Synthesis of Electrocatalytic Tungsten Carbide Nanoparticles by High-Pressure and High-Temperature Treatment of Organotungsten Compounds
Abstract
:1. Introduction
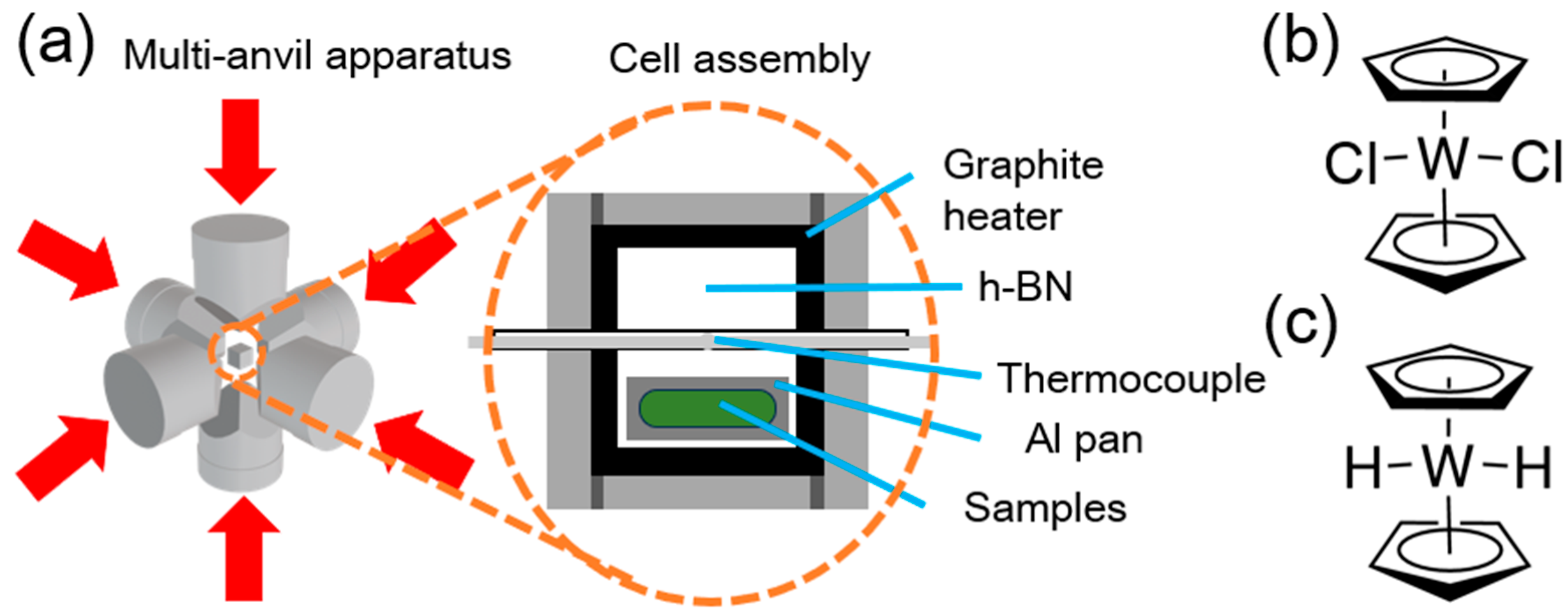
2. Materials and Methods
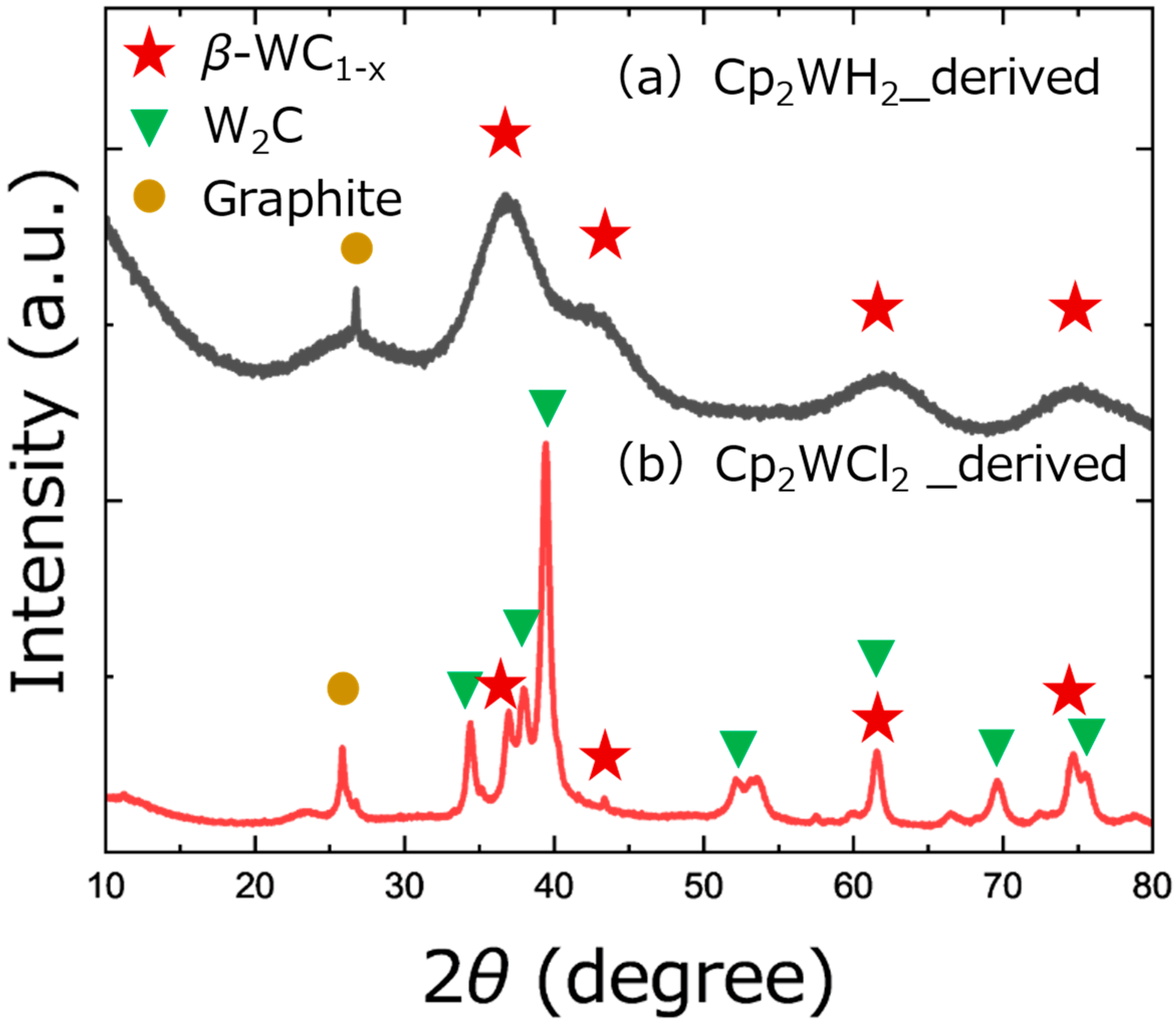
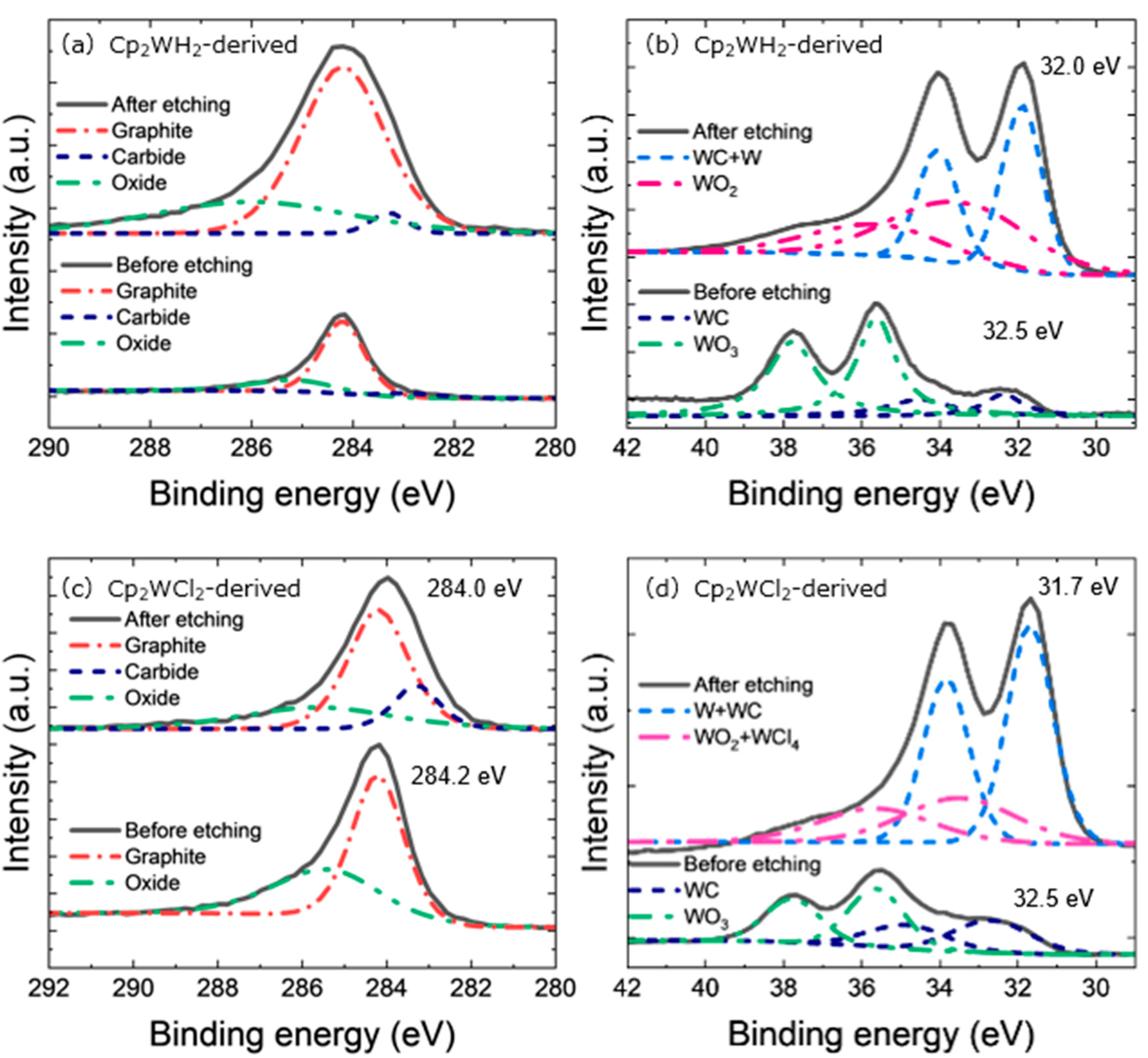
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunhiraman, A.K. Hydrogen evolution reaction catalyzed by platinum nanoislands decorated on three-dimensional nanocarbon hybrid. Ionics 2019, 25, 3787–3797. [Google Scholar] [CrossRef]
- Gómez-Marín, A.; Feliu, J.M.; Edson, T. Reaction Mechanism for Oxygen Reduction on Platinum: Existence of a Fast Initial Chemical Step and a Soluble Species Different from H2O2. ACS Catal. 2018, 8, 7931–7943. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. Engl. 2016, 55, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, L.; Zhang, X.; Wang, L.; Zou, J. Rational Design and Construction of Cocatalysts for Semiconductor-Based Photo-Electrochemical Oxygen Evolution: A Comprehensive Review. Adv. Sci. 2019, 6, 1801505. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Regmi, Y.N.; Waetzig, G.R.; Duffee, K.D.; Schmuecker, S.M.; Thode, J.M.; Leonard, B.M. Carbides of group IVA, VA and VIA transition metals as alternative HER and ORR catalysts and support materials. J. Mater. Chem. A 2015, 3, 10085–10091. [Google Scholar] [CrossRef]
- Oyama, S.T. Preparation and Catalytic Properties of Transition Metal Carbides and Nitrides. Catal. Today 1992, 15, 179–200. [Google Scholar] [CrossRef]
