GO/CdS Heterojunctions for Accelerated Photocatalytic Antibiotic Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Samples
2.2.1. Synthesis of GO/CdS Nanocomposites
2.2.2. Synthesis of CdS
2.3. Material Characterization
2.4. Photocatalytic Degradation Experiments
3. Results and Discussion
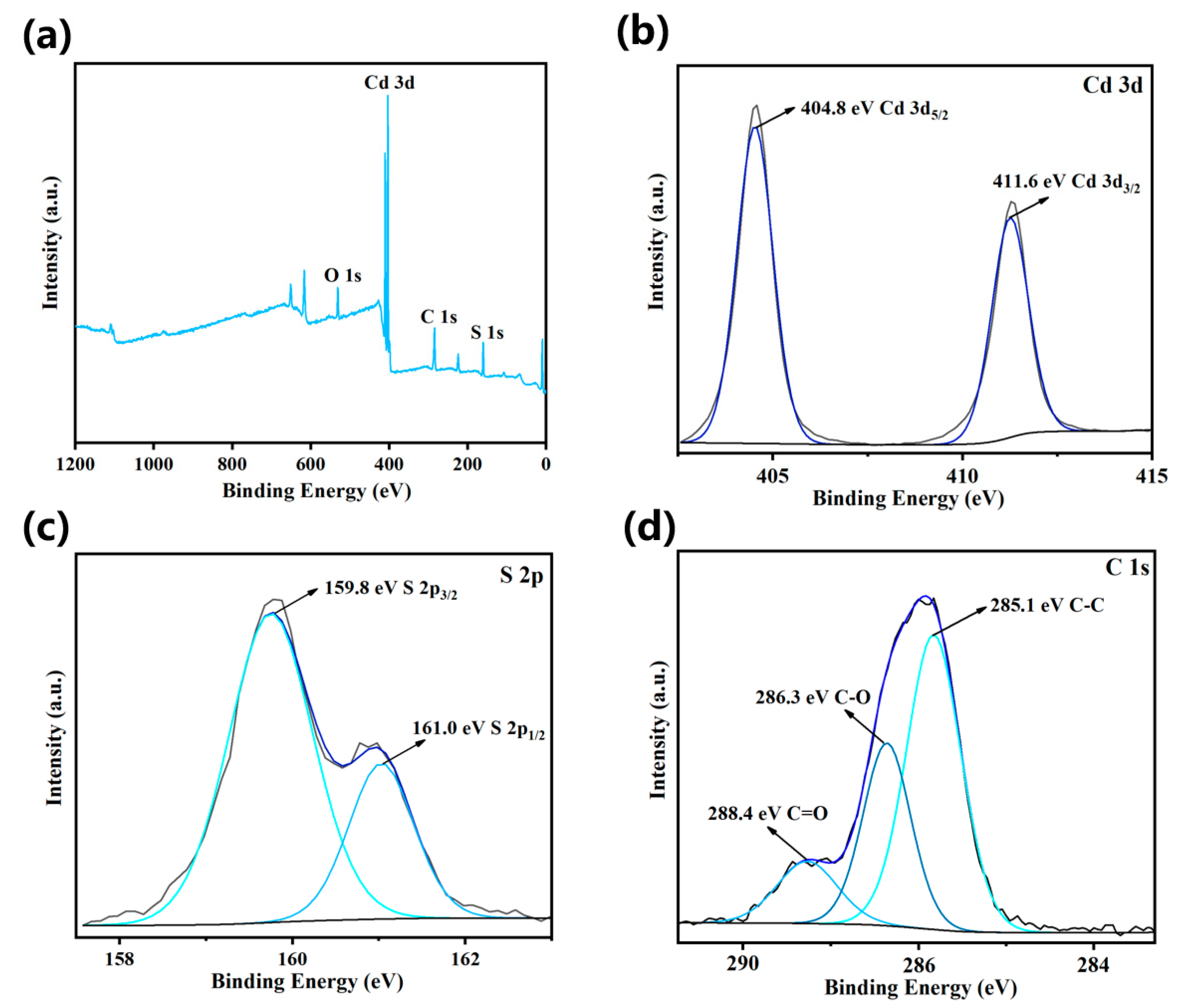
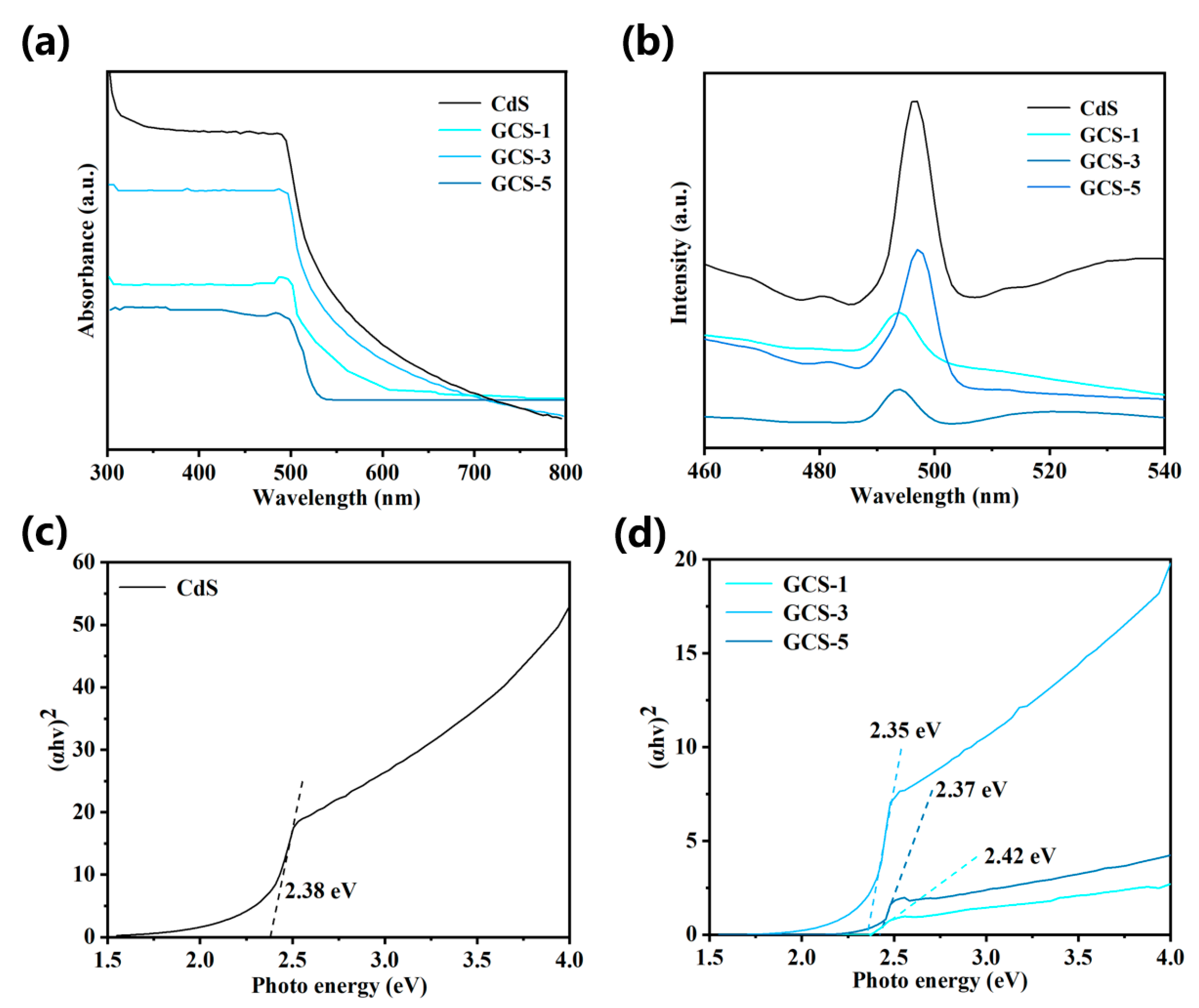
3.1. Characterization Results
3.2. Photocatalytic Degradation Performance
3.3. Photocatalytic Degradation Mechanism
3.3.1. Active Species Identification
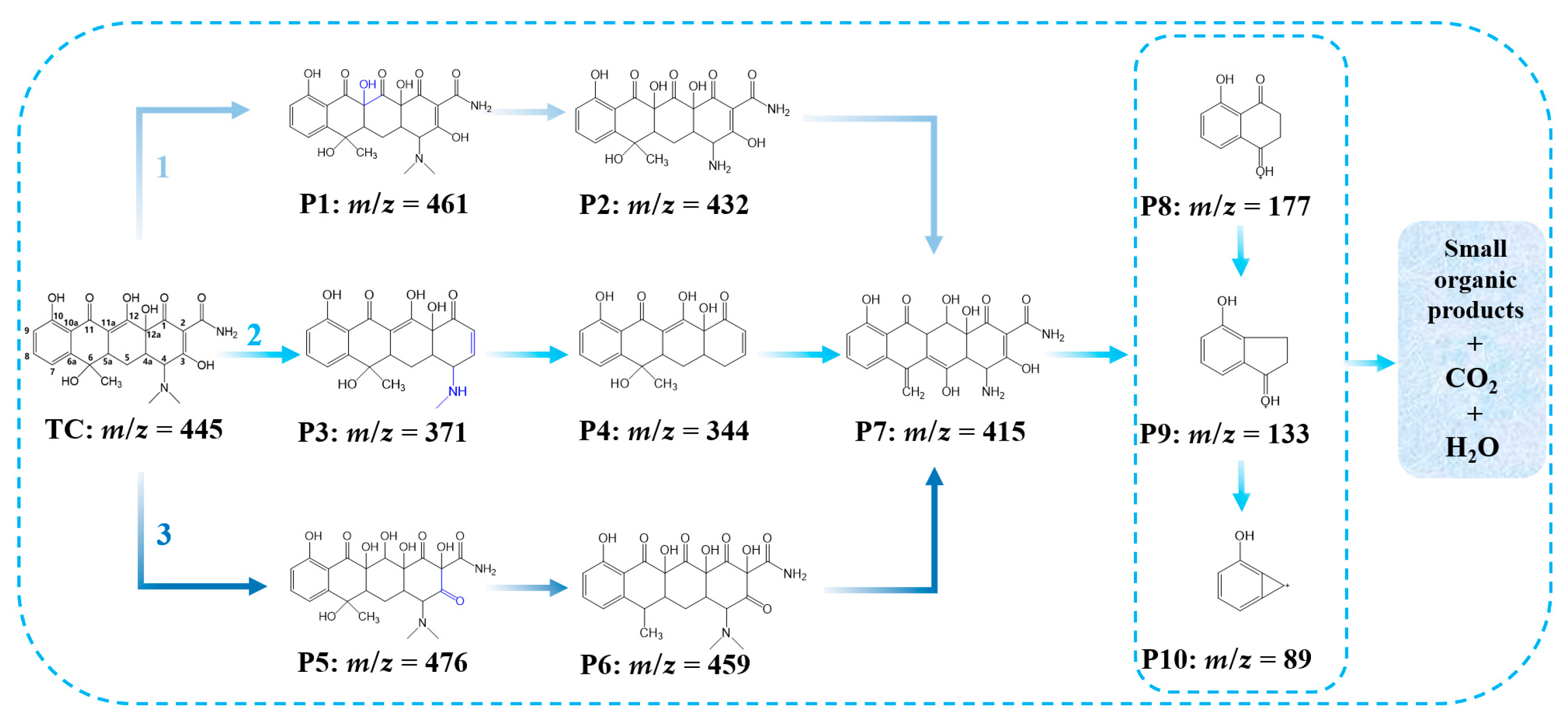
3.3.2. Proposed Degradation Pathways
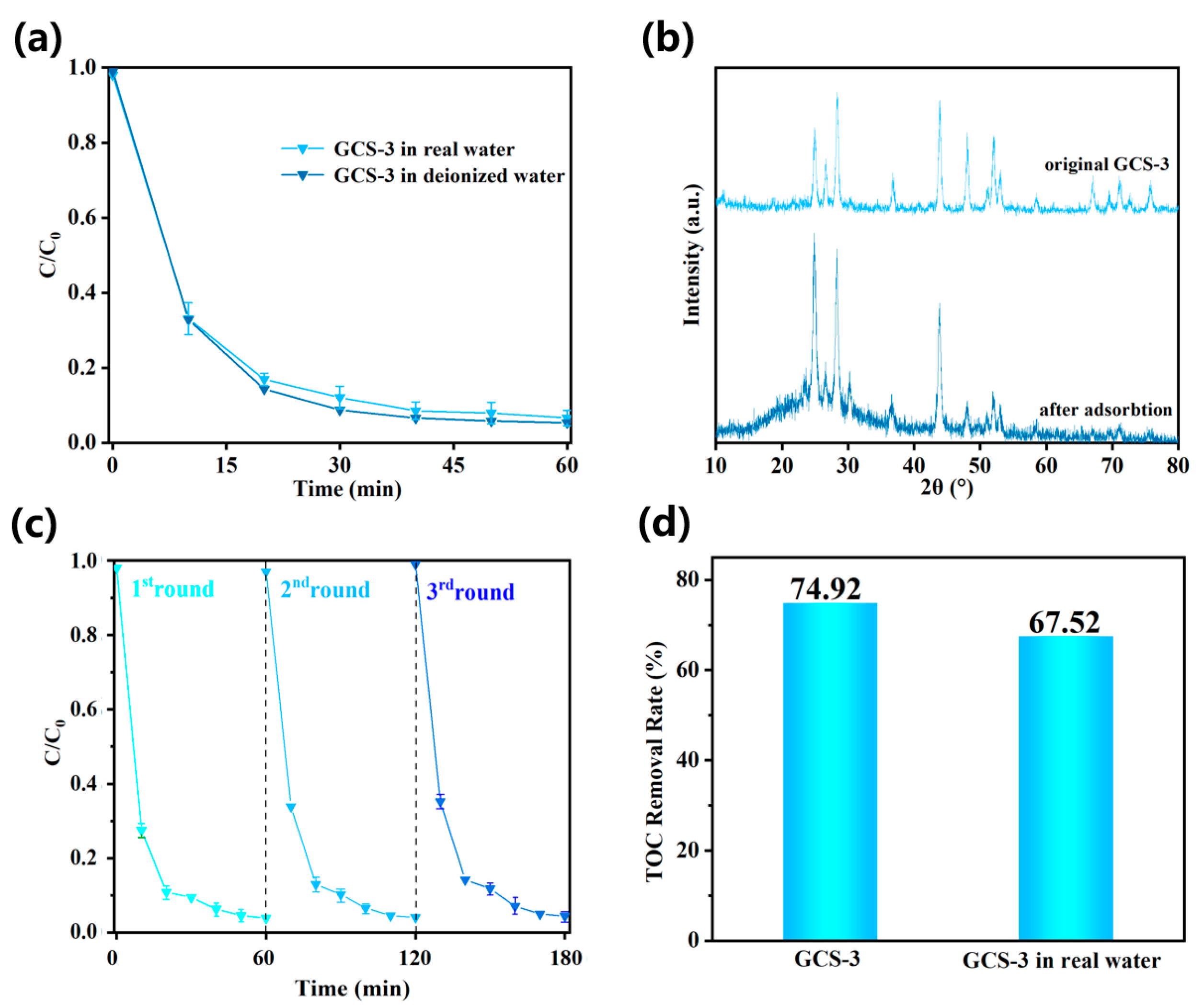
3.4. Photocatalytic Performance in Real Water Systems and Recyclability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; Jery, A.E.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar]
- Li, D.W.; Yu, S.Y.; Geng, H.J.; Zhou, W.; Mu, D.D.; Liu, S.T. The (002) exposing facets of WO3 boosting photocatalytic degradation of nitrobenzene. Appl. Surf. Sci. 2023, 607, 154996. [Google Scholar]
- Galloni, M.G.; Falletta, E.; Mahdi, M.; Cerrato, G.; Giordana, A.; Boffito, D.C.; Bianchi, C.L. An Innovative Sunlight-Driven Device for Photocatalytic Drugs Degradation: From laboratory- to real-Scale Application. A First Step Toward Vulnerable Communities. Adv. Sustain. Syst. 2024, 8, 2300565. [Google Scholar]
- Han, J.; He, S.S.; Lichtfouse, E. Waves of pharmaceutical waste. Environ. Chem. Lett. 2023, 21, 1251–1255. [Google Scholar] [PubMed]
- Velpandian, T.; Halder, N.; Nath, M.; Das, U.; Moksha, L.; Gowtham, L.; Batta, S.P. Un-segregated waste disposal: An alarming threat of antimicrobials in surface and ground water sources in Delhi. Environ. Sci. Pollut. Res. 2018, 25, 29518–29528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, C.S.; Luo, Y.; Lv, J.P.; Zhang, Y.; Lin, H.X.; Wang, L.; Xu, J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016, 213, 833–840. [Google Scholar] [CrossRef]
- Javid, A.; Mesdaghinia, A.; Nasseri, S.; Mahvi, A.H.; Alimohammadi, M.; Gharibi, H. Assessment of tetracycline contamination in surface and groundwater resources proximal to animal farming houses in Tehran, Iran. J. Environ. Health Sci. Eng. 2016, 14, 4. [Google Scholar]
- Liu, S.A.; Zhao, X.R.; Sun, H.Y.; Li, R.P.; Fang, Y.F.; Huang, Y.P. The degradation of tetracycline in a photo-electro-Fenton system. Chem. Eng. J. 2013, 231, 441–448. [Google Scholar]
