Galleria mellonella (Greater Wax Moth) as a Reliable Animal Model to Study the Efficacy of Nanomaterials in Fighting Pathogens
Abstract
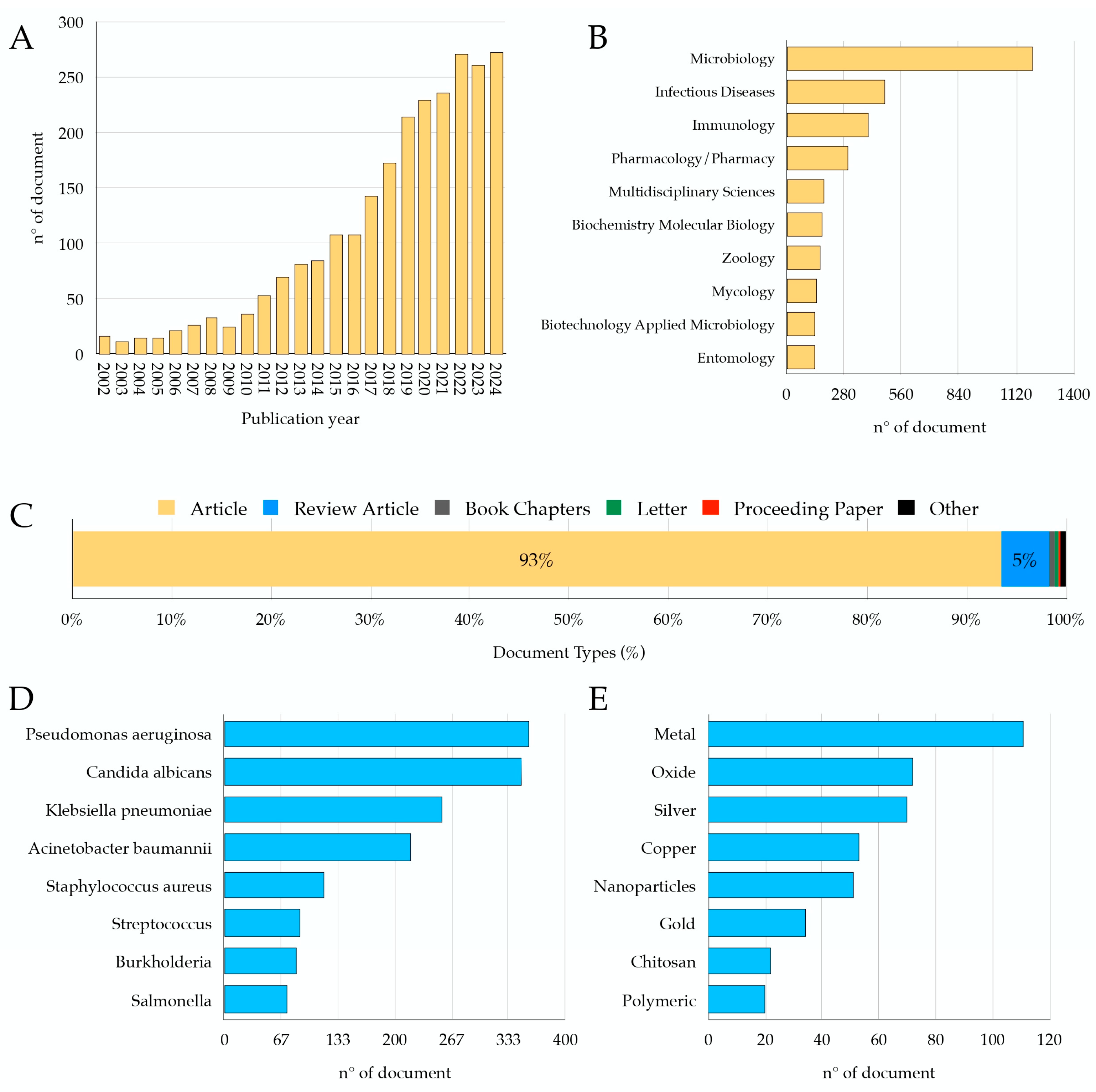
1. Introduction
2. G. mellonella as an Animal Model of Infection: Quantitative Data
2.1. A General Overview of the Innate Immunity in Insects
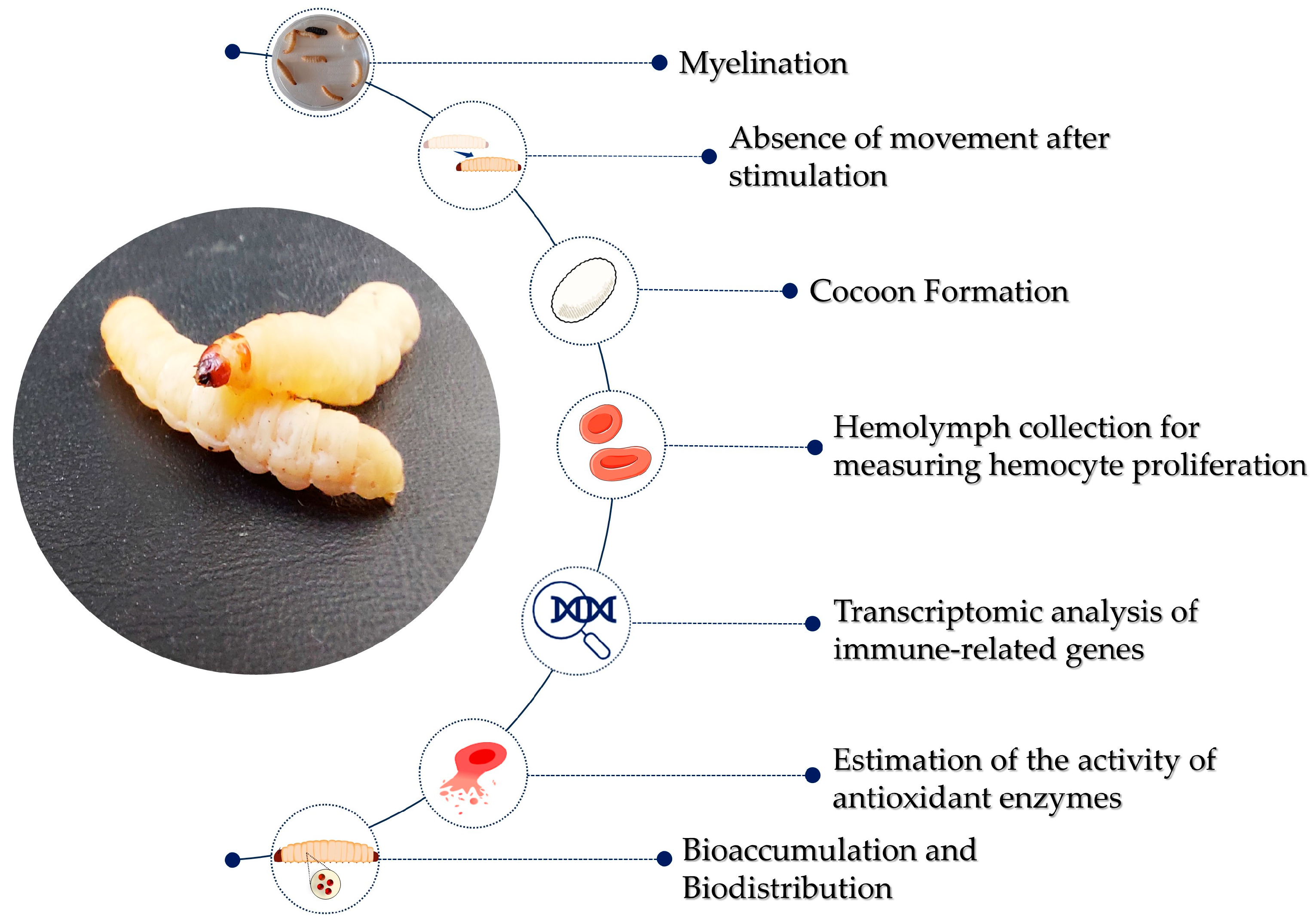
2.2. Monitoring Infection in the Context of the Immune System of G. mellonella
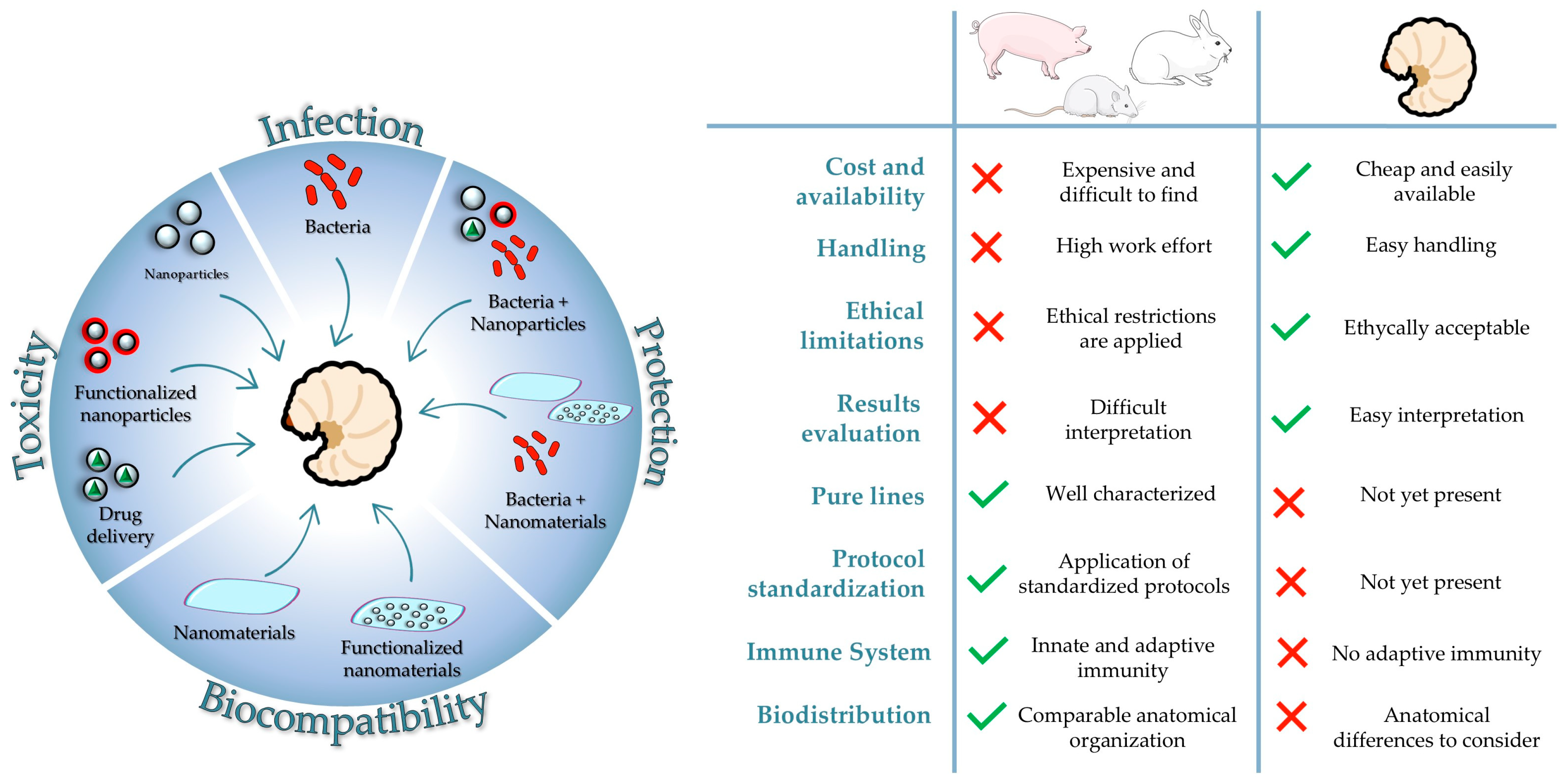
3. G. mellonella Larvae as an Animal Model for Nanotoxicity Studies
Nanotoxicity Assessment in G. mellonella Larvae
4. G. mellonella Model to Investigate Antimicrobial Properties of Nanoparticles, Their Toxicity, and the Underlying Mechanisms
| Type | Synthesis Method | NPs’ Average Size (nm) | In Vivo Infection | Toxicity Assessment | Ref. | |
|---|---|---|---|---|---|---|
| Metal NPs | Ag | Green| water kefir liquor | 20 | P. aeruginosa | Mortality–THC–bacterial load–phenoloxidase activity–nodulation assay | [154] |
| Green|C. latifolia tan Biological|A. flavus | 20 | - * | Mortality | [155] | ||
| Biological| A. tubingensis | 35 | - * | Mortality | [156] | ||
| Biological|B. ochroleuca | 8–21 | - * | Mortality | [157] | ||
| Ag–chitosan | Chemical | 11 | - * | Mortality | [158] | |
| Aptamer–AgNCs | Chemical | Unspecified | P. aeruginosa | Mortality | [159] | |
| Inorganic NPs | Graphite oxide–Ag | Chemical | Unspecified | S. aureus | Mortality | [164] |
| Mg(OH)2 | Green|rosehip extract | 90 | S. aureus | Mortality | [165] | |
| Metal Oxide NPs | CuO | Commercial|Nanokar | 38 | - * | LC50 and LC90–THC– hemocyte viability | [173] |
| Commercial| Sigma-Aldrich | <50 | - * | LC10 and LC30—metabolic and biochemical parameters–THC | [136] | ||
| Commercial| Sigma-Aldrich | <50 | - * | Bioaccumulation–metabolic enzyme activity | [174] | ||
| TiO2 | Commercial| Degussa P25 | 29 | - * | Bioaccumulation–metabolic enzymes activity–total protein dosage–THC | [135] | |
| ZnO | Commercial| Alfa Aesar | 70 | - * | LC–THC | [134] | |
| Commercial| Sigma-Aldrich | 100 × 15 | C. albicans | Mortality–histopathological analysis–THC–bacterial load—SEM-phenoloxidase assay—Phagocytosis assays | [175] | ||
| Polymeric NPs | Lipid-core Nanocapsules | Chemical | 150–190 | - * | Mortality | [176] |
In Vivo Administration of Functionalized Nanoparticles for Toxicity Modulation and Drug Delivery
| Type | Synthesis Method | Functionalization | NPs’ Average Size (nm) | In Vivo Infection | Toxicity Assessment | Ref. | |
|---|---|---|---|---|---|---|---|
| Inorganic NPs | Ag | Biological| F. oxysporum | Phenothiazinium photosensitizers | 16–33 | - * | Mortality | [180] |
| Biological| L. acidophilus | Cinnamaldehyde | 9 | EAEC | LC50–mortality–bacterial load–THC–LDH cytotoxicity assay | [181] | ||
| Ag, CuO, ZnO | Commercial| DENANA NanoBEL- Chemical | Biomolecule coronas | 10–50 | A. fumigatus | Mortality | [182] | |
| Au nanostar | Chemical | Amikacin | 104 | K. pneumoniae | Mortality | [183] | |
| Silica | Chemical | Lycopene | Unspecified | - * | Mortality–cocoon formation | [184] | |
| Polymeric NPs | Alginate | Chemical| external gelation method | Miltefosine | ~300 | C. albicans–C. neoformans–C. gattii | Mortality–fungal burden–histopathological analysis | [185] |
| Chemical| external gelation method | Miltefosine | ~300 | C. auris | Mortality–fungal burden–histopathological analysis | [186] | ||
| Lipid-based | Chemical | Itraconazole | 216 | S. brasiliensis–C. albicans | Mortality | [187] | |
| Chemical| dry liquid film technique | Anidulafungin | ~100 | C. albicans | Mortality | [188] | ||
| Chemical| hot emulsification | L. sidoides essential oil | 213–445 | - * | Mortality | [189] | ||
| PLGA | Chemical| nanoprecipitation | Coumarin 6–pterostilbene | 50 | A. brasiliensis | Mortality | [190] | |
| Chemical| water-in-oil-in-water | Gentamicin | 227 | K. pneumoniae | Mortality–THC–bacterial load–histopathological analysis | [192] | ||
| PBCA | Chemical| in situ anionic polymerization method | Propolis | ~195 | C. neoformans | Mortality | [191] | |
| PCL | Chemical| nanoprecipitation | [Ag(I)] complex | 155–162 | - * | Mortality | [193] | |
| Alginate/chitosan | Chemical | Tobramycin–dornase alfa | ~500 | P. aeruginosa | Mortality | [194] | |
| Acetylated cashew gum NPs | Chemical| nanoprecipitation | Lycopene | 160–270 | - * | Mortality | [195] | |
| Cationic nanoemulsion | Chemical | C6 ceramide | ~40 | - * | Mortality | [196] | |
| Lecithin/chitosan | Chemical | Melatonin | 160–207 | - * | Mortality | [197] |
5. G. mellonella Model for Biocompatibility Assessment of Materials and Nanocomposites
6. Standardization in Larvae Rearing and Experimental Protocols
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Renwick, M.J.; Brogan, D.M.; Mossialos, E. A Systematic Review and Critical Assessment of Incentive Strategies for Discovery and Development of Novel Antibiotics. J. Antibiot. 2016, 69, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009–2018. JAMA 2020, 323, 844. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, X.; Li, M.; Yu, Z.; Cheng, W.; Yu, Z.; Zhang, W.; Zhang, Y.; Shen, A.; Sun, H.; et al. Global Spread of Carbapenem-Resistant Enterobacteriaceae: Epidemiological Features, Resistance Mechanisms, Detection and Therapy. Microbiol. Res. 2023, 266, 127249. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Młynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Młynarczyk, G.; Majewski, S. Multiresistant Neisseria gonorrhoeae: A New Threat in Second Decade of the XXI Century. Med. Microbiol. Immunol. 2020, 209, 95–108. [Google Scholar] [CrossRef]
- Whiteway, C.; Breine, A.; Philippe, C.; Van der Henst, C. Acinetobacter baumannii . Trends Microbiol. 2022, 30, 199–200. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Qamar, F.N.; Hussain, W.; Qureshi, S. Salmonellosis Including Enteric Fever. Pediatr. Clin. N. Am. 2022, 69, 65–77. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Seixas, A.M.M.; Sousa, S.A.; Leitão, J.H. Antibody-Based Immunotherapies as a Tool for Tackling Multidrug-Resistant Bacterial Infections. Vaccines 2022, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, M.; Jeguirim, I.; Tredici, S.M.; Damiano, F.; Alifano, P. Spiramycin Disarms Pseudomonas aeruginosa without Inhibiting Growth. Antibiotics 2023, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Mühlen, S.; Dersch, P. Anti-Virulence Strategies to Target Bacterial Infections. Curr. Top. Microbiol. Immunol. 2016, 398, 147–183. [Google Scholar] [CrossRef]
