Investigating Benzoic Acid Derivatives as Potential Atomic Layer Deposition Inhibitors Using Nanoscale Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Atomic Layer Deposition on Monolayer Substrates
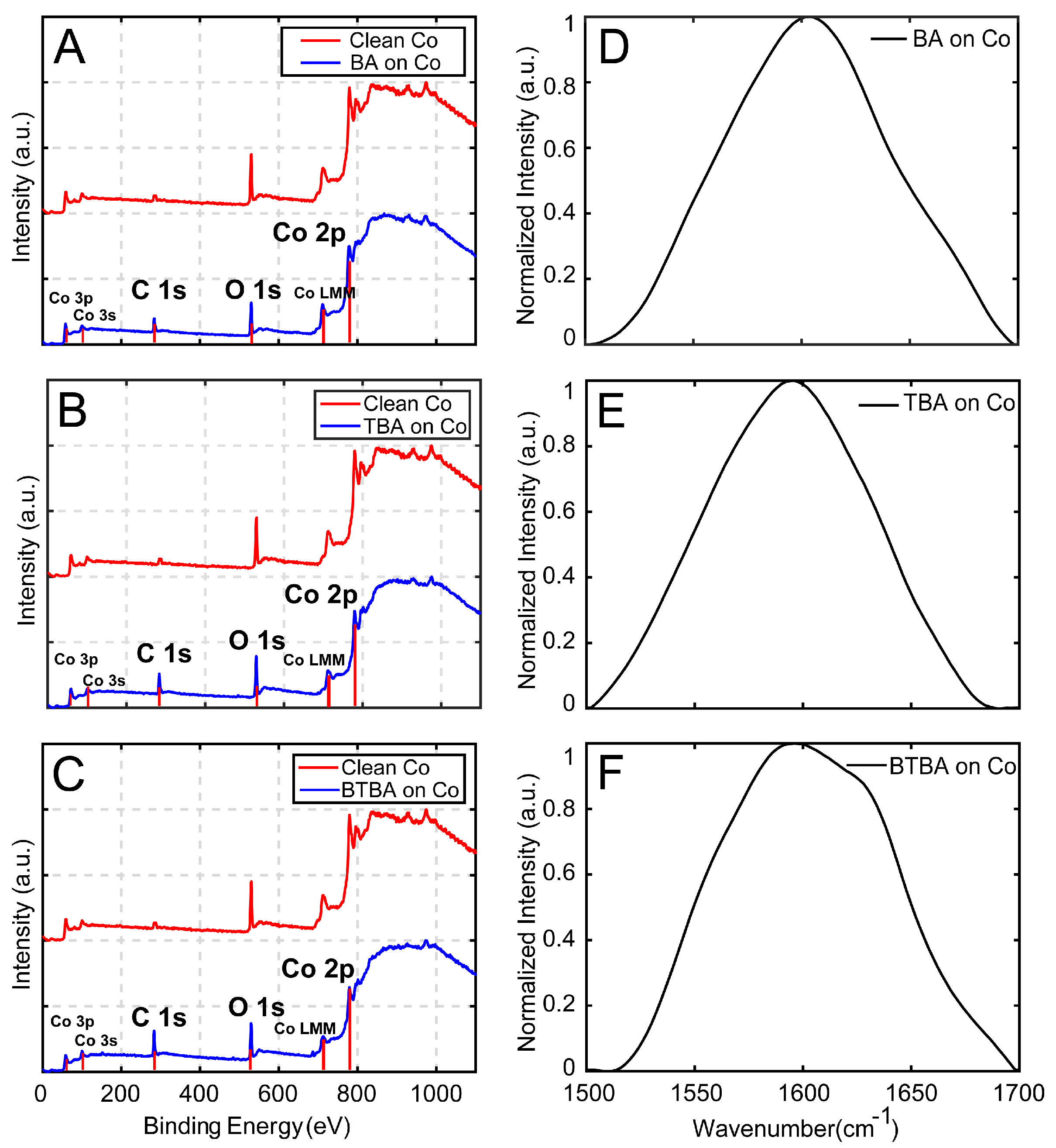
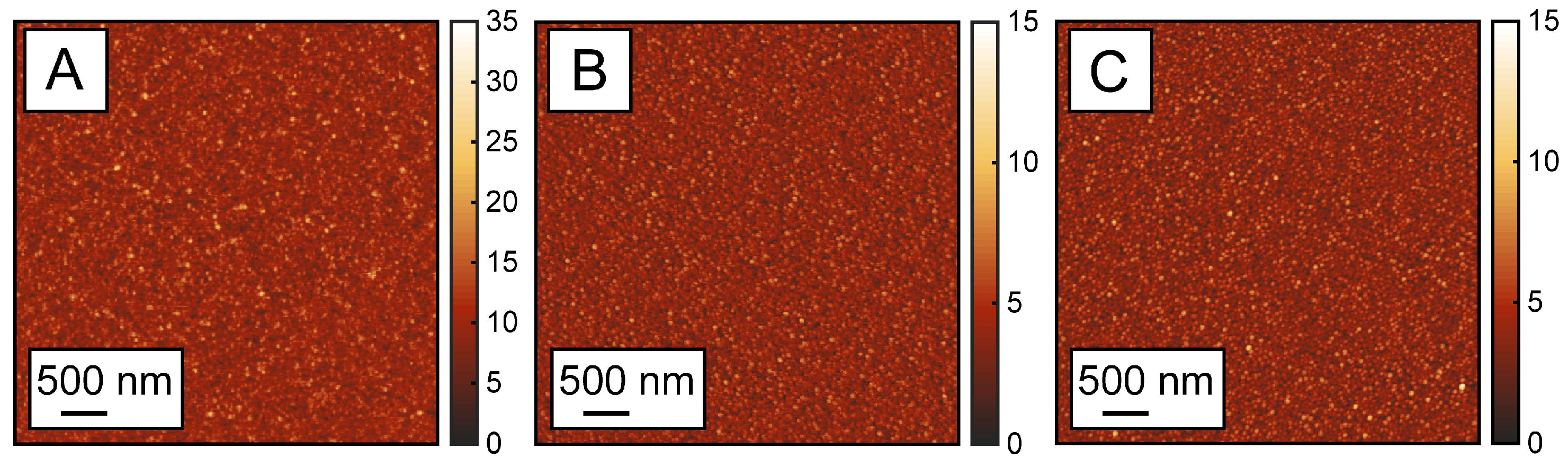
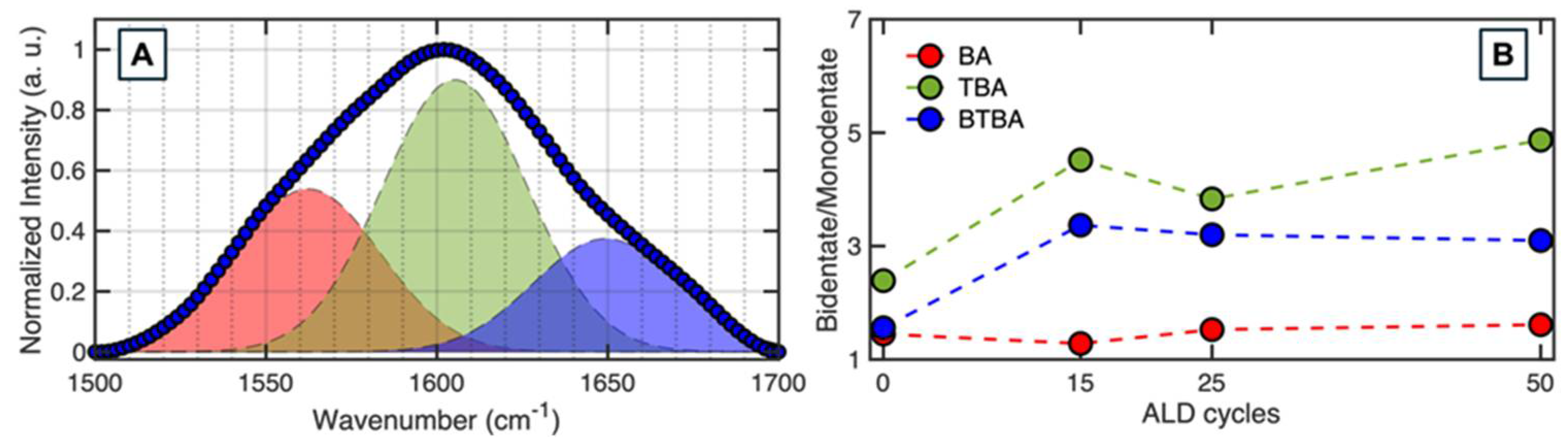
2.4. Characterization of Monolayers
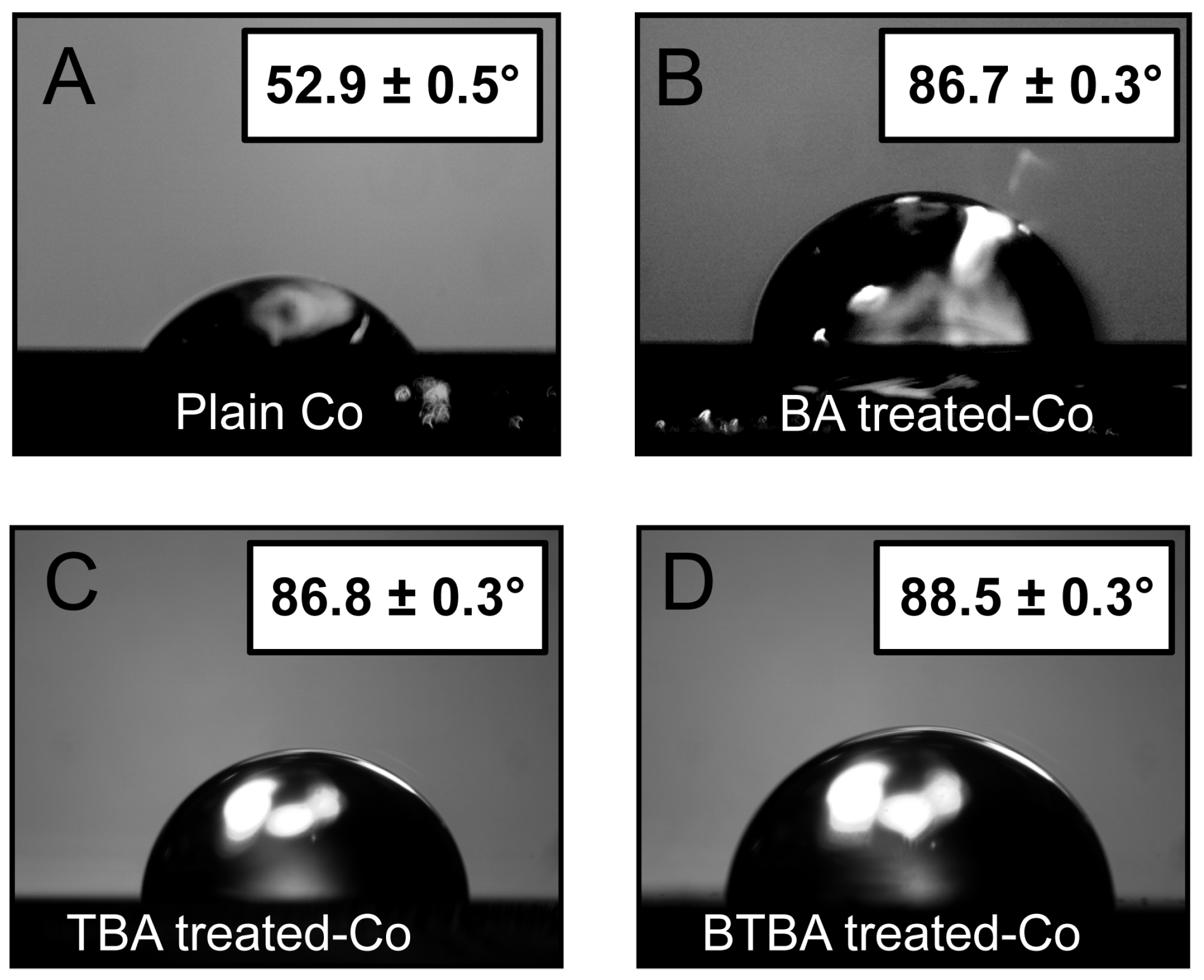
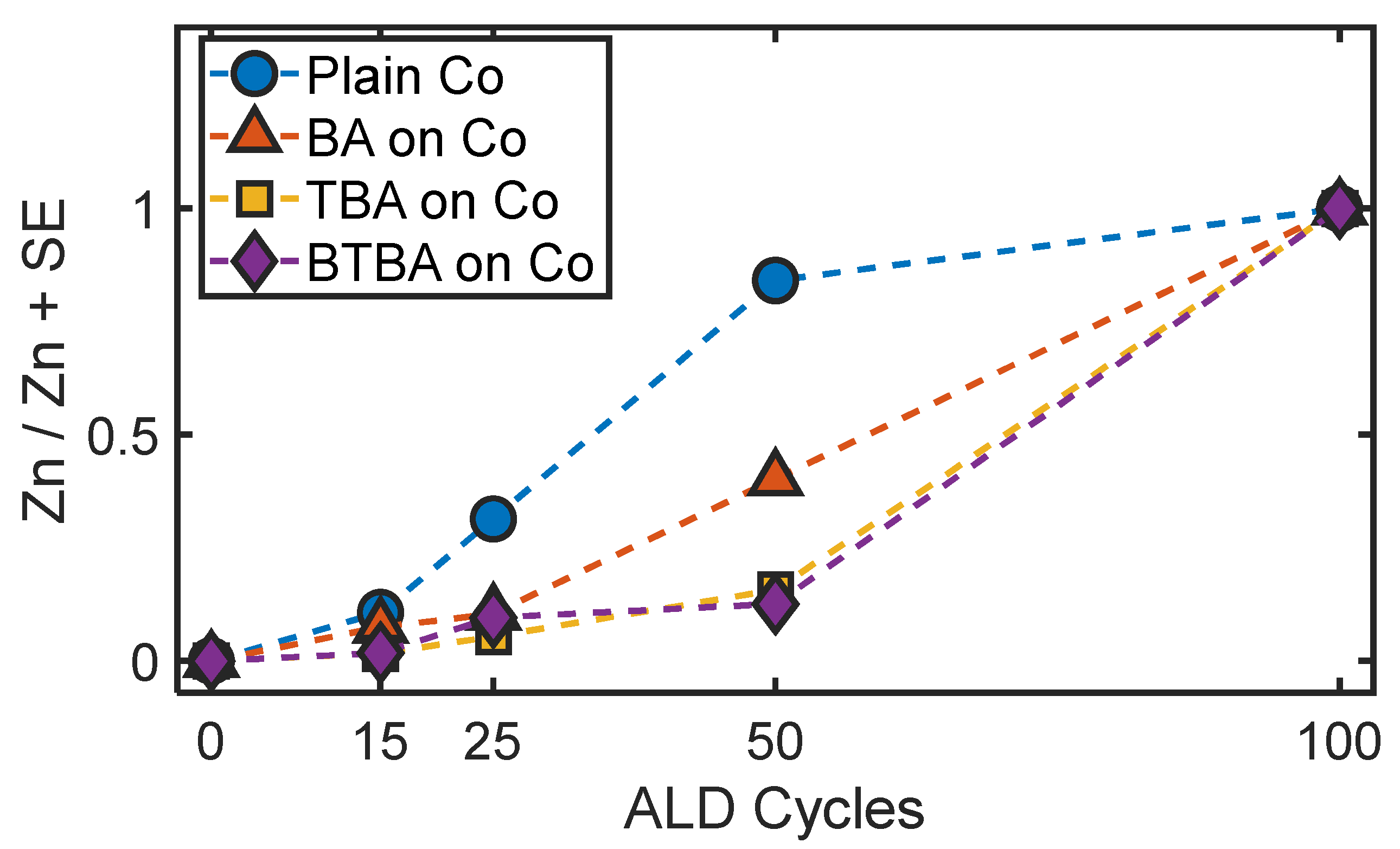
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Mackus, A.J.; Bol, A.A.; Kessels, W.M. The use of atomic layer deposition in advanced nanopatterning. Nanoscale 2014, 6, 10941–10960. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.S.; Chen, L.D.; Thomas, D.F. Vibrational spectrum perturbations of alkanethiol self-assembled monolayers with noble gases and chlorinated. Can. J. Chem. 2024, 102, 682–695. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, K.; Zhang, Y.; Wei, T.; Sun, Y.; Chen, X.; Dai, N. Application of three-dimensionally area-selective atomic layer deposition for selectively coating the vertical surfaces of standing nanopillars. Sci. Rep. 2014, 4, 4458. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, S.M.P.; Tang, E.X.; Moser, H.O.; Breese, M.B.H.; Turaga, S.P.; Kasi, H.; Heussler, S.P. Wafer scale manufacturing of high precision micro-optical components through X-ray lithography yielding 1800 Gray Levels in a fingertip sized chip. Sci. Rep. 2022, 12, 2730. [Google Scholar] [CrossRef] [PubMed]
- Parsons, G.N.; Clark, R.D. Area-Selective Deposition: Fundamentals, Applications, and Future Outlook. Chem. Mater. 2020, 32, 4920–4953. [Google Scholar] [CrossRef]
- Cho, T.H.; Farjam, N.; Allemang, C.R.; Pannier, C.P.; Kazyak, E.; Huber, C.; Rose, M.; Trejo, O.; Peterson, R.L.; Barton, K.; et al. Area-Selective Atomic Layer Deposition Patterned by Electrohydrodynamic Jet Printing for Additive Manufacturing of Functional Materials and Devices. ACS Nano 2020, 14, 17262–17272. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.M.; Lii-Rosales, A.; George, S.M. Thermal Atomic Layer Etching of Aluminum Nitride Using HF orXeF for Fluorination and BCl for Ligand Exchange. J. Phys. Chem. C 2022, 126, 6990–6999. [Google Scholar] [CrossRef]
- Jenkins, A.H.; Dunphy, E.E.; Toney, M.F.; Musgrave, C.B.; Medlin, J.W. Tailoring the Near-Surface Environment of Rh Single-Atom Catalysts for Selective CO Hydrogenation. Acs Catal. 2023, 13, 15340–15350. [Google Scholar] [CrossRef]
- Liu, T.L.; Bent, S.F. Nanostructure fabrication by area selective deposition: A brief review. Mater. Horiz. 2025. advance article. [Google Scholar] [CrossRef]