- Cheng, X.J.; Liao, J.H.; Xue, Y.; Lin, Q.Q.; Yang, Z.M.; Yan, G.L.; Zeng, G.Y.; Sengupta, A. Ultrahigh-flux and self-cleaning composite membrane based on BiOCl-PPy modified MXene nanosheets for contaminants removal from wastewater. J. Membr. Sci. 2022, 644, 120188. [Google Scholar]
- Bembibre, A.; Benamara, M.; Hjiri, M.; Gómez, E.; Alamri, H.R.; Dhahri, R.; Serrà, A. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem. Eng. J. 2022, 427, 132006. [Google Scholar]
- Zhang, N.Q.; Chen, J.Y.; Fang, Z.Q.; Tsang, E.P. Ceria accelerated nanoscale zerovalent iron assisted heterogenous Fenton oxidation of tetracycline. Chem. Eng. J. 2019, 369, 588–599. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Zhu, X.; Wang, J.L. Kinetic, equilibrium, and thermodynamic performance of sulfonamides adsorption onto graphene. Environ. Sci. Pollut. Res. 2018, 25, 36615–36623. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Wang, J.L. Adsorptive removal of pharmaceutical pollutants by defective metal organic framework UiO-66: Insight into the contribution of defects. Chemosphere 2021, 281, 130997. [Google Scholar] [CrossRef]
- Wang, D.B.; Jia, F.Y.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.B.; Zeng, G.M.; Li, X.M.; Yang, Q.; Yuan, X.Z. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, H.L. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Fatima, H.; Azhar, M.R.; Khiadani, M.; Zhong, Y.J.; Wang, W.; Su, C.; Shao, Z.P. Prussian blue-conjugated ZnO nanoparticles for near-infrared light-responsive photocatalysis. Mater. Today Energy 2022, 23, 100895. [Google Scholar]
- Li, J.J.; Li, Y.; Chen, M.H.; Tang, X.; Zhu, N.W.; Li, W.X.; Mei, Q.; Yue, S.J.; Tang, Y.P.; Wang, Q.Z. Construction of polynary systems by coupling Cd/CdS with magnetic recyclable CuO/Fe2O3/CuFe2O4 nanocomposite for enhancing photo-Fenton degradation of antibiotics. J. Environ. Chem. Eng. 2023, 11, 111089. [Google Scholar]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Zeng, F.G.; Chen, H.H.; Mei, Y.C.; Ye, L.B.; Zhuang, S.T.; Pu, N.; Wang, L.M. Performance and mechanism of sulfonamide-antibiotic adsorption by Ti3C2 MXene. New J. Chem. 2024, 48, 16742–16752. [Google Scholar]
- Chava, R.K.; Son, N.; Kang, M. Bismuth quantum dots anchored one-dimensional CdS as plasmonic photocatalyst for pharmaceutical tetracycline hydrochloride pollutant degradation. Chemosphere 2022, 300, 134570. [Google Scholar] [PubMed]
- Zhou, P.P.; Le, Z.G.; Xie, Y.; Fang, J.; Xu, J.W. Studies on facile synthesis and properties of mesoporous CdS/TiO2 composite for photocatalysis applications. J. Alloys Compd. 2017, 692, 170–177. [Google Scholar]
