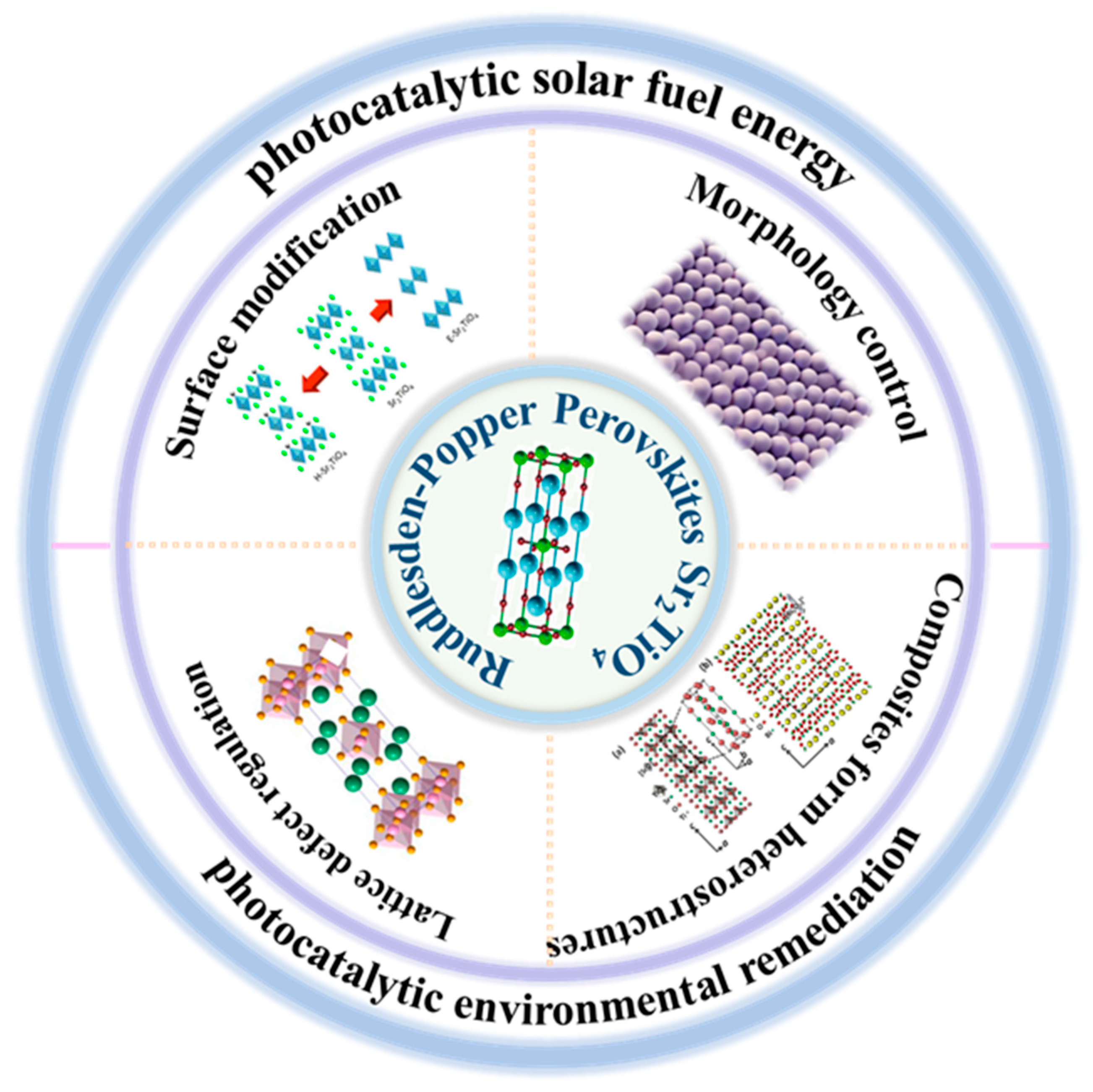
2. The Properties and Modification of Sr2TiO4
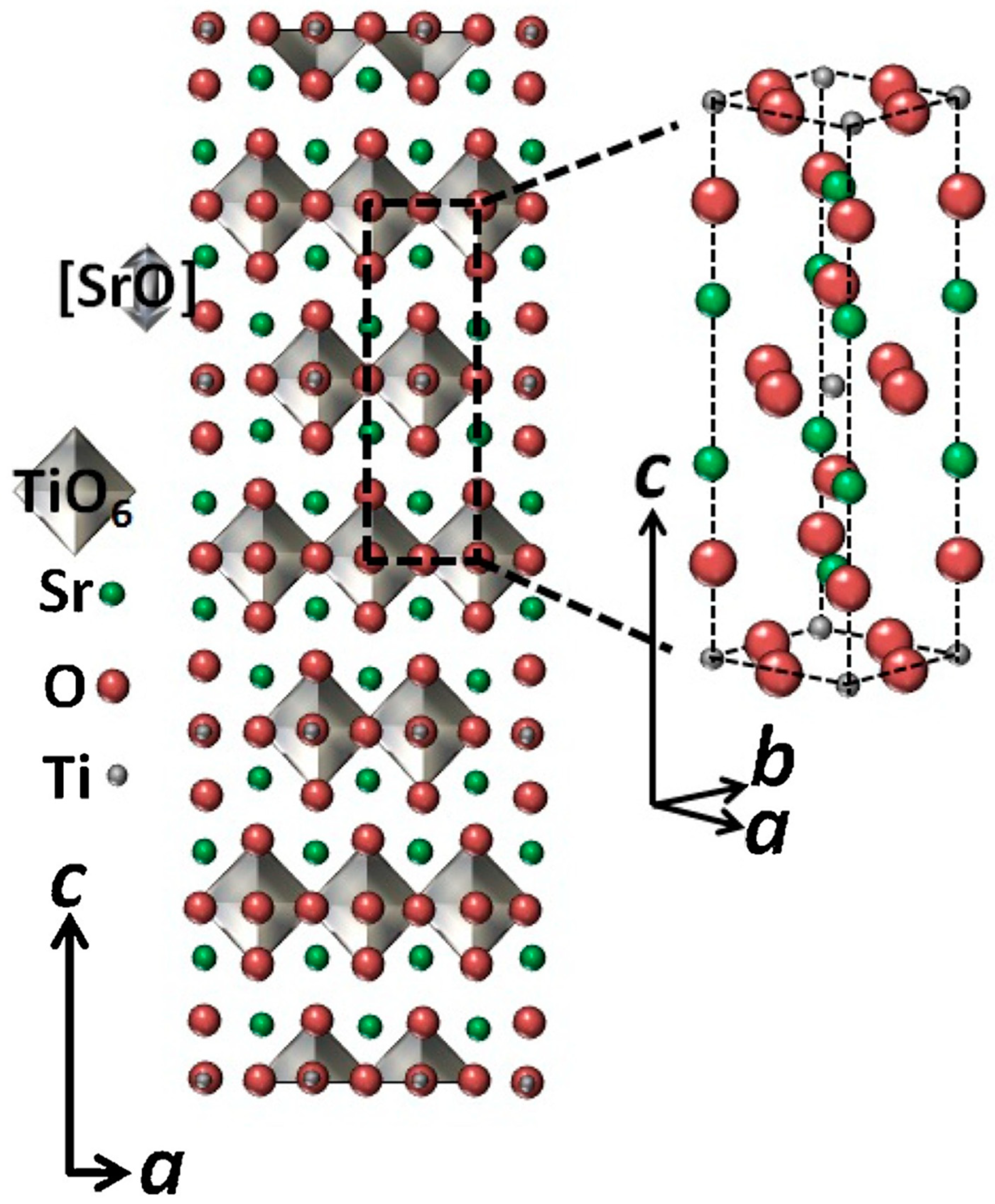
2.1. Sr2TiO4
2.2. Modification Strategies
2.2.1. Surface Modification
2.2.2. Morphology Control
2.2.3. Lattice Defect Regulation
2.2.4. Composites Form Heterostructures
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kong, W.; Xing, Z.; Fang, B.; Cui, Y.; Li, Z.; Zhou, W. Plasmon Ag/Na-doped defective graphite carbon nitride/NiFe layered double hydroxides Z-scheme heterojunctions toward optimized photothermal-photocatalytic-Fenton performance. Appl. Catal. B 2022, 304, 120969. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, F.; Song, W.; Wang, P.; Zhu, C.; Wang, X.; Li, S.; Li, X.; Li, W. Construction of surface oxygen vacancy active sites upon MnOx/AC composite catalysts for efficient coupling adsorption and oxidation of toluene. Chem. Eng. J. 2023, 464, 142740. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Zhang, J.-Y.; Song, W.; Chen, Y.; Wang, F.; Liu, Y.; Zhu, C.; Li, W. Construction of core-shell Ni-Co(OH)F@NiCo2S4 nanorods for highly efficient hydrazine-assisted hydrogen evolution. Sustain. Energy Fuels 2023, 7, 84–91. [Google Scholar] [CrossRef]
- Xiong, J.; Huang, H.X.; Lin, B.; Xia, J.X.; Di, J. Lower oxygen vacancy concentration in BiPO4 with unexpected higher photocatalytic activity. Chin. Chem. Lett. 2023, 34, 107844. [Google Scholar] [CrossRef]
- Jian, S.J.; Tian, Z.W.; Hu, J.P.; Zhang, K.Y.; Zhang, L.; Duan, G.G.; Yang, W.S.; Jiang, S.H. Enhanced visible light photocatalytic efficiency of La-doped ZnO nanofibers via electrospinning-calcination technology. Adv. Powder Mater. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Wang, P.; Song, W.; Li, W. Phase transformation strategies for the construction of heterojunction TiO2 photocatalysts. ChemCatChem 2024, 16, e202301489. [Google Scholar] [CrossRef]
- Song, P.; Du, J.; Ma, X.; Shi, Y.; Fang, X.; Liu, D.; Wei, S.; Liu, Z.; Cao, Y.; Lin, B.; et al. Design of Bi4O5Br2/g-C3N4 heterojunction for efficient photocatalytic removal of persistent organic pollutants from water. Ecoenergy 2023, 1, 197–206. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.H.; Huang, H.W. Layered photocatalytic nanomaterials for environmental applications. Chem. Eng. J. 2023, 34, 107523. [Google Scholar] [CrossRef]