- Tackett, B.M.; Sheng, W.; Chen, J.G. Opportunities and Challenges in Utilizing Metal-Modified Transition Metal Carbides as Low-Cost Electrocatalysts. Joule 2017, 1, 253–263. [Google Scholar] [CrossRef]
- Weidman, M.C.; Esposito, D.V.; Hsu, Y.-C.; Chen, J.G. Comparison of electrochemical stability of transition metal carbides (WC, W2C, Mo2C) over a wide pH range. J. Power Sources 2012, 202, 11–17. [Google Scholar] [CrossRef]
- Kim, D.-W.; Li, O.L.; Pootawang, P.; Saito, N. Solution plasma synthesis process of tungsten carbide on N-doped carbon nanocomposite with enhanced catalytic ORR activity and durability. RSC Adv. 2014, 4, 16813–16819. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Asset, T.; Tieu, P.; Gili, A.; Kulkarni, D.; De Andrade, V.; De Carlo, F.; Barnard, H.S.; et al. Catalysts by pyrolysis: Direct observation of chemical and morphological transformations leading to transition metal-nitrogen-carbon materials. Mater. Today 2021, 47, 53–68. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Nakibli, Y.; Mazal, Y.; Dubi, Y.; Wächtler, M.; Amirav, L. Size Matters: Cocatalyst Size Effect on Charge Transfer and Photocatalytic Activity. Nano Lett. 2018, 18, 357–364. [Google Scholar] [CrossRef]
- Lopez-Acevedo, O.; Kacprzak, K.A.; Akola, J.; Häkkinen, H. Quantum size effects in ambient CO oxidation catalysed by ligand-protected gold clusters. Nat. Chem. 2010, 2, 329–334. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Xu, B.-Q. Nano-size effect of Au catalyst for electrochemical reduction of oxygen in alkaline electrolyte. Chin. J. Catal. 2013, 34, 942–948. [Google Scholar] [CrossRef]