- Dou, M.Y.; Han, S.R.; Du, X.X.; Pang, D.H.; Li, L.L. Well-defined FeP/CdS heterostructure construction with the assistance of amine for the efficient H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2020, 45, 32039–32049. [Google Scholar]
- Lv, N.; Li, Y.Y.; Huang, Z.L.; Li, T.; Ye, S.Y.; Dionysiou, D.D.; Song, X.L. Synthesis of GO/TiO2/Bi2WO6 nanocomposites with enhanced visible light photocatalytic degradation of ethylene. Appl. Catal. B Environ. 2019, 246, 303–311. [Google Scholar]
- Zeng, X.K.; Wang, Z.Y.; Meng, N.; McCarthy, D.T.; Deletic, A.; Pan, J.H.; Zhang, X.W. Highly dispersed TiO2 nanocrystals and carbon dots on reduced graphene oxide: Ternary nanocomposites for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 2017, 202, 33–41. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Baek, K.W.; Paeng, C.G.; Li, L.L.; Cha, G.; Woo, J.; Kim, D.S.; Yim, C.Y.; Park, J.S.; Cho, J.S.; et al. Dual-sensitized hollow SnO2 nanospheres with rGO and Pd for highly sensitive detection of acetone in exhaled breath. Appl. Surf. Sci. 2025, 696, 162959. [Google Scholar]
- Das, D.; Nandi, P. Synthesis of CdS/GO modified ZnO heterostructure for visible light dye degradation applications. Appl. Surf. Sci. 2021, 570, 151260. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Li, M.; Wang, N.; Zhou, Z.M.; Hu, Y.; Fan, D.Y.; Pang, Y.Y.; Liu, Y.P.; Lu, Z.H.; Hai, J.F. Deposition of CdS and ZnS directly on rGO via. an emulsion-solvothermal method for excellent photocatalytic activity and stability. Appl. Surf. Sci. 2023, 612, 155844. [Google Scholar]
- Yang, H.Z.; Wan, Y.Q.; Cheng, Q.R.; Zhou, H.; Pan, Z.Q.; Liu, Y. MOF-derived CdS/CoO S-type heterojunctions for improving the efficiency of photocatalytic evolution. Dalton Trans. 2023, 29, 10013–10022. [Google Scholar] [CrossRef]
- Rahman, M.S.; Suvo, M.A.H.; Islam, M.T.; Noor, A.R.; Yeachin, N.; Bhuiyan, M.A. Fast and efficient removal of metronidazole from aqueous solution using graphene oxide (GO) supported nitrogen (N) doped zinc oxide (ZnO) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 690, 133660. [Google Scholar]
- Nguyen, C.H.; Tran, M.L.; Tran, T.T.V.; Juang, R.S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.Y.; Hou, J.; Li, J.R.; Li, X.B.; Xu, L.K.; Sun, M.X.; Zeng, R.C. Effectively enhanced photocatalytic hydrogen production performance of one-pot synthesized MoS2 clusters/CdS nanorod heterojunction material under visible light. Chem. Eng. J. 2018, 345, 404–413. [Google Scholar] [CrossRef]