- Betts, J.W.; Hornsey, M.; La Ragione, R.M. Novel Antibacterials: Alternatives to Traditional Antibiotics. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 73, pp. 123–169. ISBN 978-0-12-815190-7. [Google Scholar]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Villani, S.; De Matteis, V.; Calcagnile, M.; Cascione, M.; Pellegrino, P.; Vincenti, L.; Demitri, C.; Alifano, P.; Rinaldi, R. Tuning Antibacterial Efficacy against Pseudomonas aeruginosa by Using Green AgNPs in Chitosan Thin Films as a Plastic Alternative. Int. J. Biol. Macromol. 2025, 285, 138277. [Google Scholar] [CrossRef]
- Pal, S.; Villani, S.; Mansi, A.; Marcelloni, A.M.; Chiominto, A.; Amori, I.; Proietto, A.R.; Calcagnile, M.; Alifano, P.; Bagheri, S.; et al. Antimicrobial and Superhydrophobic CuONPs/TiO2 Hybrid Coating on Polypropylene Substrates against Biofilm Formation. ACS Omega 2024, 9, 45376–45385. [Google Scholar] [CrossRef]
- Sullivan, C.; Matty, M.A.; Jurczyszak, D.; Gabor, K.A.; Millard, P.J.; Tobin, D.M.; Kim, C.H. Infectious Disease Models in Zebrafish. Methods Cell Biol. 2017, 138, 101–136. [Google Scholar] [CrossRef]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella Infection Models for the Study of Bacterial Diseases and for Antimicrobial Drug Testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Chamilos, G.; Samonis, G.; Kontoyiannis, D.P. Drosophila Melanogaster as a Model Host for the Study of Microbial Pathogenicity and the Discovery of Novel Antimicrobial Compounds. Curr. Pharm. Des. 2011, 17, 1246–1253. [Google Scholar] [CrossRef]
- Canteri de Souza, P.; Custódio Caloni, C.; Wilson, D.; Sergio Almeida, R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio Molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, M.; Tredici, S.M.; Talà, A.; Alifano, P. Bacterial Semiochemicals and Transkingdom Interactions with Insects and Plants. Insects 2019, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Li, Y.; Newton, S.M.; Robertson, B.D.; Langford, P.R. Galleria mellonella-Intracellular Bacteria Pathogen Infection Models: The Ins and Outs. FEMS Microbiol. Rev. 2023, 47, fuad011. [Google Scholar] [CrossRef]
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria mellonella as an Infection Model: An in-Depth Look at Why It Works and Practical Considerations for Successful Application. Pathog. Dis. 2020, 78, ftaa056. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.-Y. Galleria mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef]
- Nakhleh, J.; El Moussawi, L.; Osta, M.A. The Melanization Response in Insect Immunity. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 52, pp. 83–109. ISBN 978-0-12-811775-0. [Google Scholar]
- Kavanagh, K.; Sheehan, G. The Use of Galleria mellonella Larvae to Identify Novel Antimicrobial Agents against Fungal Species of Medical Interest. J. Fungi 2018, 4, 113. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef]
- Durieux, M.-F.; Melloul, É.; Jemel, S.; Roisin, L.; Dardé, M.-L.; Guillot, J.; Dannaoui, É.; Botterel, F. Galleria mellonella as a Screening Tool to Study Virulence Factors of Aspergillus fumigatus. Virulence 2021, 12, 818–834. [Google Scholar] [CrossRef]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Cristina, A.; Prata, M.; Jorge, A.O.C.; Junqueira, J.C. From Moths to Caterpillars: Ideal Conditions for Galleria mellonella Rearing for in Vivo Microbiological Studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Ausubel, F.M. Enterococcus Infection Biology: Lessons from Invertebrate Host Models. J. Microbiol. 2014, 52, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Duma, L.; Rossez, Y. Galleria mellonella as a Good Model to Study Acinetobacter baumannii Pathogenesis. Pathogens 2021, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Robin, F. Correlation between Antimicrobial Resistance and Virulence in Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 333–341. [Google Scholar] [CrossRef]
- Papaioannou, E.; Utari, P.D.; Quax, W.J. Choosing an Appropriate Infection Model to Study Quorum Sensing Inhibition in Pseudomonas Infections. Int. J. Mol. Sci. 2013, 14, 19309–19340. [Google Scholar] [CrossRef]
- Pereira, M.F.; Rossi, C.C. Overview of Rearing and Testing Conditions and a Guide for Optimizing Galleria mellonella Breeding and Use in the Laboratory for Scientific Purposes. APMIS 2020, 128, 607–620. [Google Scholar] [CrossRef]
- Kavanagh, K.; Fallon, J.P. Galleria mellonella Larvae as Models for Studying Fungal Virulence. Fungal Biol. Rev. 2010, 24, 79–83. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a Consolidated in Vivo Model Hosts: New Developments in Antibacterial Strategies and Novel Drug Testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef]
- Rosales, C. Cellular and Molecular Mechanisms of Insect Immunity. In Insect Physiology and Ecology; Shields, V.D.C., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3033-8. [Google Scholar]
- Ali Mohammadie Kojour, M.; Han, Y.S.; Jo, Y.H. An Overview of Insect Innate Immunity. Entomol. Res. 2020, 50, 282–291. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect Hemocytes and Their Role in Immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Vlisidou, I.; Wood, W. Drosophila Blood Cells and Their Role in Immune Responses. FEBS J. 2015, 282, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Satyavathi, V.V.; Minz, A.; Nagaraju, J. Nodulation: An Unexplored Cellular Defense Mechanism in Insects. Cell. Signal. 2014, 26, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in Lepidopteran Insects. In Invertebrate Immunity; Söderhäll, K., Ed.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2010; Volume 708, pp. 181–204. ISBN 978-1-4419-8058-8. [Google Scholar]
- Tsakas, S.; Marmaras, V.J. Insect Immunity and Its Signalling: An Overview. Invertebr. Surviv. J. 2010, 7, 228–238. [Google Scholar]
