Applications of MOF-Based Nanocomposites in Heat Exchangers: Innovations, Challenges, and Future Directions
Abstract
:1. Introduction
2. MOF-Based Nanocomposites for Heat Exchanger Performance
2.1. High-Performance MOF Nanocomposites for Thermal Conductivity
2.2. MOF–Polymer and MOF–Metal Nanocomposites
2.2.1. Advantages and Applications of MOF–Polymer Composites
2.2.2. Applications and Advantages of MOF–Metal Composites
2.3. MOF-Based Nanomaterials for Enhanced Fouling Resistance
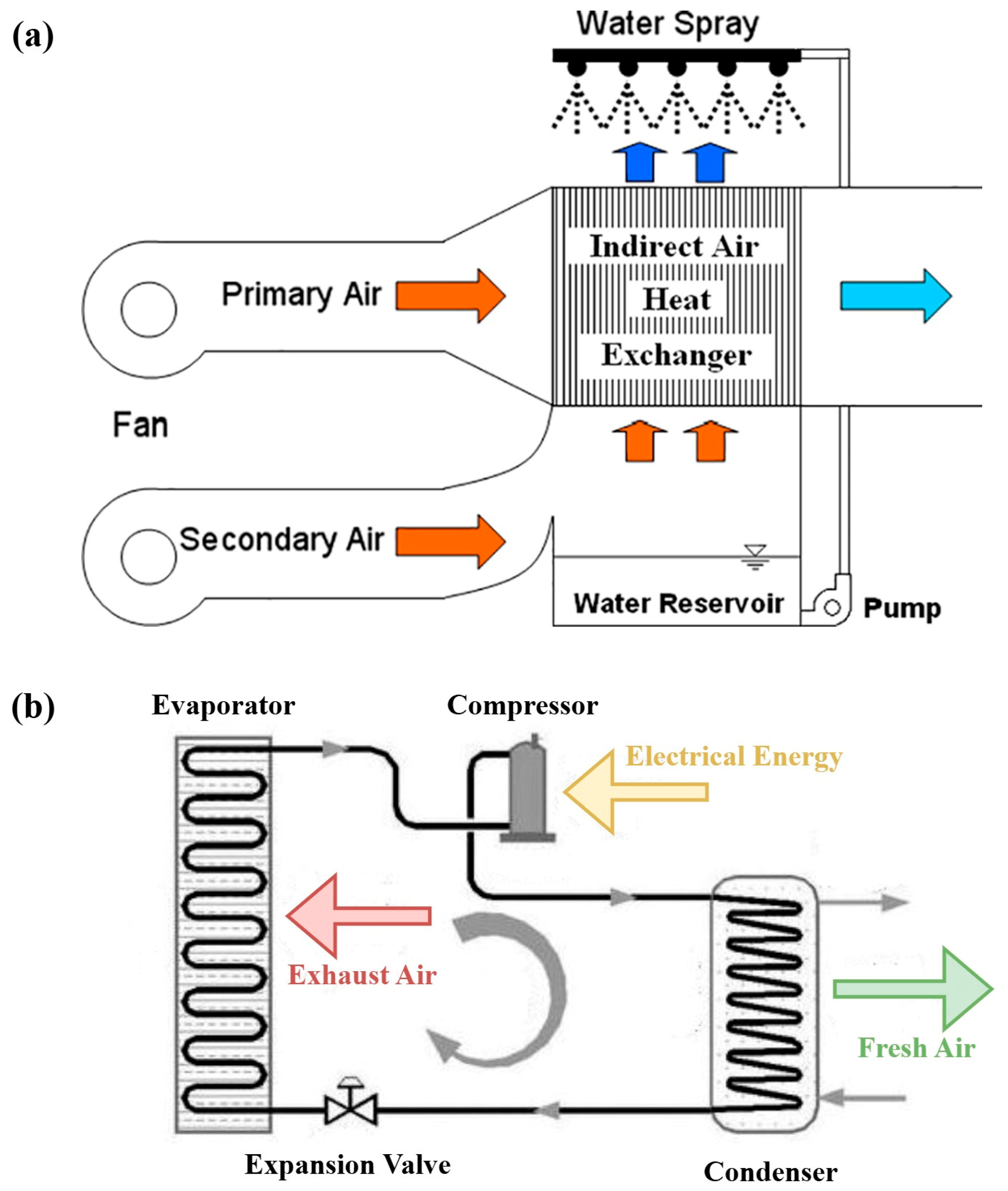
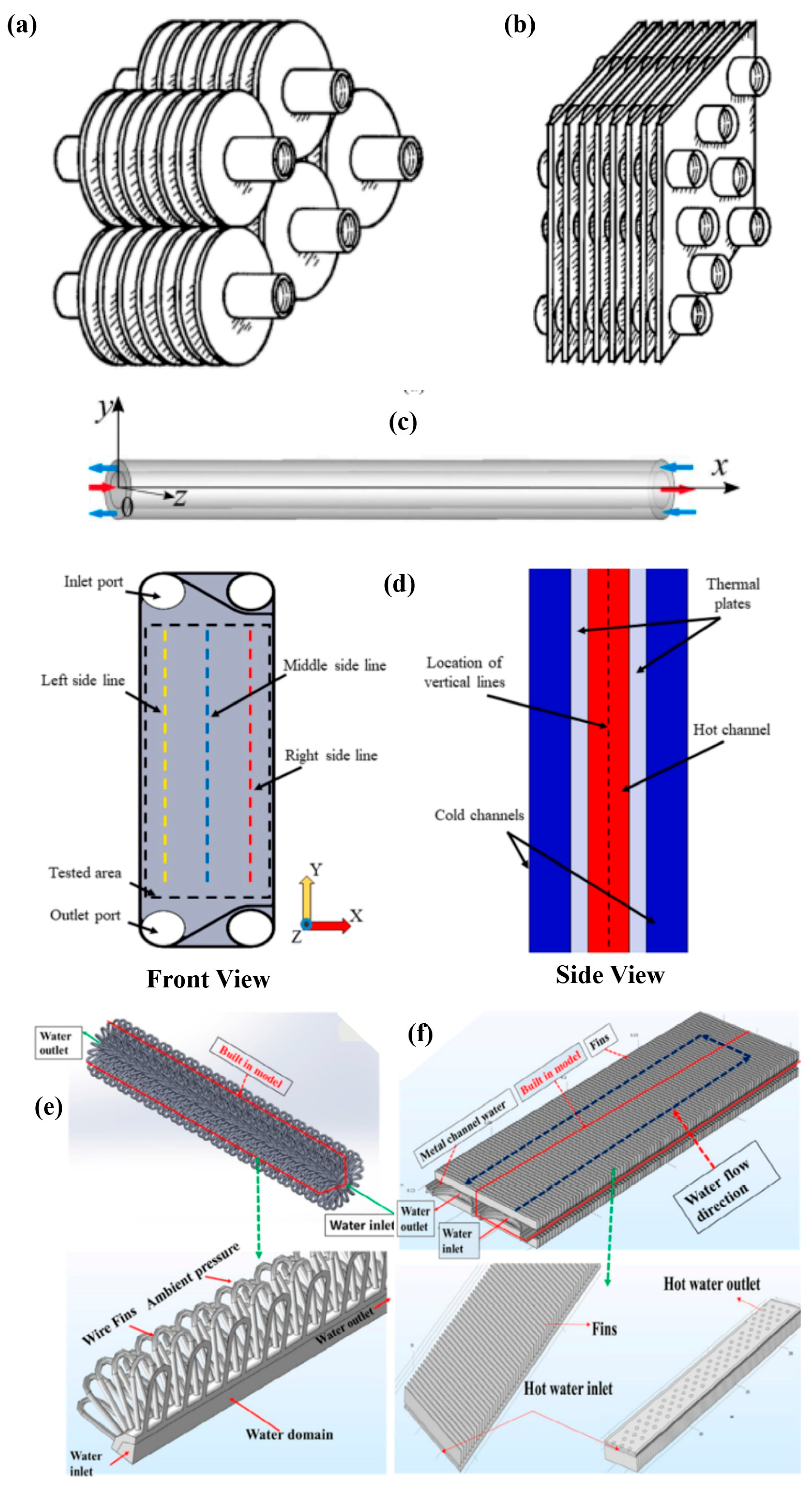
2.4. Applications in Specific Heat Exchanger Designs
2.4.1. Compact Plate Heat Exchanger
2.4.2. Fin Tube Heat Exchangers
2.4.3. Wire-Finned Tube Heat Exchangers
3. Innovations and Challenges in MOF-Based Heat Exchanger Applications
3.1. Innovations in Synthesis and Fabrication
3.1.1. Scalable Synthesis Method
3.1.2. Surface Functionalization Method
3.2. Challenges in Industrial Integration
3.2.1. Cost and Feasibility
3.2.2. Long-Term Stability and Durability
4. Research Gaps and Future Directions for MOF-Based Nanocomposites in Heat Exchangers
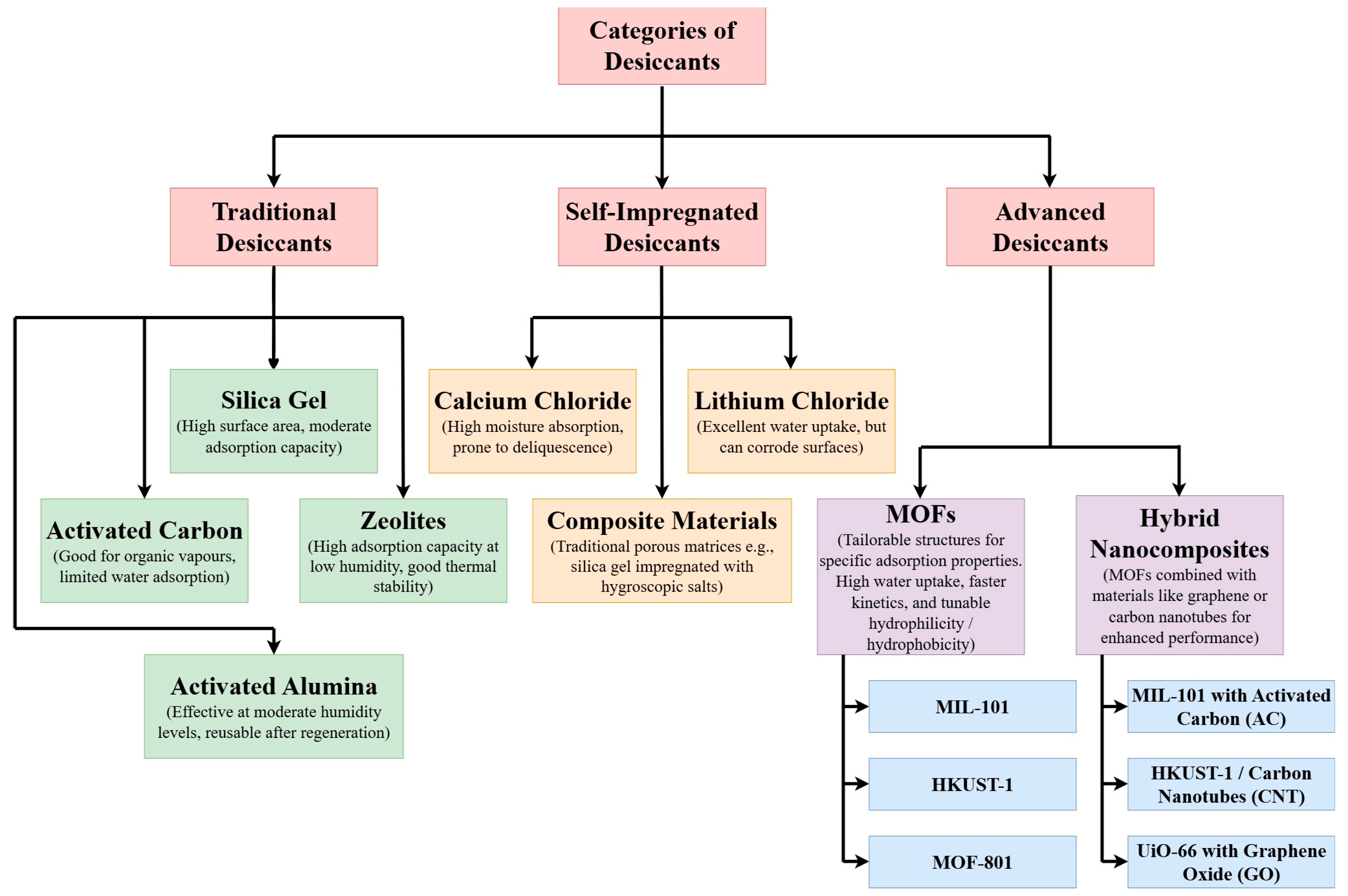
4.1. Multifunctional MOFs
4.2. Bridging Gaps Between Research and Industry
4.3. Green and Pollution-Free Preparation Methods for MOFs
- One promising approach is solvent-free synthesis, which eliminates the use of hazardous organic solvents by employing mechanochemical methods [233]. In this process, the metal precursors and organic linkers are ground together in a ball mill, enabling the formation of MOFs under ambient conditions. This method not only reduces waste but also significantly lowers energy consumption, making it a highly sustainable alternative [234].