- Li, S.J.; You, C.J.; Rong, K.; Zhuang, C.Q.; Chen, X.B.; Zhang, B. Chemically bonded Mn0.5Cd0.5S/BiOBr S-scheme photocatalyst with rich oxygen vacancies for improved photocatalytic decontamination performance. Adv. Powder Mater. 2024, 3, 100183. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y.; Dong, Y.; Han, X.; Mu, M.; Chen, Y.; Wang, W.; Wang, P.; Li, W. Fabrication of brookite@anatase heterojunction TiO2 via phase transformation from metal organic frameworks for enhanced photocatalytic hydrogen evolution and TCH degradation. Catal. Sci. Technol. 2023, 13, 3292–3303. [Google Scholar] [CrossRef]
- Li, S.J.; Cai, M.J.; Liu, Y.P.; Wang, C.C.; Yan, R.Y.; Chen, X.B. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Ding, G.; Wang, Z.; Zhang, J.; Wang, P.; Chen, L.; Liao, G. Layered double hydroxides-based Z-scheme heterojunction for photocatalysis. Ecoenergy 2024, 2, 22–44. [Google Scholar] [CrossRef]
- Chen, F.J.; Shao, C.W.; Zhao, M.N.; Bu, Z.S.; Zhang, Y.; Dai, X.N.; Zhou, G.W. Controllable synthesis and photocatalytic activities of rod-shaped mesoporous titanosilicate composites with varied aspect ratios. Chin. Chem. Lett. 2014, 25, 962–966. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Ruddlesden–Popper Perovskite Oxides for Photocatalysis-Based Water Splitting and Wastewater Treatment. Energy Fuels 2020, 34, 9208–9221. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B″O6 perovskites: A review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Barnes, P.W.; Woodward, P.M. Structure prediction of ordered and disordered multiple octahedral cation perovskites using SPuDS. Acta Cryst. 2006, 62, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F.; Yin, W.-J. Stability Trend of Tilted Perovskites. J. Phys. Chem. 2018, 122, 15214–15219. [Google Scholar] [CrossRef]
- Sun, X.; Xu, X. Efficient photocatalytic hydrogen production over La/Rh co-doped Ruddlesden-Popper compound Sr2TiO4. Appl. Catal. B 2017, 210, 149–159. [Google Scholar] [CrossRef]