- Tian, S.; Li, Y.-Z.; Li, M.-B.; Yuan, J.; Yang, J.; Wu, Z.; Jin, R. Structural isomerism in gold nanoparticles revealed by X-ray crystallography. Nat. Commun. 2015, 6, 8667. [Google Scholar] [CrossRef]
- Cuenya, B.R.; Behafarid, F. Nanocatalysis: Size- and shape-dependent chemisorption and catalytic reactivity. Surf. Sci. Rep. 2015, 70, 135–187. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. Susmat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Mahmood, A.; Zou, R.Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Yamane, I.; Sato, K.; Otomo, R.; Yanase, T.; Miura, A.; Nagahama, T.; Kamiya, Y.; Shimada, T. Ultrahigh-Pressure Preparation and Catalytic Activity of MOF-Derived Cu Nanoparticles. Nanomaterials 2021, 11, 1040. [Google Scholar] [CrossRef]
- Yamane, I.; Sato, K.; Ando, T.; Tadokoro, T.; Yokokura, S.; Nagahama, T.; Kato, Y.; Takeguchi, T.; Shimada, T. Ultrahigh pressure-induced modification of morphology and performance of MOF-derived Cu@C electrocatalysts. Nanoscale Adv. 2022, 5, 493–502. [Google Scholar] [CrossRef]
- Riedel, R. High-pressure materials synthesis—A guideline for the discovery of advanced ceramic nitrides. Ceram. Int. 2023, 49, 24102–24111. [Google Scholar] [CrossRef]
- Bykov, M.; Fedotenko, T.; Chariton, S.; Laniel, D.; Glazyrin, K.; Hanfland, M.; Smith, J.S.; Prakapenka, V.B.; Mahmood, M.F.; Goncharov, A.F.; et al. High-Pressure Synthesis of Dirac Materials: Layered van der Waals Bonded BeN4 Polymorph. Phys. Rev. Lett. 2021, 126, 175501. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Ma, Y. Materials by design at high pressures. Chem. Sci. 2022, 13, 329–344. [Google Scholar] [CrossRef]