- Marieshwari, B.N.; Bhuvaragavan, S.; Sruthi, K.; Mullainadhan, P.; Janarthanan, S. Insect Phenoloxidase and Its Diverse Roles: Melanogenesis and Beyond. J. Comp. Physiol. B 2023, 193, 1–23. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A Key Component of the Insect Immune System: Biochemical and Evolutionary Ecology of PO. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, Q.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.-X.; Ling, E. Insect Prophenoloxidase: The View beyond Immunity. Front. Physiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect Antimicrobial Peptides: Structures, Properties and Gene Regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Shen, W.; He, P.; Xiao, C.; Chen, X. From Antimicrobial Peptides to Antimicrobial Poly(α-Amino Acid)s. Adv. Healthcare Mater. 2018, 7, 1800354. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, X.; Yang, L.; Su, P.; Fu, P.; Peng, J.; Yang, N.; Guo, G. Antimicrobial Peptide AMP-17 Affects Candida albicans by Disrupting Its Cell Wall and Cell Membrane Integrity. Infect. Drug Resist. 2020, 13, 2509–2520. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ochiai, A.; Kondo, H.; Fukuda, S.; Ishiyama, Y.; Saitoh, E.; Kato, T.; Tanaka, T. Pyrrhocoricin, a Proline-Rich Antimicrobial Peptide Derived from Insect, Inhibits the Translation Process in the Cell-Free Escherichia coli Protein Synthesis System. J. Biosci. Bioeng. 2016, 121, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.G.; Roy, R.N.; Lomakin, I.B.; Florin, T.; Mankin, A.S.; Steitz, T.A. Structures of Proline-Rich Peptides Bound to the Ribosome Reveal a Common Mechanism of Protein Synthesis Inhibition. Nucleic Acids Res. 2016, 44, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.-L.; Bulet, P. Antimicrobial Peptides in Drosophila: Structures, Activities and Gene Regulation. In Chemical Immunology and Allergy; Kabelitz, D., Schröder, J.-M., Eds.; KARGER: Basel, Switzerland, 2005; pp. 1–21. ISBN 978-3-8055-7862-2. [Google Scholar]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect Antimicrobial Peptides and Their Applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef]
- Li, W.; Tailhades, J.; O’Brien-Simpson, N.M.; Separovic, F.; Otvos, L.; Hossain, M.A.; Wade, J.D. Proline-Rich Antimicrobial Peptides: Potential Therapeutics against Antibiotic-Resistant Bacteria. Amino Acids 2014, 46, 2287–2294. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect Antimicrobial Peptides: Potential Weapons to Counteract the Antibiotic Resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Innate Immunity: Impact on the Adaptive Immune Response. Curr. Opin. Immunol. 1997, 9, 4–9. [Google Scholar] [CrossRef]
- Garver, L.S.; Wu, J.; Wu, L.P. The Peptidoglycan Recognition Protein PGRP-SC1a Is Essential for Toll Signaling and Phagocytosis of Staphylococcus aureus in Drosophila. Proc. Natl. Acad. Sci. USA 2006, 103, 660–665. [Google Scholar] [CrossRef]
- Wang, L.; Weber, A.N.R.; Atilano, M.L.; Filipe, S.R.; Gay, N.J.; Ligoxygakis, P. Sensing of Gram-Positive Bacteria in Drosophila: GNBP1 Is Needed to Process and Present Peptidoglycan to PGRP-SA. EMBO J. 2006, 25, 5005–5014. [Google Scholar] [CrossRef]
- Neyen, C.; Poidevin, M.; Roussel, A.; Lemaitre, B. Tissue- and Ligand-Specific Sensing of Gram-Negative Infection in Drosophila by PGRP-LC Isoforms and PGRP-LE. J. Immunol. 2012, 189, 1886–1897. [Google Scholar] [CrossRef]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate Humoral Immune Defences in Mammals and Insects: The Same, with Differences ? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Bang, I.S. JAK/STAT Signaling in Insect Innate Immunity. Entomol. Res. 2019, 49, 339–353. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Li, J.; Zhang, Y.; Wang, Z.; Sun, S. Antifungal Activity and Potential Mechanism of Action of Caspofungin in Combination with Ribavirin against Candida albicans. Int. J. Antimicrob. Agents 2023, 61, 106709. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.; Veli, N.; Coote, P.J. Evaluation of Galleria mellonella Larvae for Measuring the Efficacy and Pharmacokinetics of Antibiotic Therapies against Pseudomonas aeruginosa Infection. Int. J. Antimicrob. Agents 2014, 43, 254–261. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Liu, Y.; Xu, S.; Wu, M.; Liu, Z.; Qi, C.; Zhang, G.; Li, J.; Huang, X. Synergistic Effect of Linezolid with Fosfomycin against Staphylococcus aureus in Vitro and in an Experimental Galleria mellonella Model. J. Microbiol. Immunol. Infect. 2020, 53, 731–738. [Google Scholar] [CrossRef]
- Cools, F.; Torfs, E.; Aizawa, J.; Vanhoutte, B.; Maes, L.; Caljon, G.; Delputte, P.; Cappoen, D.; Cos, P. Optimization and Characterization of a Galleria mellonella Larval Infection Model for Virulence Studies and the Evaluation of Therapeutics Against Streptococcus pneumoniae. Front. Microbiol. 2019, 10, 311. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Aslan Erdem, S.; Ozturk, S.; Safi Oz, Z.; Subasi, E.; Koyuncu, M.; Vlainić, J.; Kosalec, I. Antifungal and Anti-Virulent Activity of Origanum Majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model. Molecules 2022, 27, 663. [Google Scholar] [CrossRef]
- Dinh, H.; Semenec, L.; Kumar, S.S.; Short, F.L.; Cain, A.K. Microbiology’s next Top Model: Galleria in the Molecular Age. Pathog. Dis. 2021, 79, ftab006. [Google Scholar] [CrossRef]
- Mowlds, P.; Kavanagh, K. Effect of Pre-Incubation Temperature on Susceptibility of Galleria mellonella Larvae to Infection by Candida albicans. Mycopathologia 2008, 165, 5–12. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Prata, M.C.D.A.; Furlong, J.; Prezoto, F. Exigências Térmicas de Estágios Imaturos de Galleria mellonella L. (Lepidoptera: Pyralidae). Neotrop. Entomol. 2007, 36, 657–661. [Google Scholar] [CrossRef]
- Scorzoni, L.; De Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal Efficacy during Candida Krusei Infection in Non-Conventional Models Correlates with the Yeast In Vitro Susceptibility Profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, A.D.; Alekseev, A.A.; Slepneva, I.A. The Phenylthiourea Is a Competitive Inhibitor of the Enzymatic Oxidation of DOPA by Phenoloxidase. J. Enzym. Inhib. Med. Chem. 2012, 27, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Dijokaite, A.; Humbert, M.V.; Borkowski, E.; La Ragione, R.M.; Christodoulides, M. Establishing an Invertebrate Galleria mellonella Greater Wax Moth Larval Model of Neisseria gonorrhoeae Infection. Virulence 2021, 12, 1900–1920. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Ding, Y.; Yi, Y. Ultrastructural and Functional Characterization of Circulating Hemocytes from Galleria mellonella Larva: Cell Types and Their Role in the Innate Immunity. Tissue Cell 2016, 48, 297–304. [Google Scholar] [CrossRef]
- Wright, C.L.; Kavanagh, O. Galleria mellonella as a Novel In Vivo Model to Screen Natural Product-Derived Modulators of Innate Immunity. Appl. Sci. 2022, 12, 6587. [Google Scholar] [CrossRef]
- Calcagnile, M.; Tredici, M.S.; Pennetta, A.; Resta, S.C.; Talà, A.; De Benedetto, G.E.; Alifano, P. Bacillus velezensis MT9 and Pseudomonas chlororaphis MT5 as Biocontrol Agents against Citrus Sooty Mold and Associated Insect Pests. Biol. Control 2022, 176, 105091. [Google Scholar] [CrossRef]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial Activity of ZnO Nanoparticle on Gram-Positive and Gram-Negative Bacteria. Afr. J. Microbiol. Res. 2012, 5, 1368–1373. [Google Scholar] [CrossRef]
- Cavassin, E.D.; De Figueiredo, L.F.P.; Otoch, J.P.; Seckler, M.M.; De Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.S.; Costa, S.F. Comparison of Methods to Detect the in Vitro Activity of Silver Nanoparticles (AgNP) against Multidrug Resistant Bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef]
