Study on the Influence and Mechanism of Mineral Admixtures and Fibers on Frost Resistance of Slag–Yellow River Sediment Geopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Mixed Proportion
2.1.1. Raw Material
2.1.2. Mixed Proportion
2.2. Experimental Method
2.2.1. Frost Resistance Test
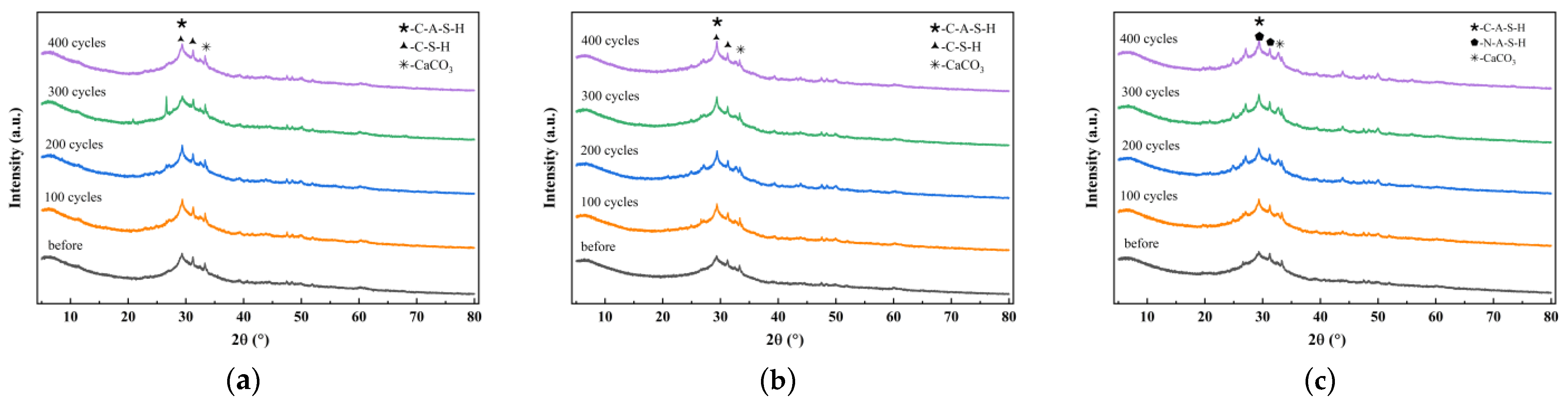
2.2.2. X-Ray Diffraction Analysis
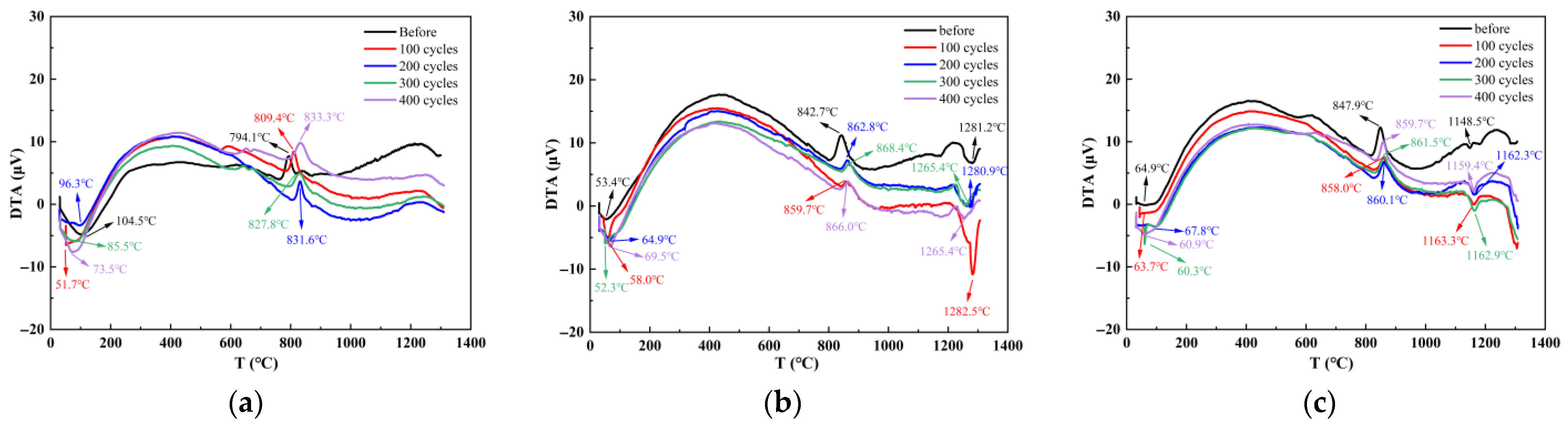
2.2.3. Thermogravimetric Analysis
