Mesoporous Nitrogen-Doped Carbon Support from ZIF-8 for Pt Catalysts in Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mesoporous ZIF-8 Based Nitrogen-Doped Carbon (Meso-ZIF-NC)
2.2. Preparation of Pt/Meso-ZIF-NC
2.3. Characterization of Materials
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Key Components in the Synthesis of Meso-ZIF-NC
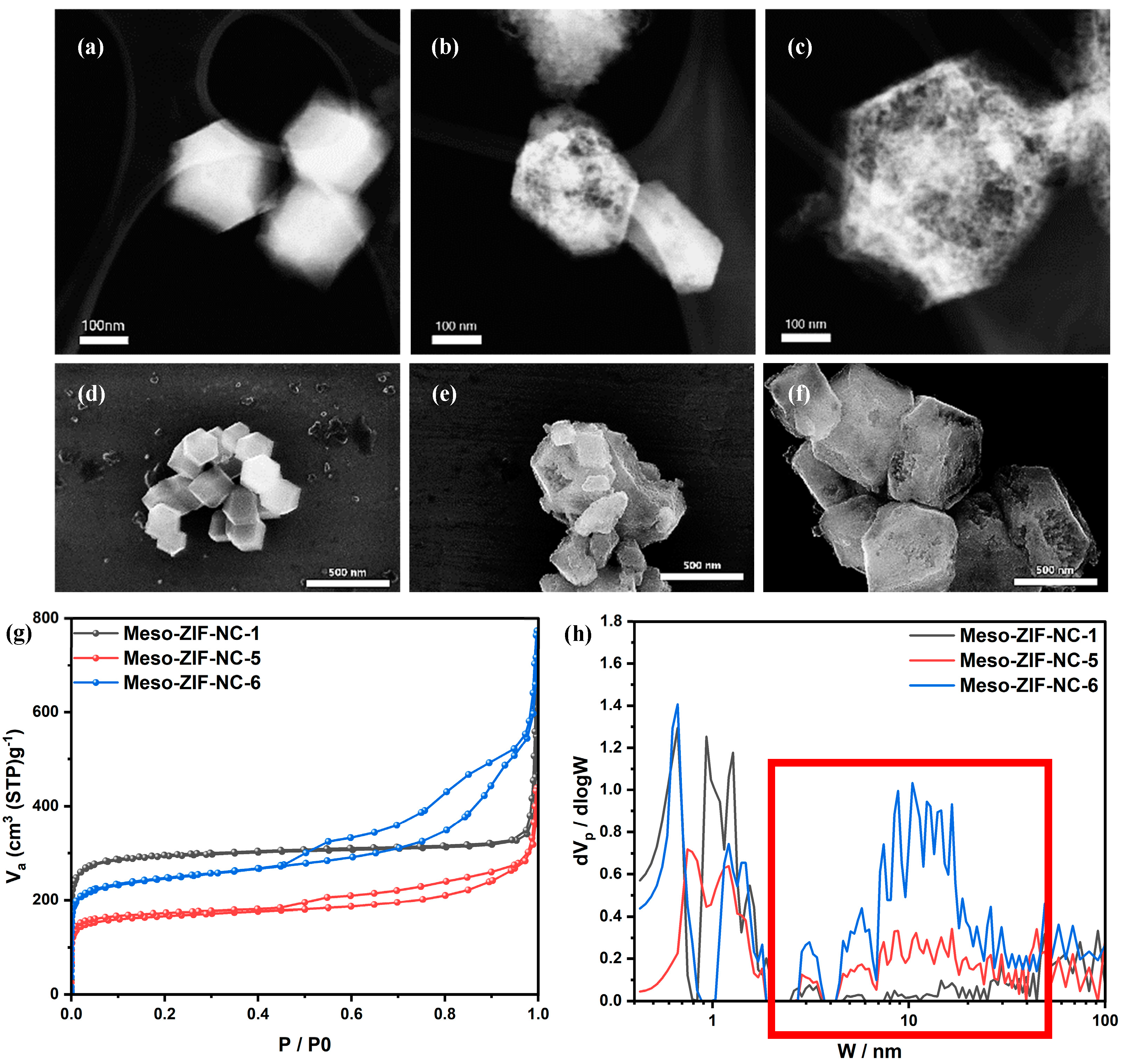
3.2. Materials Characteristics
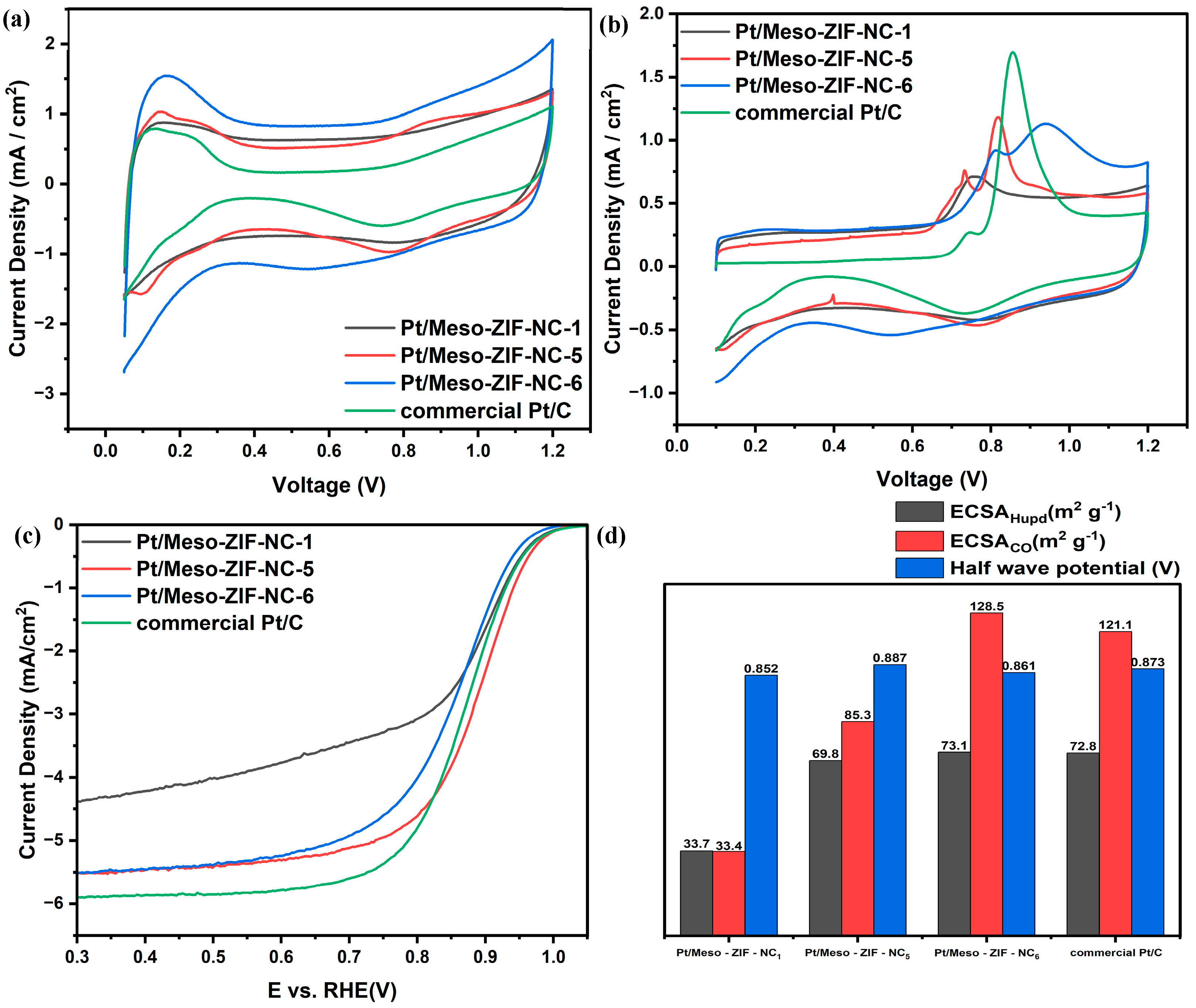
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Sun, Z.; Chen, Z. A systematic review of system modeling and control strategy of proton exchange membrane fuel cell. Energy Rev. 2024, 3, 100054. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Kim, J.G.; Cho, J.; Han, S.; Lee, H.; Yuk, E.; Bae, B.; Jang, S.S.; Pak, C. Boosting activity toward oxygen reduction reaction of a mesoporous FeCuNC catalyst via heteroatom doping-induced electronic state modulation. J. Mater. Chem. A 2022, 10, 5361–5372. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Gittleman, C.S.; Kongkanand, A.; Masten, D.; Gu, W. Materials research and development focus areas for low cost automotive proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2019, 18, 81–89. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.W.; Khan, E.; Yip, A.C.K.; Ng, Y.H.; Rinklebe, J.; Ok, Y.S. Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem. Eng. J. 2020, 401, 126136. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Garcia, M.A.S.; Gothe, M.L.; Galvan, D.; Troise, P.C.; Conte-Junior, C.A.; Vidinha, P.; Camargo, P.H.C.; Rossi, L.M. Recent advances in the use of nitrogen-doped carbon materials for the design of noble metal catalysts. Coord. Chem. Rev. 2023, 481, 215053. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, J.G.; Kim, D.H.; Pak, C. Achieving unprecedented cyclability of flowless zinc-bromine battery by nitrogen-doped mesoporous carbon on thick graphite felt electrode. Chem. Eng. J. 2024, 490, 151538. [Google Scholar] [CrossRef]