- Oshima, T.; Yokoi, T.; Eguchi, M.; Maeda, K. Synthesis and photocatalytic activity of K2CaNaNb3O10, a new Ruddlesden–Popper phase layered perovskite. Dalton Trans. 2017, 46, 10594–10601. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, I.A.; Mechtaeva, E.V.; Burovikhina, A.A.; Silyukov, O.I.; Toikka, M.A.; Zvereva, I.A. Effect of protonation on the photocatalytic activity of the K2La2Ti3O10 layered oxide in the reaction of hydrogen production. Monatshefte Chem.-Chem. Mon. 2018, 149, 475–482. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Y.; Wu, F.; Chen, H.; Lv, M.; Ni, S.; Liu, G.; Xu, X. Photocatalytic Hydrogen Production over Chromium Doped Layered Perovskite Sr2TiO4. Inorg. Chem. 2015, 54, 7445–7453. [Google Scholar] [CrossRef] [PubMed]
- Sorkh-Kaman-Zadeh, A.; Dashtbozorg, A. Facile chemical synthesis of nanosize structure of Sr2TiO4 for degradation of toxic dyes from aqueous solution. J. Mol. Liq. 2016, 223, 921–926. [Google Scholar] [CrossRef]
- Sun, X.; Mi, Y.; Jiao, F.; Xu, X. Activating Layered Perovskite Compound Sr2TiO4 via La/N Codoping for Visible Light Photocatalytic Water Splitting. ACS Catal. 2018, 8, 3209–3221. [Google Scholar] [CrossRef]
- Hu, C.; Chen, T.-S.; Huang, H.-X. Heterojunction of n-type Sr2TiO4 with p-type Bi5O7I with enhanced photocatalytic activity under irradiation of simulated sunlight. Appl. Surf. Sci. 2017, 426, 536–544. [Google Scholar] [CrossRef]
- Chupakhina, T.I.; Eremina, R.M.; Gyrdasova, O.I.; Yanchenko, M.Y.; Buldakova, L.Y.; Yatsyk, I.V.; Gavrilova, T.P.; Deeva, Y.A.; Sukhanov, A.A.; Baklanova, I.V.; et al. Perovskite-like LaxSr2-xTi1-x/2Cux/2O4 (x = 0.2, 0.3, 0.5) oxides with the K2NiF4-type structure active in visible light range: New members of the photocatalyst family. J. Korean Ceram. Soc. 2024, 61, 623–635. [Google Scholar] [CrossRef]
- Jacob, K.T.; Rajitha, G. Thermodynamic properties of strontium titanates: Sr2TiO4, Sr3Ti2O7, Sr4Ti3O10, and SrTiO3. J. Chem. Thermodyn. 2011, 43, 51–57. [Google Scholar] [CrossRef]
- Shibuya, K.; Mi, S.; Jia, C.L.; Meuffels, P.; Dittmann, R. Sr2TiO4 layered perovskite thin films grown by pulsed laser deposition. Appl. Phys. Lett. 2008, 92, 241918. [Google Scholar] [CrossRef]