- Another green technique is the use of water as a reaction medium. Aqueous-based synthesis methods utilize water as a benign solvent, reducing the ecological footprint associated with solvent disposal [235]. For instance, the production of MIL-53 [236] and MIL-100/101 [237] has been successfully demonstrated using water as the primary solvent, achieving comparable performance to their traditionally synthesized counterparts.
- Microwave-assisted synthesis is another eco-friendly method that offers rapid reaction times and reduced energy requirements [172]. This technique utilizes microwave irradiation to heat the reaction mixture uniformly, leading to faster crystallization and higher yields [238]. Moreover, the process can often be conducted in water or ethanol, further reducing the environmental impact.
- Lastly, the adoption of waste valorization strategies, such as using industrial by-products as precursors for MOF synthesis, exemplifies a circular economy approach [239]. These strategies not only reduce the cost of raw materials but also contribute to waste reduction and resource conservation [240].
5. Conclusions
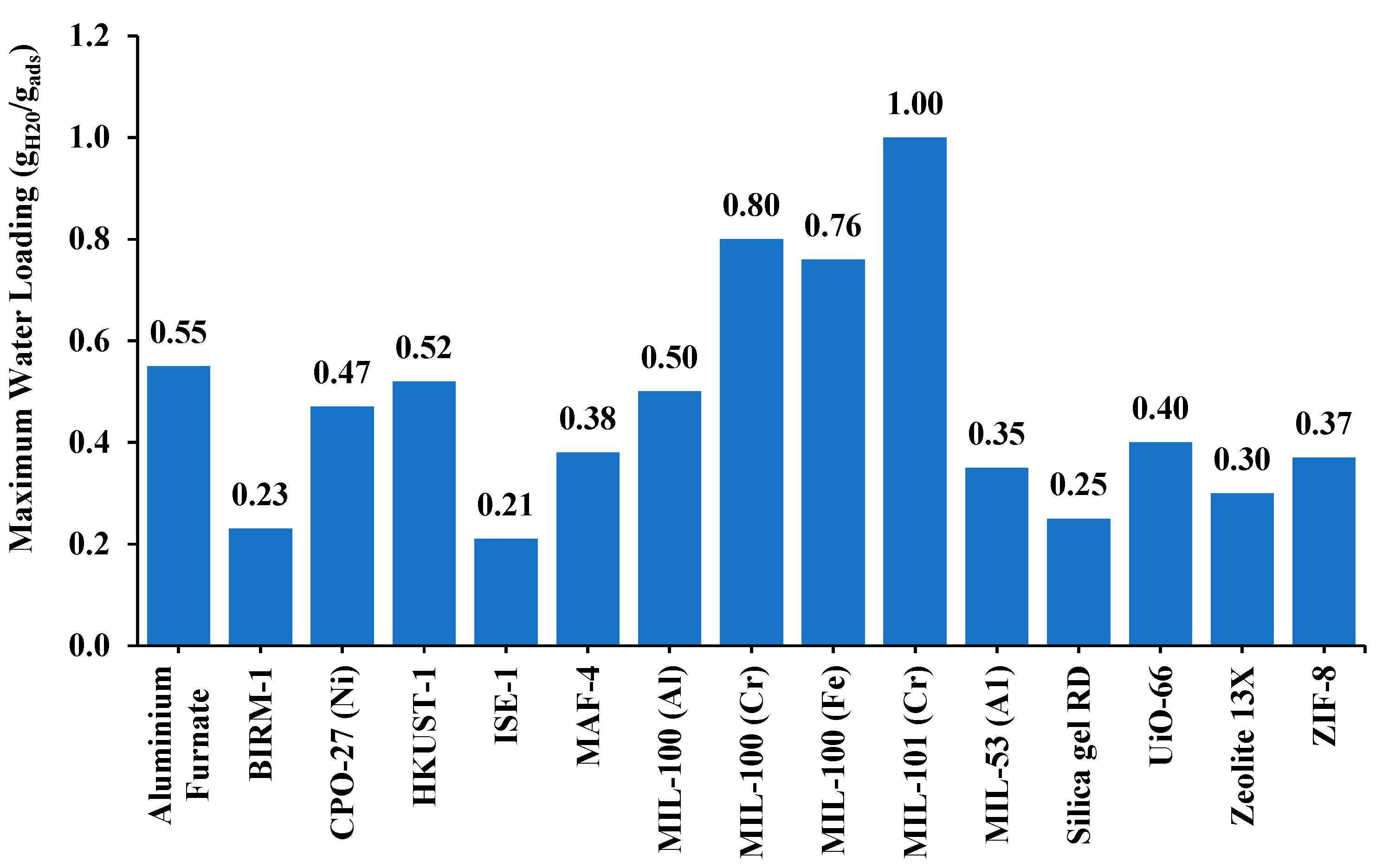
- MOFs integrated into a heat exchanger, such as wire-finned and shell-and-tube designs, have shown remarkable gains in heat transfer rates and moisture adsorption, and reduction in fouling resistance. Studies have proven that MOF-based coatings, like MOF-303/GO and HKUST-1 composites, enhance both water uptake, up to 22%, and thermal diffusivity, outperforming traditional materials such as silica gels and activated alumina. MOFs also outperformed traditional materials such as silica gel by enhancing COP as much as six times in comparison to it.