- Jayamani, E.; Rajamuthiah, R.; Larkins-Ford, J.; Fuchs, B.B.; Conery, A.L.; Vilcinskas, A.; Ausubel, F.M.; Mylonakis, E. Insect-Derived Cecropins Display Activity against Acinetobacter baumannii in a Whole-Animal High-Throughput Caenorhabditis elegans Model. Antimicrob. Agents Chemother. 2015, 59, 1728–1737. [Google Scholar] [CrossRef]
- Vilcinskas, A.; Jegorov, A.; Landa, Z.; Götz, P.; Matha, V. Effects of Beauverolide L and Cyclosporin A on Humoral and Cellular Immune Response of the Greater Wax Moth, Galleria mellonella. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 122, 83–92. [Google Scholar] [CrossRef]
- Fiolka, M.J. Immunosuppressive Effect of Cyclosporin A on Insect Humoral Immune Response. J. Invertebr. Pathol. 2008, 98, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.; Zdybicka-Barabas, A.; Cytryńska, M. A Different Repertoire of Galleria mellonella Antimicrobial Peptides in Larvae Challenged with Bacteria and Fungi. Dev. Comp. Immunol. 2010, 34, 1129–1136. [Google Scholar] [CrossRef]
- Andrejko, M.; Mak, P.; Siemińska-Kuczer, A.; Iwański, B.; Wojda, I.; Suder, P.; Kuleta, P.; Regucka, K.; Cytryńska, M. A Comparison of the Production of Antimicrobial Peptides and Proteins by Galleria mellonella Larvae in Response to Infection with Two Pseudomonas aeruginosa Strains Differing in the Profile of Secreted Proteases. J. Insect Physiol. 2021, 131, 104239. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Kim, K.N.; Lee, J.H.; Lee, H.S.; Kim, S.H.; Cho, K.Y.; Nam, M.H.; Lee, I.H. Comparative Study on Characteristics of Lysozymes from the Hemolymph of Three Lepidopteran Larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Dev. Comp. Immunol. 2002, 26, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Rosenman, M.; Harwig, S.S.S.L.; Jackson, R.; Eisenhauer, P. Ultrasensitive Assays for Endogenous Antimicrobial Polypeptides. J. Immunol. Methods 1991, 137, 167–173. [Google Scholar] [CrossRef]
- Vogel, H.; Altincicek, B.; Glöckner, G.; Vilcinskas, A. A Comprehensive Transcriptome and Immune-Gene Repertoire of the Lepidopteran Model Host Galleria mellonella. BMC Genom. 2011, 12, 308. [Google Scholar] [CrossRef]
- Lange, A.; Schäfer, A.; Bender, A.; Steimle, A.; Beier, S.; Parusel, R.; Frick, J.-S. Galleria mellonella: A Novel Invertebrate Model to Distinguish Intestinal Symbionts From Pathobionts. Front. Immunol. 2018, 9, 2114. [Google Scholar] [CrossRef]
- Oztug, M.; Martinon, D.; Weers, P.M.M. Characterization of the ApoLp-III/LPS Complex: Insight into the Mode of Binding Interaction. Biochemistry 2012, 51, 6220–6227. [Google Scholar] [CrossRef]
- Halwani, A.E.; Niven, D.F.; Dunphy, G.B. Apolipophorin-III and the Interactions of Lipoteichoic Acids with the Immediate Immune Responses of Galleria mellonella. J. Invertebr. Pathol. 2000, 76, 233–241. [Google Scholar] [CrossRef]
- Whitten, M.M.A.; Tew, I.F.; Lee, B.L.; Ratcliffe, N.A. A Novel Role for an Insect Apolipoprotein (Apolipophorin III) in β-1,3-Glucan Pattern Recognition and Cellular Encapsulation Reactions. J. Immunol. 2004, 172, 2177–2185. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Sowa-Jasiłek, A.; Stączek, S.; Jakubowicz, T.; Cytryńska, M. Different Forms of Apolipophorin III in Galleria mellonella Larvae Challenged with Bacteria and Fungi. Peptides 2015, 68, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kordaczuk, J.; Sułek, M.; Mak, P.; Zdybicka-Barabas, A.; Śmiałek, J.; Wojda, I. Cationic Protein 8 Plays Multiple Roles in Galleria mellonella Immunity. Sci. Rep. 2022, 12, 11737. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Shin, Y.P.; Noh, M.Y.; Jo, Y.H.; Han, Y.S.; Seong, Y.S.; Lee, I.H. An Insect Multiligand Recognition Protein Functions as an Opsonin for the Phagocytosis of Microorganisms. J. Biol. Chem. 2010, 285, 25243–25250. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Taniguchi, N.; Arakawa, C.; Kobayashi, T. On the Basic Concept of Nano-Technology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26 August 1974; pp. 18–23. [Google Scholar]
- Rauscher, H.; Sokull-Klüttgen, B.; Stamm, H. The European Commission’s Recommendation on the Definition of Nanomaterial Makes an Impact. Nanotoxicology 2013, 7, 1195–1197. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on Nanomaterials: Synthesis and Applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- O’Callaghan, D.; Vergunst, A. Non-Mammalian Animal Models to Study Infectious Disease: Worms or Fly Fishing? Curr. Opin. Microbiol. 2010, 13, 79–85. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, L.; Zhang, L.; Shen, X.; Kong, L.; Wu, T. Research Advances on the Adverse Effects of Nanomaterials in a Model Organism, Caenorhabditis elegans. Environ. Toxicol. Chem. 2021, 40, 2406–2424. [Google Scholar] [CrossRef]
- Ng, C.T.; Yu, L.E.; Ong, C.N.; Bay, B.H.; Baeg, G.H. The Use of Drosophila Melanogaster as a Model Organism to Study Immune-Nanotoxicity. Nanotoxicology 2019, 13, 429–446. [Google Scholar] [CrossRef]
- Bai, C.; Tang, M. Toxicological Study of Metal and Metal Oxide Nanoparticles in Zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Calisi, A.; Lorusso, C.; Gallego-Urrea, J.A.; Hassellöv, M.; Dondero, F. Ecotoxicological Effects of Silver Nanoparticles in Marine Mussels. Sci. Total Environ. 2022, 851, 158113. [Google Scholar] [CrossRef] [PubMed]
- Saggese, I.; Sarà, G.; Dondero, F. Silver Nanoparticles Affect Functional Bioenergetic Traits in the Invasive Red Sea Mussel Brachidontes pharaonis. BioMed Res. Int. 2016, 2016, 1872351. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Avila, P.E.; Rangel Mendoza, A.; Pichardo Molina, J.L.; Flores Villavicencio, L.L.; Castruita Dominguez, J.P.; Chilakapati, M.K.; Sabanero Lopez, M. Biological Response of HeLa Cells to Gold Nanoparticles Coated with Organic Molecules. Toxicol. In Vitro 2017, 42, 114–122. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Gutiérrez, J.; Villacorta, A.; Arribas Arranz, J.; Romero-Andrada, I.; Lacoma, A.; Marcos, R.; Hernández, A.; Rubio, L. Polylactic Acid Nanoplastics (PLA-NPLs) Induce Adverse Effects on an in Vitro Model of the Human Lung Epithelium: The Calu-3 Air-Liquid Interface (ALI) Barrier. J. Hazard. Mater. 2024, 475, 134900. [Google Scholar] [CrossRef]
- Ude, V.C.; Brown, D.M.; Viale, L.; Kanase, N.; Stone, V.; Johnston, H.J. Impact of Copper Oxide Nanomaterials on Differentiated and Undifferentiated Caco-2 Intestinal Epithelial Cells; Assessment of Cytotoxicity, Barrier Integrity, Cytokine Production and Nanomaterial Penetration. Part. Fibre Toxicol. 2017, 14, 31. [Google Scholar] [CrossRef]