- Zhang, H.; Ni, S.; Mi, Y.; Xu, X. Ruddlesden-Popper compound Sr2TiO4 co-doped with La and Fe for efficient photocatalytic hydrogen production. J. Catal. 2018, 359, 112–121. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Zhao, Y.; Zeng, X. Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light. Int. J. Mol. Sci. 2022, 23, 10927. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, J.; Zhang, F. Morphology-controlled fabrication of Nb5+ doped SrTiO3 for promoting photocatalytic reduction of Cr(VI). Mol. Catal. 2024, 553, 113754. [Google Scholar] [CrossRef]
- Chen, Z.B.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–6. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Xie, J.; Lu, Z.J.; Hu, J.D.; Hao, A.Z.; Cao, Y.L. Insight into the effect of OH modification on the piezo-photocatalytic hydrogen production activity of SrTiO3. J. Colloid Interface Sci. 2022, 612, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, S.H.; Wang, Y.H.; Hu, Z.F.; Zhao, H.; Xie, W. Ethanol-Quenching Introduced Oxygen Vacancies in Strontium Titanate Surface and the Enhanced Photocatalytic Activity. Nanomaterials 2019, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Gu, W.; Li, H.J.; Zou, W.; Liu, H.; Zhang, Y.Y.; Wu, Q.M.; Fu, Z.P.; Lu, Y.L. Enhancing the photocatalytic hydrogen production performance of SrTiO3 by coating with a hydrophilic poloxamer. Appl. Surf. Sci. 2020, 528, 146937. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wang, Z.L.; Qiao, L.; Liu, S.W.; Zhao, H.J.; Yan, Y.N. Photocatalytic Surface Modification of PI Film for Electroless Copper Plating. Adv. Condens. Matter Phys. 2018, 8, 1619581. [Google Scholar] [CrossRef]
- Xu, X.W.; Huang, Z.X.; Tan, L.J.; Zhang, Z.Y.; Chen, B.H.; Xia, X.H.; Cheng, G.; Chen, X.P. Surface modification of g-C3N4 for enhanced photocatalytic activity via a simple illumination in pure water. Appl. Surf. Sci. 2024, 672, 160794. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.H.; Wang, X.L.; Han, J.F.; Han, H.X.; Li, C. Effects of surface modification on photocatalytic activity of CdS nanocrystals studied by photoluminescence spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 535–560. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, S.; Ivanova, Y.; Tsybulya, S.; Chesalov, Y.; Nartova, A.; Suprun, E.; Isupova, L. Sr2TiO4 Prepared Using Mechanochemical Activation: Influence of the Initial Compounds’ Nature on Formation, Structural and Catalytic Properties in Oxidative Coupling of Methane. Catalysts 2022, 12, 929. [Google Scholar] [CrossRef]