- This research also focused on the innovations and challenges surrounding MOF-based heat exchanger applications. Innovations in scalable synthesis methods, such as microwave-assisted synthesis and surface functionalization techniques, are key to reducing costs while ensuring consistent material quality. These advancements have the potential to make MOFs more economically viable for large-scale industrial applications. Furthermore, surface functionalization has been shown to enhance the thermal conductivity, mechanical stability, and chemical resilience of MOFs, making them more suitable for demanding environments. Yet, issues such as long-term stability under high temperatures, pressure, and corrosive conditions still need to be addressed, as shown by ongoing research into the durability of various MOF composites under operational stresses.
- The research gaps and future directions for MOF-based nanocomposites in heat exchanger systems are also studied in this research. The integration of multifunctional MOF hybrids, combining materials like GO, AC, and CNT, presents a promising path forward for developing heat exchangers with enhanced performance across multiple domains, such as heat transfer, gas adsorption, and catalysis. The multifunctional MOFs of CNT enhanced the thermal conductivity up to seven times in comparison to the actual MOFs. However, these hybrid solutions will require further collaboration between academia and industry to optimize material properties and develop scalable production processes.
- Real-world industrial case studies have also been analyzed such as BASF’s innovation in adsorptive dehumidification, which leverages MOFs to reduce energy consumption in air conditioning systems by 50–60%, offering higher volumetric energy density and a 27% improvement in COP compared to silica gel. While MOFs require energy for regeneration, this can be sourced from low-temperature heat like solar or waste heat. Scaling production is expected to lower costs, broadening their applicability and enhancing energy efficiency. Similarly, NuMat Technologies exemplifies the transition of MOFs from research to industrial applications by developing cost-effective, scalable production methods for gas storage systems. Their innovations demonstrate the potential of MOFs in clean energy and industrial processes, emphasizing their role in advancing sustainable and economically viable solutions.
- Green synthesis methods for MOFs include solvent-free mechanochemical techniques, which eliminate hazardous solvents and reduce energy consumption by grinding metal precursors and linkers. Aqueous-based synthesis uses water as a benign solvent, lowering environmental impact. Methods for production of MIL-53 and MIL-100/101 using water as the primary solvent have displayed commendable performance in comparison to traditional synthesis counterparts. Microwave-assisted synthesis offers rapid, energy-efficient crystallization, often using eco-friendly solvents.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, A.R.J.; Alahyari, A.A.; Eastman, S.A.; Thibaud-Erkey, C.; Johnston, S.; Sobkowicz, M.J. Review of polymers for heat exchanger applications: Factors concerning thermal conductivity. Appl. Therm. Eng. 2017, 113, 1118–1127. [Google Scholar] [CrossRef]
- Abdullah, S.; Zubir, M.N.B.M.; Muhamad, M.R.B.; Newaz, K.M.S.; Öztop, H.F.; Alam, M.S.; Shaikh, K. Technological development of evaporative cooling systems and its integration with air dehumidification processes: A review. Energy Build. 2023, 283, 112805. [Google Scholar] [CrossRef]
- Sajjad, U.; Abbas, N.; Hamid, K.; Abbas, S.; Hussain, I.; Ammar, S.M.; Sultan, M.; Ali, H.M.; Hussain, M.; Rehman, T.U.; et al. A review of recent advances in indirect evaporative cooling technology. Int. Commun. Heat Mass Transf. 2021, 122, 105140. [Google Scholar] [CrossRef]
- Salhein, K.; Kobus, C.J.; Zohdy, M.; Annekaa, A.M.; Alhawsawi, E.Y.; Salheen, S.A. Heat Transfer Performance Factors in a Vertical Ground Heat Exchanger for a Geothermal Heat Pump System. Energies 2024, 17, 5003. [Google Scholar] [CrossRef]
- Ismail, M.; Yebiyo, M.; Chaer, I. A Review of Recent Advances in Emerging Alternative Heating and Cooling Technologies. Energies 2021, 14, 502. [Google Scholar] [CrossRef]
- GaneshKumar, P.; VinothKumar, S.; Vigneswaran, V.S.; Kim, S.C.; Ramkumar, V. Advancing heat exchangers for energy storage: A comprehensive review of methods and techniques. J. Energy Storage 2024, 99, 113334. [Google Scholar] [CrossRef]
- Shirmohammadi, R.; Gilani, N. Effectiveness enhancement and performance evaluation of indirect-direct evaporative cooling system for a wide variety of climates. Environ. Prog. Sustain. Energy 2019, 38, 13032. [Google Scholar] [CrossRef]
- Kang, Z.; Zhou, X.; Wang, R.; Cheng, X.; Zhao, S. Research Status of Heat Recovery Device in a Train Station of Severe Cold Area. Procedia Eng. 2017, 205, 1705–1710. [Google Scholar] [CrossRef]
- T’Joen, C.; Park, Y.; Wang, Q.; Sommers, A.; Han, X.; Jacobi, A. A review on polymer heat exchangers for HVAC&R applications. Int. J. Refrig. 2009, 32, 763–779. [Google Scholar]
- Shah, R.K.; Sekulić, D.P. Classification of Heat Exchangers. In Fundamentals of Heat Exchanger Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 1–77. [Google Scholar]
- Huu-Quan, D.; Rostami, A.M.; Rad, M.S.; Izadi, M.; Hajjar, A.; Xiong, Q. 3D numerical investigation of turbulent forced convection in a double-pipe heat exchanger with flat inner pipe. Appl. Therm. Eng. 2021, 182, 116106. [Google Scholar] [CrossRef]
- Al Zahrani, S.; Islam, M.S.; Saha, S.C. Heat transfer enhancement of modified flat plate heat exchanger. Appl. Therm. Eng. 2021, 186, 116533. [Google Scholar] [CrossRef]
- Saleh, M.M.; Elsayed, E.; Al-Dadh, R.; Mahmoud, S. Experimental testing of wire finned heat exchanger coated with aluminium fumarate MOF material for adsorption desalination application. Therm. Sci. Eng. Prog. 2022, 28, 101050. [Google Scholar] [CrossRef]
- Patel, A. Heat Exchangers in Industrial Applications: Efficiency and Optimization Strategies. Int. J. Eng. Res. Technol. (IJERT) 2023, 12, 1–10. [Google Scholar]
- Kaur, I.; Singh, P. State-of-the-art in heat exchanger additive manufacturing. Int. J. Heat Mass Transf. 2021, 178, 121600. [Google Scholar] [CrossRef]
- Zhang, X.; Keramati, H.; Arie, M.; Singer, F.; Tiwari, R.; Shooshtari, A.; Ohadi, M. Recent developments in high temperature heat exchangers: A review. Front. Heat Mass Transf. 2018, 11, 1–14. [Google Scholar]
- Soltan, Y.I.; Nasser, M.S.; Almomani, F.; Mahmoud, K.A.; Onaizi, S.A. Thermal conductivity of different materials nanofluids Nanofluids of MXenes, metal organic frameworks, and other Nanostructured materials in heat transfer applications: Review. J. Mater. Res. Technol. 2024, 31, 2723–2761. [Google Scholar] [CrossRef]