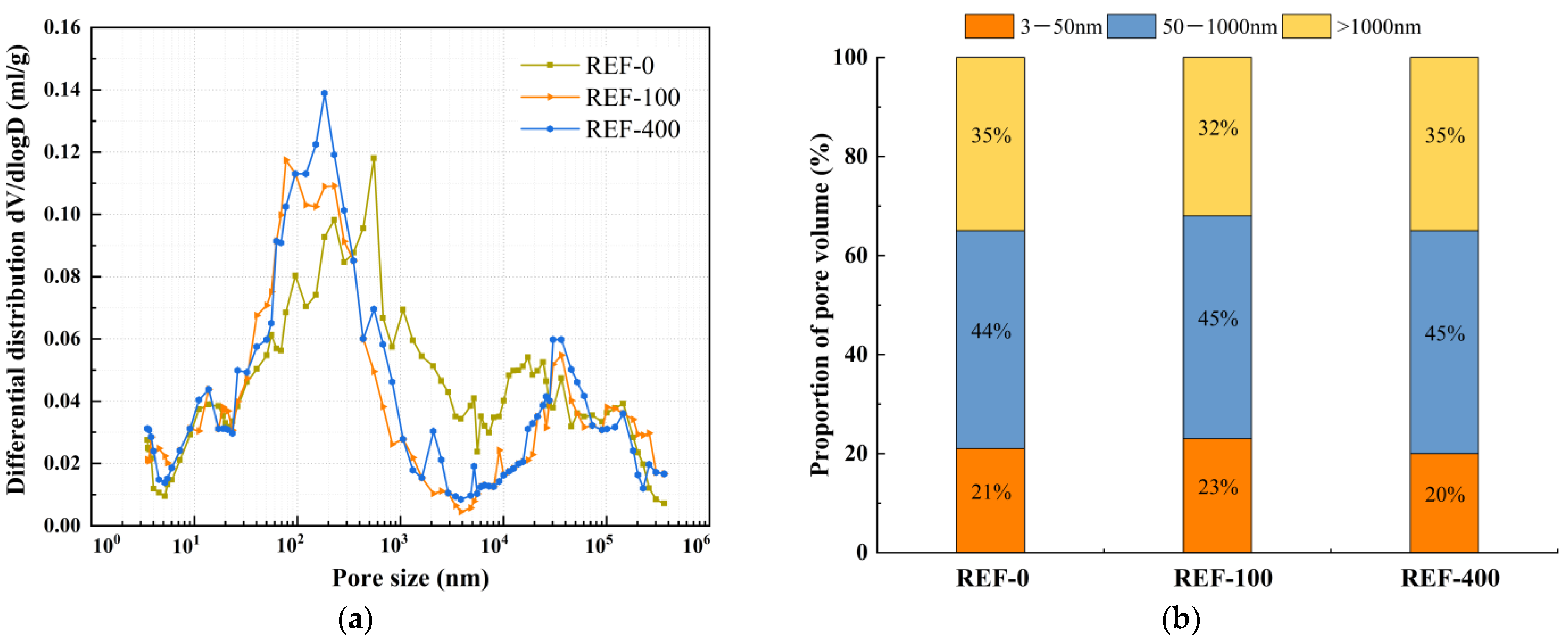
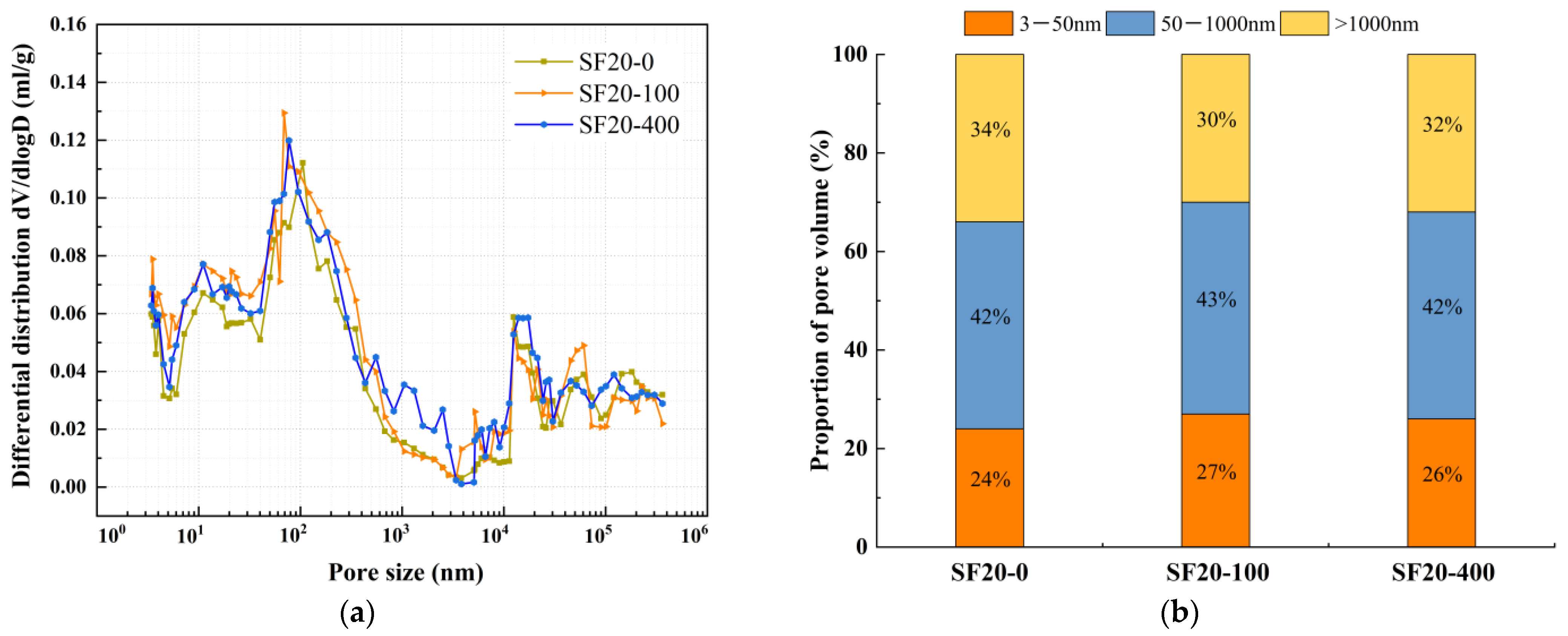
2.2.4. Porosity Test
2.2.5. Scanning Electron Microscopy Test
3. Experiment Results and Analysis
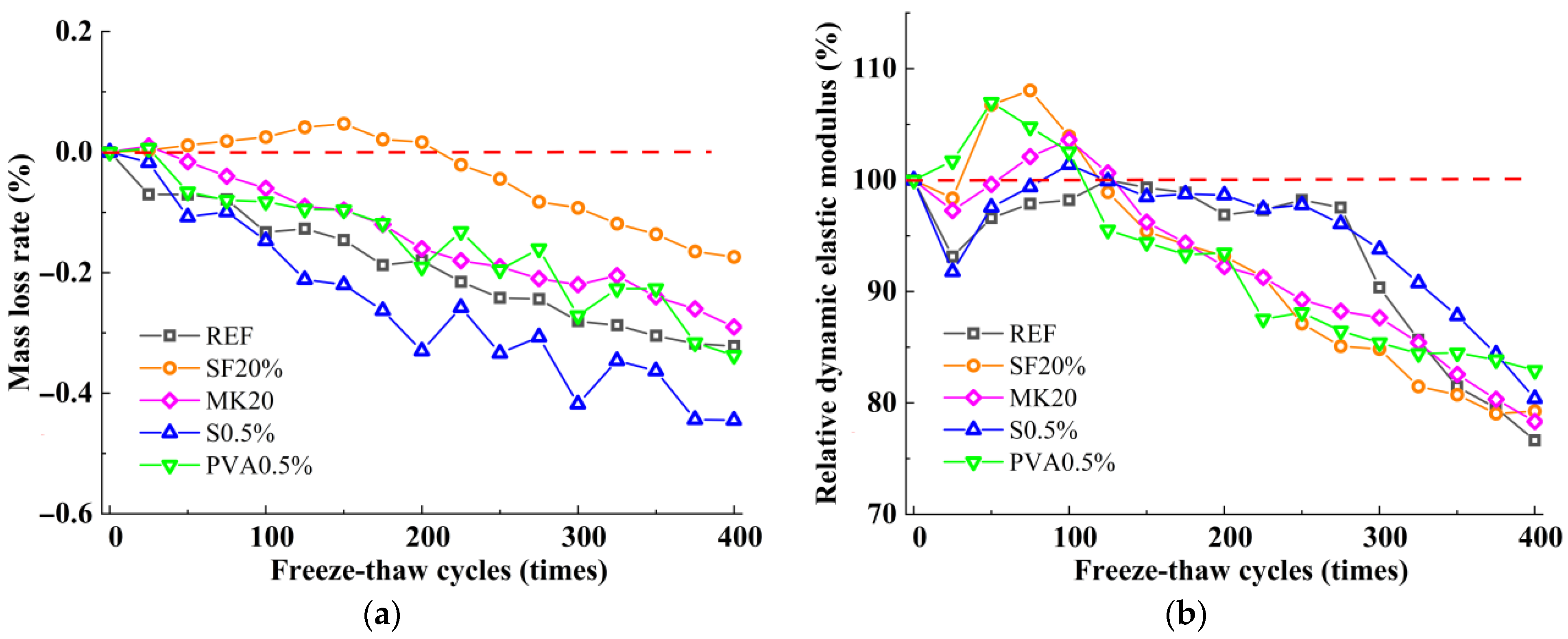
3.1. Mass Loss Rate and Relative Dynamic Elastic Modulus
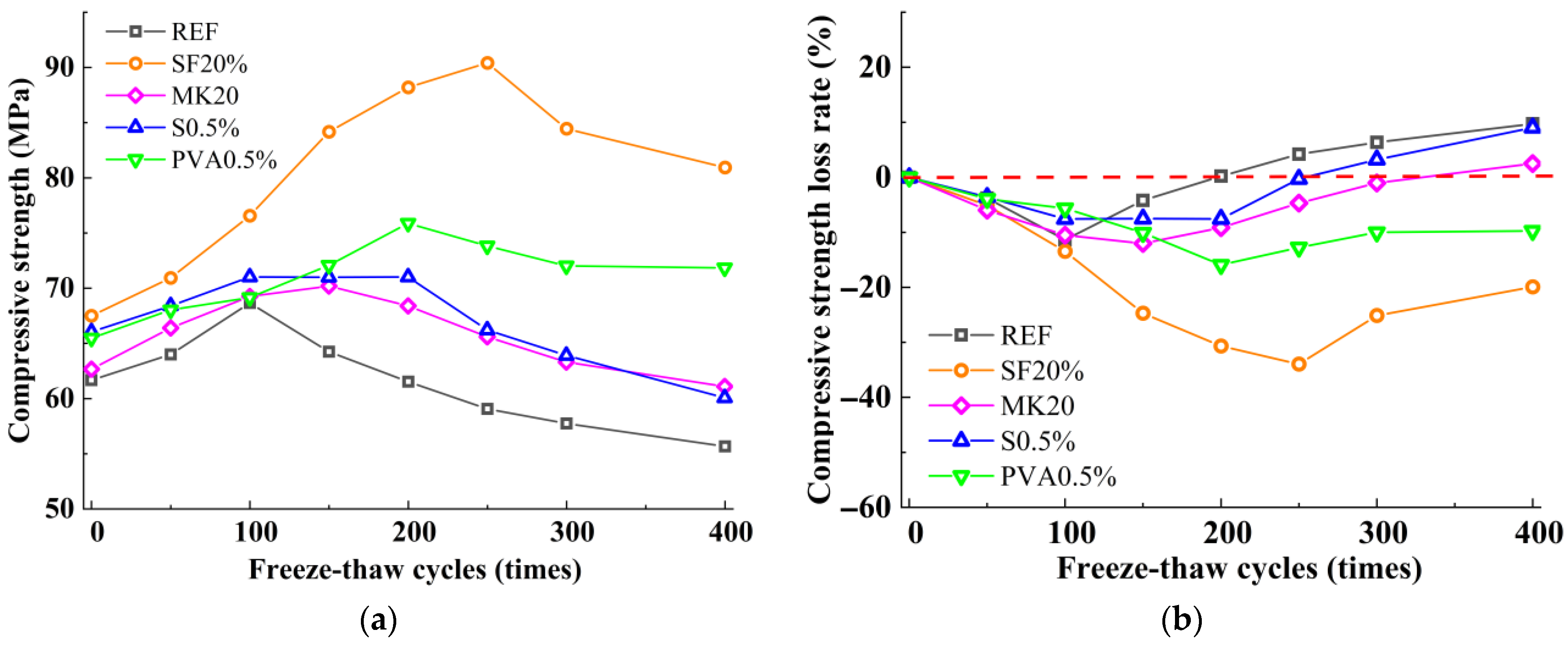
3.2. Compressive Strength
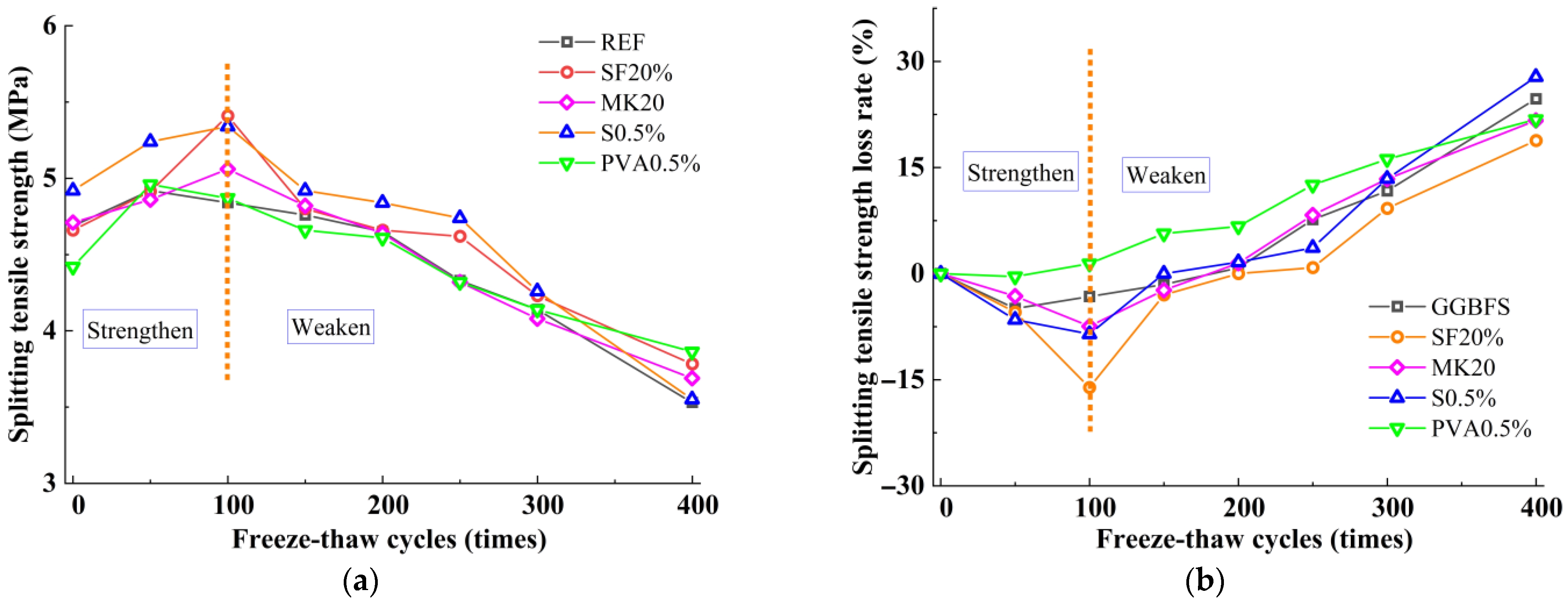
3.3. Splitting Tensile Strength
3.4. Tension-Compression Ratio