- Gorkusha, A.S.; Tsybulya, S.V.; Cherepanova, S.V.; Gerasimov, E.Y.; Pavlova, S.N. Nonstoichiometry Defects in Double Oxides of the A2BO4-Type. Materials 2022, 15, 7642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy 2022, 23, 100899. [Google Scholar] [CrossRef]
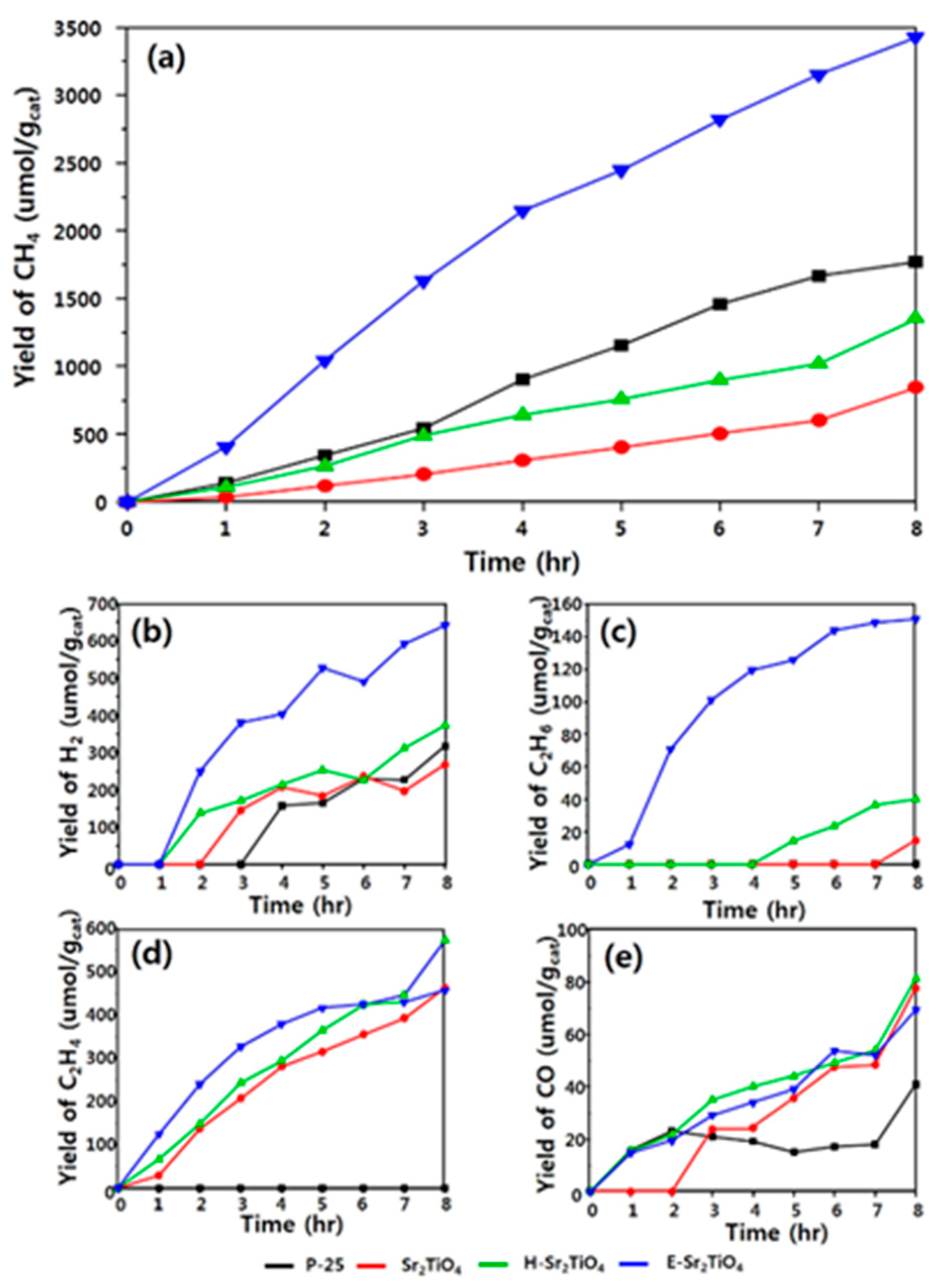
- Kwak, B.S.; Do, J.Y.; Park, N.-K.; Kang, M. Surface modification of layered perovskite Sr2TiO4 for improved CO2 photoreduction with H2O to CH4. Sci. Rep. 2017, 7, 16370. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.N.; Zhu, F.; Liu, Z.F.; Wang, L.C.; Liu, L.Y.; Lu, G.Z.; Zhao, Z. Morphology-controlled synthesis of SrTiO3/TiO2 heterostructures and their photocatalytic performance for water splitting. RSC Adv. 2016, 6, 21118. [Google Scholar] [CrossRef]
- Dong, H.J.; Zhang, X.X.; Li, J.M.; Zhou, P.J.; Yu, S.Y.; Song, N.; Liu, C.B.; Che, G.B.; Li, C.M. Construction of morphology-controlled nonmetal 2D/3D homojunction towards enhancing photocatalytic activity and mechanism insight. Appl. Catal. B 2020, 263, 118270. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.L.; Wang, Z.G.; Wu, S.F.; Ma, J.X. Cellulose controlled zinc oxide nanoparticles with adjustable morphology and their photocatalytic performances. Carbohydr. Polym. 2021, 259, 117752. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Bairagi, K.; Vaidya, S. Relating the structure, properties, and activities of nanostructured SrTiO3 and SrO-(SrTiO3)n (n=1 and 2) for photocatalytic hydrogen evolution. Mater. Adv. 2022, 3, 5055–5063. [Google Scholar] [CrossRef]
- Ivanova, Y.A.; Sutormina, E.F.; Rudina, N.A.; Nartova, A.V.; Isupova, L.A. Effect of preparation route on Sr2TiO4 catalyst for the oxidative coupling of methane. Catal. Commun. 2018, 117, 43–48. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Slater, P.R.; Gover, R.K.B. Synthesis and structure of the new oxide fluoride Sr2TiO3F2 from the low temperature fluorination of Sr2TiO4: An example of a staged fluorine substitution/insertion reaction. J. Mater. Chem. 2002, 12, 291–294. [Google Scholar] [CrossRef]