- Kapustenko, P.; Klemeš, J.J.; Arsenyeva, O. Plate heat exchangers fouling mitigation effects in heating of water solutions: A review. Renew. Sustain. Energy Rev. 2023, 179, 113283. [Google Scholar] [CrossRef]
- Shoar, Z.K.; Pourpasha, H.; Heris, S.Z.; Mousavi, S.B.; Mohammadpourfard, M. The effect of heat transfer characteristics of macromolecule fouling on heat exchanger surface: A dynamic simulation study. Can. J. Chem. Eng. 2023, 101, 5802–5817. [Google Scholar] [CrossRef]
- Garcia, S.; Trueba, A. Fouling in Heat Exchanger. In Inverse Heat Conduction and Heat Exchangers; IntechOpen Limited: London, UK, 2020. [Google Scholar]
- Müller-Steinhagen, H.; Malayeri, M.R.; Watkinson, A.P. Heat Exchanger Fouling: Mitigation and Cleaning Strategies. Heat Transf. Eng. 2011, 32, 189–196. [Google Scholar] [CrossRef]
- Li, M.; Xiao, W.; Yin, Z.; Chen, Y.; Luo, Y.; Hong, Z.; Xue, M. Construction of a robust MOF-based superhydrophobic composite coating with the excellent performance in antifouling, drag reduction, and organic photodegradation. Prog. Org. Coat. 2024, 186, 108086. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Yusaf, T. Biofouling in RO system: Mechanisms, monitoring and controlling. Desalination 2012, 302, 1–23. [Google Scholar] [CrossRef]
- Suckeveriene, R.Y. Grafting of polyaniline by a dynamic inverse emulsion polymerization technique onto reverse osmosis membranes as an antibiofouling agent. Polym. Adv. Technol. 2019, 30, 1759–1766. [Google Scholar] [CrossRef]
- Farh, H.M.H.; Seghier, M.E.A.B.; Taiwo, R.; Zayed, T. Analysis and ranking of corrosion causes for water pipelines: A critical review. NPJ Clean Water 2023, 6, 65. [Google Scholar] [CrossRef]
- Agala, A.; Khan, M.; Starr, A. Degradation mechanisms associated with metal pipes and the effective impact of LDMs and LLMs in water transport and distribution. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 237, 1855–1876. [Google Scholar] [CrossRef]
- De Almeida, C.F.; Saget, M.; Delaplace, G.; Jimenez, M.; Fierro, V.; Celzard, A. Innovative fouling-resistant materials for industrial heat exchangers: A review. Rev. Chem. Eng. 2021, 39, 71–104. [Google Scholar] [CrossRef]
- Weiss, S.; Ben-Shmuel, A.; Chajanovsky, I.; Mizrahi, D.M.; Suckeveriene, R.Y. Hybrid PANI-halamine design, synthesis and antibacterial activity. J. Water Process Eng. 2023, 56, 104539. [Google Scholar] [CrossRef]
- Faes, W.; Lecompte, S.; Ahmed, Z.Y.; Van Bael, J.; Salenbien, R.; Verbeken, K.; De Paepe, M. Corrosion and corrosion prevention in heat exchangers. Corros. Rev. 2019, 37, 131–155. [Google Scholar] [CrossRef]
- Viswanathan, R.; Stringer, J. Failure Mechanisms of High Temperature Components in Power Plants. J. Eng. Mater. Technol. 2000, 122, 246–255. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.; Zhou, L.; Wang, L.; Zhang, J.; Liu, Y.; Lei, J. Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem. Eng. J. 2022, 427, 131503. [Google Scholar] [CrossRef]
- Hayat, A.; Rauf, S.; Al Alwan, B.; El Jery, A.; Almuqati, N.; Melhi, S.; Amin, M.A.; Al-Hadeethi, Y.; Sohail, M.; Orooji, Y.; et al. Recent advance in MOFs and MOF-based composites: Synthesis, properties, and applications. Mater. Today Energy 2024, 41, 101542. [Google Scholar] [CrossRef]
- Mohseni, M.M.; Jouyandeh, M.; Sajadi, S.M.; Hejna, A.; Habibzadeh, S.; Mohaddespour, A.; Rabiee, N.; Daneshgar, H.; Akhavan, O.; Asadnia, M.; et al. Metal-organic frameworks (MOF) based heat transfer: A comprehensive review. Chem. Eng. J. 2022, 449, 137700. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Zabihi, O.; Ahmadi, M.; Li, Q.; Blanchard, P.; Kiziltas, A.; Naebe, M. Metal–organic framework structure–property relationships for high-performance multifunctional polymer nanocomposite applications. J. Mater. Chem. A 2021, 9, 4348–4378. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, S.; Zhuang, X.; Zhang, G.; Tang, Y.; Pang, H. Recent progress of MOF-functionalized nanocomposites: From structure to properties. Adv. Colloid Interface Sci. 2024, 323, 103050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Vo, P.; Haranczyl, M. Insights into Thermal Conductivity at the MOF-Polymer Interface. ACS Appl. Mater. Interfaces 2024, 16, 56221–56231. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Marales, D.M.; Dubal, D.P.; Nanjudan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Luo, X.; Min, X.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef]
- Jrad, A.; Damacet, P.; Yaghi, Z.; Ahmad, M.; Hmadeh, M. Zr-Based Metal–Organic Framework Nanocrystals for Water Remediation. ACS Appl. Nano Mater. 2022, 5, 10795–10808. [Google Scholar] [CrossRef]
- Kalauni, K.; Vedrtnam, A.; Wdowin, M.; Chaturvedi, S. ZIF for CO2 Capture: Structure, Mechanism, Optimization, and Modeling. Processes 2022, 10, 2689. [Google Scholar] [CrossRef]
- Mirkatuli, F.S.; Nosratinia, F.; Rohani, A.A.; Rashidi, A.M.; Ardjamand, M. Enhancing CO2 adsorption with modified zeolite imidazolate frameworks (ZIF-8) functionalized with amine groups. J. Environ. Chem. Eng. 2024, 12, 114770. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in Metal–Organic Frameworks: A fundamental understanding. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, L.; Zhang, G.; Xu, L.; Xu, Z.; Meng, Q. Preparation of stable multilayer PDMS composite pervaporation membrane incorporated with in-situ transformed metal organic frameworks for enhanced butanol recovery. J. Membr. Sci. 2024, 700, 122727. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Haris, M.H.; Ghadiri, A.M.; Moghaddam, F.M.; Fatahi, Y.; Dinarvand, R.; Jarahiyan, A.; Ahmadi, S.; Shokouhimehr, M. Polymer-Coated NH2-UiO-66 for the Codelivery of DOX/pCRISPR. ACS Appl. Mater. Interfaces 2021, 13, 10796–10811. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Mozafari, M.; Shokouhimehr, M.; Varma, R.S. Natural Polymers Decorated MOF-MXene Nanocarriers for Co-delivery of Doxorubicin/pCRISPR. ACS Appl. Bio Mater. 2021, 4, 5106–5121. [Google Scholar] [CrossRef]
- Karmakar, A.; Prabakaran, V.; Zhao, D.; Chua, K.J. A review of metal-organic frameworks (MOFs) as energy-efficient desiccants for adsorption driven heat-transformation applications. Appl. Energy 2020, 269, 115070. [Google Scholar] [CrossRef]
- Cui, S.; Qin, M.; Marandi, A.; Steggles, V.; Wang, S.; Feng, X.; Nouar, F.; Serre, C. Metal-Organic Frameworks as advanced moisture sorbents for energy-efficient high temperature cooling. Sci. Rep. 2018, 8, 15284. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Fan, H.; Hong, R.; Li, W. Construction of MOF-based superhydrophobic composite coating with excellent abrasion resistance and durability for self-cleaning, corrosion resistance, anti-icing, and loading-increasing research. Chem. Eng. J. 2021, 408, 127343. [Google Scholar] [CrossRef]