- Tang, Y.F.; Liu, X.L.; Ma, C.C.; Zhou, M.J.; Huo, P.W.; Yu, L.B.; Pan, J.M.; Shi, W.D.; Yan, Y.S. Enhanced photocatalytic degradation of tetracycline antibiotics by reduced graphene oxide–CdS/ZnS heterostructure photocatalysts. New J. Chem. 2015, 39, 5150–5160. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhao, C.X.; Zou, Z.Y.; Chen, Z.P.; Tian, L.; Xu, H.T.; Tang, H.; Liu, Q.Q.; Lin, Z.X.; Yang, X.F. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118382. [Google Scholar] [CrossRef]
- Wang, X.W.; Tian, H.W.; Yang, Y.; Wang, H.; Wang, S.M.; Zheng, W.T.; Liu, Y.C. Reduced graphene oxide/CdS for efficiently photocatalystic degradation of methylene blue. J. Alloys Compd. 2012, 524, 5–12. [Google Scholar] [CrossRef]
- Jiang, L.S.; Xie, Y.; He, F.; Ling, Y.; Zhao, J.S.; Ye, H.; Li, S.Q.; Wang, J.L.; Hou, Y. Facile synthesis of GO as middle carrier modified flower-like BiOBr and C3N4 nanosheets for simultaneous treatment of chromium (VI) and tetracycline. Chin. Chem. Lett. 2021, 32, 2187–2191. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Li, M.; Liu, Y.T.; Wang, X.D.; You, D.D. Fabrication of the hierarchical CdS flowers assembled from flakes with (0001) facets exposed as efficient photocatalyst for H2 production. Solid State Sci. 2021, 112, 106514. [Google Scholar] [CrossRef]
- Hassan, A.; Liaquat, R.; Iqbal, N.; Ali, G.; Fan, X.; Hu, Z.L.; Anwar, M.; Ahmad, A. Photo-electrochemical water splitting through graphene-based ZnS composites for H2 production. J. Electroanal. Chem. 2021, 889, 115223. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Visible-light-responsive photocatalyst based on ZnO/CdS nanocomposite for photodegradation of reactive red azo dye and ofloxacin antibiotic. Mater. Sci. Semicond. Process. 2021, 123, 105558. [Google Scholar] [CrossRef]
- Mautschke, H.H.; Drache, F.; Senkovska, I.; Kaskel, S.; Xamena, F.X.L.I. Catalytic properties of pristine and defect-engineered Zr-MOF-808 metal organic frameworks. Catal. Sci. Technol. 2018, 8, 3610–3616. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhuang, Y.B.; Fu, X.Z. New Insight for Enhanced Photocatalytic Activity of TiO2 by Doping Carbon Nanotubes: A Case Study on Degradation of Benzene and Methyl Orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Imboon, T.; Khumphon, J.; Yotkuna, K.; Tang, I.M.; Thongmee, S. Enhancement of photocatalytic by Mn3O4 spinel ferrite decorated graphene oxide nanocomposites. SN Appl. Sci. 2021, 3, 653. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, S.; Baskey (Sen), M. Decorating mechanism of Mn3O4 nanoparticles on reduced graphene oxide surface through reflux condensation method to improve photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 17860–17870. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, L.; Sung, M.Y.; Lin, J.H.; Liu, C.J.; Kuo, C.J.; Cho, E.C.; Lee, K.C. Environment-friendly organic coordination design of Z-scheme heterojunction N-BOB/BiOIO3 for efficient LED-light-driven photocatalytic and electrochemical performance. Chemosphere 2023, 341, 140101. [Google Scholar] [CrossRef]
- Yan, J.J.; Wang, K.; Xu, H.; Qian, J.; Liu, W.; Yang, X.W.; Li, H.M. Visible-light photocatalytic efficiencies and anti-photocorrosion behavior of CdS/graphene nanocomposites: Evaluation using methylene blue degradation. Chin. J. Catal. 2013, 34, 1876–1882. [Google Scholar] [CrossRef]
- Wang, H.J.; Li, J.Z.; Wan, Y.; Nazir, A.; Song, X.H.; Huo, P.W.; Wang, H.Q. Synthesis of AgInS2 QDs-MoS2/GO composite with enhanced interfacial charge separation for efficient photocatalytic degradation of tetracycline and CO2 reduction. J. Alloys Compd. 2023, 954, 170159. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Sonu Raizada, P.; Nguyen, V.H.; Le, Q.V.; Ahamad, T.; Thakur, S.; Hussain, C.M.; Singh, P. Integrating K and P co-doped g-C3N4 with ZnFe2O4 and graphene oxide for S-scheme-based enhanced adsorption coupled photocatalytic real wastewater treatment. Chemosphere 2023, 337, 139267. [Google Scholar] [CrossRef] [PubMed]
- Perumal, K.; Shanavas, S.; Ahamad, T.; Karthigeyan, A.; Murugakoothan, P. Construction of Ag2CO3/BiOBr/CdS ternary composite photocatalyst with improved visible-light photocatalytic activity on tetracycline molecule degradation. J. Environ. Sci. 2023, 125, 47–60. [Google Scholar] [CrossRef]
- Soori, M.M.; Ghahramani, E.; Kazemian, H.; Al-Musawi, T.J.; Zarrabi, M. Intercalation of tetracycline in nano sheet layered double hydroxide: An insight into UV/VIS spectra analysis. J. Taiwan Inst. Chem. Eng. 2016, 63, 271–285. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Geng, J.J.; Wang, X.R.; Gu, X.Y.; Gao, S.X. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, Y.F.; Zhang, L.Y.; Cheng, B.; Yu, J.G.; Fan, J.J. Step-scheme CdS/TiO2 nanocomposite hollow microsphere with enhanced photocatalytic CO2 reduction activity. J. Mater. Sci. Technol. 2020, 56, 143–150. [Google Scholar]