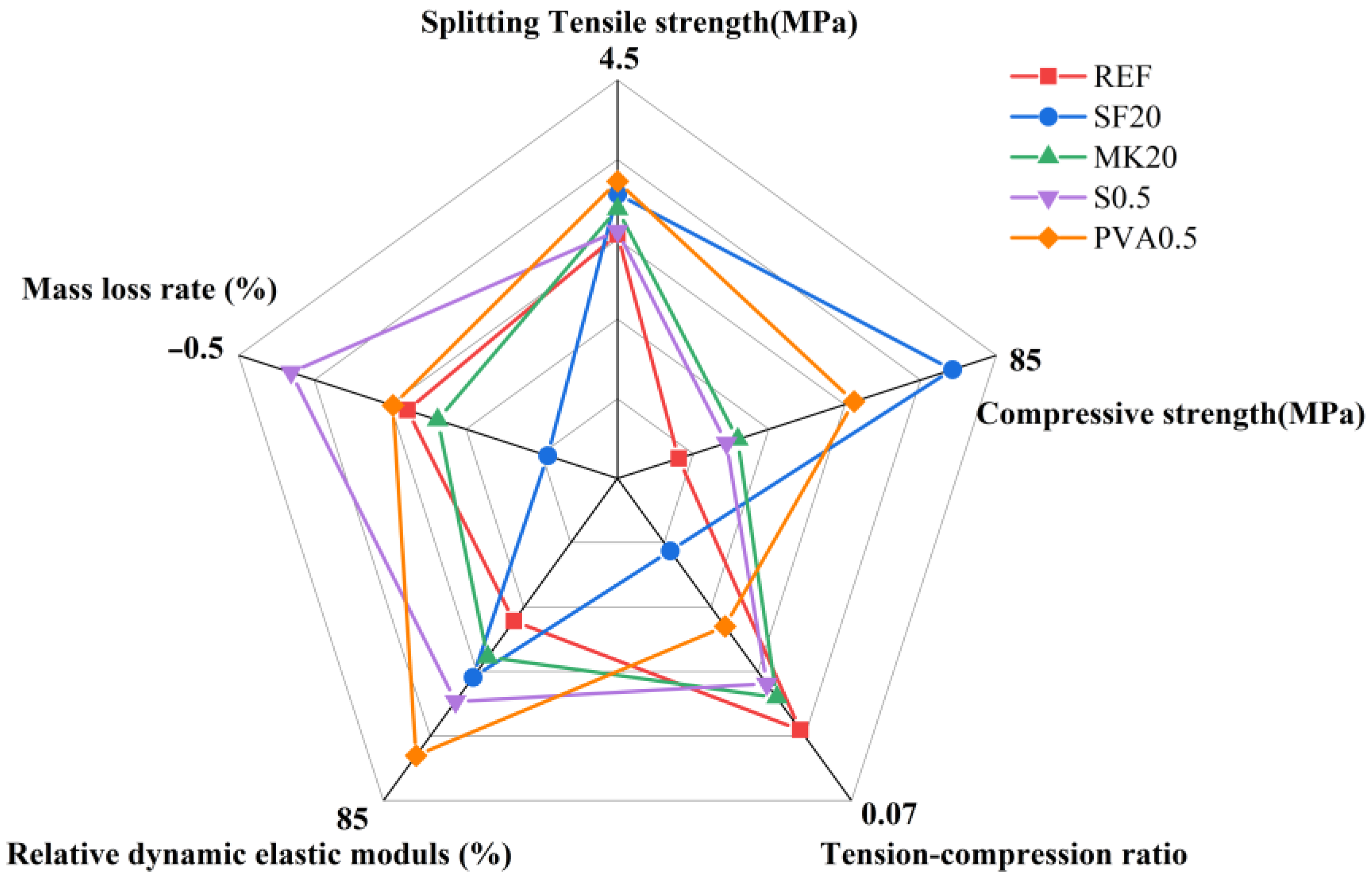
3.5. Five-Dimensional Evaluation Diagram
4. Influence Mechanism Analysis
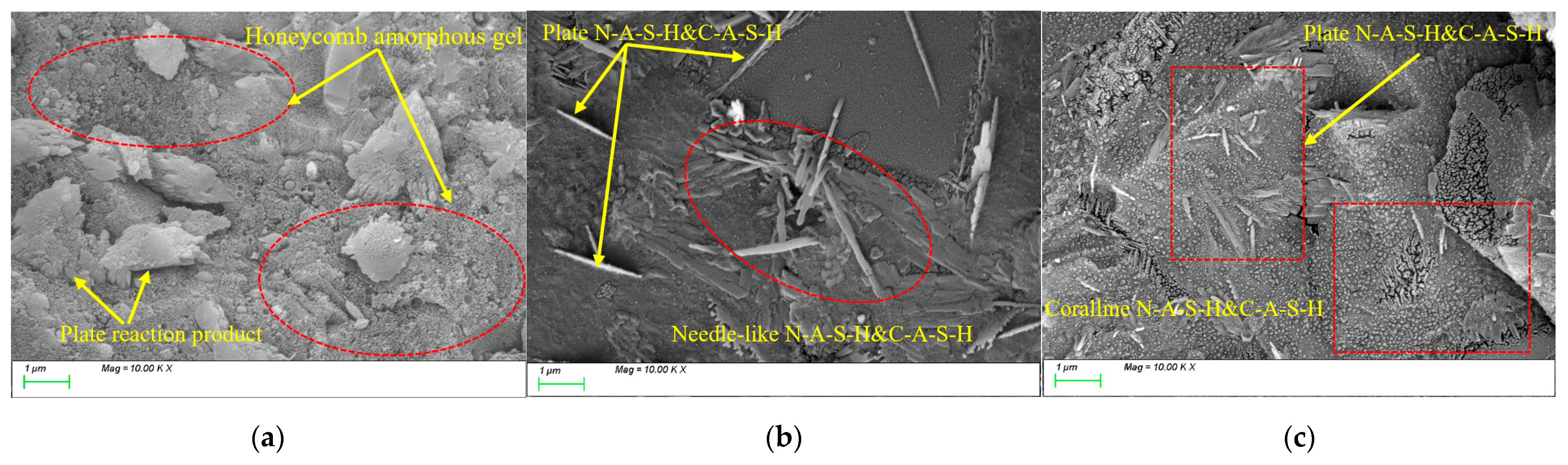
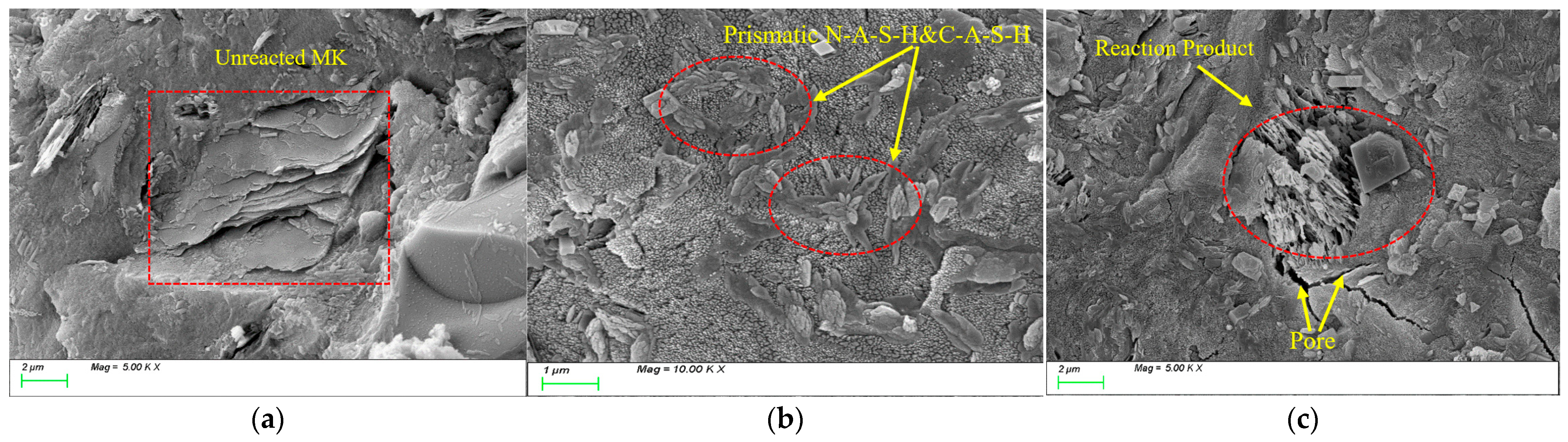
4.1. Characteristic Reaction Products
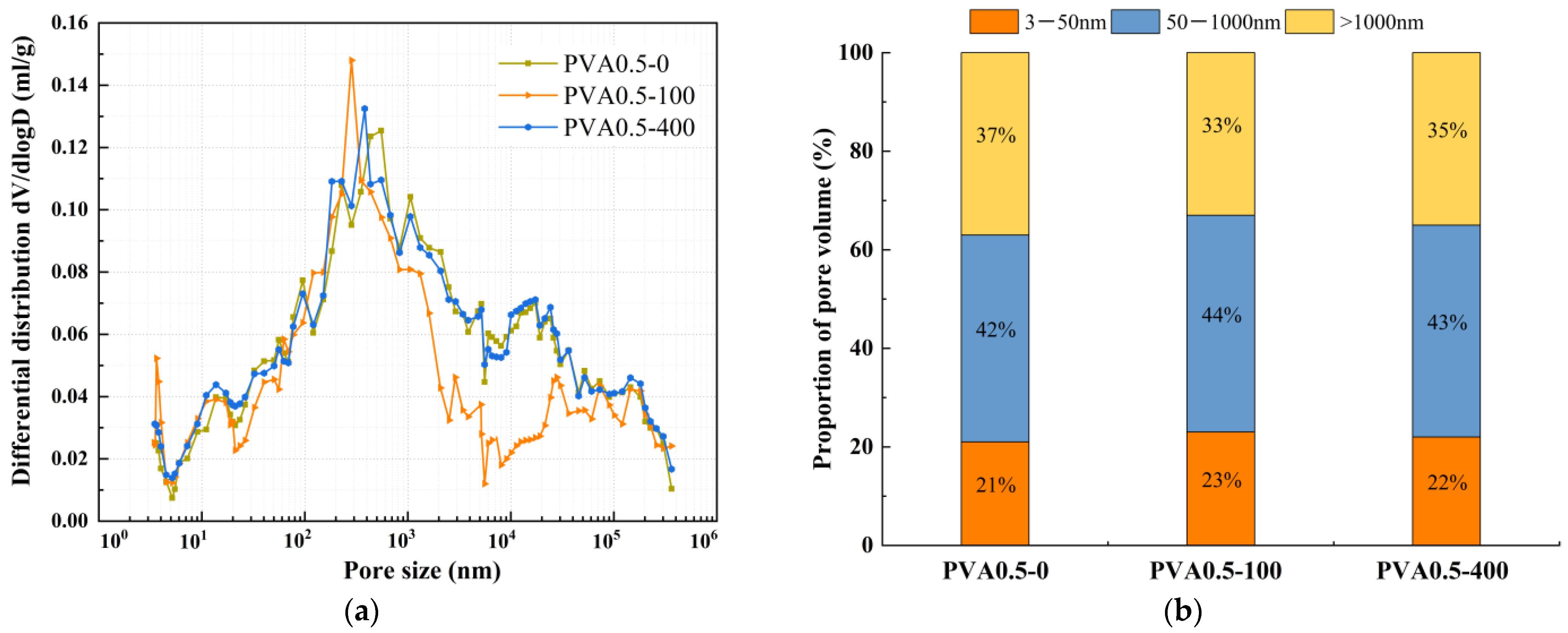
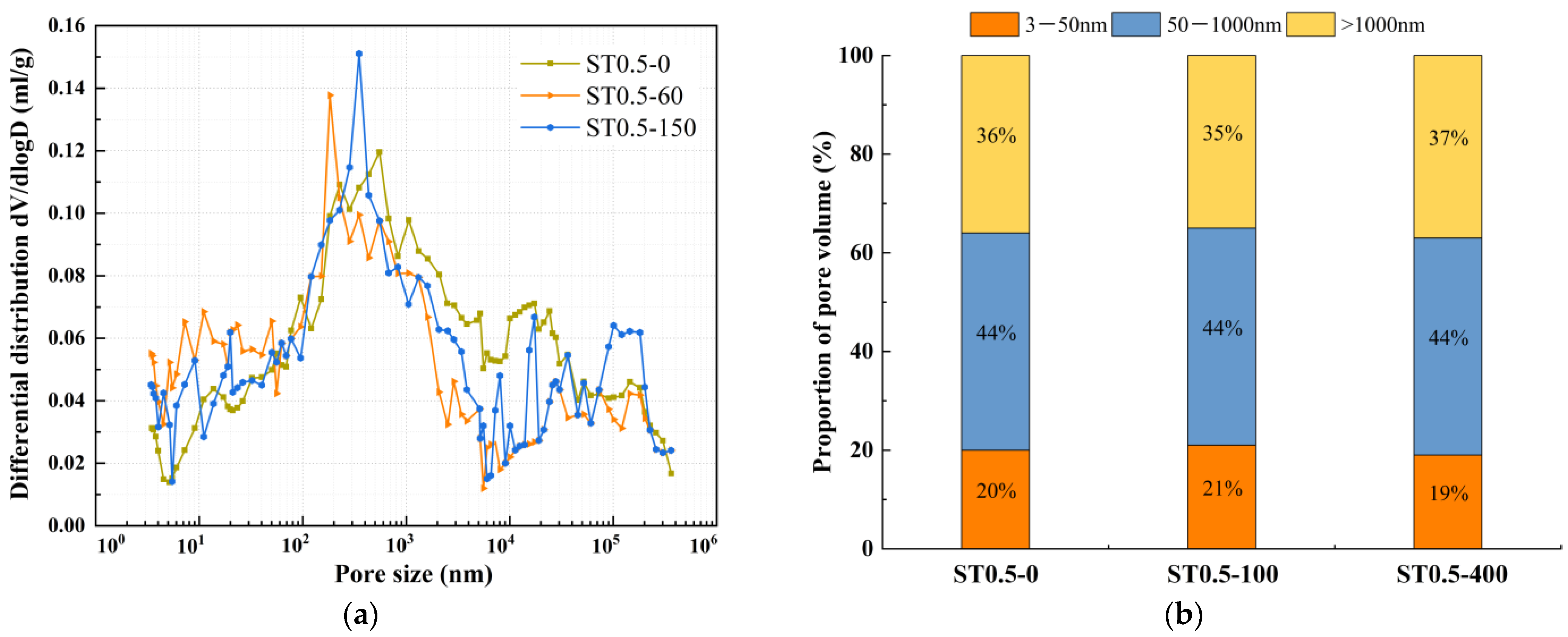
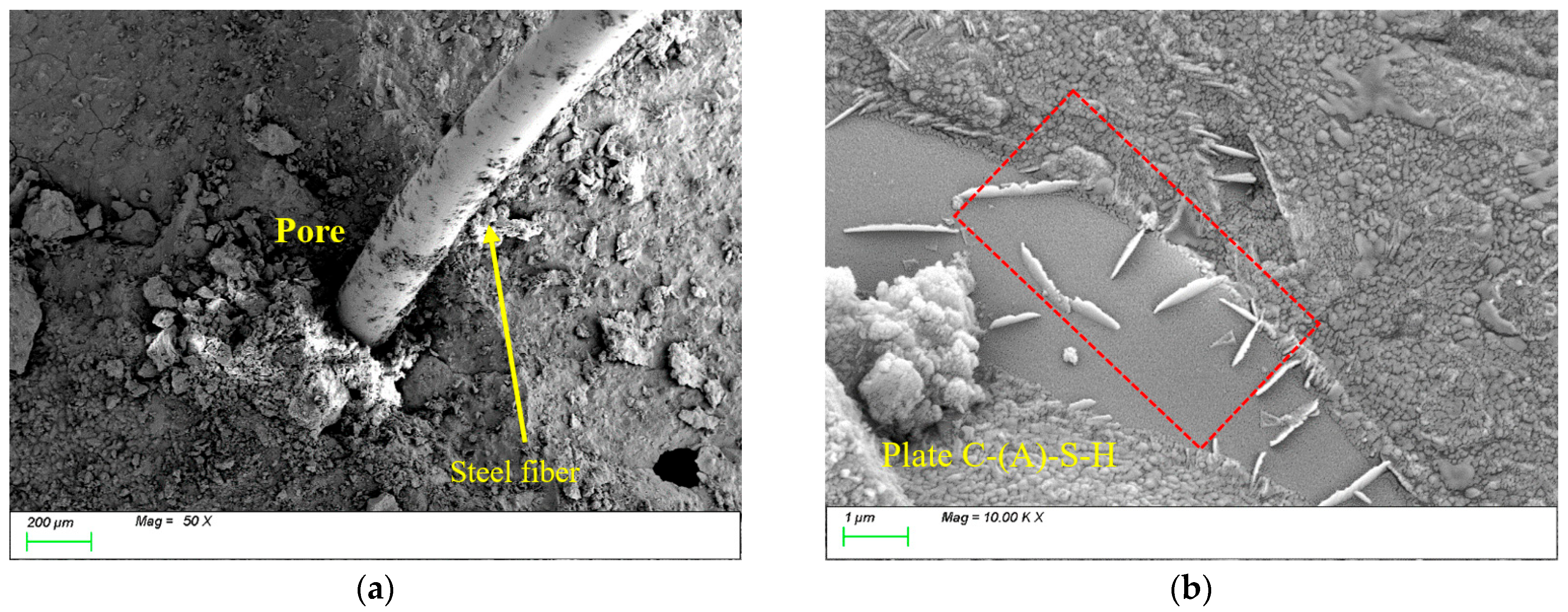
4.2. Pore Structure
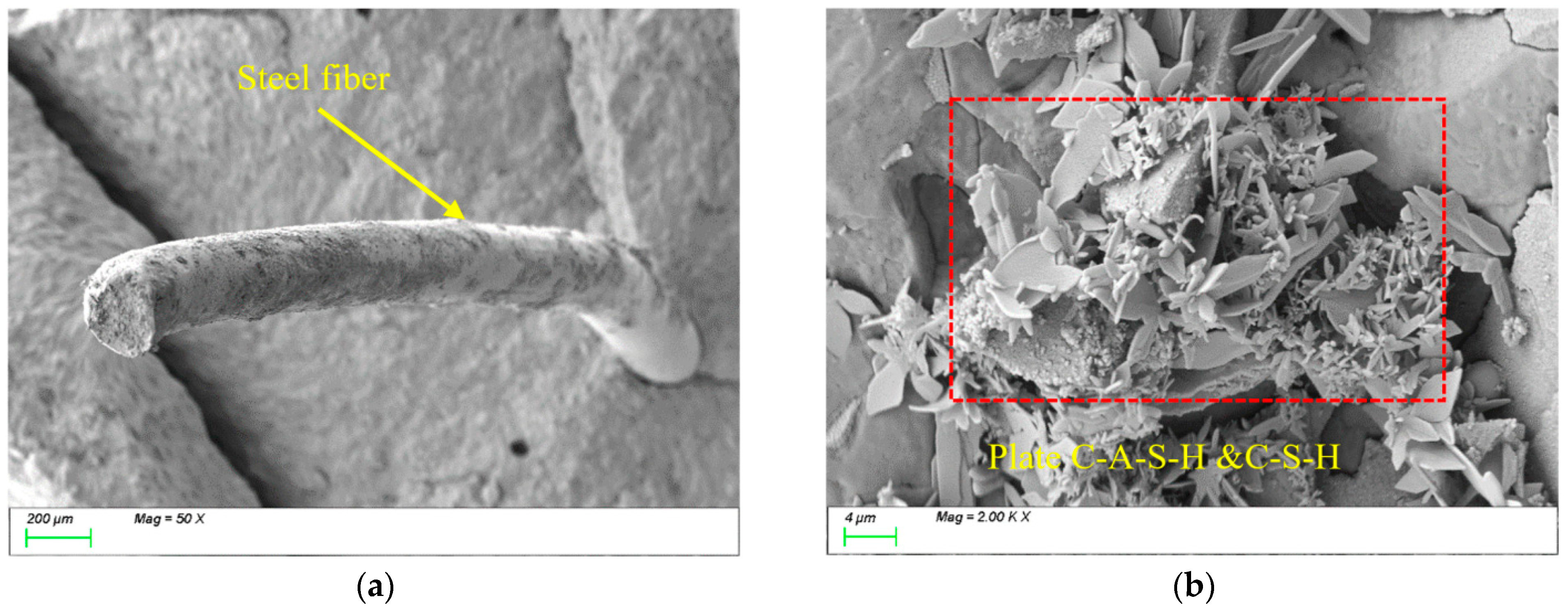