- Xiao, C.; He, H.; Li, J.; Zhu, W. Kapitza resistance for nanoscale crystalline and amorphous silicon carbide. In Proceedings of the 19th International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), Toulouse, France, 15–18 April 2018. [Google Scholar]
- Bebek, M.B.; Stanley, C.M.; Gibbons, T.M.; Estreicher, S.K. Temperature dependence of phonon-defect interactions: Phonon scattering vs. phonon trapping. Sci. Rep. 2016, 6, 32150. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, L.; Milanesi, A.; Khlebtsov, B.N.; Centi, S.; Ratto, F.; Khlebtsov, N.G.; Pini, R. Impact of Kapitza resistance on the stability and efficiency of photoacoustic conversion from gold nanorods. J. Colloid Interface Sci. 2020, 578, 358–365. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Mortazavi, B.; Zhuang, X.; Rabczuk, T. Modeling Kapitza resistance of two-phase composite material. Compos. Struct. 2016, 152, 939–946. [Google Scholar] [CrossRef]
- Giri, A.; Hopkins, P.E.; Wessel, J.G.; Duda, J.C. Kapitza resistance and the thermal conductivity of amorphous superlattices. J. Appl. Phys. 2015, 118, 165303. [Google Scholar] [CrossRef]
- Su, Y.; Li, J.J.; Weng, G.J. Theory of thermal conductivity of graphene-polymer nanocomposites with interfacial Kapitza resistance and graphene-graphene contact resistance. Carbon 2018, 137, 222–233. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Zhou, J.; Li, B. Interfacial thermal resistance: Past, present, and future. Rev. Mod. Phyics 2022, 94, 025002. [Google Scholar] [CrossRef]
- Schelling, P.K.; Phillpot, S.R.; Keblinski, P. Kapitza conductance and phonon scattering at grain boundaries by simulation. J. Appl. Phys. 2004, 95, 6082–6091. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Chen, G. Recent Progress in Designing Thermoelectric Metal–Organic Frameworks. Small 2021, 17, 2100505. [Google Scholar] [CrossRef]
- Ma, H.; Gao, B.; Wang, M.; Yuan, Z.; Shen, J.; Zhao, J.; Feng, Y. Strategies for enhancing thermal conductivity of polymer-based thermal interface materials: A review. J. Mater. Sci. 2020, 56, 1064–1086. [Google Scholar] [CrossRef]
- Suckeveriene, R. Chapter 4: Polymers and Nanocomposites with High. In Importance & Applications of Nanotechnology (Volume 7); MedDocs Publishers: Reno, NV, USA, 2021; pp. 26–32. [Google Scholar]
- Ge, L.; Feng, Y.; Wu, J.; Wang, R.; Ge, T. Performance evaluation of MIL-101(Cr) based desiccant-coated heat exchangers for efficient dehumidification. Energy 2024, 289, 130049. [Google Scholar] [CrossRef]
- Wee, M.G.V.; Chinnappan, A.; Shang, R.; Lee, P.S.; Ramakrishna, S. Enhanced moisture sorption through regulated MIL-101(Cr) synthesis and its integration onto heat exchangers. J. Mater. Chem. A 2024, 12, 824–839. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Chen, G. Constructing flexible metal-organic framework/polymer/carbon nanotubes ternary composite films with enhanced thermoelectric properties for heat-to-electricity conversion. Compos. Commun. 2022, 29, 100997. [Google Scholar] [CrossRef]
- Yang, S.; Karve, V.V.; Justin, A.; Kochetygov, I.; Espín, J.; Asgari, M.; Trukhina, O.; Sun, D.T.; Peng, L.; Queen, W.L. Enhancing MOF performance through the introduction of polymer guests. Coord. Chem. Rev. 2021, 427, 213525. [Google Scholar] [CrossRef]
- Kim, H.K.; Yun, W.S.; Kim, M.-B.; Kim, J.Y.; Bae, Y.-S.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal–Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Thakur, A. Applications of copper based metal organic frameworks. Mater. Today Proc. 2022, 50, 1906–1911. [Google Scholar] [CrossRef]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as Adsorbents for Low Temperature Heating and Cooling Applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef]
- Ehrenmann, J.; Henninger, S.K.; Janiak, C. Water Adsorption Characteristics of MIL-101 for Heat-Transformation Applications of MOFs. Eur. J. Inorg. Chem. 2011, 2011, 471–474. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Janiak, C. MOFs for Use in Adsorption Heat Pump Processes. Eur. J. Inorg. Chem. 2012, 2012, 2625–2634. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schossig, P.; Henning, H.-M. Novel Sorption Materials for Solar Heating and Cooling. Energy Procedia 2012, 30, 279–288. [Google Scholar] [CrossRef]
- Janiak, C.; Henninger, S.K. Porous Coordination Polymers as Novel Sorption Materials for Heat Transformation Processes. CHIMIA 2013, 67, 419. [Google Scholar] [CrossRef]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF. RSC Adv. 2014, 4, 24073. [Google Scholar] [CrossRef]
- Jeremias, F.; Khutia, A.; Henninger, S.K.; Janiak, C. MIL-100 (Al, Fe) as water adsorbents for heat transformation purposes—A promising application. J. Mater. Chem. 2012, 22, 10148–10151. [Google Scholar] [CrossRef]
- Jeremias, F.; Lozan, V.; Henninger, S.K.; Janiak, S.K. Programming MOFs for water sorption: Amino-functionalized MIL-125 and UiO-66 for heat transformation and heat storage applications. Dalton Trans. 2013, 42, 15967–15973. [Google Scholar] [CrossRef] [PubMed]
- Khutia, A.; Rammelberg, H.U.; Schmidt, T.; Henninger, S.; Janiak, C. Water Sorption Cycle Measurements on Functionalized MIL-101Cr for Heat Transformation Application. Chem. Mater. 2013, 25, 790–798. [Google Scholar] [CrossRef]
- Elsayed, E.; Saleh, M.M.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Aluminium fumarate metal-organic framework coating for adsorption cooling application: Experimental study. Int. J. Refrig. 2021, 130, 288–304. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Experimental investigation of metal organic frameworks characteristics for water adsorption chillers. Proc. Inst. Mech. Eng. Part C: J. Mech. Eng. Sci. 2012, 227, 992–1005. [Google Scholar] [CrossRef]
- Shi, B.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A.; Elsayed, E. CPO-27(Ni) metal–organic framework based adsorption system for automotive air conditioning. Appl. Therm. Eng. 2016, 106, 325–333. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Characterisation of metal organic frameworks for adsorption cooling. Int. J. Heat Mass Transf. 2012, 55, 7366–7374. [Google Scholar] [CrossRef]
- Sundari, S.S.K.; Rishwana, S.S.; Kotresh, T.M.; Ramani, R.; Shekar, R.I.; Vijayakumar, C.T. Effect of structural variation on the thermal degradation of nanoporous aluminum fumarate metal organic framework (MOF). J. Therm. Anal. Calorim. 2021, 147, 5067–5085. [Google Scholar] [CrossRef]
- Tannert, N.; Jansen, C.; Nießing, S.; Janiak, C. Robust synthesis routes and porosity of the Al-based metal–organic frameworks Al-fumarate, CAU-10-H and MIL-160. Dalton Trans. 2019, 48, 2967–2976. [Google Scholar] [CrossRef]