- Li, J.; Han, K.; Wang, D.; Teng, Z.; Cao, Y.; Qi, J.; Li, M.; Wang, M. Fabrication of high performance structural N-doped hierarchical porous carbon for supercapacitors. Carbon 2020, 164, 42–50. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kurtyka, K.; Majumder, M.; Yang, X.; Ta, H.Q.; Bachmatiuk, A.; Liu, L.; Trzebicka, B.; Rummeli, M.H. Recent Advances in Boron- and Nitrogen-Doped Carbon-Based Materials and Their Various Applications. Adv. Mater. Interfaces 2022, 9, 2101964. [Google Scholar] [CrossRef]
- Sun, J.-F.; Xu, Q.-Q.; Qi, J.-L.; Zhou, D.; Zhu, H.-Y.; Yin, J.-Z. Isolated Single Atoms Anchored on N-Doped Carbon Materials as a Highly Efficient Catalyst for Electrochemical and Organic Reactions. ACS Sustain. Chem. Eng. 2020, 8, 14630–14656. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Zhao, Y.; Wang, K.; Chao, Y.; Chen, H.; Wang, C.; Wallace, G.G. A Nitrogen-Doped Porous Carbon Supported Copper Catalyst from a Scalable One-Step Method for Efficient Carbon Dioxide Electroreduction. ChemElectroChem 2023, 10, e202200817. [Google Scholar] [CrossRef]
- Agour, A.M.; Elkersh, E.; Khedr, G.E.; El-Aqapa, H.G.; Allam, N.K. Fe-Single-Atom Catalysts on Nitrogen-Doped Carbon Nanosheets for Electrochemical Conversion of Nitrogen to Ammonia. ACS Appl. Nano Mater. 2023, 6, 15980–15989. [Google Scholar] [CrossRef]
- Li, H.-F.; Wu, F.; Wang, C.; Zhang, P.-X.; Hu, H.-Y.; Xie, N.; Pan, M.; Zeng, Z.; Deng, S.; Wu, M.H.; et al. One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials 2018, 8, 700. [Google Scholar] [CrossRef]
- Hammi, N.; Chen, S.; Dumeignil, F.; Royer, S.; El Kadib, A. Chitosan as a sustainable precursor for nitrogen-containing carbon nanomaterials: Synthesis and uses. Mater. Today Sustain. 2020, 10, 100053. [Google Scholar] [CrossRef]
- Lofek, M.; Ryłko, A.; Grzybek, G.; Ejsmont, A.; Darvishzad, T.; Goscianska, J.; Kotarba, A.; Stelmachowski, P. Electrocatalytic activity in the oxygen evolution reaction of nitrogen-doped mesoporous carbon-supported cobalt oxide nanoparticles. Catal. Today 2024, 441, 114878. [Google Scholar] [CrossRef]