- Park, H.H.; Fermin, D.J. Recent Developments in Atomic Layer Deposition of Functional Overlayers in Perovskite Solar Cells. Nanomaterials 2023, 13, 3112. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, D.G.; Kim, Y.S.; Kim, M.; Park, J.S. Atomic layer deposition for nanoscale oxide semiconductor thin film transistors: Review and outlook. Int. J. Extrem. Manuf. 2023, 5, 012006. [Google Scholar] [CrossRef]
- Jussila, T.; Philip, A.; Tripathi, T.; Nielsch, K.; Karppinen, M. Atomic layer deposition of magnetic thin films: Basic processes, engineering efforts, and road forward. Appl. Phys. Rev. 2023, 10, 041313. [Google Scholar] [CrossRef]
- Chen, M.; Nijboer, M.P.; Kovalgin, A.Y.; Nijmeijer, A.; Roozeboom, F.; Luiten-Olieman, M.W.J. Atmospheric-pressure atomic layer deposition: Recent applications and new emerging applications in high-porosity/3D materials. Dalton Trans. 2023, 52, 10254–10277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Li, Y.C.; Cao, K.; Chen, R. Advances in Atomic Layer Deposition. Nanomanuf. Metrol. 2022, 5, 191–208. [Google Scholar] [CrossRef]
- Krishtab, M.; Armini, S.; Meersschaut, J.; De Gendt, S.; Ameloot, R. Cyclic Plasma Halogenation of Amorphous Carbon for Defect-Free Area-Selective Atomic Layer Deposition of Titanium Oxide. ACS Appl. Mater. Interfaces 2021, 13, 32381–32392. [Google Scholar] [CrossRef] [PubMed]
- Abd EL-Moaz, Y.; Mohamed, W.; Rifai, M.; Morgan, N.; Ghany, N.A. Rf-Plasma Protective Coating on Silver- Copper Alloys Using Hdmso/O2/Ar Precursors. Egypt. J. Archaeol. Res. 2023, 13, 61–74. [Google Scholar] [CrossRef]
- Kaur, D.; Physics, R.; Vashishtha, P.; Gupta, G.; Sarkar, S.; Kumar, M. Surface Nanopatterning of Amorphous Gallium Oxide Thin Film for Enhanced Solar-blind Photodetection. Nanotechnology 2022, 33, 375302. [Google Scholar] [CrossRef]
- Vazquez, L.; Redondo-Cubero, A.; Lorenz, K.; Palomares, F.J.; Cuerno, R. Surface nanopatterning by ion beam irradiation: Compositional effects. J. Phys. Condens. Matter 2022, 34, 333002. [Google Scholar] [CrossRef] [PubMed]
- Demontis, V.; Zannier, V.; Sorba, L.; Rossella, F. Surface Nano-Patterning for the Bottom-Up Growth of III-V Semiconductor Nanowire Ordered Arrays. Nanomaterials 2021, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Klement, P.; Anders, D.; Gumbel, L.; Bastianello, M.; Michel, F.; Schormann, J.; Elm, M.T.; Heiliger, C.; Chatterjee, S. Surface Diffusion Control Enables Tailored-Aspect-Ratio Nanostructures in Area-Selective Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2021, 13, 19398–19405. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Shu, Z.W.; Zhang, S.; Zeng, P.; Liang, H.K.; Zheng, M.J.; Duan, H.G. Sub-10 nm fabrication: Methods and applications. Int. J. Extrem. Manuf. 2021, 3, 032002. [Google Scholar] [CrossRef]