- Schlüsener, C.; Jordan, D.N.; Xhinovci, M.; Ntep, T.J.M.M.; Schmitz, A.; Giesen, B.; Janiak, C. Probing the limits of linker substitution in aluminum MOFs through water vapor sorption studies: Mixed-MOFs instead of mixed-linker CAU-23 and MIL-160 materials. Dalton Trans. 2020, 49, 7373–7383. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, M.; Askalany, A.A.; Ibrahim, E.M.M.; Mohamed, A.S.A.; Ali, E.S.; Al-Dadah, R. Solar powered adsorption desalination system employing CPO-27(Ni). J. Energy Storage 2022, 53, 105174. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Franco, A.M.M. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron- Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. Mater. Int. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Huo, S.-H.; Yan, X.-P. Metal–organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Yılmaz, E.; Sert, E.; Atalay, F.S. Synthesis, characterization of a metal organic framework: MIL-53 (Fe) and adsorption mechanisms of methyl red onto MIL-53 (Fe). J. Taiwan Inst. Chem. Eng. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Abid, H.R.; Rada, Z.H.; Shang, J.; Wang, S. Synthesis, characterization, and CO2 adsorption of three metal-organic frameworks (MOFs): MIL-53, MIL-96, and amino-MIL-53. Polyhedron 2016, 120, 103–111. [Google Scholar] [CrossRef]
- Zheng, Z.; Nguyen, H.L.; Hanikel, N.; Li, K.K.-Y.; Zhou, Z.; Ma, T.; Yaghi, O.M. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air. Nat. Protoc. 2023, 18, 136–156. [Google Scholar] [CrossRef]
- Solovyeva, M.; Gordeeva, L.; Krieger, T.; Aristov, Y. MOF-801 as a promising material for adsorption cooling: Equilibrium and dynamics of water adsorption. Energy Convers. Manag. 2018, 174, 356–363. [Google Scholar] [CrossRef]
- Luu, C.L.; Nguyen, T.T.V.; Nguyen, T.; Hoang, T.C. Synthesis, characterization and adsorption ability of UiO-66-NH2. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025004. [Google Scholar] [CrossRef]
- Vahabi, A.H.; Norouzi, F.; Sheibani, E.; Rahimi-Nasrabadi, M. Functionalized Zr-UiO-67 metal-organic frameworks: Structural landscape and application. Coord. Chem. Rev. 2021, 445, 214050. [Google Scholar] [CrossRef]
- Ye, X.; Liu, D. Metal–Organic Framework UiO-68 and Its Derivatives with Sufficiently Good Properties and Performance Show Promising Prospects in Potential Industrial Applications. Cryst. Growth Des. 2021, 21, 4780–4804. [Google Scholar] [CrossRef]
- Butova, V.; Budnyk, A.; Bulanova, E.; Lamberti, C.; Soldatov, A. Hydrothermal synthesis of high surface area ZIF-8 with minimal use of TEA. Solid State Sci. 2017, 69, 13–21. [Google Scholar] [CrossRef]
- Laurenz, E.; Füldner, G.; Velte, A.; Schnabel, L.; Schmidtz, G. Frequency response analysis for the determination of thermal conductivity and water transport in MOF adsorbent coatings for heat transformation. Int. J. Heat Mass Transf. 2021, 169, 120921. [Google Scholar] [CrossRef]
- Huang, J.; Xia, X.; Hu, X.; Li, S.; Liu, K. A general method for measuring the thermal conductivity of MOF crystals. Int. J. Heat Mass Transf. 2019, 138, 11–16. [Google Scholar] [CrossRef]
- Babaei, H.; DeCoster, M.E.; Jeong, M.; Hassan, Z.M.; Islamoglu, T.; Baumgart, H.; McGaughey, A.J.H.; Redel, E.; Farha, O.K.; Hopkins, P.E.; et al. Observation of reduced thermal conductivity in a metal-organic framework due to the presence of adsorbates. Nat. Commun. 2020, 11, 4010. [Google Scholar] [CrossRef]
- Babaei, H.; McGhaughey, A.J.H.; Wilmer, C.E. Effect of pore size and shape on the thermal conductivity of metal-organic frameworks. Chem. Sci. 2017, 8, 583–589. [Google Scholar] [CrossRef]
- Yin, Y.; Shao, J.; Zhang, L.; Zui, Q.; Wang, H. Experimental Study on Heat Transfer and Adsorption Cooling Performance of MIL-101/Few Layer Graphene Composite. Energies 2021, 14, 4970. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Veenstra, M.; Dixon, C. Room temperature thermal conductivity measurements of neat MOF-5 compacts with high pressure hydrogen and helium. Int. J. Hydrogen Energy 2016, 41, 4690–4702. [Google Scholar] [CrossRef]
- Huang, B.; Ni, Z.; Millward, A.; McGaughey, A.; Uher, C.; Kaviany, M.; Yaghi, O. Thermal conductivity of a metal-organic framework (MOF-5): Part II. Measurement. Int. J. Heat Mass Transf. 2007, 50, 405–411. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liao, B.; Sheberla, D.; Kraemer, D.; Zhou, J.; Stach, E.A.; Zakharov, D.; Stavila, V.; Talin, A.A.; Ge, Y.; et al. A Microporous and Naturally Nanostructured Thermoelectric Metal-Organic Framework with Ultralow Thermal Conductivity. Joule 2017, 1, 168–177. [Google Scholar] [CrossRef]
- Erickson, K.J.; Léonard, F.; Stavila, V.; Foster, M.E.; Spataru, C.D.; Jones, R.E.; Foley, B.M.; Hopkins, P.E.; Allendorf, M.D.; Talin, A.A. Thin Film Thermoelectric Metal–Organic Framework with High Seebeck Coefficient and Low Thermal Conductivity. Adv. Mater. 2015, 27, 3341–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, J. Thermal Conductivity of Zeolitic Imidazolate Framework-8: A Molecular Simulation Study. J. Phys. Chem. C 2013, 117, 18441–18447. [Google Scholar] [CrossRef]
- Ying, P.; Zhang, J.; Zhang, X.; Zhong, Z. Impacts of Functional Group Substitution and Pressure on the Thermal Conductivity of ZIF-8. J. Phys. Chem. C 2020, 124, 6274–6283. [Google Scholar] [CrossRef]
- Cui, B.; Audu, C.O.; Liao, Y.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T.; Grayson, M. Thermal conductivity of ZIF-8 thin-film under ambient gas pressure. ACS Appl. Mater. Interfaces 2017, 9, 28139–28143. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Ji, H.; Hwang, S.; Kim, K.; Kim, C.; Jeong, N.C. Direct in Situ Conversion of Metals into Metal–Organic Frameworks: A Strategy for the Rapid Growth of MOF Films on Metal Substrates. ACS Appl. Mater. Interfaces 2016, 8, 32414–32420. [Google Scholar] [CrossRef]
- Peterson, G.W.; Lee, D.T.; Barton, H.F.; Epps, T.H.; Parsons, G.N. Fibre-based composites from the integration of metal–organic frameworks and polymers. Nat. Rev. Mater. 2021, 6, 605–621. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Depauw, H.; Coudert, F.X.; Vreilinck, H.; Voort, P.V.D.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chakraborty, A. Recent advances in metal-organic frameworks for adsorption heat transformations. Renew. Sustain. Energy Rev. 2024, 198, 114411. [Google Scholar] [CrossRef]