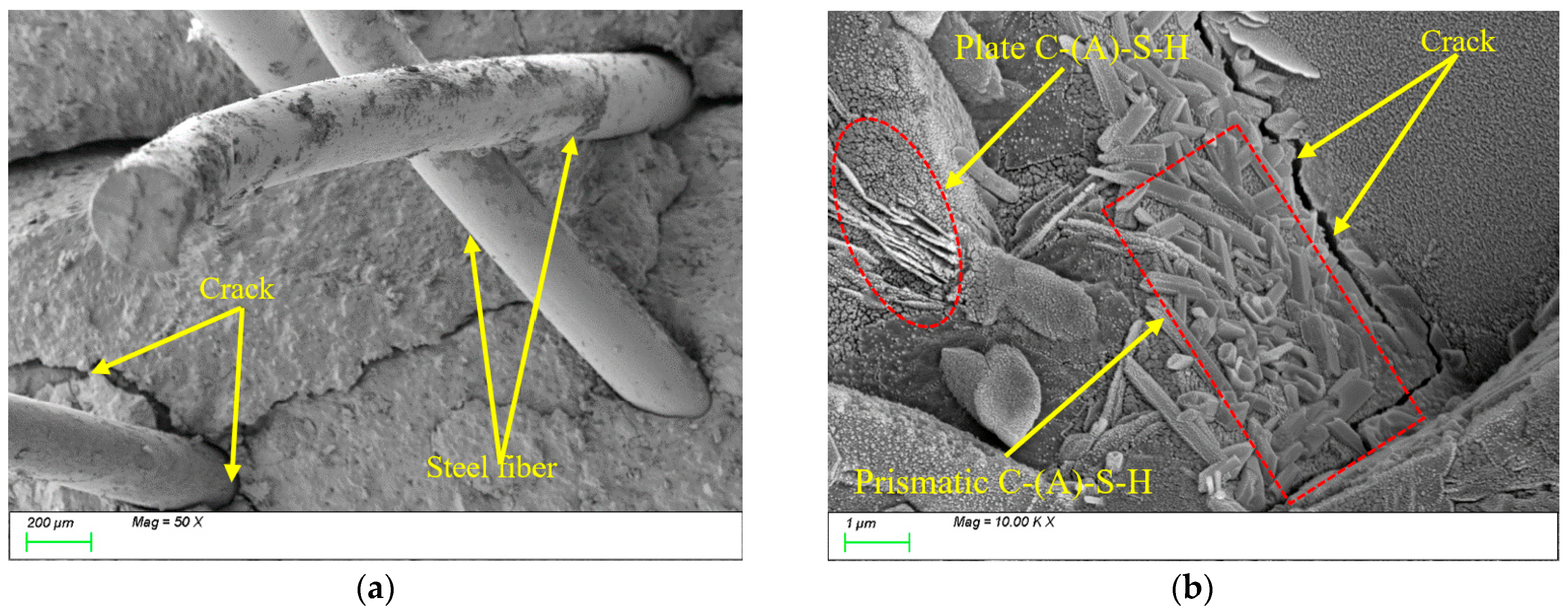
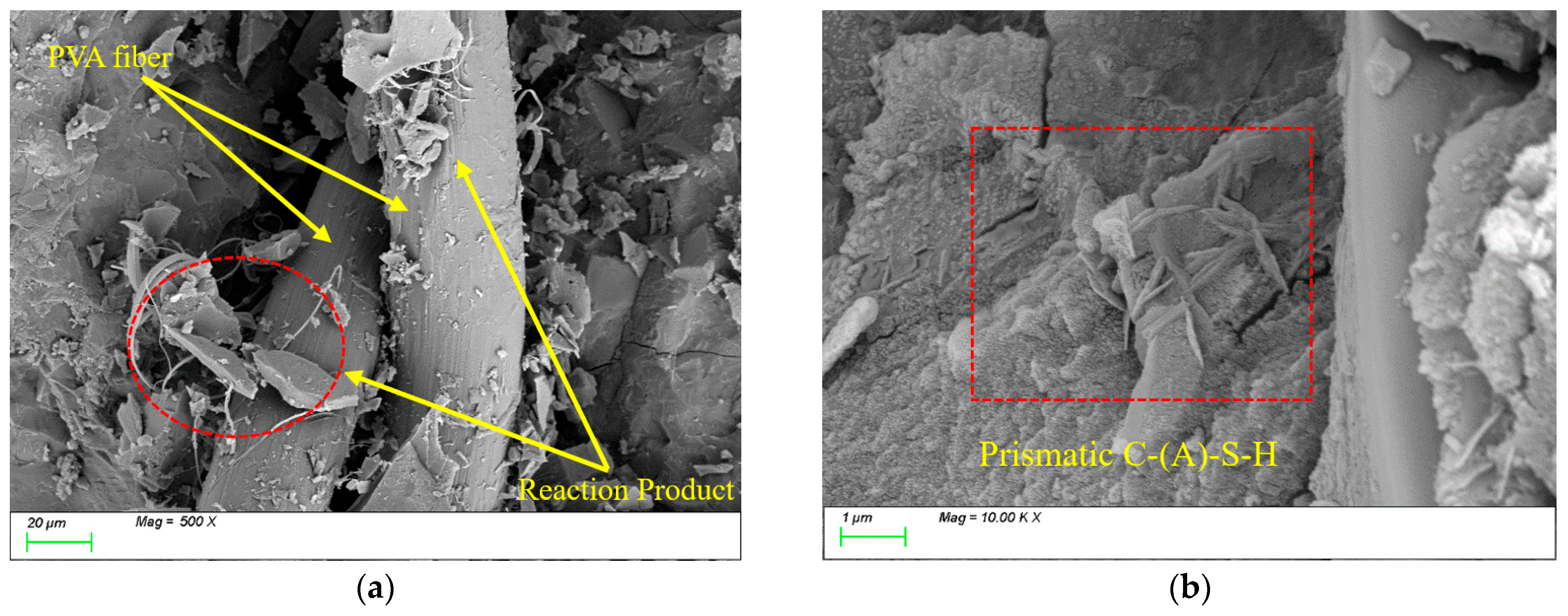
4.3. Matrix Microstructure
5. Conclusions
- (1)
- The incorporation of silica fume and metakaolin significantly enhances the frost resistance of slag-Yellow River sediment geopolymers. Silica fume promotes secondary hydration reactions through its highly reactive SiO2, generating low calcium-to-silica ratio C-(A)-S-H gels that refine pore structures, resulting in a 19.9% increase in compressive strength after 400 freeze-thaw cycles. Metakaolin reinforces matrix stability by forming a three-dimensional N-A-S-H network structure, effectively delaying freeze-thaw damage, with a tensile strength loss rate of only 2.51% following 400 freeze-thaw cycles.
- (2)
- The incorporation of fibers significantly influences the freeze-thaw performance of the material. Leveraging its high elasticity and deformation capacity, PVA fibers effectively mitigate frost heave stress and suppress microcrack propagation, with compressive strength and splitting tensile strength loss rates (6.18% and 21.79%) notably lower than those of steel fibers (9.03% and 27.81%). Due to their significant thermal expansion coefficient mismatch with the matrix, steel fibers are prone to interfacial crack formation under thermal cycling, thereby weakening the reinforcement effect.