- Du, K.; Jiang, Y.H.; Huang, P.S.; Ding, J.J.; Gao, T.C.; Choi, C.H. Self-formation of polymer nanostructures in plasma etching: Mechanisms and applications. J. Micromech. Microeng. 2018, 28, 014006. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, L.; Cullen, D.A.; Aireddy, D.R.; Ding, K. Construction of Inverse Metal-Zeolite Interfaces via Area-Selective Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2021, 13, 51759–51766. [Google Scholar] [CrossRef]
- Merkx, M.J.M.; Angelidis, A.; Mameli, A.; Li, J.; Lemaire, P.C.; Sharma, K.; Hausmann, D.M.; Kessels, W.M.M.; Sandoval, T.E.; Mackus, A.J.M. Relation between Reactive Surface Sites and Precursor Choice for Area-Selective Atomic Layer Deposition Using Small Molecule Inhibitors. J. Phys. Chem. C 2022, 126, 4845–4853. [Google Scholar] [CrossRef] [PubMed]
- Pavel, E.; Marinescu, V.; Lungulescu, M. Nanopatterning of monolayer graphene by quantum optical lithography. Appl. Opt. 2021, 60, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Cao, K.; Lan, Y.X.; Zhang, J.M.; Gong, M.; Wen, Y.W.; Shan, B.; Chen, R. Inherently Area-Selective Atomic Layer Deposition of Manganese Oxide through Electronegativity-Induced Adsorption. Molecules 2021, 26, 3056. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Y.; Lan, E.; Banatao, D.R.; Forse, G.J.; Lu, J.; Blom, H.O.; Yeates, T.O.; Dunn, B.; Chang, J.P. Generation of oxide nanopatterns by combining self-assembly of S-layer proteins and area-selective atomic layer deposition. J. Am. Chem. Soc. 2008, 130, 16908–16913. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Haeve, M.G.N.; Lemaire, P.C.; Sharma, K.; Hausmann, D.M.; Agarwal, S. Functionalization of the SiO(2) Surface with Aminosilanes to Enable Area-Selective Atomic Layer Deposition of Al(2)O(3). Langmuir 2022, 38, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Baek, G.; Kim, H.M.; Kim, Y.H.; Park, J.S. Facile rearrangement of molecular layer deposited metalcone thin films by electron beam irradiation for area selective atomic layer deposition. Dalton Trans. 2021, 50, 9958–9967. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; He, W.; Spampinato, V.; Franquet, A.; Sergeant, S.; Gendt, S.; Armini, S. Area-Selective Atomic Layer Deposition of TiN Using Trimethoxy(octadecyl)silane as a Passivation Layer. Langmuir 2020, 36, 13144–13154. [Google Scholar] [CrossRef]
- Mameli, A.; Merkx, M.J.M.; Karasulu, B.; Roozeboom, F.; Kessels, W.; Mackus, A.J.M. Area-Selective Atomic Layer Deposition of SiO(2) Using Acetylacetone as a Chemoselective Inhibitor in an ABC-Type Cycle. ACS Nano 2017, 11, 9303–9311. [Google Scholar] [CrossRef] [PubMed]