- Fizeșan, I.; Cambier, S.; Moschini, E.; Chary, A.; Nelissen, I.; Ziebel, J.; Audinot, J.-N.; Wirtz, T.; Kruszewski, M.; Pop, A.; et al. In Vitro Exposure of a 3D-Tetraculture Representative for the Alveolar Barrier at the Air-Liquid Interface to Silver Particles and Nanowires. Part. Fibre Toxicol. 2019, 16, 14. [Google Scholar] [CrossRef]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. In Nanotoxicity; Zhang, Q., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1894, pp. 1–29. ISBN 978-1-4939-8915-7. [Google Scholar]
- Talà, A.; Guerra, F.; Resta, S.C.; Calcagnile, M.; Barca, A.; Tredici, S.M.; De Donno, M.D.; Vacca, M.; Liso, M.; Chieppa, M.; et al. Phenotyping of Fecal Microbiota of Winnie, a Rodent Model of Spontaneous Chronic Colitis, Reveals Specific Metabolic, Genotoxic, and Pro-Inflammatory Properties. Inflammation 2022, 45, 2477–2497. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The Pig: A Model for Human Infectious Diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Pinheiro, A.; Esteves, P.J. The Rabbit as an Animal Model to Study Innate Immunity Genes: Is It Better than Mice? Front. Immunol. 2022, 13, 981815. [Google Scholar] [CrossRef] [PubMed]
- Esteves, P.J.; Abrantes, J.; Baldauf, H.-M.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The Wide Utility of Rabbits as Models of Human Diseases. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, M.; Tredici, S.M.; Alifano, P. A Comprehensive Review on Probiotics and Their Use in Aquaculture: Biological Control, Efficacy, and Safety through the Genomics and Wet Methods. Heliyon 2024, 10, e40892. [Google Scholar] [CrossRef]
- Arrazuria, R.; Kerscher, B.; Huber, K.E.; Hoover, J.L.; Lundberg, C.V.; Hansen, J.U.; Sordello, S.; Renard, S.; Aranzana-Climent, V.; Hughes, D.; et al. Variability of Murine Bacterial Pneumonia Models Used to Evaluate Antimicrobial Agents. Front. Microbiol. 2022, 13, 988728. [Google Scholar] [CrossRef]
- Herzog, M.K.-M.; Cazzaniga, M.; Peters, A.; Shayya, N.; Beldi, L.; Hapfelmeier, S.; Heimesaat, M.M.; Bereswill, S.; Frankel, G.; Gahan, C.G.M.; et al. Mouse Models for Bacterial Enteropathogen Infections: Insights into the Role of Colonization Resistance. Gut Microbes 2023, 15, 2172667. [Google Scholar] [CrossRef]
- Kubelkova, K.; Benuchova, M.; Kozakova, H.; Sinkora, M.; Krocova, Z.; Pejchal, J.; Macela, A. Gnotobiotic Mouse Model’s Contribution to Understanding Host–Pathogen Interactions. Cell. Mol. Life Sci. 2016, 73, 3961–3969. [Google Scholar] [CrossRef]
- Santos, C.F.; Andrade, S.M.; Mil-Homens, D.; Montemor, M.F.; Alves, M.M. Antibacterial Activity of ZnO Nanoparticles in a Staphylococcus-aureus-Infected Galleria mellonella Model Is Tuned by Different Apple-Derived Phytocargos. J. Funct. Biomater. 2023, 14, 463. [Google Scholar] [CrossRef]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue Distribution and Acute Toxicity of Silver after Single Intravenous Administration in Mice: Nano-Specific and Size-Dependent Effects. Part. Fibre Toxicol. 2015, 13, 12. [Google Scholar] [CrossRef]
- Pratt, I.S. Global Harmonisation of Classification and Labelling of Hazardous Chemicals. Toxicol. Lett. 2002, 128, 5–15. [Google Scholar] [CrossRef]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella Larvae Allow the Discrimination of Toxic and Non-Toxic Chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Meccatti, V.M.; Figueiredo-Godoi, L.M.A.; Pereira, T.C.; de Lima, P.M.N.; Abu Hasna, A.; Senna, L.B.; Marcucci, M.C.; Junqueira, J.C.; de Oliveira, L.D. The Biocompatibility and Antifungal Effect of Rosmarinus officinalis against Candida albicans in Galleria mellonella Model. Sci. Rep. 2022, 12, 15611. [Google Scholar] [CrossRef] [PubMed]
- Kishore, J.; Goel, M.; Khanna, P. Understanding Survival Analysis: Kaplan-Meier Estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar] [CrossRef]
- Moya-Andérico, L.; Vukomanovic, M.; del Mar Cendra, M.; Segura-Feliu, M.; Gil, V.; del Río, J.A.; Torrents, E. Utility of Galleria mellonella Larvae for Evaluating Nanoparticle Toxicology. Chemosphere 2021, 266, 129235. [Google Scholar] [CrossRef]
- Çelik Uzuner, S. Development of a Direct Trypan Blue Exclusion Method to Detect Cell Viability of Adherent Cells into ELISA Plates. Celal Bayar Univ. J. Sci. 2018, 14, 99–104. [Google Scholar] [CrossRef]
- Eskin, A.; Öztürk, Ş.; Körükçü, M. Determination of the Acute Toxic Effects of Zinc Oxide Nanoparticles (ZnO NPs) in Total Hemocytes Counts of Galleria mellonella (Lepidoptera: Pyralidae) with Two Different Methods. Ecotoxicology 2019, 28, 801–808. [Google Scholar] [CrossRef]
- Tuncsoy, B.; Mese, Y. Influence of Titanium Dioxide Nanoparticles on Bioaccumulation, Antioxidant Defense and Immune System of Galleria mellonella L. Environ. Sci. Pollut. Res. 2021, 28, 38007–38015. [Google Scholar] [CrossRef]
- Sezer Tuncsoy, B.; Tuncsoy, M.; Gomes, T.; Sousa, V.; Teixeira, M.R.; Bebianno, M.J.; Ozalp, P. Effects of Copper Oxide Nanoparticles on Tissue Accumulation and Antioxidant Enzymes of Galleria mellonella L. Bull. Environ. Contam. Toxicol. 2019, 102, 341–346. [Google Scholar] [CrossRef]
- Coskun, M.; Kayis, T.; Yilmaz, M.; Dursun, O.; Emre, I. Copper and Zinc Impact on Stress Biomarkers and Growth Parameters in a Model Organism, Galleria mellonella Larvae. Biometals 2021, 34, 1263–1273. [Google Scholar] [CrossRef]
- Mese, Y.; Tuncsoy, B.; Ozalp, P. Effects of Cu, Zn and Their Mixtures on Bioaccumulation and Antioxidant Enzyme Activities in Galleria mellonella L. (Lepidoptera: Pyralidae). Ecotoxicology 2022, 31, 649–656. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris Tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, S.N.; Sisubalan, N.; Vijayan, A.; Karthikeyan, C.; Gnanaraj, M.; Gideon, D.A.M.; Jebastin, T.; Varaprasad, K.; Sadiku, R. Recent Advances in Green Synthesized Nanoparticles for Bactericidal and Wound Healing Applications. Heliyon 2023, 9, e13128. [Google Scholar] [CrossRef] [PubMed]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- He, M.; Han, Z.; Liang, Y.; Zhao, H.; Ji, X.; Ma, G.; Cui, Y.; Wang, L. Green Synthesis of Ag Nanoparticles Using Elm Pod Polysaccharide for Catalysis and Bacteriostasis. Int. J. Biol. Macromol. 2022, 213, 1078–1087. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial Activity and Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa. Int. J. Nanomedicine 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomedicine 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Green Synthesis of Flower-Like Carrageenan-Silver Nanoparticles and Elucidation of Its Physicochemical and Antibacterial Properties. Molecules 2023, 28, 907. [Google Scholar] [CrossRef]