- Liao, M.J.; Zheng, Z.L.; Jiang, H.Y.; Ma, M.Y.; Wang, L.M.; Wang, Y.; Zhuang, S.T. MXenes as emerging adsorbents for removal of environmental pollutants. Sci. Total Environ. 2024, 912, 169014. [Google Scholar]
- Guan, Z.L.; Li, X.M.; Wu, Y.; Chen, Z.; Huang, X.D.; Wang, D.B.; Yang, Q.; Liu, J.L.; Tian, S.H.; Chen, X.Y.; et al. AgBr nanoparticles decorated 2D/2D GO/Bi2WO6 photocatalyst with enhanced photocatalytic performance for the removal of tetracycline hydrochloride. Chem. Eng. J. 2021, 410, 128283. [Google Scholar]
- Firouzi, F.; Pirbazari, A.E.; Saraei, F.E.K.; Yazdi, F.S.T.; Esmaeili, A.; Khodaee, Z. Simultaneous adsorption-photocatalytic degradation of tetracycline by CdS/TiO2 nanosheets/graphene nanocomposites: Experimental study and modeling. J. Environ. Chem. Eng. 2021, 9, 106795. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, Y.Y.; Yin, H.F.; Chen, X.B. Construction of CdS/CdSnO3 direct Z-scheme heterostructure for efficient tetracycline hydrochloride photodegradation. Mater. Sci. Semicond. Process. 2022, 152, 107044. [Google Scholar]
- Du, C.Y.; Zhang, Z.; Tan, S.Y.; Yu, G.L.; Chen, H.; Zhou, L.; Yu, L.; Su, Y.H.; Zhang, Y.; Deng, F.F.; et al. Construction of Z-scheme g-C3N4/MnO2/GO ternary photocatalyst with enhanced photodegradation ability of tetracycline hydrochloride under visible light radiation. Environ. Res. 2021, 200, 111427. [Google Scholar]
- Zeng, Y.; Liu, S.T.; Zhu, G.P.; Yang, X.T.; Yu, H.W. Fe(III)-doped ZnIn2S4-modified graphene oxide in-situ for the degradation of tetracycline hydrochloride in simulated sunlight. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135053. [Google Scholar]
- Liu, C.; Yao, A.R.; Li, W.; Xu, Q.; Yang, L.; Ge, Y.Q.; Lan, J.W.; Lin, S.J.; Qiu, J.H. Design of GO@TiO2 and PDA@CNC decorated gelatin aerogel for efficient adsorption and photocatalytic degradation of organic pollutants. J. Water Process Eng. 2025, 75, 108024. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, F.; Zhu, Z.Y.; Zhan, S.; He, Q.C. Enhanced photocatalytic activities of Ag3PO4/GO in tetracycline degradation. Chem. Phys. Lett. 2019, 72, 90–95. [Google Scholar] [CrossRef]
- Panbude, U.; Palwe, V.; Khairkar, R.V.; Ravi, M.; Nagababu, P. Enhanced antibiotic degradation using rGO composites under visible light photocatalysis. J. Environ. Chem. Eng. 2024, 12, 114547. [Google Scholar] [CrossRef]