- Xi, W.; Shen, M.; Yin, X.; Gao, B.; He, L.; Chen, Y.; Lin, B. Molten-salt confined synthesis of nitrogen-doped carbon nanosheets supported Co3O4 nanoparticles as a superior oxygen electrocatalyst for rechargeable Zn-air battery. J. Power Sources 2023, 560, 232692. [Google Scholar] [CrossRef]
- Zhong, L.; Huang, Q.; Ding, J.; Guo, Y.; Wang, X.; Chai, L.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Abundant Co-Nx sites onto hollow MOF-Derived nitrogen-doped carbon materials for enhanced oxygen reduction. J. Power Sources 2021, 492, 229632. [Google Scholar] [CrossRef]
- Liu, F.; Liu, S.; Meng, J.; Xia, F.; Xiao, Z.; Liu, Z.; Li, Q.; Wu, J.; Mai, L. Stabilizing conversion reaction electrodes by MOF derived N-doped carbon shell for highly reversible lithium storage. Nano Energy 2020, 73, 104758. [Google Scholar] [CrossRef]
- Marpaung, F.; Kim, M.; Khan, J.H.; Konstantinov, K.; Yamauchi, Y.; Hossain, M.S.A.; Na, J.; Kim, J. Metal-Organic Framework (MOF)-Derived Nanoporous Carbon Materials. Chem. Asian J. 2019, 14, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, T.; Xu, J.; Shao, L.; Shi, X.; Sun, Z. Metal-organic frameworks based solid-state electrolytes for lithium metal batteries: Modifications and future prospects. Next Energy 2025, 6, 100191. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Li, Y.; Henzie, J.; Park, T.; Wang, J.; Young, C.; Xie, H.; Yi, J.W.; Li, J.; Kim, M.; Kim, J.; et al. Fabrication of Flexible Microsupercapacitors with Binder-Free ZIF-8 Derived Carbon Films via Electrophoretic Deposition. Bull. Chem. Soc. Jpn. 2019, 93, 176–181. [Google Scholar] [CrossRef]
- Wan, L.; Shamsaei, E.; Easton, C.D.; Yu, D.; Liang, Y.; Chen, X.; Abbasi, Z.; Akbari, A.; Zhang, X.; Wang, H. ZIF-8 derived nitrogen-doped porous carbon/carbon nanotube composite for high-performance supercapacitor. Carbon 2017, 121, 330–336. [Google Scholar] [CrossRef]
- He, H.; Feng, D.; Zhang, X.; Feng, Y. Transport properties of ZIF-8 nanocrystals for hydrogen adsorption: Molecular dynamics study. J. Energy Storage 2023, 72, 108270. [Google Scholar] [CrossRef]