- Kumkoon, T.; Srisaisap, M.; Boonserm, P. Biosynthesized Silver Nanoparticles Using Morus alba (White Mulberry) Leaf Extract as Potential Antibacterial and Anticancer Agents. Molecules 2023, 28, 1213. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, V.; Srećković, N.; Nedić, Z.P.; Dimitrijević, S.; Matić, M.; Obradović, A.; Selaković, D.; Rosić, G.; Katanić Stanković, J.S. Green Synthesis of Silver Nanoparticles Using Salvia verticillata and Filipendula ulmaria Extracts: Optimization of Synthesis, Biological Activities, and Catalytic Properties. Molecules 2023, 28, 808. [Google Scholar] [CrossRef] [PubMed]
- Al-Soub, A.; Khleifat, K.; Al-Tarawneh, A.; Al-limoun, M.; Alfarrayeh, I.; Sarayreh, A.A.; Qaisi, Y.A.; Qaralleh, H.; Alqaraleh, M.; Albashaireh, A. Silver Nanoparticles Biosynthesis Using an Airborne Fungal Isolate, Aspergillus Flavus: Optimization, Characterization and Antibacterial Activity. Iran. J. Microbiol. 2022, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Khodeer, D.M.; Nasr, A.M.; Swidan, S.A.; Shabayek, S.; Khinkar, R.M.; Aldurdunji, M.M.; Ramadan, M.A.; Badr, J.M. Characterization, Antibacterial, Antioxidant, Antidiabetic, and Anti-Inflammatory Activities of Green Synthesized Silver Nanoparticles Using Phragmanthera Austroarabica A. G. Mill and J. A. Nyberg Extract. Front. Microbiol. 2023, 13, 1078061. [Google Scholar] [CrossRef]
- Dua, T.K.; Giri, S.; Nandi, G.; Sahu, R.; Shaw, T.K.; Paul, P. Green Synthesis of Silver Nanoparticles Using Eupatorium adenophorum Leaf Extract: Characterizations, Antioxidant, Antibacterial and Photocatalytic Activities. Chem. Pap. 2023. [Google Scholar] [CrossRef]
- Thomaz, L.; Gustavo de Almeida, L.; Silva, F.R.O.; Cortez, M.; Taborda, C.P.; Spira, B. In Vivo Activity of Silver Nanoparticles Against Pseudomonas aeruginosa Infection in Galleria mellonella. Front. Microbiol. 2020, 11, 582107. [Google Scholar] [CrossRef]
- Campo-Beleño, C.; Villamizar-Gallardo, R.A.; López-Jácome, L.E.; González, E.E.; Muñoz-Carranza, S.; Franco, B.; Morales-Espinosa, R.; Coria-Jimenez, R.; Franco-Cendejas, R.; Hernández-Durán, M.; et al. Biologically Synthesized Silver Nanoparticles as Potent Antibacterial Effective against Multidrug-Resistant Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2022, 75, 680–688. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Maria, D.A.; Gonçalves, P.J.R.D.O.; de Araújo, W.L.; de Souza, A.O. Biogenic Aspergillus tubingensis Silver Nanoparticles’ in Vitro Effects on Human Umbilical Vein Endothelial Cells, Normal Human Fibroblasts, HEPG2, and Galleria mellonella. Toxicol. Res. 2019, 8, 789–801. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Romano de Oliveira Gonçalves, P.J.; Ottoni, C.A.; de Cássia Ruiz, R.; Morgano, M.A.; de Araújo, W.L.; de Melo, I.S.; De Souza, A.O. Functional Textiles Impregnated with Biogenic Silver Nanoparticles from Bionectria ochroleuca and Its Antimicrobial Activity. Biomed. Microdevices 2019, 21, 56. [Google Scholar] [CrossRef]
- Artunduaga Bonilla, J.J.; Honorato, L.; Cordeiro de Oliveira, D.F.; Araújo Gonçalves, R.; Guimarães, A.; Miranda, K.; Nimrichter, L. Silver Chitosan Nanocomposites as a Potential Treatment for Superficial Candidiasis. Med. Mycol. 2021, 59, 993–1005. [Google Scholar] [CrossRef]
- Soundy, J.; Day, D. Delivery of Antibacterial Silver Nanoclusters to Pseudomonas aeruginosa Using Species-Specific DNA Aptamers. J. Med. Microbiol. 2020, 69, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Simberg, D. Interactions of Nanoparticles with Plasma Proteins: Implication on Clearance and Toxicity of Drug Delivery Systems. Expert Opin. Drug Deliv. 2011, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; He, Q. The Interaction of Nanoparticles with Plasma Proteins and the Consequent Influence on Nanoparticles Behavior. Expert Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Barbir, R.; Capjak, I.; Crnković, T.; Debeljak, Ž.; Domazet Jurašin, D.; Ćurlin, M.; Šinko, G.; Weitner, T.; Vinković Vrček, I. Interaction of Silver Nanoparticles with Plasma Transport Proteins: A Systematic Study on Impacts of Particle Size, Shape and Surface Functionalization. Chem.-Biol. Interact. 2021, 335, 109364. [Google Scholar] [CrossRef]
- de Macedo, E.F.; Santos, N.S.; Nascimento, L.S.; Mathey, R.; Brenet, S.; de Moura, M.S.; Hou, Y.; Tada, D.B. Interaction between Nanoparticles, Membranes and Proteins: A Surface Plasmon Resonance Study. Int. J. Mol. Sci. 2022, 24, 591. [Google Scholar] [CrossRef]
- Morka, K.D.; Wernecki, M.; Kędziora, A.; Książczyk, M.; Dudek, B.; Gerasymchuk, Y.; Lukowiak, A.; Bystroń, J.; Bugla-Płoskońska, G. The Impact of Graphite Oxide Nanocomposites on the Antibacterial Activity of Serum. Int. J. Mol. Sci. 2021, 22, 7386. [Google Scholar] [CrossRef]
- Alves, M.M.; Batista, C.; Mil-Homens, D.; Grenho, L.; Fernandes, M.H.; Santos, C.F. Enhanced Antibacterial Activity of Rosehip Extract-Functionalized Mg(OH)2 Nanoparticles: An in Vitro and in Vivo Study. Colloids Surf. B Biointerfaces 2022, 217, 112643. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Killian, M.S.; Raghu, S.N.V.; Schmuki, P.; Mazare, A.; Cimpean, A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. J. Funct. Biomater. 2022, 13, 274. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A. Emerging Applications of Biocompatible Phytosynthesized Metal/Metal Oxide Nanoparticles in Healthcare. J. Drug Deliv. Sci. Technol. 2020, 56, 101516. [Google Scholar] [CrossRef]
- Webster, T.J.; Seil, I. Antimicrobial Applications of Nanotechnology: Methods and Literature. Int. J. Nanomedicine 2012, 2767. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The Antibacterial Effects of Silver, Titanium Dioxide and Silica Dioxide Nanoparticles Compared to the Dental Disinfectant Chlorhexidine on Streptococcus mutans Using a Suite of Bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Atri, A.; Echabaane, M.; Bouzidi, A.; Harabi, I.; Soucase, B.M.; Ben Chaâbane, R. Green Synthesis of Copper Oxide Nanoparticles Using Ephedra alata Plant Extract and a Study of Their Antifungal, Antibacterial Activity and Photocatalytic Performance under Sunlight. Heliyon 2023, 9, e13484. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, R.; Raorane, C.J.; Kim, S.-C.; Lee, Y.R. One Pot Synthesis of Copper Oxide Nanoparticles for Efficient Antibacterial Activity. Materials 2022, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, R.; Raorane, C.J.; Kim, S.-C.; Ashokkumar, S.; Lee, Y.R. Novel Microwave Synthesis of Copper Oxide Nanoparticles and Appraisal of the Antibacterial Application. Micromachines 2023, 14, 456. [Google Scholar] [CrossRef]