- Ziati, M.; Bekkioui, N.; Ez-Zahraouy, H. Ruddlesden-Popper compound Sr2TiO4 doped with chalcogens for optoelectronic applications: Insights from first-principle calculations. Chem. Phys. 2021, 548, 111221. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Anjaneyulu, O.; Shanker, V. Synthesis of Cr and La-codoped SrTiO3 nanoparticles for enhanced photocatalytic performance under sunlight irradiation. Phys. Chem. Chem. Phys. 2014, 16, 23819–23828. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, T.; Yan, S.; Li, Z.; Yu, T.; Zou, Z. Highly efficient visible light photocatalytic activity of Cr–La codoped SrTiO3 with surface alkalinization: An insight from DFT calculation. Comput. Mater. Sci. 2013, 79, 87–94. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.; Jing, Y.; Yao, Y.; Ma, J.; Sun, J. Effect of non-metal elements (B, C, N, F, P, S) mono-doping as anions on electronic structure of SrTiO3. Comput. Mater. Sci. 2013, 79, 69–74. [Google Scholar] [CrossRef]
- Comes, R.B.; Sushko, P.V.; Heald, S.M.; Colby, R.J.; Bowden, M.E.; Chambers, S.A. Band-Gap Reduction and Dopant Interaction in Epitaxial La,Cr Co-doped SrTiO3 Thin Films. Chem. Mater. 2014, 26, 7073–7082. [Google Scholar] [CrossRef]
- Yun, J.N.; Zhang, Z.Y.; Yan, J.F.; Zhao, W. First-principles study of Sc-doping effect on the stability, electronic structure and photocatalytic properties of Sr2TiO4. Thin Solid Film. 2013, 542, 276–280. [Google Scholar] [CrossRef]
- Yun, J.-N.; Zhang, Z.-Y.; Yan, J.-F.; Zhang, F.-C. Effect of In-Doping on Electronic Structure and Optical Properties of Sr2TiO4. Chin. Phys. Lett. 2009, 26, 067102. [Google Scholar]
- Ouyang, S.; Tong, H.; Umezawa, N.; Cao, J.; Li, P.; Bi, Y.; Zhang, Y.; Ye, J. Surface-Alkalinization-Induced Enhancement of Photocatalytic H2 Evolution over SrTiO3-Based Photocatalysts. J. Am. Chem. Soc. 2012, 134, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, K.; Kudo, A. Rh-Doped SrTiO3 Photocatalyst Electrode Showing Cathodic Photocurrent for Water Splitting under Visible-Light Irradiation. J. Am. Chem. Soc. 2011, 133, 13272–13275. [Google Scholar] [CrossRef]
- Yu, J.; Xu, X. Fluorination over Cr doped layered perovskite Sr2TiO4 for efficient photocatalytic hydrogen production under visible light illumination. J. Energy Chem. 2020, 51, 30–38. [Google Scholar] [CrossRef]