- Chao, S.; Zou, F.; Wan, F.; Dong, X.; Wang, Y.; Wang, Y.; Guan, Q.; Wang, G.; Li, W. Nitrogen-doped Carbon Derived from ZIF-8 as a High-performance Metal-free Catalyst for Acetylene Hydrochlorination. Sci. Rep. 2017, 7, 39789. [Google Scholar] [CrossRef]
- Chen, H.; You, S.; Ma, Y.; Zhang, C.; Jing, B.; Cai, Z.; Tang, B.; Ren, N.; Zou, J. Carbon Thin-Layer-Protected Active Sites for ZIF-8-Derived Nitrogen-Enriched Carbon Frameworks/Expanded Graphite as Metal-Free Catalysts for Oxygen Reduction in Acidic Media. Chem. Mater. 2018, 30, 6014–6025. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, C.; Li, B.; Liang, J.; Zachman, M.J.; Cullen, D.A.; Hermann, R.P.; Alp, E.E.; Lavina, B.; Karakalos, S.; et al. Tuning the thermal activation atmosphere breaks the activity–stability trade-off of Fe–N–C oxygen reduction fuel cell catalysts. Nat. Catal. 2023, 6, 1215–1227. [Google Scholar] [CrossRef]
- Roh, J.; Cho, A.; Kim, S.; Lee, K.-S.; Shin, J.; Choi, J.S.; Bak, J.; Lee, S.; Song, D.; Kim, E.-J.; et al. Transformation of the Active Moiety in Phosphorus-Doped Fe–N–C for Highly Efficient Oxygen Reduction Reaction. ACS Catal. 2023, 13, 9427–9441. [Google Scholar] [CrossRef]
- Yi, S.Y.; Choi, E.; Jang, H.Y.; Lee, S.; Park, J.; Choi, D.; Jang, Y.; Kang, H.; Back, S.; Jang, S.; et al. Insight into Defect Engineering of Atomically Dispersed Iron Electrocatalysts for High-Performance Proton Exchange Membrane Fuel Cell. Adv. Mater. 2023, 35, 2302666. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Kwon, H.C.; Han, M.H.; Oh, H.-S.; Jaouen, F.; Choi, C.H. Single-Site-Level Deciphering of the Complexity of Electrochemical Oxygen Reduction on Fe–N–C Catalysts. ACS Catal. 2024, 14, 8184–8192. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal-organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Liu, F.; Lu, X.; Shi, C.; Sun, Z. Metal-Organic Framework Materials as Bifunctional Electrocatalyst for Rechargeable Zn-Air Batteries. Batter. Supercaps 2024, 7, e202400402. [Google Scholar] [CrossRef]