- Babu, A.M.; Varghese, A. Electrochemical deposition for metal organic Frameworks: Advanced Energy, Catalysis, sensing and separation applications. J. Electroanal. Chem. 2023, 937, 117417. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.R.C.; Li, M.; Stappers, L.; Dincă, M.; Denayer, J.F.M.; Binnemans, K.; De Vos, D.E.; Fransaer, J. On the electrochemical deposition of metal–organic frameworks. J. Mater. Chem. A 2016, 4, 3914–3925. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochemical deposition of metal–organic framework films and their applications. J. Mater. Chem. A 2020, 8, 7569. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef]
- Muresan, L.M. Nanocomposite Coatings for Anti-Corrosion Properties of Metallic Substrates. Materials 2023, 16, 5092. [Google Scholar] [CrossRef]
- Wei, L.; Dongxue, J.; Wenhui, L.; Yu, Z. Recent advances in the applications of metal-organic frameworks-based molecularly imprinted materials. Chin. J. Chromatogr. 2023, 41, 651–661. [Google Scholar]
- Mahdi, E.M.; Tan, J.C. Mixed-matrix membranes of zeolitic imidazolate framework (ZIF-8)/Matrimid nanocomposite: Thermo-mechanical stability and viscoelasticity underpinning membrane separation performance. J. Membr. Sci. 2016, 498, 276–290. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S., Jr.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Zhang, J.; Xiang, T. Recent advances on ZIF-8 composites for adsorption and photocatalytic wastewater pollutant removal: Fabrication, applications and perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar] [CrossRef]
- Carter, D.; Tezel, F.H.; Kruczek, B.; Kalipcilar, H. Investigation and comparison of mixed matrix membranes composed of polyimide matrimid with ZIF—8, silicalite, and SAPO—34. J. Membr. Sci. 2017, 544, 35–46. [Google Scholar] [CrossRef]
- Li, T.T.; Cen, X.; Ren, H.T.; Wu, L.; Peng, H.K.; Wang, W.; Gao, B.; Lou, C.W.; Lin, J.H. Zeolitic Imidazolate Framework-8/Polypropylene–Polycarbonate Barklike Meltblown Fibrous Membranes by a Facile in Situ Growth Method for Efficient PM2.5 Capture. ACS Appl. Mater. Interfaces 2020, 12, 8730–8739. [Google Scholar] [CrossRef] [PubMed]
- Albdoor, A.K.; Ma, Z.; Cooper, P.; Al-Ghazzawi, F.; Liu, J.; Richardson, C.; Wagner, P. Air-to-air enthalpy exchangers: Membrane modification using metal-organic frameworks, characterisation and performance assessment. J. Clean. Prod. 2021, 293, 126157. [Google Scholar] [CrossRef]
- Chung, C.K. Development of Dual-Layer Membrane and Metal Organic Framework for Gas Separation to Improve Indoor Air Quality; Universiti Tunku Abdul Rahman (UTAR): Jaya, Malaysia, 2023. [Google Scholar]
- Kim, E.; Patel, R. Recent Advances in Metal Organic Framework based Thin Film Nanocomposite Membrane for Nanofiltration. Membr. J. 2021, 31, 35–51. [Google Scholar] [CrossRef]
- Iqbal, A.; Cevik, E.; Mustafa, A.; Qahtan, T.F.; Zeeshan, M.; Bozkurt, A. Emerging developments in polymeric nanocomposite membrane-based filtration for water purification: A concise overview of toxic metal removal. Chem. Eng. J. 2024, 481, 148760. [Google Scholar] [CrossRef]
- Guo, F.; Li, B.; Ding, R.; Li, D.; Jiang, X.; He, G.; Xiao, W. A Novel Composite Material UiO-66@HNT/Pebax Mixed Matrix Membranes for Enhanced CO2/N2 Separation. Membranes 2021, 11, 693. [Google Scholar] [CrossRef]
- Giliopoulus, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2019, 25, 185. [Google Scholar] [CrossRef]
- Khosrojerdi, S.; Gholami, L.; Khazaei, M.; Hashemzadeh, A.; Darroudi, M.; Oskuee, R.K. Synthesis and evaluation of gene delivery vectors based on PEI-modified metal-organic framework (MOF) nanoparticles. Iran. J. Basic Med. Sci. 2023, 27, 203–213. [Google Scholar]
- Singh, V.; Saini, I. Recent Developments in MOF-Polymer Composites. In Metal-Organic Frameworks-Based Hybrid Materials for Environmental Sensing and Monitoring; CRC Press: Boca Raton, FL, USA, 2022; pp. 20–32. [Google Scholar]
- Yang, X.; Cheng, T.C.; Morris, A.J. Polymer-grafted metal–organic frameworks: Design, synthesis, and application. J. Mater. Chem. C 2024, 12, 4562–4592. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Tian, N.; Gao, Y.; Wu, J.; Luo, S.; Dai, W. Water-resistant HKUST-1 functionalized with polydimethylsiloxane for efficient rubidium ion capture. New J. Chem. 2019, 43, 15339–15547. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Wang, H.; Li, L.; Zhang, X.; Zhao, R.; Han, J.; Wang, L. MOF-derived hollow octahedral CoxP/MOF-801 p-n heterojunction for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2024, 81, 66–74. [Google Scholar] [CrossRef]
- Lenzen, D.; Zhao, J.; Ernst, S.-J.; Wahiduzzaman, M.; Inge, A.K.; Fröhlich, D.; Xu, H.; Bart, H.-J.; Janiak, C.; Henninger, S.; et al. A metal–organic framework for efficient water-based ultra-low-temperature-driven cooling. Nat. Commun. 2019, 10, 3025. [Google Scholar] [CrossRef] [PubMed]
- Chaemchuen, S.; Xiao, X.; Klomkliang, N.; Yusubov, M.S.; Verpoort, F. Tunable Metal–Organic Frameworks for Heat Transformation Applications. Nanomaterials 2018, 8, 661. [Google Scholar] [CrossRef]
- Rocchetti, A.; Lippi, M.; Socci, L.; Gullo, P.; Khorshidi, V.; Talluri, L. Metal-Organic Framework Adsorbent Materials in HVAC Systems: General Survey and Theoretical Assessment. Energies 2022, 15, 8908. [Google Scholar] [CrossRef]
- Emam, H.E.; Abdelhameed, R.M.; Ahmed, H.B. Adsorptive Performance of MOFs and MOF Containing Composites for Clean Energy and Safe Environment. J. Environ. Chem. Eng. 2020, 8, 104386. [Google Scholar] [CrossRef]
- Chang, C.; Luo, W.; Lu, C.; Cheng, Y.; Tsai, B.; Lin, Z. Effects of process air conditions and switching cycle period on dehumidification performance of desiccant-coated heat exchangers. Sci. Technol. Built Environ. 2017, 23, 81–90. [Google Scholar] [CrossRef]
- Hou, P.; Zu, K.; Qin, M.; Cui, S. A novel metal-organic frameworks based humidity pump for indoor moisture control. Build. Environ. 2021, 187, 107396. [Google Scholar] [CrossRef]
- Aziz, A.N.; Mahmoud, S.; Al-Dadah, R.; Taskin, A.; Ismail, M.A.; Fahmy, Y.M.; Rashid, M.M. Novel MOF-303/G coated wire-finned heat exchanger for dehumidification applications–Experimental investigation. Energy 2024, 305, 132333. [Google Scholar] [CrossRef]
- Albaik, I.; Elsheniti, M.B.; Al-Dadah, R.; Mahmoud, S.; Solmaz, I. Numerical and experimental investigation of multiple heat exchanger. Energy Convers. Manag. 2022, 251, 114934. [Google Scholar] [CrossRef]
- Shahvari, S.Z.; Kalkhorani, V.A.; Clark, J.D. Performance evaluation of a metal organic frameworks based combined dehumidification and indirect evaporative cooling system in different climates. Int. J. Refrig. 2022, 140, 186–197. [Google Scholar] [CrossRef]
- Harrouz, J.P.; Katramiz, E.; Ghali, K.; Ouahrani, D.; Ghaddar, N. Life cycle assessment of desiccant—Dew point evaporative cooling systems with water reclamation for poultry houses in hot and humid climate. Appl. Therm. Eng. 2022, 210, 118419. [Google Scholar] [CrossRef]