- Li, J.; Kuang, Y.; Meng, Y.; Tian, X.; Hung, W.-H.; Zhang, X.; Li, A.; Xu, M.; Zhou, W.; Ku, C.-S.; et al. Electroreduction of CO2 to Formate on a Copper-Based Electrocatalyst at High Pressures with High Energy Conversion Efficiency. J. Am. Chem. Soc. 2020, 142, 7276–7282. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhao, S.; Li, J.; Chen, Z.; Peng, W.; He, Y.; Zhang, L.; Yan, S.; Wu, L.; Ahuja, R.; et al. Pressure-promoted highly-ordered Fe-doped-Ni2B for effective oxygen evolution reaction and overall water splitting. J. Mater. Chem. A 2021, 9, 6469–6475. [Google Scholar] [CrossRef]
- Xu, S.; Gao, X.; Deshmukh, A.; Zhou, J.; Chen, N.; Peng, W.; Gong, Y.; Yao, Z.; Finkelstein, K.D.; Wan, B.; et al. Pressure-promoted irregular CoMoP2 nanoparticles activated by surface reconstruction for oxygen evolution reaction electrocatalysts. J. Mater. Chem. A 2020, 8, 2001–2007. [Google Scholar] [CrossRef]
- Peng, W.; Lv, Z.; Zhou, W.; Yan, B.; Zhao, S.; Wu, L.; Zhou, J.; Gou, H. High-pressure synthesis of trimetal phosphide CFNP as highly efficient bifunctional catalyst for alkaline water splitting. Fuel 2025, 380, 133110. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, G.; Ma, S.; Hu, J.; Zhu, P.; Cui, T.; Li, Z.; Zou, Z. Hydrogen evolution reaction of γ-Mo0.5W0.5C achieved by high pressure high temperature synthesis. Catalysts 2016, 6, 208. [Google Scholar] [CrossRef]