- Li, J.; You, S.; Liu, M.; Zhang, P.; Dai, Y.; Yu, Y.; Ren, N.; Zou, J. ZIF-8-derived carbon-thin-layer protected WC/W24O68 micro-sized rods with enriched oxygen vacancies as efficient Pt co-catalysts for methanol oxidation and oxygen reduction. Appl. Catal. B. 2020, 265, 118574. [Google Scholar] [CrossRef]
- Zhou, F.; Ruan, Y.; Zhu, M.; Gao, X.; Guo, W.; Liu, X.; Wang, W.; Chen, M.; Wu, G.; Yao, T.; et al. Coupling Single-Atom Sites and Ordered Intermetallic PtM Nanoparticles for Efficient Catalysis in Fuel Cells. Small 2023, 19, 2302328. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Cheng, S.; Li, B.; Zeng, Y.; Xie, Z.; Meng, Q.; Ma, L.; Kisslinger, K.; Tong, X.; et al. Atomic Structure Evolution of Pt–Co Binary Catalysts: Single Metal Sites versus Intermetallic Nanocrystals. Adv. Mater. 2021, 33, 2106371. [Google Scholar] [CrossRef]
- Xu, X.; Dong, X.; Li, D.; Qi, M.; Huang, H. ZIF-8-Derived Three-Dimensional Nitrogen-Doped Porous Carbon as a Pt Catalyst Support for Electrocatalytic Oxidation of Glucose in a Glucose Fuel Cell. ACS Appl. Energy Mater. 2023, 6, 2886–2896. [Google Scholar] [CrossRef]
- Kim, J.G.; Han, S.; Pak, C. Pore Modification and Phosphorus Doping Effect on Phosphoric Acid-Activated Fe-N-C for Alkaline Oxygen Reduction Reaction. Nanomaterials 2021, 11, 1519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Wan, K.; Tan, A.-d.; Yu, Z.-p.; Liang, Z.-x.; Piao, J.-h.; Tsiakaras, P. 2D nitrogen-doped hierarchically porous carbon: Key role of low dimensional structure in favoring electrocatalysis and mass transfer for oxygen reduction reaction. Appl. Catal. B 2017, 209, 447–454. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; Wang, X.; Zhang, Z.; Li, Y.; Qin, Y.; Wang, B.; Han, A.; Liu, J. Hollow mesoporous atomically dispersed metal-nitrogen-carbon catalysts with enhanced diffusion for catalysis involving larger molecules. Nat. Commun. 2022, 13, 2900. [Google Scholar] [CrossRef]