- Junige, M.; Loffler, M.; Geidel, M.; Albert, M.; Bartha, J.W.; Zschech, E.; Rellinghaus, B.; Dorp, W.F.V. Area-selective atomic layer deposition of Ru on electron-beam-written Pt(C) patterns versus SiO(2) substratum. Nanotechnology 2017, 28, 395301. [Google Scholar] [CrossRef]
- Hui, L.; Nixon, R.; Tolman, N.; Mukai, J.; Bai, R.; Wang, R.; Liu, H. Area-Selective Atomic Layer Deposition of Metal Oxides on DNA Nanostructures and Its Applications. ACS Nano 2020, 14, 13047–13055. [Google Scholar] [CrossRef]
- Hashemi, F.S.M.; Bent, S.F. Sequential Regeneration of Self-Assembled Monolayers for Highly Selective Atomic Layer Deposition. Adv. Mater. Interfaces 2016, 3, 1600464. [Google Scholar] [CrossRef]
- Hashemi, F.S.M.; Prasittichai, C.; Bent, S.F. Self-Correcting Process for High Quality Patterning by Atomic Layer Deposition. ACS Nano 2015, 9, 8710–8717. [Google Scholar] [CrossRef]
- Hashemi, F.S.M.; Birchansky, B.R.; Bent, S.F. Selective Deposition of Dielectrics: Limits and Advantages of Alkanethiol Blocking Agents on Metal-Dielectric Patterns. Acs Appl. Mater. Interfaces 2016, 8, 33264–33272. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Yeo, B.C.; Han, S.S.; Yoon, C.M.; Yang, J.Y.; Yoon, J.; Yoo, C.; Kim, H.J.; Lee, Y.B.; Lee, S.J.; et al. Reaction Mechanism of Area-Selective Atomic Layer Deposition for Al2O3 Nanopatterns. Acs Appl. Mater. Interfaces 2017, 9, 41607–41617. [Google Scholar] [CrossRef]
- Minjauw, M.M.; Rijckaert, H.; Van Driessche, I.; Detavemier, C.; Dendooven, J. Nucleation Enhancement and Area-Selective Atomic Layer Deposition of Ruthenium Using RuO4 and H2 Gas. Chem. Mater. 2019, 31, 1491–1499. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Merkx, M.J.M.; Kessels, W.M.M. From the Bottom-Up: Toward Area-Selective Atomic Layer Deposition with High Selectivity. Chem. Mater. 2019, 31, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Zeng, L.; Nardi, K.L.; Hausmann, D.M.; Bent, S.F. Characterizing Self-Assembled Monolayer Breakdown in Area-Selective Atomic Layer Deposition. Langmuir 2021, 37, 11637–11645. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.; Pieck, F.; Shearer, A.B.; Maue, P.; Tonner-Zech, R.; Bent, S.F. Area-Selective Atomic Layer Deposition of Al2O3 with a Methanesulfonic Acid Inhibitor. Chem. Mater. 2023, 35, 5963–5974. [Google Scholar] [CrossRef]
- Fickenscher, G.; Fromm, L.; Görling, A.; Libuda, J. Atomic Layer Deposition of HfS2 on Oxide Interfaces: A Model Study on the Initial Nucleation Processes. J. Phys. Chem. C 2022, 126, 21596–21605. [Google Scholar] [CrossRef]