- Song, C.J.; Li, X.Y.; Wang, L.P.; Shi, W.D. Fabrication, Characterization and Response Surface Method (RSM) Optimization for Tetracycline Photodegration by Bi3.84W0.16O6.24- graphene oxide (BWO-GO). Sci. Rep. 2016, 6, 37466. [Google Scholar] [CrossRef]
- Huo, P.W.; Zhou, M.J.; Tang, Y.F.; Liu, X.L.; Ma, C.C.; Yu, L.B.; Yan, Y.S. Incorporation of N–ZnO/CdS/Graphene oxide composite photocatalyst for enhanced photocatalytic activity under visible light. J. Alloys Compd. 2016, 670, 198–209. [Google Scholar] [CrossRef]
- Li, K.Q.; Jiang, Y.Q.; Rao, W.; Li, Y.D.; Liu, X.; Zhang, J.; Xu, X.Z.; Lin, K.F. Cooperative coupling strategy for constructing 0D/2D carbon nitride composites with strengthened chemical interaction for enhanced photocatalytic applications. Chem. Eng. J. 2022, 431, 134075. [Google Scholar] [CrossRef]
- Siddhardhan, E.V.; Surender, S.; Arumanayagam, T. Degradation of tetracycline drug in aquatic environment by visible light active CuS/CdS photocatalyst. Inorg. Chem. Commun. 2023, 147, 110244. [Google Scholar] [CrossRef]
- Cao, Y.M.; Yue, L.; Li, Z.X.; Han, Y.H.; Lian, J.; Qin, H.P.; He, S.Y. Construction of Sn-Bi-MOF/Ti3C2 Schottky junction for photocatalysis of tetracycline: Performance and degradation mechanism. Appl. Surf. Sci. 2023, 609, 155191. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.X.; Chen, X.X.; Tian, S.H.; Chen, D.S.; He, C.; Xiong, Y. Efficient mineralization of aqueous antibiotics by simultaneous catalytic ozonation and photocatalysis using MgMnO3 as a bifunctional catalyst. Chem. Eng. J. 2019, 358, 48–57. [Google Scholar] [CrossRef]
- Ji, Y.F.; Shi, Y.Y.; Dong, W.; Wen, X.; Jiang, M.D.; Lu, J.H. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Khan, M.H.; Bae, H.; Jung, J.Y. Tetracycline degradation by ozonation in the aqueous phase: Proposed degradation intermediates and pathway. J. Hazard. Mater. 2010, 181, 659–665. [Google Scholar] [CrossRef]
- Yang, R.X.; Zhu, Z.J.; Hu, C.Y.; Zhong, S.; Zhang, L.S.; Liu, B.J.; Wang, W. One-step preparation (3D/2D/2D) BiVO4/FeVO4@rGO heterojunction composite photocatalyst for the removal of tetracycline and hexavalent chromium ions in water. Chem. Eng. J. 2020, 390, 124522. [Google Scholar]
- Ma, Y.; Gao, N.Y.; Li, C. Degradation and Pathway of Tetracycline Hydrochloride in Aqueous Solution by Potassium Ferrate. Environ. Eng. Sci. 2012, 29, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lahootifar, Z.; Yangjeh, A.H.; Khataee, A. One-pot decoration of CdS and CdMoO4 nanoparticles on g-C3N4 nanoplates: Boosted photocatalytic degradation of tetracycline. J. Alloys Compd. 2023, 969, 172481. [Google Scholar] [CrossRef]