- Kim, M.; Xu, X.; Xin, R.; Earnshaw, J.; Ashok, A.; Kim, J.; Park, T.; Nanjundan, A.K.; El-Said, W.A.; Yi, J.W.; et al. KOH-Activated Hollow ZIF-8 Derived Porous Carbon: Nanoarchitectured Control for Upgraded Capacitive Deionization and Supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 52034–52043. [Google Scholar] [CrossRef]
- Huang, Z.; Rath, J.; Zhou, Q.; Cherevan, A.; Naghdi, S.; Eder, D. Hierarchically Micro- and Mesoporous Zeolitic Imidazolate Frameworks Through Selective Ligand Removal. Small 2024, 20, 2307981. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, J.G.; Han, S.; Cho, Y.; Pak, C. A Gram Scale Soft-Template Synthesis of Heteroatom Doped Nanoporous Hollow Carbon Spheres for Oxygen Reduction Reaction. Nanomaterials 2023, 13, 2555. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, V.; Khosravi, H.S.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; et al. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, G.; Zhang, H.; Zhang, Z.; Liu, Y.; Gao, F.; Shang, Z.; Gao, C.; Zhou, Y.; Fu, S.; et al. Soft template-induced self-assembly strategy for sustainable production of porous carbon spheres as anode towards advanced sodium-ion batteries. Chem. Eng. J. 2024, 495, 153646. [Google Scholar] [CrossRef]
- Hu, F.; Lin, Y.; Qiu, Y.; Wen, B.; Zheng, Y.; Yang, H. Synthesis of mesoporous hollow carbon microcages by combining hard and soft template method for high performance supercapacitors. Ceram. Int. 2021, 47, 5968–5976. [Google Scholar] [CrossRef]
- Yang, J.; Gong, M.; Xia, F.; Tong, Y.; Gu, J. Hofmeister Effect Promoted the Introduction of Tunable Large Mesopores in MOFs at Low Temperature for Femtomolar ALP Detection. Adv. Sci. 2024, 11, 2305786. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, Y.; Yang, J.; Gu, J. Nanoemulsion-directed growth of MOFs with versatile architectures for the heterogeneous regeneration of coenzymes. Nat. Commun. 2022, 13, 1879. [Google Scholar] [CrossRef]