- Clerix, J.W.J.; Dianat, G.; Delabie, A.; Parsons, G.N. In situ analysis of nucleation reactions during TiCl4/H2O atomic layer deposition on SiO2 and H-terminated Si surfaces treated with a silane small molecule inhibitor. J. Vac. Sci. Technol. A 2023, 41, 032406. [Google Scholar] [CrossRef]
- Hofmaier, M.; Flemming, P.; Guskova, O.; Münch, A.S.; Uhlmann, P.; Müller, M. Swelling and Orientation Behavior of End-Grafted Polymer Chains by In Situ Attenuated Total Reflection Fourier Transform Infrared Spectroscopy Complementing Ellipsometry. Langmuir 2023, 39, 16219–16230. [Google Scholar] [CrossRef]
- Foadi, F.; Etminan, M.; Aghamir, F.M.; Mohammadizadeh, M.R. Role of surface morphological parameters on wettability of obliquely deposited Cu thin films in a plasma focus device. J. Mater. Res. 2023, 38, 3666–3676. [Google Scholar] [CrossRef]
- Alkarsifi, R.; Buffeteau, T.; Labrugère-Sarroste, C.; Hirsch, L.; Bassani, D.M.; Toupance, T. Optimization of fluorinated interfacial layers with minimal surface coverage for hybrid perovskite materials. J. Mater. Chem. C 2023, 11, 16056–16065. [Google Scholar] [CrossRef]
- Bracesco, A.E.A.; Jansen, J.W.P.; Xue, H.B.; Zardetto, V.; Brocks, G.; Kessels, W.M.M.; Tao, S.X.; Creatore, M. In Situ IR Spectroscopy Studies of Atomic Layer-Deposited SnO on Formamidinium-Based Lead Halide Perovskite. Acs Appl. Mater. Interfaces 2023, 15, 38018–38028. [Google Scholar] [CrossRef]
- Azzam, W.; Subaihi, A.; Al-Rawashdeh, N.A.F.; Al-Nawaiseh, A. Imaging of Terphenyldithiol Molecules in Highly Orientated and Ordered Aromatic Dithiol SAMs on Au(111). J. Phys. Chem. C 2024, 128, 4093–4103. [Google Scholar] [CrossRef]
- Huang, C.P.; Guan, C.K.; Lin, B.Q.; Huang, S.H.; Huang, B.J.; Su, W.F.; Xu, L. Effect of Laser Scribing on Coating, Drying, and Crystallization of Absorber Layer of Perovskite Solar Cells. Sol. RRL 2023, 7, 2200945. [Google Scholar] [CrossRef]
- McCormick, J.A.; Cloutier, B.L.; Weimer, A.W.; George, S.M. Rotary reactor for atomic layer deposition on large quantities of nanoparticles. J. Vac. Sci. Technol. A 2007, 25, 67–74. [Google Scholar] [CrossRef]
- Mameli, A.; Verheijen, M.A.; Mackus, A.J.M.; Kessels, W.M.M.; Roozeboom, F. Isotropic Atomic Layer Etching of ZnO Using Acetylacetone and O2 Plasma. Acs Appl. Mater. Interfaces 2018, 10, 38588–38595. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.F.; Mattson, E.C.; Nanayakkara, C.E.; Oyekan, K.A.; Mallikarjunan, A.; Chandra, H.; Xiao, M.C.; Lei, X.J.; Pearlstein, R.M.; Derecskei-Kovacs, A.; et al. In Situ Infrared Absorption Study of Plasma-Enhanced Atomic Layer Deposition of Silicon Nitride. Langmuir 2018, 34, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Merkx, M.J.M.; Tezsevin, I.; Yu, P.M.; Janssen, T.; Heinemans, R.H.G.M.; Lengers, R.J.; Chen, J.R.; Jezewski, C.J.; Clendenning, S.B.; Kessels, W.M.M.; et al. In situ formation of inhibitor species through catalytic surface reactions during area-selective atomic layer deposition of TaN. J. Chem. Phys. 2024, 160, 204701. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.X.; Gasvoda, R.J.; Lemaire, P.C.; Sharma, K.; Hausmann, D.M.; Agarwal, S. Area-selective atomic layer deposition of Al2O3 on SiNx with SiO2 as the nongrowth surface. J. Vac. Sci. Technol. A 2022, 40, 012403. [Google Scholar] [CrossRef]
- Satyarthy, S.; Ul Iqbal, M.H.; Abida, F.; Nahar, R.; Hauser, A.J.; Cheng, M.M.C.; Ghosh, A. Stearic Acid as an Atomic Layer Deposition Inhibitor: Spectroscopic Insights from AFM-IR. Nanomaterials 2023, 13, 2713. [Google Scholar] [CrossRef] [PubMed]
- Soulié, J.P.; Sankaran, K.; Van Troeye, B.; Lesniewska, A.; Pedreira, O.V.; Oprins, H.; Delie, G.; Fleischmann, C.; Boakes, L.; Rolin, C.; et al. Selecting alternative metals for advanced interconnects. J. Appl. Phys. 2024, 136, 171101. [Google Scholar] [CrossRef]
- Pedreira, O.V.; Croes, K.; Leśniewska, A.; Wu, C.; Van Der Veen, M.H.; De Messemaeker, J.; Vandersmissen, K.; Jourdan, N.; Wen, L.G.; Adelmann, C.; et al. Reliability Study on Cobalt and Ruthenium as Alternative Metals for Advanced Interconnects. In Proceedings of the 2017 IEEE International Reliability Physics Symposium (IRPS), Monterey, CA, USA, 2–6 April 2017; pp. 6B-2.1–6B-2.8. [Google Scholar]
- Kim, C.; Kang, G.; Jung, Y.; Kim, J.Y.; Lee, G.B.; Hong, D.; Lee, Y.; Hwang, S.G.; Jung, I.H.; Joo, Y.C. Robust Co alloy design for Co interconnects using a self-forming barrier layer. Sci. Rep. 2022, 12, 12291. [Google Scholar] [CrossRef]
- Jung, H.K.; Lee, H.B.; Tsukasa, M.; Jung, E.; Yun, J.H.; Lee, J.M.; Choi, G.H.; Choi, S.; Chung, C. Formation of highly reliable Cu low-k interconnects by using CVD Co barrier in dual damascene structures. In Proceedings of the 2011 International Reliability Physics Symposium, Monterey, CA, USA, 10–14 April 2011; pp. 3E.2.1–3E.2.5. [Google Scholar]
- Hai, Z.Y.; Gao, L.B.; Zhang, Q.; Xu, H.Y.; Cui, D.F.; Zhang, Z.X.; Tsoukalas, D.; Tang, J.; Yan, S.B.; Xue, C.Y. Facile synthesis of core shell structured PANI-CoO nanocomposites with superior electrochemical performance in supercapacitors. Appl. Surf. Sci. 2016, 361, 57–62. [Google Scholar] [CrossRef]
- Grafia, A.L.; Barbosa, S.E. Polyethylene Film Surface Modification via Benzoic Acid Grafting. Polymers 2024, 16, 1291. [Google Scholar] [CrossRef]