- (3)
- During freeze-thaw cycling, the material’s pore structure exhibits a two-stage evolution characterized by “refinement followed by coarsening.” In the early stages of freeze-thaw cycles, pore refinement is primarily driven by the post-reaction of mineral admixtures filling pores, leading to an increased proportion of gel pores, reduced macropores, and enhanced mechanical strength. In the late stages of freeze-thaw cycles, due to the continuous effects of temperature alternation and ice expansion stress, microcrack propagation occurs, which reduces the compactness of the matrix and causes a rebound in the proportion of macropores. This ultimately leads to the deterioration of mechanical properties at the macroscopic scale, with splitting tensile strength exhibiting more pronounced degradation.
- (4)
- Through a five-dimensional analysis, it is evident that SF20 exhibits excellent durability in freeze-thaw environments. On the one hand, it demonstrates the lowest strength loss rate. It maintains a stable pore structure while also showing balanced performance in mass loss rate and relative dynamic elastic modulus. These properties make it suitable for frequent freeze-thaw environments such as road foundations and underground engineering. Additionally, this study provides a feasible solution for the resource utilization of Yellow River silty fine sand and industrial solid waste, offering both environmental and economic benefits.
- (5)
- This study found that although the incorporation of fibers does not alter the types of characteristic hydration products such as C-(A)-S-H and N-(A)-S-H gels during freeze-thaw cycles, it significantly influences their reaction processes and spatial distribution patterns, thereby exerting a pronounced impact on matrix durability. This effect primarily depends on three critical factors: (1) the distribution of fibers within the matrix; (2) physical characteristic parameters of fibers, including thermal expansion coefficient, diameter, and aspect ratio; and (3) hydrophilicity and chemical adsorption effects. However, constrained by current experimental sample sizes and environmental simulation conditions, the long-term interaction mechanisms and practical implications require further in-depth investigation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Liu, C.Y.; Hao, Y.F.; Zhang, Y.; Chen, L.; Li, Z. Experimental investigation of engineered geopolymer composite for structural strengthening against blast loads. Def. Technol. 2024, 32, 496–509. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.Q.; He, X.Y.; Su, Y.; Tang, Y.Z.; Qi, H.H.; Yang, C.; Xiong, G.Q. Using superabsorbent polymer to mitigate the fast setting and high autogenous shrinkage of carbide slag and sodium silicate activated ultrafine GGBS based composites. Sustain. Chem. Pharm. 2024, 39, 101550. [Google Scholar] [CrossRef]
- Cao, B.S.; Li, Y.; Li, P.P. Synergistic Effect of Blended Precursors and Silica Fume on Strength and High Temperature Resistance of Geopolymer. Materials 2024, 17, 2975. [Google Scholar] [CrossRef]
- Yang, N.; Xuan, Q.D.; Fu, Y.; Ma, X.; Lei, D.Y.; Niu, J.L.; Dai, J.G. Phosphate activated geopolymer-based coating with high temperature resistance for sub-ambient radiative cooling Phosphate activated geopolymer-based coating with high temperature resistance for sub-ambient radiative cooling. Sustain. Cities Soc. 2024, 100, 104992. [Google Scholar] [CrossRef]
- Wang, Z.K.; Fu, C.H.; Wang, K.; Zhao, J.; Shumuye, E.D.; Yang, Z.H. Effect of geopolymer concrete cover on improving tensile and transverse shear behaviors of BFRP bars after exposure to high temperature. Case Stud. Constr. Mater. 2024, 20, e02862. [Google Scholar] [CrossRef]
- Chen, Z.M.; Liu, H.; Zhu, P.H.; Li, H.C.; Ge, T.Z.; Yang, L.; Chen, C.H.; Dong, Y.L. Effect of Curing Mechanism on Sulfuric Acid Corrosion Resistance of Geopolymer Recycled Aggregate Concrete. Ksce J. Civ. Eng. 2024, 28, 1173–1182. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Yu, Y.; Guo, W.H.; Xing, F.; Guo, M.H. Development of inorganic anticorrosive coatings for steel bars: Corrosion resistance testing and design. Cem. Concr. Compos. 2024, 152, 105612. [Google Scholar] [CrossRef]
- Cui, L.J.; Xiang, T.F.; Hu, B.J.; Lv, Y.J.; Rong, H.; Liu, D.E.; Zhang, S.Q.; Guo, M.L.; Lv, Z.; Chen, D.P. Design of monolithic super hydrophobic concrete with excellent anti-corrosion and self-cleaning properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133345. [Google Scholar] [CrossRef]
- Alzeer, M.I.M.; MacKenzie, K.J.D. Chapter 5—Fiber composites of inorganic polymers (geopolymers) reinforced with natural fibers. In Composite Materials; Low, I.-M., Dong, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 117–147. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Nuaklong, P.; Worawatnalunart, P.; Jongvivatsakul, P.; Tangaramvong, S.; Pothisiri, T.; Likitlersuang, S. Pre- and post-fire mechanical performances of high calcium fly ash geopolymer concrete containing granite waste. J. Build. Eng. 2021, 44, 103265. [Google Scholar] [CrossRef]
- Figiela, B.; Bak, A.; Hebda, M.; Korniejenko, K. Eco-friendly production of foamed geopolymers based on mine waste. J. Achiev. Mater. Manuf. Eng. 2023, 121, 341–349. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The role of inorganic polymer technology in the development of green concrete. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Dong, C.H.; Li, T.; Zhang, Y.M.; Liu, J. Damage process and performance of PVA fiber-reinforced alkali-activated slag mortar plate under bending. J. Southeast Univ. (Engl. Ed.) 2018, 34, 229–236. [Google Scholar] [CrossRef]
- Zhang, S.Z.; He, S.; Ghiassi, B.; Breugel, K.V.; Ye, G. Interface bonding properties of polyvinyl alcohol (PVA) fiber in alkali-activated slag/fly ash. Cem. Concr. Res. 2023, 173, 107308. [Google Scholar] [CrossRef]
- Sarker, P.K.; Haque, R.; Ramgolam, K.V. Fracture behaviour of heat cured fly ash based geopolymer concrete. Mater. Des. 2013, 44, 580–586. [Google Scholar] [CrossRef]
- Pan, Z.; Sanjayan, J.G.; Rangan, B.V. Fracture properties of geopolymer paste and concrete. Mag. Concr. 2011, 63, 763–771. [Google Scholar] [CrossRef]
- Thomas, R.J.; Peethamparan, S. Alkali-activated concrete: Engineering propertie sand stress strain behavior. Constr. Build. Mater. 2015, 93, 49–56. [Google Scholar] [CrossRef]
- Atis, C.D.; Bilim, C.; Celik, O.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Lee, M.; Kim, K.; Chung, C.W.; Kim, W.; Jeong, Y.; Lee, J. Mechanical characterization of recycled-PET fiber reinforced mortar composites treated with nano-SiO2 and mixed with seawater. Constr. Build. Mater. 2023, 392, 131882. [Google Scholar] [CrossRef]
- Lee, B.Y.; Cho, C.G.; Lim, H.J.; Song, J.K.; Yang, K.H.; Li, V.C. Strain hardening fiber-reinforced alkali-activated mortar—A feasibility study. Constr. Build. Mater. 2012, 37, 15–20. [Google Scholar] [CrossRef]
- Natali, A.; Manzi, S.; Bignozzi, M.C. Novel fiber-reinforced composite materials based on sustainable geopolymer matrix. Procedia Eng. 2011, 21, 1124–1131. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Li, Z.; Zhou, X.; Eddie; Chau, C. Impact properties of geopolymer based extrudates incorporated with fly ash and PVA short fiber. Constr. Build. Mater. 2008, 22, 370–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Li, Z.; Zhou, X. Geopolymer extruded composites with incorporated fly ash and polyvinyl alcohol short fiber. ACI Mater. J. 2009, 106, 3–10. [Google Scholar]
- Akturk, B.; Akca, A.H.; Kizilkanat, A.B. Fracture response of fiber-reinforced sodium carbonate activated slag mortar. Constr. Build. Mater. 2020, 241, 118128. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.M.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Wang, B.M.; Li, G.N.; Han, J.N.; Zheng, Y.; Liu, H.; Song, W.Z. Study on the properties of artificial flood-prevention stone made by Yellow River silt. Constr. Build. Mater. 2017, 144, 484–492. [Google Scholar] [CrossRef]
- Song, Y.B.; Xu, J.X.; Xu, X.; Wang, P. Alkali-activated preparation and frost resistance of artificial flood-prevention stone material. Hydro-Sci. Eng. 2023, 5, 149–157. Available online: https://link.cnki.net/urlid/32.1613.tv.20230505.1240.006 (accessed on 3 July 2025).