- Wei, W. Hofmeister Effects Shine in Nanoscience. Adv. Sci. 2023, 10, 2302057. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Guo, Y.; Qi, R.; Xu, X.; Jiang, D.; Wang, T.; Sugahara, Y.; He, J.; Yamauchi, Y. Surface curvature effect on single-atom sites for the oxygen reduction reaction: A model of mesoporous MOF-derived carbon. Chem. Eng. J. 2023, 477, 146841. [Google Scholar] [CrossRef]
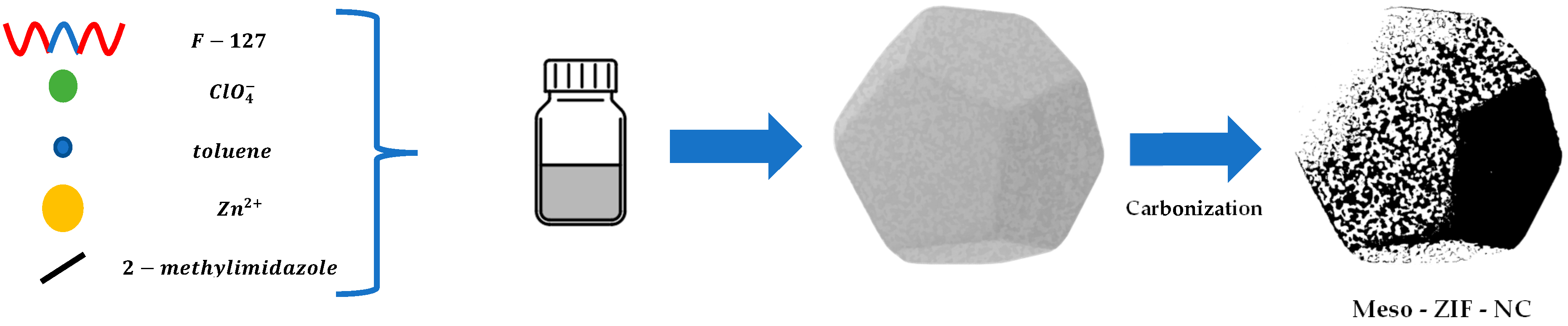


| Sample | F-127 (mol) | NaClO4 (mol) | Toluene (mol) |
|---|---|---|---|
| Meso-ZIF-NC-1 | 0 | 0 | 0 |
| Meso-ZIF-NC-2 | 3.17 × 10−5 | 8.54 × 10−3 | 3.76 × 10−3 |
| Meso-ZIF-NC-3 | 6.34 × 10−5 | 8.54 × 10−3 | 3.76 × 10−3 |
| Meso-ZIF-NC-4 | 3.17 × 10−5 | 0.017 | 3.76 × 10−3 |
| Meso-ZIF-NC-5 | 3.17 × 10−5 | 8.54 × 10−3 | 7.52 × 10−3 |
| Meso-ZIF-NC-6 | 6.34 × 10−5 | 0.017 | 7.52 × 10−3 |
| Sample | SBET a (m2/g) | Vp b (cm3/g) | Vmicro c (cm3/g) | Vmeso d (cm3/g) | Vmacro e (cm3/g) |
|---|---|---|---|---|---|
| Meso-ZIF-NC-1 | 1042 | 0.561 | 0.447 | 0.063 | 0.051 |
| Meso-ZIF-NC-2 | 941 | 0.826 | 0.356 | 0.393 | 0.077 |
| Meso-ZIF-NC-3 | 808 | 0.787 | 0.307 | 0.363 | 0.117 |
| Meso-ZIF-NC-4 | 883 | 0.928 | 0.319 | 0.492 | 0.117 |
| Meso-ZIF-NC-5 | 589 | 0.49 | 0.225 | 0.211 | 0.054 |
| Meso-ZIF-NC-6 | 873 | 0.916 | 0.318 | 0.513 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, J.G.; Cho, Y.; Pak, C. Mesoporous Nitrogen-Doped Carbon Support from ZIF-8 for Pt Catalysts in Oxygen Reduction Reaction. Nanomaterials 2025, 15, 128. https://doi.org/10.3390/nano15020128
Park S, Kim JG, Cho Y, Pak C. Mesoporous Nitrogen-Doped Carbon Support from ZIF-8 for Pt Catalysts in Oxygen Reduction Reaction. Nanomaterials. 2025; 15(2):128. https://doi.org/10.3390/nano15020128
Chicago/Turabian StylePark, Sangyeup, Jong Gyeong Kim, Youngin Cho, and Chanho Pak. 2025. "Mesoporous Nitrogen-Doped Carbon Support from ZIF-8 for Pt Catalysts in Oxygen Reduction Reaction" Nanomaterials 15, no. 2: 128. https://doi.org/10.3390/nano15020128
APA StylePark, S., Kim, J. G., Cho, Y., & Pak, C. (2025). Mesoporous Nitrogen-Doped Carbon Support from ZIF-8 for Pt Catalysts in Oxygen Reduction Reaction. Nanomaterials, 15(2), 128. https://doi.org/10.3390/nano15020128