- Eskin, A.; Bozdoğan, H. Effects of the Copper Oxide Nanoparticles (CuO NPs) on Galleria mellonella Hemocytes. Drug Chem. Toxicol. 2022, 45, 1870–1880. [Google Scholar] [CrossRef]
- Tunçsoy, B.; Sugeçti, S.; Büyükgüzel, E.; Özalp, P.; Büyükgüzel, K. Effects of Copper Oxide Nanoparticles on Immune and Metabolic Parameters of Galleria mellonella L. Bull. Environ. Contam. Toxicol. 2021, 107, 412–420. [Google Scholar] [CrossRef]
- Xu, M.; Li, L.; Pan, W.; Zheng, H.; Wang, M.; Peng, X.; Dai, S.; Tang, Y.; Zeng, K.; Huang, X. Zinc Oxide Nanoparticles Prime a Protective Immune Response in Galleria mellonella to Defend Against Candida albicans. Front. Microbiol. 2021, 12, 766138. [Google Scholar] [CrossRef]
- Cé, R.; Silva, R.C.; Trentin, D.S.; Marchi, J.G.B.D.; Paese, K.; Guterres, S.S.; Macedo, A.J.; Pohlmann, A.R. Galleria mellonella Larvae as an In Vivo Model to Evaluate the Toxicity of Polymeric Nanocapsules. J. Nanosci. Nanotechnol. 2020, 20, 1486–1494. [Google Scholar] [CrossRef]
- Arsene, M.; Viktorovna, P.; Davares, A. Galleria mellonella (Greater Wax Moth) as an Eco-Friendly in Vivo Approach for the Assessment of the Acute Toxicity of Medicinal Plants: Application to Some Plants from Cameroon. Open Vet. J. 2021, 11, 651. [Google Scholar] [CrossRef]
- Maguire, R.; Duggan, O.; Kavanagh, K. Evaluation of Galleria mellonella Larvae as an in Vivo Model for Assessing the Relative Toxicity of Food Preservative Agents. Cell Biol. Toxicol. 2016, 32, 209–216. [Google Scholar] [CrossRef]
- Costabile, G.; Provenzano, R.; Azzalin, A.; Scoffone, V.C.; Chiarelli, L.R.; Rondelli, V.; Grillo, I.; Zinn, T.; Lepioshkin, A.; Savina, S.; et al. PEGylated Mucus-Penetrating Nanocrystals for Lung Delivery of a New FtsZ Inhibitor against Burkholderia cenocepacia Infection. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102113. [Google Scholar] [CrossRef] [PubMed]
- Rigotto Caruso, G.; Tonani, L.; Marcato, P.D.; von Zeska Kress, M.R. Phenothiazinium Photosensitizers Associated with Silver Nanoparticles in Enhancement of Antimicrobial Photodynamic Therapy. Antibiotics 2021, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Prasastha Ram, V.; Yasur, J.; Abishad, P.; Unni, V.; Purushottam Gourkhede, D.; Nishanth, M.A.D.; Niveditha, P.; Vergis, J.; Singh Malik, S.V.; Kullaiah, B.; et al. Antimicrobial Efficacy of Green Synthesized Nanosilver with Entrapped Cinnamaldehyde against Multi-Drug-Resistant Enteroaggregative Escherichia coli in Galleria mellonella. Pharmaceutics 2022, 14, 1924. [Google Scholar] [CrossRef] [PubMed]
- Stauber, R.H.; Westmeier, D.; Wandrey, M.; Becker, S.; Docter, D.; Ding, G.-B.; Thines, E.; Knauer, S.K.; Siemer, S. Mechanisms of Nanotoxicity—Biomolecule Coronas Protect Pathological Fungi against Nanoparticle-Based Eradication. Nanotoxicology 2020, 14, 1157–1174. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; García-Álvarez, R.; Mediero, A.; Esteban, J.; Vallet-Regí, M. Effect of Gold Nanostars Plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms. Biology 2022, 11, 162. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Marena, G.D.; Leonardi, G.R.; Sábio, R.M.; Corrêa, I.; Chorilli, M.; Bauab, T.M. Lycopene, Mesoporous Silica Nanoparticles and Their Association: A Possible Alternative against Vulvovaginal Candidiasis? Molecules 2022, 27, 8558. [Google Scholar] [CrossRef]
- Spadari, C.d.C.; de Bastiani, F.W.M.d.S.; Lopes, L.B.; Ishida, K. Alginate Nanoparticles as Non-Toxic Delivery System for Miltefosine in the Treatment of Candidiasis and Cryptococcosis. Int. J. Nanomedicine 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- Barreto, T.L.; Rossato, L.; de Freitas, A.L.D.; Meis, J.F.; Lopes, L.B.; Colombo, A.L.; Ishida, K. Miltefosine as an Alternative Strategy in the Treatment of the Emerging Fungus Candida auris. Int. J. Antimicrob. Agents 2020, 56, 106049. [Google Scholar] [CrossRef]
- Passos, J.S.; de Martino, L.C.; Dartora, V.F.C.; de Araujo, G.L.B.; Ishida, K.; Lopes, L.B. Development, Skin Targeting and Antifungal Efficacy of Topical Lipid Nanoparticles Containing Itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar] [CrossRef]
- Vera-González, N.; Bailey-Hytholt, C.M.; Langlois, L.; Camargo Ribeiro, F.; Souza Santos, E.L.; Junqueira, J.C.; Shukla, A. Anidulafungin Liposome Nanoparticles Exhibit Antifungal Activity against Planktonic and Biofilm Candida albicans. J. Biomed. Mater. Res. 2020, 108, 2263–2276. [Google Scholar] [CrossRef]
- Baldim, I.; Paziani, M.H.; Grizante Barião, P.H.; Kress, M.R.V.Z.; Oliveira, W.P. Nanostructured Lipid Carriers Loaded with Lippia sidoides Essential Oil as a Strategy to Combat the Multidrug-Resistant Candida auris. Pharmaceutics 2022, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, A.; Palocci, C.; Chronopoulou, L.; De Angelis, G.; Badiali, C.; Petruccelli, V.; D’Angeli, S.; Pasqua, G.; Simonetti, G. Poly-(Lactic-Co-Glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri. Molecules 2022, 27, 5424. [Google Scholar] [CrossRef] [PubMed]
- Thammasit, P.; Tharinjaroen, C.S.; Tragoolpua, Y.; Rickerts, V.; Georgieva, R.; Bäumler, H.; Tragoolpua, K. Targeted Propolis-Loaded Poly (Butyl) Cyanoacrylate Nanoparticles: An Alternative Drug Delivery Tool for the Treatment of Cryptococcal Meningitis. Front. Pharmacol. 2021, 12, 723727. [Google Scholar] [CrossRef]
- Jiang, L.; Greene, M.K.; Insua, J.L.; Pessoa, J.S.; Small, D.M.; Smyth, P.; McCann, A.P.; Cogo, F.; Bengoechea, J.A.; Taggart, C.C.; et al. Clearance of Intracellular Klebsiella pneumoniae Infection Using Gentamicin-Loaded Nanoparticles. J. Control. Release 2018, 279, 316–325. [Google Scholar] [CrossRef]
- Almeida Furquim de Camargo, B.; Soares Silva, D.E.; Noronha da Silva, A.; Campos, D.L.; Machado Ribeiro, T.R.; Mieli, M.J.; Borges Teixeira Zanatta, M.; Bento da Silva, P.; Pavan, F.R.; Gallina Moreira, C.; et al. New Silver(I) Coordination Compound Loaded into Polymeric Nanoparticles as a Strategy to Improve In Vitro Anti- Helicobacter Pylori Activity. Mol. Pharm. 2020, 17, 2287–2298. [Google Scholar] [CrossRef]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial Efficacy of Tobramycin Polymeric Nanoparticles for Pseudomonas aeruginosa Infections in Cystic Fibrosis: Formulation, Characterisation and Functionalisation with Dornase Alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef]
- de Andrades, E.O.; da Costa, J.M.A.R.; de Lima Neto, F.E.M.; de Araujo, A.R.; de Oliveira Silva Ribeiro, F.; Vasconcelos, A.G.; de Jesus Oliveira, A.C.; Sobrinho, J.L.S.; de Almeida, M.P.; Carvalho, A.P.; et al. Acetylated Cashew Gum and Fucan for Incorporation of Lycopene Rich Extract from Red Guava (Psidium guajava L.) in Nanostructured Systems: Antioxidant and Antitumor Capacity. Int. J. Biol. Macromol. 2021, 191, 1026–1037. [Google Scholar] [CrossRef]