- Okunaka, S.; Tokudome, H.; Abe, R. Z-scheme Water Splitting into H2 and O2 under Visible Light over Photocatalyst Panels Consisting of Rh-doped SrTiO3 and BiVO4 Fine Particles. Chem. Lett. 2016, 45, 57–59. [Google Scholar] [CrossRef]
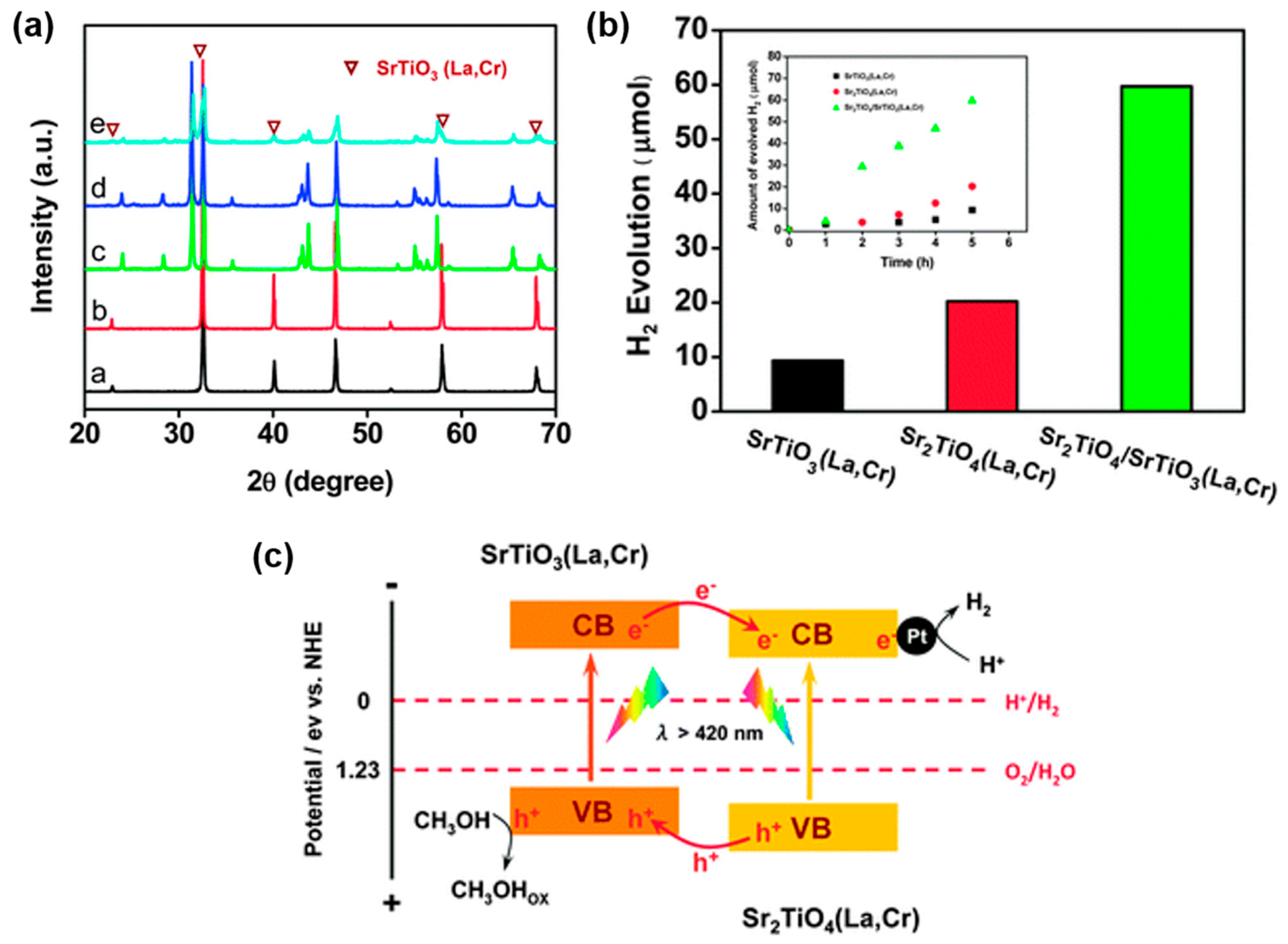
- Jia, Y.; Shen, S.; Wang, D.; Wang, X.; Shi, J.; Zhang, F.; Han, H.; Li, C. Composite Sr2TiO4/SrTiO3(La,Cr) heterojunction based photocatalyst for hydrogen production under visible light irradiation. J. Mater. Chem. A 2013, 1, 7905–7912. [Google Scholar] [CrossRef]
- Ida, S.; Takashiba, A.; Koga, S.; Hagiwara, H.; Ishihara, T. Potential Gradient and Photocatalytic Activity of an Ultrathin p–n Junction Surface Prepared with Two-Dimensional Semiconducting Nanocrystals. J. Am. Chem. Soc. 2014, 136, 1872–1878. [Google Scholar] [CrossRef]
- Chen, C.-J.; Liao, C.-H.; Hsu, K.-C.; Wu, Y.-T.; Wu, J.C.S. P–N junction mechanism on improved NiO/TiO2 photocatalyst. Catal. Commun. 2011, 12, 1307–1310. [Google Scholar] [CrossRef]
- Hou, D.; Hu, X.; Hu, P.; Zhang, W.; Zhang, M.; Huang, Y. Bi4Ti3O12 nanofibers–BiOI nanosheets p–n junction: Facile synthesis and enhanced visible-light photocatalytic activity. Nanoscale 2013, 5, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, G.; Wang, X.; Jia, D.; Tang, P.; Lv, C. A facile approach to construct BiOI/Bi5O7I composites with heterostructures: Efficient charge separation and enhanced photocatalytic activity. RSC Adv. 2015, 5, 74174–74179. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Zhou, Z. First-principles studies on facet-dependent photocatalytic properties of bismuth oxyhalides (BiOXs). RSC Adv. 2012, 2, 9224–9229. [Google Scholar] [CrossRef]
- Han, A.; Sun, J.; Lin, X.; Yuan, C.-H.; Chuah, G.K.; Jaenicke, S. Influence of facets and heterojunctions in photoactive bismuth oxyiodide. RSC Adv. 2015, 5, 88298–88305. [Google Scholar] [CrossRef]
- Hu, C.; Chen, T.-S.; Teng, C.-Y.; Yeh, T.-F.; Xiao, Y.-K.; Teng, H. Ag@Sr2TiO4/Bi5O7I Heterostructured Composite for Solar-Driven Photoelectrochemical Analysis. ECS J. Solid State Sci. Technol. 2018, 7, Q70. [Google Scholar] [CrossRef]

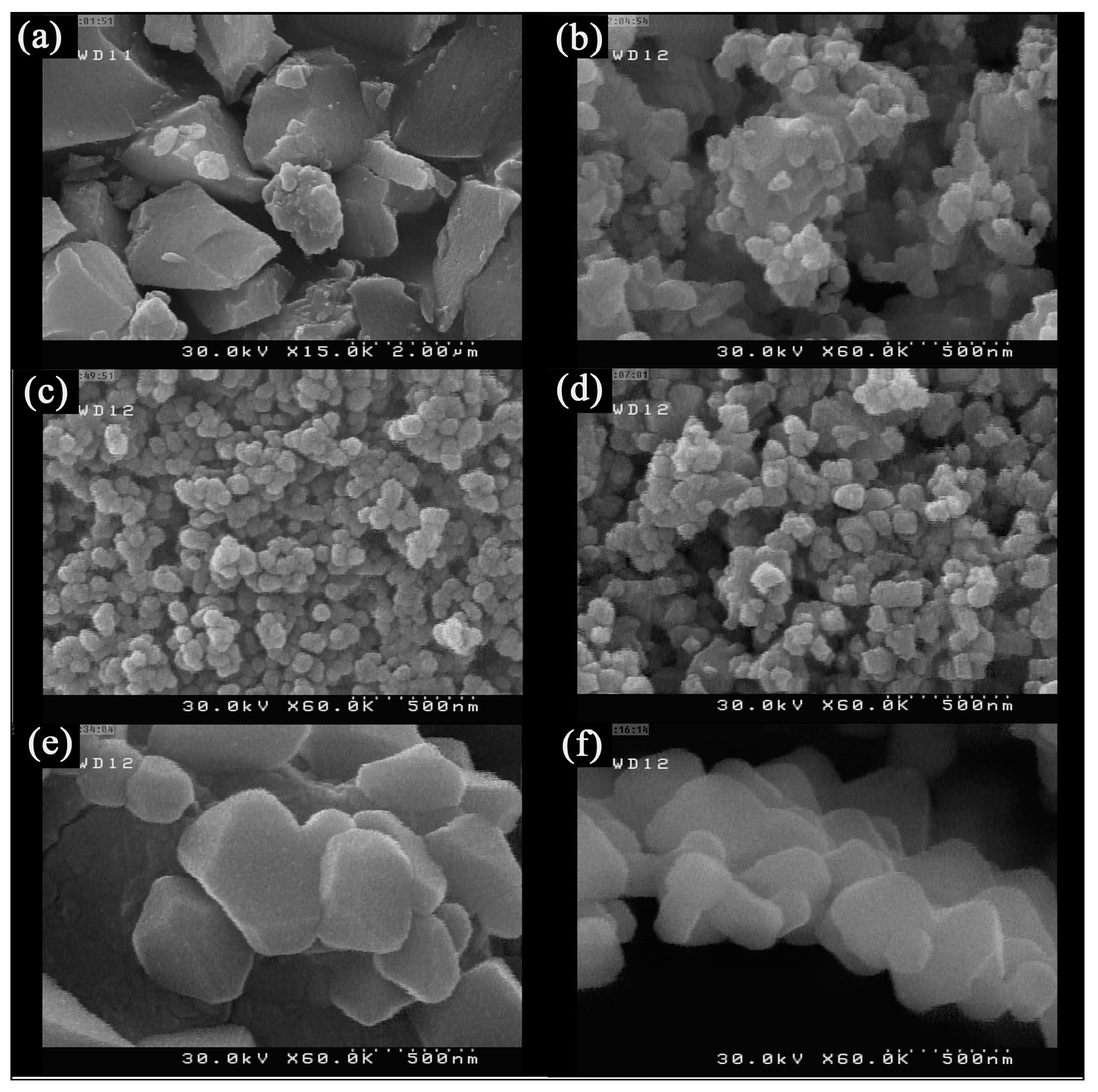
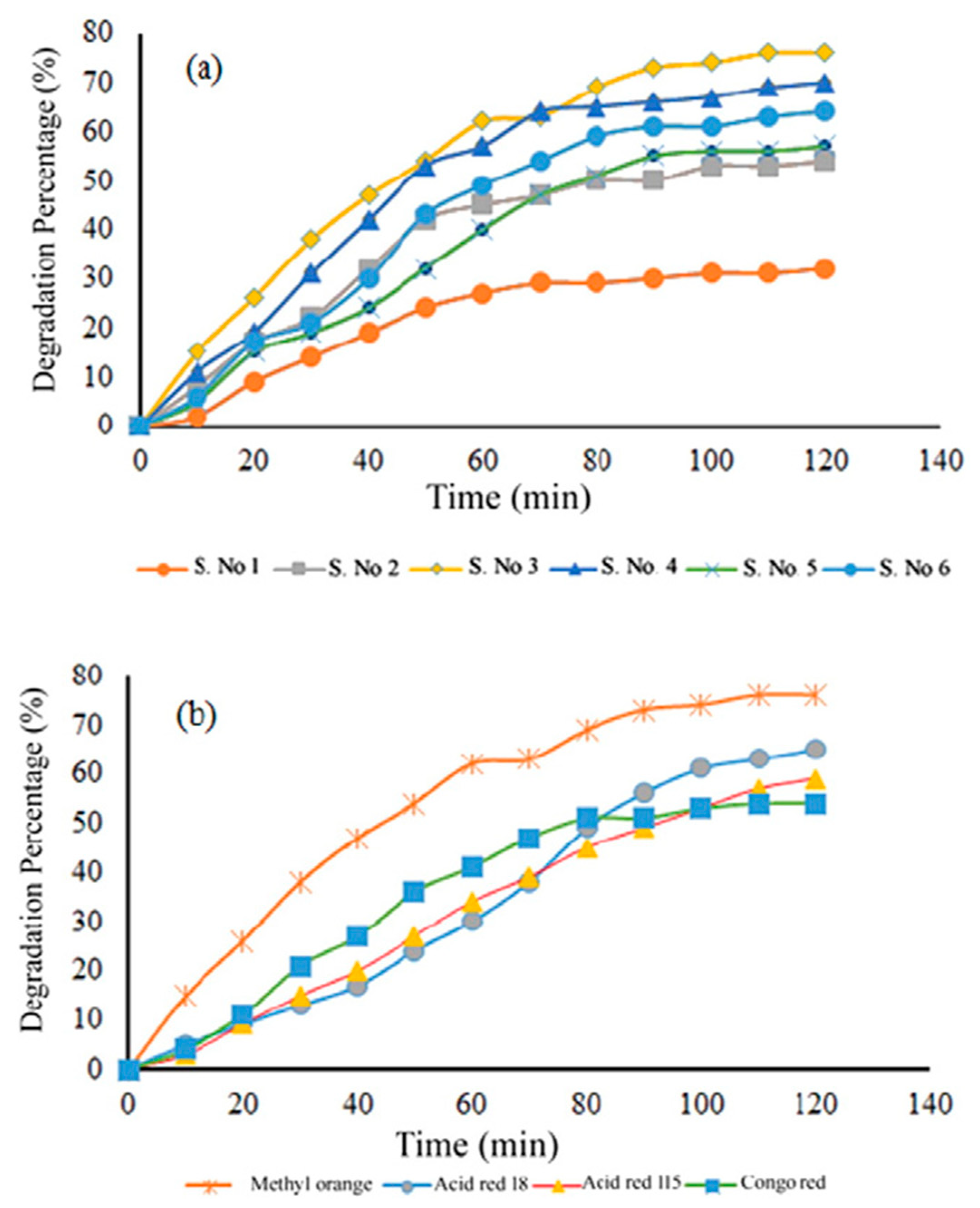