- Yamada, I.; Fujii, H.; Takamatsu, A.; Ikeno, H.; Wada, K.; Tsukasaki, H.; Kawaguchi, S.; Mori, S.; Yagi, S. Bifunctional Oxygen Reaction Catalysis of Quadruple Manganese Perovskites. Adv. Mater. 2017, 29, 1603004. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, D.; Chen, Y.; Cheng, J.; You, C.; Wang, X.; Dong, S.; Tao, Q.; Zhu, P. Surface Modification towards Integral Bulk Catalysts of Transition Metal Borides for Hydrogen Evolution Reaction. Catalysts 2022, 12, 222. [Google Scholar] [CrossRef]
- Candland, C.T.; Decker, D.L.; Vanfleet, H.B. Interstitial Diffusion of Copper in Lead at Pressures up to 56 kbar. Phys. Rev. B 1972, 5, 2085–2094. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B.; Williams, Q.; Manghnani, M.H. Short- and Intermediate-Range Structure and Dynamics of Fe-Ni-C Liquid Under Compression. Front. Earth Sci. 2019, 7, 258. [Google Scholar] [CrossRef]
- Sano, N.; Akazawa, H.; Kikuchi, T.; Kanki, T. Separated synthesis of iron-included carbon nanocapsules and nanotubes by pyrolysis of ferrocene in pure hydrogen. Carbon 2003, 41, 2159–2162. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- El Mrabet, S.; Abad, M.D.; López-Cartes, C.; Martínez-Martínez, D.; Sánchez-López, J.C. Thermal evolution of WC/C nanostructured coatings by raman and In Situ XRD analysis. Plasma Process. Polym. 2009, 6, S444–S449. [Google Scholar] [CrossRef]