- Li, J.; Niu, J.J.; Kim, Y.; Chae, H. Surface wettability control and fluorination modeling of amorphous carbon films fluorinated with CF4 plasma. Appl. Surf. Sci. 2023, 635, 157668. [Google Scholar] [CrossRef]
- Zhong, Q.F.; Gao, B.B.; Weng, Y.X.; Zhang, S.D. Adopting Intrinsic Hydrophilic Thermoplastic Starch Composites to Fabricate Antifogging Sustainable Films with High Antibiosis and Transparency. Acs Sustain. Chem. Eng. 2022, 10, 3661–3672. [Google Scholar] [CrossRef]
- Ding, X.; Wang, H.; Miao, Y.; Chen, C.; Zhai, M.; Yang, C.; Wang, B.; Tian, Y.; Cheng, M. Bi(trifluoromethyl) Benzoic Acid-Assisted Shallow Defect Passivation for Perovskite Solar Cells with an Efficiency Exceeding 21. ACS Appl. Mater. Interfaces 2022, 14, 3930–3938. [Google Scholar] [CrossRef]
- Mertens, M.; Mohr, M.; Brühne, K.; Fecht, H.J.; Lojkowski, M.; Swieszkowski, W.; Lojkowski, W. Patterned hydrophobic and hydrophilic surfaces of ultra-smooth nanocrystalline diamond layers. Appl. Surf. Sci. 2016, 390, 526–530. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Manhas, B.S.; Trikha, A.K. Relationships between the Direction of Shifts in the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. J. Indian Chem. Soc. 1982, 59, 315–319. [Google Scholar]
- Malinsky, M.D.; Kelly, K.L.; Schatz, G.C.; Van Duyne, R.P. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J. Am. Chem. Soc. 2001, 123, 1471–1482. [Google Scholar] [CrossRef]
- Bieri, M.; Bürgi, T. Probing enantiospecific interactions between proline and an L-glutathione self-assembled monolayer by modulation excitation ATR-IR spectroscopy. J. Phys. Chem. B 2005, 109, 10243–10250. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.; Shearer, A.B.; Bent, S.F. Next generation nanopatterning using small molecule inhibitors for area-selective atomic layer deposition. J. Vac. Sci. Technol. A 2021, 39, 021002. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, S.H.; Lee, J.H.; Kwak, J.; Lee, J.H.; Kim, W.H. Enhanced Deposition Selectivity of High-Dielectrics by Vapor Dosing and Selective Removal of Phosphonic Acid Inhibitors. Acs Appl. Mater. Interfaces 2024, 16, 37157–37166. [Google Scholar] [CrossRef]
- Chen, P.J.; Wallace, R.M.; Henck, S.A. Thermal properties of perfluorinated n-alkanoic acids self-assembled on native aluminum oxide surfaces. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 16, 700–706. [Google Scholar] [CrossRef]
- Lim, M.S.; Feng, K.; Chen, X.Q.; Wu, N.Q.; Raman, A.; Nightingale, J.; Gawalt, E.S.; Korakakis, D.; Hornak, L.A.; Timperman, A.T. Adsorption and desorption of stearic acid self-assembled monolayers on aluminum oxide. Langmuir 2007, 23, 2444–2452. [Google Scholar] [CrossRef]