- Li, F.; Qiang, Z.M.; Chen, S.Q.; Wei, J.Y.; Li, T.H.; Zhang, D.B. Synthesis of CdS-loaded (CuC10H26N6)3(PW12O40)2 for enhanced photocatalytic degradation of tetracycline under simulated solar light irradiation. RSC Adv. 2020, 61, 37072–37079. [Google Scholar]
- Zhuang, S.T.; Zhu, X.; Wang, J.L. Adsorptive removal of plasticizer (dimethyl phthalate) and antibiotic (sulfamethazine) from municipal wastewater by magnetic carbon nanotubes. J. Mol. Liq. 2020, 319, 114267. [Google Scholar] [CrossRef]






| Photocatalyst | BET Surface (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| CdS | 5 | 0.026 | 20.40 |
| GCS-3 | 6 | 0.043 | 28.61 |
| Photocatalysts | Photocatalytic Conditions | Degradation Rate (%) | Ref. | ||
|---|---|---|---|---|---|
| C0 (mg L−1) | Dosage of Catalysts (mg) | Time (min) | |||
| GO/CdS | 20 | 10 | 60 | 95 | This work |
| CdS/CdSnO3 | 30 | 20 | 60 | 92 | [56] |
| CMG-10 | 10 | 5 | 60 | 91 | [57] |
| KPCN/GO/ZnFe2O4 | 36 | 60 | 60 | 87 | [47] |
| GZF-25 | 10 | 5 | 60 | 87 | [58] |
| AgBr/GO/Bi2WO6 | 20 | 40 | 60 | 84 | [54] |
| CdS-TNs/rGO | 15 | 75 | 180 | 84 | [55] |
| GTPCG-20 | 20 | 50 | 120 | 82 | [59] |
| rGO–CdS/ZnS | 10 | 50 | 60 | 80 | [27] |
| Ag3PO4/GO | 10 | 25 | 30 | 75 | [60] |
| rGO@PANI-Ca:Mg | 10 | 2 | 60 | 71 | [61] |
| 2 wt% BWO-GO | 10 | 100 | 60 | 63 | [62] |
| N–ZnO/CdS/GO | 20 | 50 | 60 | 4 | [63] |
| The Physicochemical Properties of Fangzhou Lake (Beijing, China) | |
|---|---|
| pH | 7.1 |
| COD (mg L−1) | 17.3 |
| T (°C) | 25.0 |
| TC concentration (mg L−1) | 20.0 |
| Color | Clear and transparent with suspended particles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liu, K.; Zhuang, S.; Mu, Y. GO/CdS Heterojunctions for Accelerated Photocatalytic Antibiotic Degradation. Nanomaterials 2025, 15, 1475. https://doi.org/10.3390/nano15191475
Zhou Y, Liu K, Zhuang S, Mu Y. GO/CdS Heterojunctions for Accelerated Photocatalytic Antibiotic Degradation. Nanomaterials. 2025; 15(19):1475. https://doi.org/10.3390/nano15191475
Chicago/Turabian StyleZhou, Yutao, Kun Liu, Shuting Zhuang, and Yunsong Mu. 2025. "GO/CdS Heterojunctions for Accelerated Photocatalytic Antibiotic Degradation" Nanomaterials 15, no. 19: 1475. https://doi.org/10.3390/nano15191475
APA StyleZhou, Y., Liu, K., Zhuang, S., & Mu, Y. (2025). GO/CdS Heterojunctions for Accelerated Photocatalytic Antibiotic Degradation. Nanomaterials, 15(19), 1475. https://doi.org/10.3390/nano15191475