- Wang, Z.K. Multi-Sized Yellow River Sediment Activation and Its Application Research. Master’s thesis, North China University of Water Resources and Electric Power, Zhengzhou, China, 2023. [Google Scholar] [CrossRef]
- Raza, A.; Zhang, J.J.; Xu, S.W.; Umar, M.; Yuan, C.F. Experimental analysis of frost resistance and failure models in engineered cementitious composites with the integration of Yellow River sand. Sci. Eng. Compos. Mater. 2024, 31, 20240017. [Google Scholar] [CrossRef]
- GB/T 50082-2024; Standard for Test Methods of Long-Term Performance and Durability of Concrete. Ministry of Housing and Urban-Rural Development: Beijing, China, 2024.
- Zhang, Y.K.; Raza, A.; Umar, M.; Chen, Y.; Yuan, C.F. Study on Frost Resistance and Interface Bonding Performance Through the Integration of Recycled Brick Powder in Ultra-High-Performance Concrete for Structural Reinforcement. Materials 2023, 16, 6999. [Google Scholar] [CrossRef] [PubMed]
- Razzak, A.; Memon, B.A.; Oad, M.; Raza, A. Effects of height to diameter ratio on compressive strength of recycled aggregate concrete. QUEST Res. J. 2020, 18, 60–65. [Google Scholar] [CrossRef]
- Yuan, C.F.; Zhang, J.J.; Raza, A.; Fu, W.C. Residual and damage properties of recycled brick powder-UHPFRC after high temperature. Proc. Inst. Civ. Eng.-Constr. Mater. 2025, 178, 3–19. [Google Scholar] [CrossRef]
- Huang, B.T.; Wu, J.; Yu, J.; Dai, J.G.; Leung, C.K.; Li, V.C. Seawater sea-sand engineered/strain-hardening cementitious composites (ECC/SHCC): Assessment and modeling of crack characteristics. Cem. Concr. Res. 2021, 140, 106292. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, E.H.; Li, K.P.; Shi, H.W.; Chen, C. Study on the Influence and Mechanism of Steel, Polyvinyl Alcohol, and Polyethylene Fibers on Slag–Yellow River Sediment Geopolymers. Polymers 2025, 17, 1072. [Google Scholar] [CrossRef]
- Ibrahim, M.; Johari, M.A.M.; Rahman, M.K.; Maslehuddin, M.K. Effect of alkaline activators and binder content on the properties of natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2017, 147, 648–660. [Google Scholar] [CrossRef]
- Song, P.P.; Liu, Y.Z.; Kong, L.J.; Tang, Z.Y.; Sun, G.W. Research on design and optimization for compositions of ultra-high-performance geopolymer concrete. J. Build. Eng. 2025, 100, 111750. [Google Scholar] [CrossRef]
- Yu, S.W.; Xia, M.; Sanjayan, J.; Yang, L.; Xiao, J.Z.; Du, H.J. Microstructural characterization of 3D printed concrete. J. Build. Eng. 2021, 44, 102948. [Google Scholar] [CrossRef]
- Huang, H.F.; An, M.Z.; Wang, Y.; Yu, Z.R.; Ji, W.Y. Effect of environmental thermal fatigue on concrete performance based on mesostructural and microstructural analyses. Constr. Build. Mater. 2019, 207, 450–462. [Google Scholar] [CrossRef]
- An, M.Z.; Huang, H.F.; Wang, Y.; Zhao, G.Y. Effect of thermal cycling on the properties of high-performance concrete: Microstructure and mechanism. Constr. Build. Mater. 2020, 243, 118310. [Google Scholar] [CrossRef]





| Minerals | SiO2 | CaO | Al2O3 | Fe2O3 | K2O | TiO2 | MgO | Other |
|---|---|---|---|---|---|---|---|---|
| YRS | 68.64 | 8.40 | 12.33 | 3.25 | 2.55 | 0.74 | 2.05 | 2.04 |
| Slag | 32.47 | 41.06 | 14.52 | 0.28 | 0.44 | 1.25 | 7.08 | 2.9 |
| SF | 98.86 | 0.42 | 0.78 | 0.06 | 0.77 | — | 0.35 | 0.76 |
| MK | 53.57 | — | 44.40 | 0.94 | 0.73 | 0.19 | 0.09 | 0.08 |
| Fiber Type | Diameter (mm) | Density (g/cm−3) | Length (mm) | Tensile Strength (MPa) | Elasticity (GPa) | Elongation (%) |
|---|---|---|---|---|---|---|
| ST | 0.22 | 7.9 | 13 | 2800 | 210 | 5 |
| PVA | 0.04 | 1.3 | 12 | 1560 | 41 | 6.5 |
| SiO2/(%) | Na2O/(%) | H2O/(%) | Density/(g/cm3) | Modulus | Beaume |
|---|---|---|---|---|---|
| 30 | 13.5 | 56.5 | 1.51 | 2.3 | 50 |
| No. | Sand | NaOH | SS | GGBFS | SF | MK | ST | PVA | Water |
|---|---|---|---|---|---|---|---|---|---|
| REF | 1.000 | 0.020 | 0.128 | 0.660 | — | — | — | — | 0.192 |
| SF20 | 1.000 | 0.020 | 0.128 | 0.528 | 0.132 | — | — | — | 0.192 |
| MK20 | 1.000 | 0.020 | 0.128 | 0.528 | — | 0.132 | — | — | 0.192 |
| ST0.5 | 1.000 | 0.020 | 0.128 | 0.660 | — | — | 0.032 | — | 0.192 |
| PVA0.5 | 1.000 | 0.020 | 0.128 | 0.660 | — | — | — | 0.006 | 0.192 |
| Properties | Performance Index | Specimen Size | Quantity |
|---|---|---|---|
| Frost resistance | mass loss rate | 100 mm × 100 mm × 400 mm | 15 |
| dynamic elastic modulus | 100 mm × 100 mm × 400 mm | 15 | |
| compressive strength | 100 mm × 100 mm × 100 mm | 120 | |
| splitting tensile strength | 100 mm × 100 mm × 100 mm | 120 | |
| Characteristic products | Thermogravimetric analysis | 40 mm × 40 mm × 40 mm | 45 |
| X-ray diffraction analysis | 40 mm × 40 mm × 40 mm | 45 | |
| Microstructural properties | porosity | 40 mm × 40 mm × 40 mm | 45 |
| scanning electron microscopy | 40 mm × 40 mm × 40 mm | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Shi, H.; Li, K.; Li, J.; Jiang, E.; Yuan, C.; Chen, C. Study on the Influence and Mechanism of Mineral Admixtures and Fibers on Frost Resistance of Slag–Yellow River Sediment Geopolymers. Nanomaterials 2025, 15, 1051. https://doi.org/10.3390/nano15131051
Zhang G, Shi H, Li K, Li J, Jiang E, Yuan C, Chen C. Study on the Influence and Mechanism of Mineral Admixtures and Fibers on Frost Resistance of Slag–Yellow River Sediment Geopolymers. Nanomaterials. 2025; 15(13):1051. https://doi.org/10.3390/nano15131051
Chicago/Turabian StyleZhang, Ge, Huawei Shi, Kunpeng Li, Jialing Li, Enhui Jiang, Chengfang Yuan, and Chen Chen. 2025. "Study on the Influence and Mechanism of Mineral Admixtures and Fibers on Frost Resistance of Slag–Yellow River Sediment Geopolymers" Nanomaterials 15, no. 13: 1051. https://doi.org/10.3390/nano15131051
APA StyleZhang, G., Shi, H., Li, K., Li, J., Jiang, E., Yuan, C., & Chen, C. (2025). Study on the Influence and Mechanism of Mineral Admixtures and Fibers on Frost Resistance of Slag–Yellow River Sediment Geopolymers. Nanomaterials, 15(13), 1051. https://doi.org/10.3390/nano15131051