- Thissen, P.; Valtiner, M.; Grundmeier, G. Stability of Phosphonic Acid Self-Assembled Monolayers on Amorphous and Single-Crystalline Aluminum Oxide Surfaces in Aqueous Solution. Langmuir 2010, 26, 156–164. [Google Scholar] [CrossRef]
- Thennakoon, C.A.; Rajapakshe, R.B.S.D.; Malikaramage, A.U.; Rajapakse, R.M.G. Factors Affecting the Hydrophobic Property of Stearic Acid Self- Assembled on the TiO2 Substrate. Acs Omega 2022, 7, 48184–48191. [Google Scholar] [CrossRef]
- Dunn, G.; McDonald, R. Infrared spectra of aqueous sodium benzoates and salicylates in the carboxyl-stretching region: Chelation in aqueous sodium salicylates. Can. J. Chem. 1969, 47, 4577–4588. [Google Scholar] [CrossRef]
- Barth, A.; Zscherp, C. What vibrations tell about proteins. Q. Rev. Biophys. 2002, 35, 369–430. [Google Scholar] [CrossRef]
- Bobb-Semple, D.; Nardi, K.L.; Draeger, N.; Hausmann, D.M.; Bent, S.F. Area-Selective Atomic Layer Deposition Assisted by Self-Assembled Monolayers: A Comparison of Cu, Co, W, and Ru. Chem. Mater. 2019, 31, 1635–1645. [Google Scholar] [CrossRef]
- Lecordier, L.; Herregods, S.; Armini, S. Vapor-deposited octadecanethiol masking layer on copper to enable area selective Hf3N4 atomic layer deposition on dielectrics studied by in situ spectroscopic ellipsometry. J. Vac. Sci. Technol. A 2018, 36, 031605. [Google Scholar] [CrossRef]
- Kim, C.; Choi, M.; Sim, J.; Kim, H.; Choi, C. Area-selective atomic layer deposition (AS-ALD) of low temperature (300 °C) cobalt thin film using octadecyltrichlorosilane (ODTS) self-assembled monolayers (SAMs). Appl. Surf. Sci. 2024, 663, 160033. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satyarthy, S.; Cheng, M.; Ghosh, A. Investigating Benzoic Acid Derivatives as Potential Atomic Layer Deposition Inhibitors Using Nanoscale Infrared Spectroscopy. Nanomaterials 2025, 15, 164. https://doi.org/10.3390/nano15030164
Satyarthy S, Cheng M, Ghosh A. Investigating Benzoic Acid Derivatives as Potential Atomic Layer Deposition Inhibitors Using Nanoscale Infrared Spectroscopy. Nanomaterials. 2025; 15(3):164. https://doi.org/10.3390/nano15030164
Chicago/Turabian StyleSatyarthy, Saumya, Mark Cheng, and Ayanjeet Ghosh. 2025. "Investigating Benzoic Acid Derivatives as Potential Atomic Layer Deposition Inhibitors Using Nanoscale Infrared Spectroscopy" Nanomaterials 15, no. 3: 164. https://doi.org/10.3390/nano15030164
APA StyleSatyarthy, S., Cheng, M., & Ghosh, A. (2025). Investigating Benzoic Acid Derivatives as Potential Atomic Layer Deposition Inhibitors Using Nanoscale Infrared Spectroscopy. Nanomaterials, 15(3), 164. https://doi.org/10.3390/nano15030164