- Prima, A.; Egorova, Y.; Pushkarev, A.; Matryenin, S. Modification of the WC-Co carbide surface with high-intensity pulsed ion beam. J. Physics Conf. Ser. 2020, 1588, 012045. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Tahini, H.A.; Lin, Q.; Chen, Y.; Guan, D.; Zhou, C.; Hu, Z.; Lin, H.-J.; Chan, T.-S.; et al. Single-phase perovskite oxide with super-exchange induced atomic-scale synergistic active centers enables ultrafast hydrogen evolution. Nat. Commun. 2020, 11, 5657. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Dash, T.; Nayak, B. Preparation of Multi-phase Composite of Tungsten Carbide, Tungsten Boride and Carbon by Arc Plasma Melting: Characterization of Melt-cast Product. Ceram. Int. 2016, 42, 445–459. [Google Scholar] [CrossRef]
- Debus, J.; Schindler, J.J.; Waldkirch, P.; Goeke, S.; Brümmer, A.; Biermann, D.; Bayer, M. Indication of worn WC/C surface locations of a dry-running twin-screw rotor by the oxygen incorporation in tungsten-related Raman modes. Appl. Phys. Lett. 2016, 109, 171601. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Beck, W., Jr.; Varanda, L.C.; Ticianelli, E.A. Electrocatalytic activity of platinum nanoparticles supported on different phases of tungsten carbides for the oxygen reduction reaction. Int. J. Hydrog. Energy 2017, 42, 20677–20688. [Google Scholar] [CrossRef]
- Huang, K.; Bi, K.; Xu, J.C.; Liang, C.; Lin, S.; Wang, W.J.; Yang, T.Z.; Du, Y.X.; Zhang, R.; Yang, H.J.; et al. Novel graphite-carbon encased tungsten carbide nanocomposites by solid-state reaction and their ORR electrocatalytic performance in alkaline medium. Electrochimica Acta 2015, 174, 172–177. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, J.; Chen, P.; Wei, B.; Gao, D.; Xu, D. Novel combustion-carbonization preparation of mesoporous tungsten carbide as a highly active catalyst for oxygen reduction. New J. Chem. 2020, 44, 4004. [Google Scholar] [CrossRef]
- Sohn, Y.; Jung, J.Y.; Kim, P. Facile synthesis of tungsten carbide-carbon composites for oxygen reduction reaction. Korean J. Chem. Eng. 2017, 34, 2162–2168. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, Z.; Chen, M.; Cao, H.; Li, K.; Cai, Y.; Wang, F. Highly porous composite based on tungsten carbide and N-doped carbon aerogels for electrocatalyzing oxygen reduction reaction in acidic and alkaline media. Electrochim. Acta 2017, 236, 154–160. [Google Scholar] [CrossRef]
- Esposito, D.V.; Chen, J.G. Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: Opportunities and limitations. Energy Environ. Sci. 2011, 4, 3900–3912. [Google Scholar] [CrossRef]
- Zhu, X.; He, M.; Chen, X.; Zhou, Y.; Xu, C.; Li, X.; Luo, Q.; Yang, J. First-principles insights into tungsten semicarbide-based single-atom catalysts: Single-atom migration and mechanisms in oxygen reduction. J. Phys. Chem. Lett. 2024, 15, 2815–2824. [Google Scholar] [CrossRef]
- Bai, J.; Hao, M.; Han, X.; Zhou, P.; Yao, H.; Bian, L.; Yang, G.; Liang, J.; Laine, R.M.; Wang, F. Halloysite-derived hierarchical cobalt silicate hydroxide hollow nanorods assemble by nanosheets for highly efficient electrocatalytic oxygen evolution reaction. J. Mater. Sci. Technol. 2025, 216, 139–149. [Google Scholar] [CrossRef]