- Migotto, A.; Carvalho, V.F.M.; Salata, G.C.; da Silva, F.W.M.; Yan, C.Y.I.; Ishida, K.; Costa-Lotufo, L.V.; Steiner, A.A.; Lopes, L.B. Multifunctional Nanoemulsions for Intraductal Delivery as a New Platform for Local Treatment of Breast Cancer. Drug Deliv. 2018, 25, 654–667. [Google Scholar] [CrossRef]
- Lopes Rocha Correa, V.; Assis Martins, J.; Ribeiro de Souza, T.; de Castro Nunes Rincon, G.; Pacheco Miguel, M.; Borges de Menezes, L.; Correa Amaral, A. Melatonin Loaded Lecithin-Chitosan Nanoparticles Improved the Wound Healing in Diabetic Rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef]
- Tien, N.D.; Lyngstadaas, S.P.; Mano, J.F.; Blaker, J.J.; Haugen, H.J. Recent Developments in Chitosan-Based Micro/Nanofibers for Sustainable Food Packaging, Smart Textiles, Cosmeceuticals, and Biomedical Applications. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Smart Stimuli-Responsive Chitosan Hydrogel for Drug Delivery: A Review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Tang, Q.; Qiu, P.; Gou, D.; Zhao, J. Chitosan-Based Materials: An Overview of Potential Applications in Food Packaging. Foods 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.R.; Kurliuk, A.V.; Rubanik, V.V.; Kirichuk, A.A.; Khubiev, O.; Golubev, R.; Lobanov, N.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies. Molecules 2022, 27, 8865. [Google Scholar] [CrossRef]
- Chou, C.-M.; Mi, F.-L.; Horng, J.-L.; Lin, L.-Y.; Tsai, M.-L.; Liu, C.-L.; Lu, K.-Y.; Chu, C.-Y.; Chen, Y.-T.; Lee, Y.-L.A.; et al. Characterization and Toxicology Evaluation of Low Molecular Weight Chitosan on Zebrafish. Carbohydr. Polym. 2020, 240, 116164. [Google Scholar] [CrossRef]
- Arias, L.S.; Butcher, M.C.; Short, B.; McKloud, E.; Delaney, C.; Kean, R.; Monteiro, D.R.; Williams, C.; Ramage, G.; Brown, J.L. Chitosan Ameliorates Candida auris Virulence in a Galleria mellonella Infection Model. Antimicrob. Agents Chemother. 2020, 64, e00476-20. [Google Scholar] [CrossRef]
- Rwegasila, E.; Mubofu, E.; Nyandoro, S.; Erasto, P.; Munissi, J. Preparation, Characterization and in Vivo Antimycobacterial Studies of Panchovillin-Chitosan Nanocomposites. Int. J. Mol. Sci. 2016, 17, 1559. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Lee, M.; Megaw, J.; McEvoy, J.; Coulter, S.M.; Pentlavalli, S.; Laverty, G. Investigating the In Vivo Antimicrobial Activity of a Self-Assembling Peptide Hydrogel Using a Galleria mellonella Infection Model. ACS Omega 2019, 4, 2584–2589. [Google Scholar] [CrossRef]
- Maslova, E.; Shi, Y.; Sjöberg, F.; Azevedo, H.S.; Wareham, D.W.; McCarthy, R.R. An Invertebrate Burn Wound Model That Recapitulates the Hallmarks of Burn Trauma and Infection Seen in Mammalian Models. Front. Microbiol. 2020, 11, 998. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological Properties of Centella Asiatica Hydrogel in Accelerating Wound Healing in Rabbits. BMC Complement. Altern. Med. 2019, 19, 213. [Google Scholar] [CrossRef]
- Egro, F.; Repko, A.; Narayanaswamy, V.; Ejaz, A.; Kim, D.; Schusterman, M.A.; Loughran, A.; Ayyash, A.; Towsend, S.M.; Baker, S.; et al. Soluble Chitosan Derivative Treats Wound Infections and Promotes Wound Healing in a Novel MRSA-Infected Porcine Partial-Thickness Burn Wound Model. PLoS ONE 2022, 17, e0274455. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.H.S.; Park, J.; Kim, D.; Choi, D.; De Zoysa, M. Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 13525. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef] [PubMed]
- Petrov, L.; Stoilova, O.; Pramatarov, G.; Kanzova, H.; Tsvetanova, E.; Andreeva, M.; Georgieva, A.; Atanasova, D.; Philipov, S.; Alexandrova, A. Effect of Chitosan-Diosgenin Combination on Wound Healing. Int. J. Mol. Sci. 2023, 24, 5049. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wareham, D.W.; Yuan, Y.; Deng, X.; Mata, A.; Azevedo, H.S. Polymyxin B-Triggered Assembly of Peptide Hydrogels for Localized and Sustained Release of Combined Antimicrobial Therapy. Adv. Healthc. Mater. 2021, 10, 2101465. [Google Scholar] [CrossRef]
- Coughlan, A.; Scanlon, K.; Mahon, B.P.; Towler, M.R. Zinc and Silver Glass Polyalkenoate Cements: An Evaluation of Their Antibacterial Nature. Bio-Med. Mater. Eng. 2010, 20, 99–106. [Google Scholar] [CrossRef]
- Mannala, G. Galleria mellonella as an Alternative in Vivo Model to Study Bacterial Biofilms on Stainless Steel and Titanium Implants. ALTEX 2020. [Google Scholar] [CrossRef]
- Villani, S.; Kunjalukkal Padmanabhan, S.; Stoppa, M.; Nisi, R.; Calcagnile, M.; Alifano, P.; Demitri, C.; Licciulli, A. Neem-Hypericum-Bacterial Cellulose Wound Care Paste Characterized in Vitro and in Galleria mellonella in Vivo Model. Carbohydr. Polym. Technol. Appl. 2024, 7, 100431. [Google Scholar] [CrossRef]
- Jander, G.; Rahme, L.G.; Ausubel, F.M. Positive Correlation between Virulence of Pseudomonas aeruginosa Mutants in Mice and Insects. J. Bacteriol. 2000, 182, 3843–3845. [Google Scholar] [CrossRef]
- Wojda, I.; Taszłow, P. Heat Shock Affects Host–Pathogen Interaction in Galleria mellonella Infected with Bacillus thuringiensis. J. Insect Physiol. 2013, 59, 894–905. [Google Scholar] [CrossRef]
- Cotter, G.; Doyle, S.; Kavanagh, K. Development of an Insect Model for the in Vivo Pathogenicity Testing of Yeasts. FEMS Immunol. Med. Microbiol. 2000, 27, 163–169. [Google Scholar] [CrossRef]
- Champion, O.L.; Titball, R.W.; Bates, S. Standardization of G. Mellonella Larvae to Provide Reliable and Reproducible Results in the Study of Fungal Pathogens. J. Fungi 2018, 4, 108. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villani, S.; Calcagnile, M.; Demitri, C.; Alifano, P. Galleria mellonella (Greater Wax Moth) as a Reliable Animal Model to Study the Efficacy of Nanomaterials in Fighting Pathogens. Nanomaterials 2025, 15, 67. https://doi.org/10.3390/nano15010067
Villani S, Calcagnile M, Demitri C, Alifano P. Galleria mellonella (Greater Wax Moth) as a Reliable Animal Model to Study the Efficacy of Nanomaterials in Fighting Pathogens. Nanomaterials. 2025; 15(1):67. https://doi.org/10.3390/nano15010067
Chicago/Turabian StyleVillani, Stefania, Matteo Calcagnile, Christian Demitri, and Pietro Alifano. 2025. "Galleria mellonella (Greater Wax Moth) as a Reliable Animal Model to Study the Efficacy of Nanomaterials in Fighting Pathogens" Nanomaterials 15, no. 1: 67. https://doi.org/10.3390/nano15010067
APA StyleVillani, S., Calcagnile, M., Demitri, C., & Alifano, P. (2025). Galleria mellonella (Greater Wax Moth) as a Reliable Animal Model to Study the Efficacy of Nanomaterials in Fighting Pathogens. Nanomaterials, 15(1), 67. https://doi.org/10.3390/nano15010067