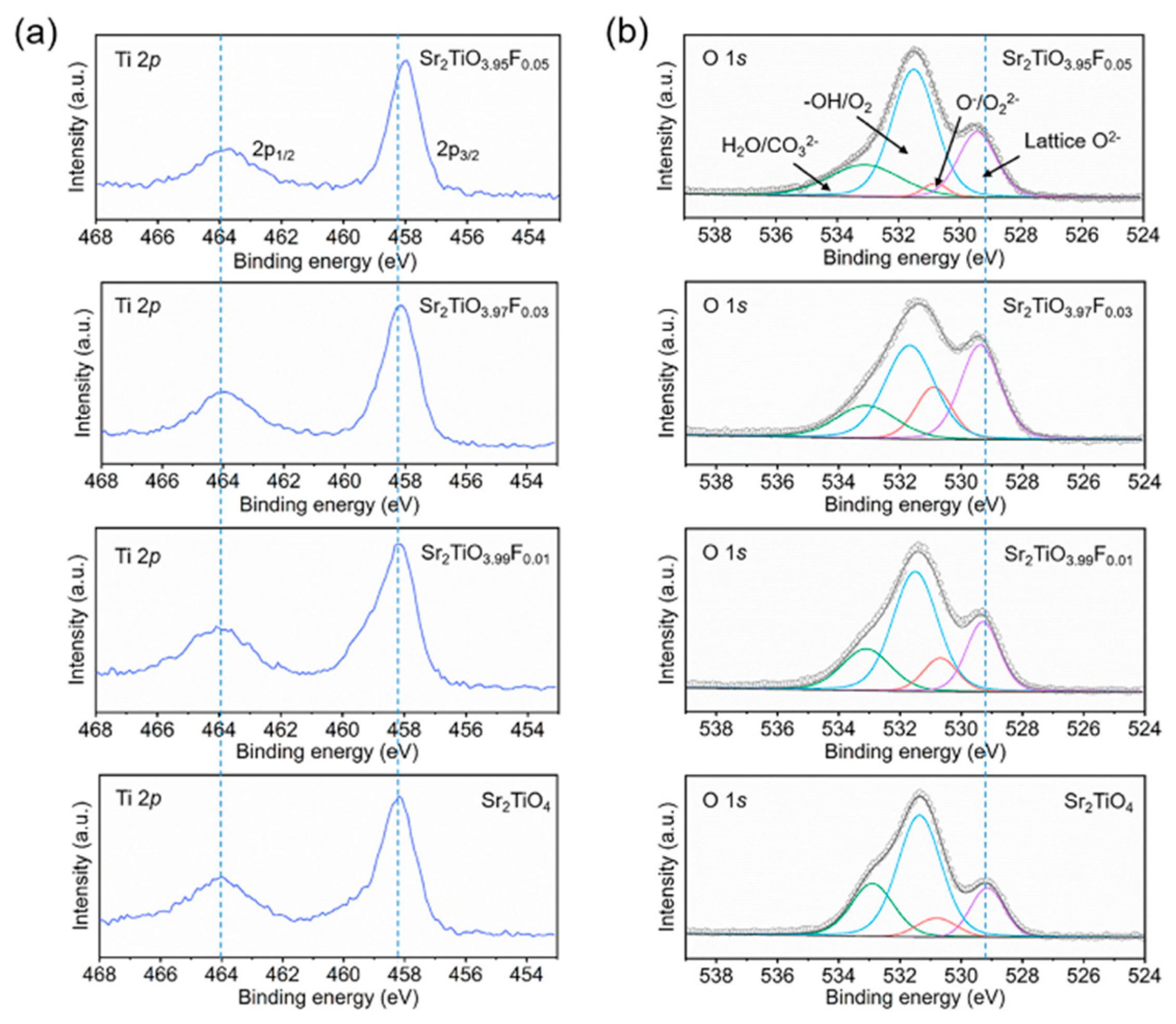

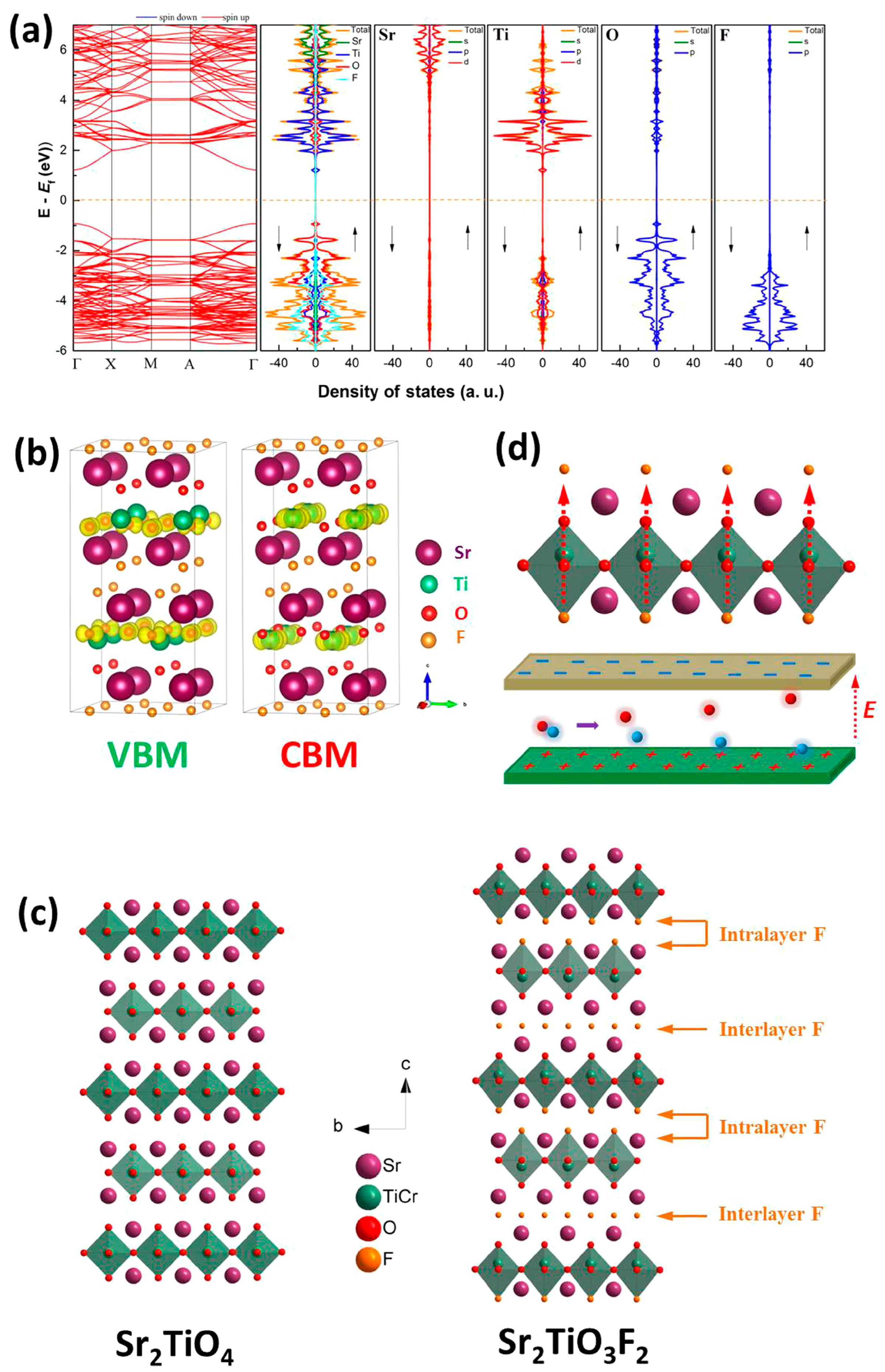
| Synthesis Methods | Calcination Temperature | Morphology | Size | Ref. |
|---|---|---|---|---|
| Polymerized complex | 900 °C | nanoparticles | 200 nm | [21] |
| sol–gel | 700 °C | cubic rod-like particles | 3–6 μm | [27] |
| sol–gel | 1000 °C | nanoparticles | 43 nm | [32] |
| sol–gel | 900 °C | nanoparticles | 185 nm | [44] |
| polymeric and hydrothermal | 1000 °C | nanosheets | 350 nm | [49] |
| sol-precipitation | 1100 °C | spheroidal nanoparticles | 5 μm | [50] |
| sol-precipitation | 1100 °C | flat particles | 1.9–5.7 μm | [50] |
| co-precipitation | 1100 °C | circular particles | 4.7 μm | [50] |
| mechanochemical | 1100 °C | rough particles | 2–10 μm | [50] |
| sol–gel | 1000 °C | nanoparticles | 248 nm | [51] |
| Doping Elements | Doping Site | Doping Content | Reactions | Eg (eV) | Ref. |
|---|---|---|---|---|---|
| Ag | A | 2.5 at% | hydrogen evolution | 3.05 | [44] |
| F | O | 3 at% | hydrogen evolution | 3.20 | [51] |
| La/Rh | A/B | 1.5 at%/3 at% | hydrogen evolution | 2.40 | [21] |
| La/N | A/O | 10 at%/- | water splitting | 2.20 | [26] |
| La/Fe | A/B | 1.5 at%/3 at% | hydrogen evolution | 2.80 | [31] |
| Nb/N | B/O | - | degradation of TC | 2.95 | [32] |
| Cr/F | B/O | 5 at%/40 at% | hydrogen evolution | 2.48 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Liao, L.; Chu, H.; Xie, Y.; Li, Z.; Zhou, W. Recent Advances in Ruddlesden–Popper Phase-Layered Perovskite Sr2TiO4 Photocatalysts. Nanomaterials 2025, 15, 20. https://doi.org/10.3390/nano15010020
Wang P, Liao L, Chu H, Xie Y, Li Z, Zhou W. Recent Advances in Ruddlesden–Popper Phase-Layered Perovskite Sr2TiO4 Photocatalysts. Nanomaterials. 2025; 15(1):20. https://doi.org/10.3390/nano15010020
Chicago/Turabian StyleWang, Pei, Lijun Liao, Hongqi Chu, Ying Xie, Zhenzi Li, and Wei Zhou. 2025. "Recent Advances in Ruddlesden–Popper Phase-Layered Perovskite Sr2TiO4 Photocatalysts" Nanomaterials 15, no. 1: 20. https://doi.org/10.3390/nano15010020
APA StyleWang, P., Liao, L., Chu, H., Xie, Y., Li, Z., & Zhou, W. (2025). Recent Advances in Ruddlesden–Popper Phase-Layered Perovskite Sr2TiO4 Photocatalysts. Nanomaterials, 15(1), 20. https://doi.org/10.3390/nano15010020