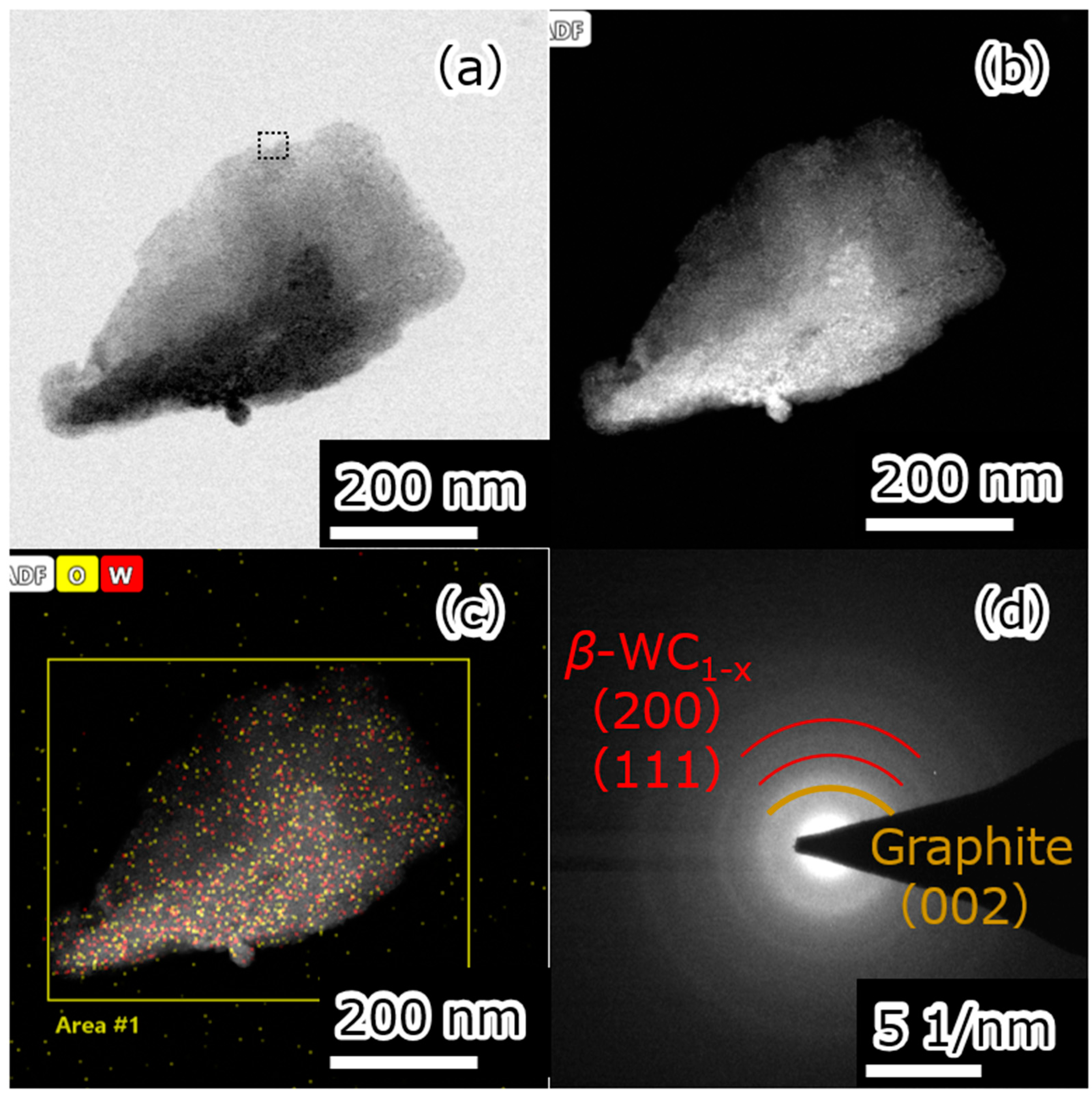
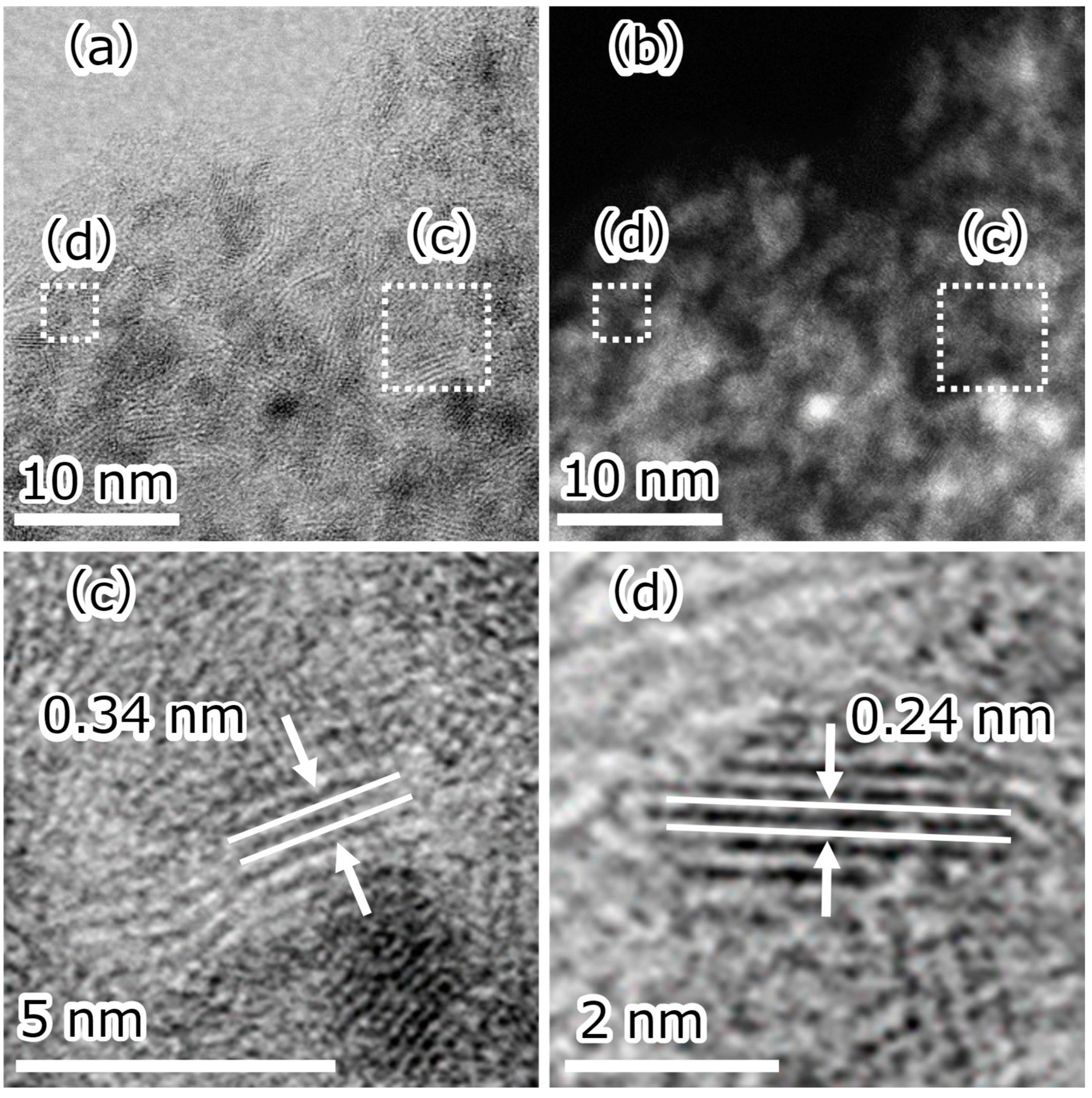
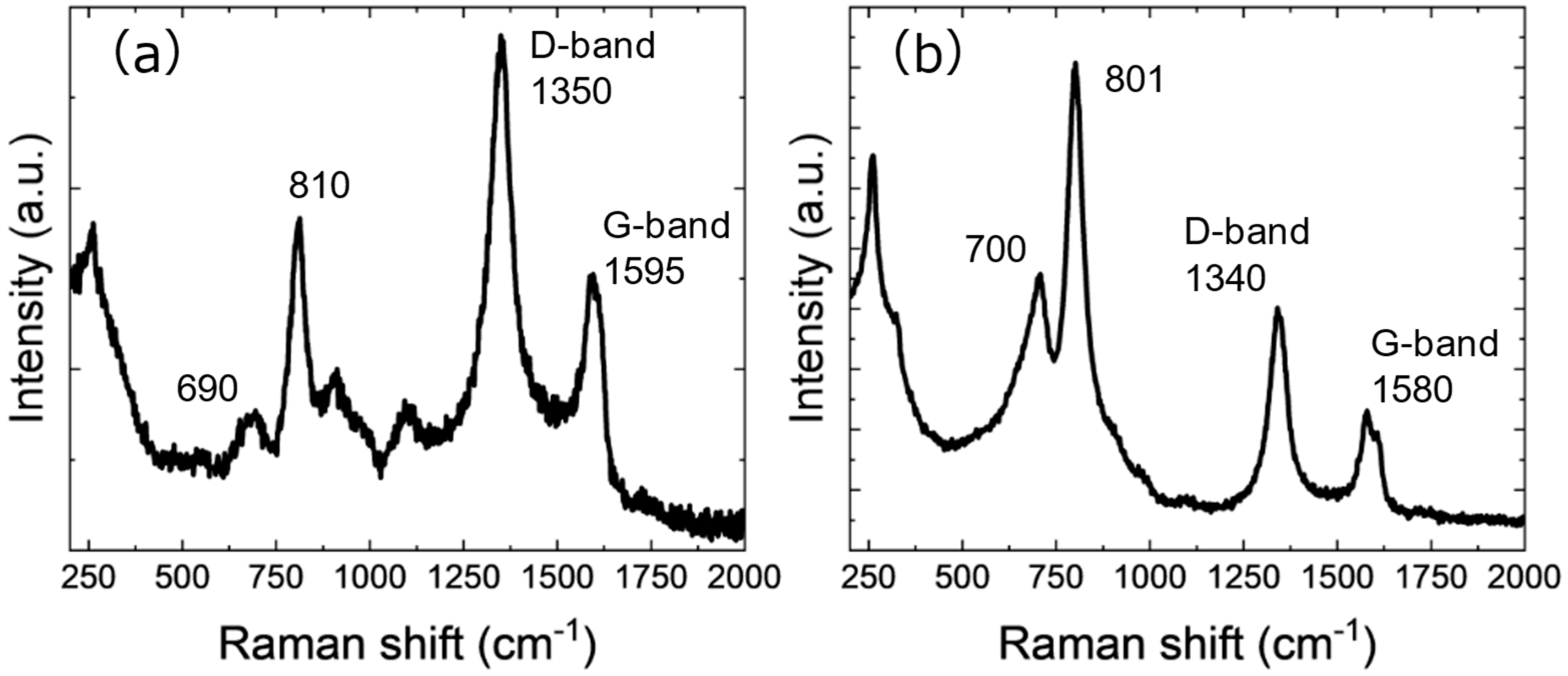



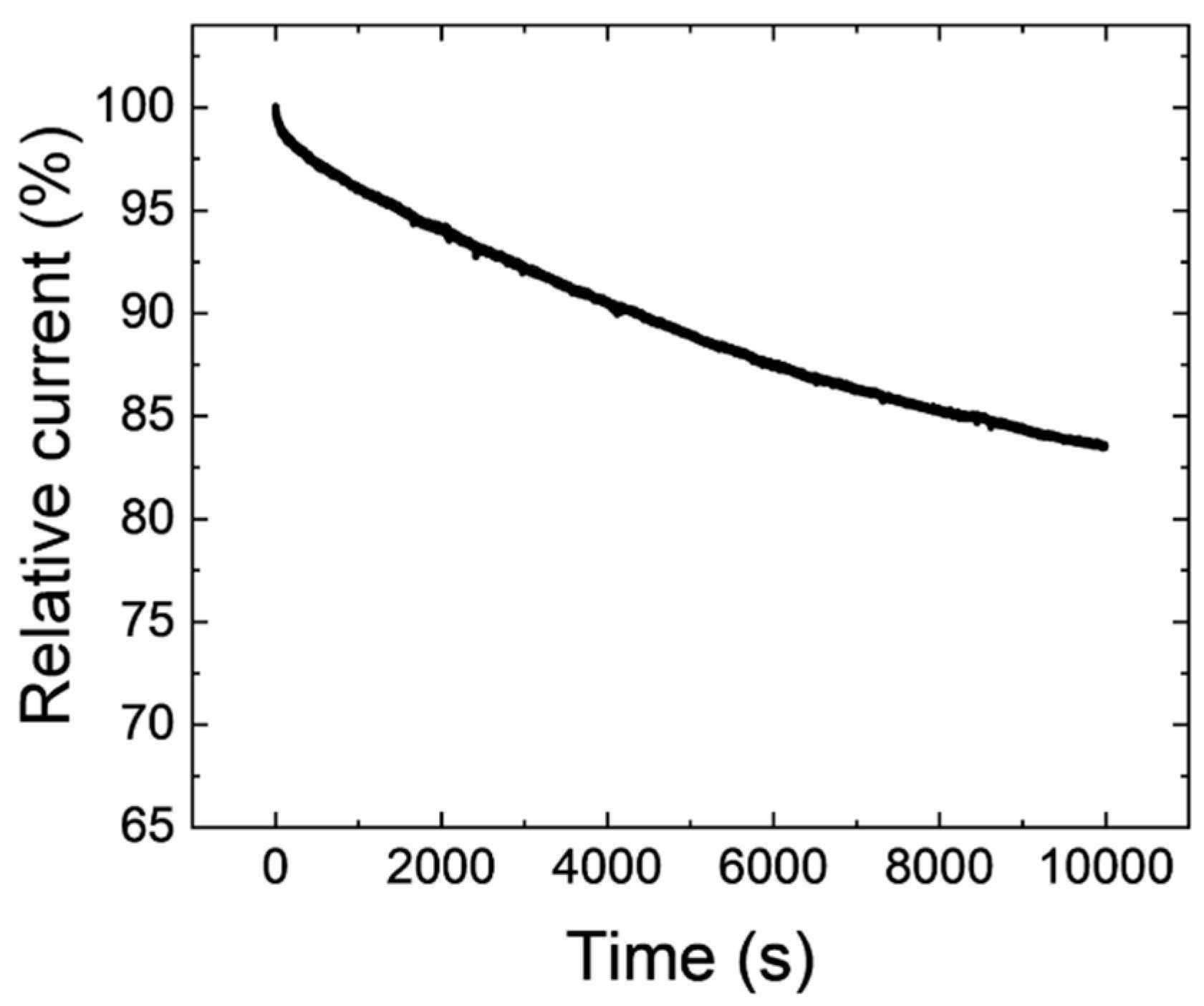
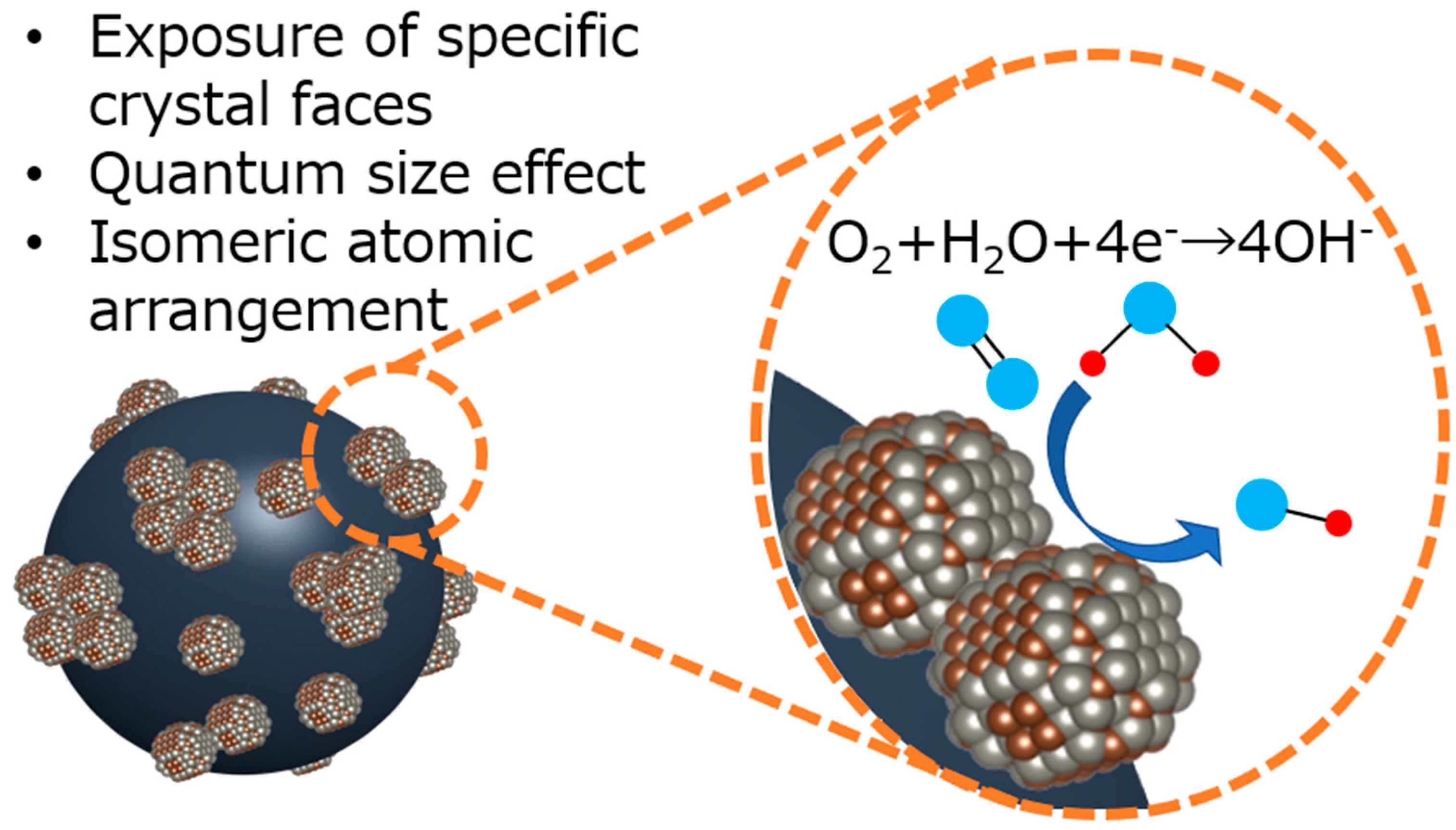
| Element | Atomic Fraction/% |
|---|---|
| W | 5.57 |
| C | 68.1 |
| O | 21.5 |
| Cu (from Cu TEM grid) | 4.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadokoro, T.; Sato, S.; Yamane, I.; Waizumi, H.; Yokokura, S.; Shimada, T. Synthesis of Electrocatalytic Tungsten Carbide Nanoparticles by High-Pressure and High-Temperature Treatment of Organotungsten Compounds. Nanomaterials 2025, 15, 170. https://doi.org/10.3390/nano15030170
Tadokoro T, Sato S, Yamane I, Waizumi H, Yokokura S, Shimada T. Synthesis of Electrocatalytic Tungsten Carbide Nanoparticles by High-Pressure and High-Temperature Treatment of Organotungsten Compounds. Nanomaterials. 2025; 15(3):170. https://doi.org/10.3390/nano15030170
Chicago/Turabian StyleTadokoro, Taijiro, Sota Sato, Ichiro Yamane, Hiroki Waizumi, Seiya Yokokura, and Toshihiro Shimada. 2025. "Synthesis of Electrocatalytic Tungsten Carbide Nanoparticles by High-Pressure and High-Temperature Treatment of Organotungsten Compounds" Nanomaterials 15, no. 3: 170. https://doi.org/10.3390/nano15030170
APA StyleTadokoro, T., Sato, S., Yamane, I., Waizumi, H., Yokokura, S., & Shimada, T. (2025). Synthesis of Electrocatalytic Tungsten Carbide Nanoparticles by High-Pressure and High-Temperature Treatment of Organotungsten Compounds. Nanomaterials, 15(3), 170. https://doi.org/10.3390/nano15030170