- Aziz, A.N.; Mahmoud, S.; Al-Dadah, R.; Ismail, M.A.; Al Mesfer, M.K. Numerical and experimental investigation of desiccant cooling system using metal organic framework materials. Appl. Therm. Eng. 2022, 215, 118940. [Google Scholar] [CrossRef]
- Ye, L.; Rupam, T.H.; Islam, M.A.; Saha, B.B. Study of metal–organic framework (MOF)/water pairs for adsorption heat transformer applications. Therm. Sci. Eng. Prog. 2024, 51, 102595. [Google Scholar] [CrossRef]
- Gholami, F.; Zinadini, S.; Zinatizadeh, A.; Abbasi, A.R. TMU-5 metal-organic frameworks (MOFs) as a novel nanofiller for flux increment and fouling mitigation in PES ultrafiltration membrane. Sep. Purif. Technol. 2018, 194, 272–280. [Google Scholar] [CrossRef]
- Campagnol, N.; Assche, T.V.; Boudewijns, T.; Denayer, J.; Binnemans, K.; De Vos, D.; Fransaer, J. High pressure, high temperature electrochemical synthesis of metal–organic frameworks: Films of MIL-100 (Fe) and HKUST-1 in different morphologies. J. Mater. Hemistry A 2013, 1, 5827–5830. [Google Scholar] [CrossRef]
- Li, H.; Yan, M.; Zhao, W. Designing a MOF-based slippery lubricant-infused porous surface with dual functional anti-fouling strategy. J. Colloid Interface Sci. 2022, 607, 1424–1435. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Zahng, X.; Li, Q.; Zhang, G.; Pu, S.; Liu, F.Q. UV-curable PBMA coating containing CuZn-MOF-74 for fouling-resistance. Microporous Mesoporous Mater. 2024, 368, 113020. [Google Scholar] [CrossRef]
- Moaness, M.; El-Sayed, S.A.M.; Beherei, H.H.; Mabrouk, M. Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies. J. Funct. Biom. 2024, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zi, Y.; Zhu, J.; Huang, W.; Zhang, Z.; Zhang, H. Construction of super-hydrophobic PDMS@MOF@Cu mesh for reduced drag, anti-fouling and self-cleaning towards marine vehicle applications. Chem. Eng. J. 2021, 417, 129265. [Google Scholar] [CrossRef]
- Arsenyeva, O.; Tovazhnyanskyy, L.; Kapustenko, P.; Klemeš, J.J.; Varbanov, P.S. Review of Developments in Plate Heat Exchanger Heat Transfer Enhancement for Single-Phase Applications in Process Industries. Energies 2023, 16, 4976. [Google Scholar] [CrossRef]
- Venegas, T.; Qu, M.; Nawaz, K.; Wang, L. Critical review and future prospects for desiccant coated heat exchangers: Materials, design, and manufacturing. Renew. Sustain. Energy Rev. 2021, 151, 111531. [Google Scholar] [CrossRef]
- Edries, E.; Petrov, A. Types of heat exchangers in industry, their advantages and disadvantages, and the study of their parameters. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Moscow, Russia, 8 September 2020. [Google Scholar]
- Zhang, J.; Zhu, X.; Mondejar, M.E.; Haglind, F. A review of heat transfer enhancement techniques in plate heat exchangers. Renew. Sustain. Energy Rev. 2019, 101, 305–328. [Google Scholar] [CrossRef]
- Sun, X.; Chen, J.; Zhao, Y.; Li, X.; Ge, T.; Wang, C.; Dai, Y. Experimental investigation on a dehumidification unit with heat recovery using desiccant coated heat exchanger in waste to energy system. Appl. Therm. Eng. 2021, 185, 116342. [Google Scholar] [CrossRef]
- Pinheiro, J.M.; Salústio, S.; Geraldes, V.; Valente, A.A.; Silva, C.M. Copper foam coated with CPO-27(Ni) metal–organic framework for adsorption heat pump: Simulation study using OpenFOAM. Appl. Therm. Eng. 2020, 178, 115498. [Google Scholar] [CrossRef]
- Zu, K.; Qin, M. Optimization of the hygrothermal performance of novel metal-organic framework (MOF) based humidity pump: A CFD approach. Energy 2022, 259, 125073. [Google Scholar] [CrossRef]
- Hua, Z.; Cai, S.; Xu, H.; Yuan, W.; Li, S.; Tu, Z. Investigations of Silica/MOF composite coating and its dehumidification performance on a desiccant-coated heat exchanger. Energy 2024, 307, 132576. [Google Scholar] [CrossRef]
- Kummer, H.; Jeremias, F.; Warlo, A.; Füldner, G.; Fröhlich, D.; Janiak, C.; Gläser, R.; Henninger, S.K. A Functional Full-Scale Heat Exchanger Coated with Aluminum Fumarate Metal–Organic Framework for Adsorption Heat Transformation. Ind. Eng. Chem. Res. 2017, 56, 8393–8398. [Google Scholar] [CrossRef]
- Lee, J.-G.; Bae, K.J.; Kwon, O.K. Experimental investigation of the solid desiccant dehumidification system with metal organic frameworks. Int. J. Refrig. 2021, 130, 179–186. [Google Scholar] [CrossRef]
- Albaik, I.; Diab, K.E.; Saleh, M.; Al-Dadah, R.; Mahmoud, S.; Elsheniti, M.B.; Solmaz, I.; Salama, E.; Hassan, H.S.; Elkadi, M.F. MOF based coated adsorption system for water desalination and cooling integrated with Pre-treatment unit. Sustain. Energy Technol. Assess. 2023, 56, 103006. [Google Scholar] [CrossRef]
- Albaik, I.; Al-Dadah, R.; Mahmoud, S.; Solmaz, I. Non-equilibrium numerical modelling of finned tube heat exchanger for adsorption desalination/cooling system using segregated solution approach. Appl. Therm. Eng. 2021, 183, 116171. [Google Scholar] [CrossRef]
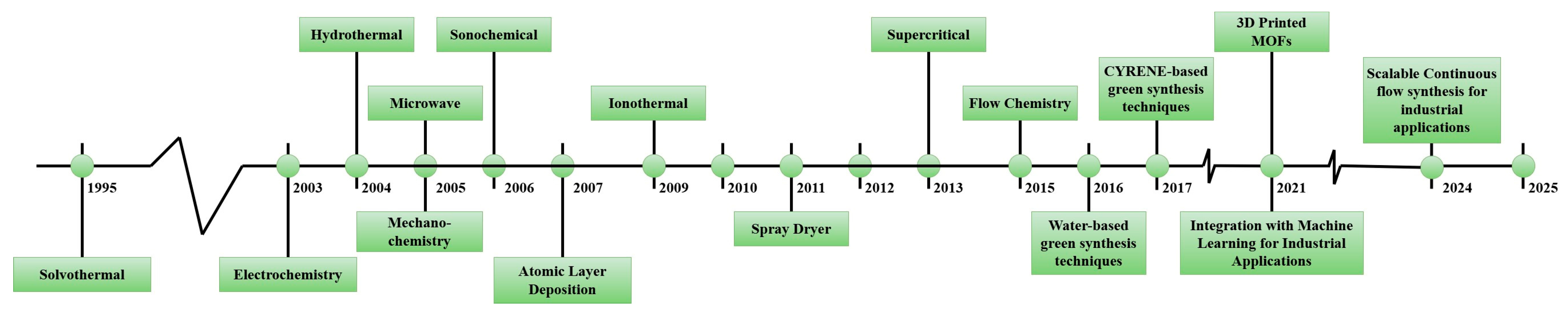
- Sud, S.; Kaur, G. A comprehensive review on synthetic approaches for metal-organic frameworks: From traditional solvothermal to greener protocols. Polyhedron 2021, 193, 114897. [Google Scholar] [CrossRef]
- Phan, P.T.; Hong, J.; Tran, N.; Le, T.H. The Properties of Microwave-Assisted Synthesis of Metal–Organic Frameworks and Their Applications. Nanomaterials 2023, 13, 352. [Google Scholar] [CrossRef]
- Klinowski, J.; Paz, F.A.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal–Organic Frameworks. Dalton Trans. 2011, 321, 40. [Google Scholar] [CrossRef]
- Chakraborty, D.; Yurdusen, A.; Mouchaham, G.; Nouar, F.; Serre, C. Large-Scale Production of Metal–Organic Frameworks. Adv. Funct. Mater. 2023, 34, 2309089. [Google Scholar] [CrossRef]
- Paul, T.; Juma, A.; Alqerem, R.; Karanikolos, G.; Arafat, H.A.; Dumée, L.F. Scale-up of metal-organic frameworks production: Engineering strategies and prospects towards sustainable manufacturing. J. Environ. Chem. Eng. 2023, 11, 111112. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal–organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Kustov, L.M. Microwave activation as an alternative production of metal-organic frameworks. Russ. Chem. Bull. 2016, 65, 2103–2114. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R.I. Rapid Production of Metal−Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, X.; Zhu, Q.; Dong, R.; Yang, R.; Cheng, Y.; He, C. Microwave-Assisted Rapid Synthesis of Well-Shaped MOF-74 (Ni) for CO2 Efficient Capture. Inorg. Chem. 2019, 58, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.K.; Chatterjee, T.; Alam, S.M. Chapter 4—Microwave-assisted synthesis of metal–organic frameworks. In Synthesis of Metal-Organic Frameworks Via Water-Based Routes—A Green and Sustainable Approach; Elsevier Inc.: Amsterdam, The Netherlands, 2024; pp. 51–72. [Google Scholar]
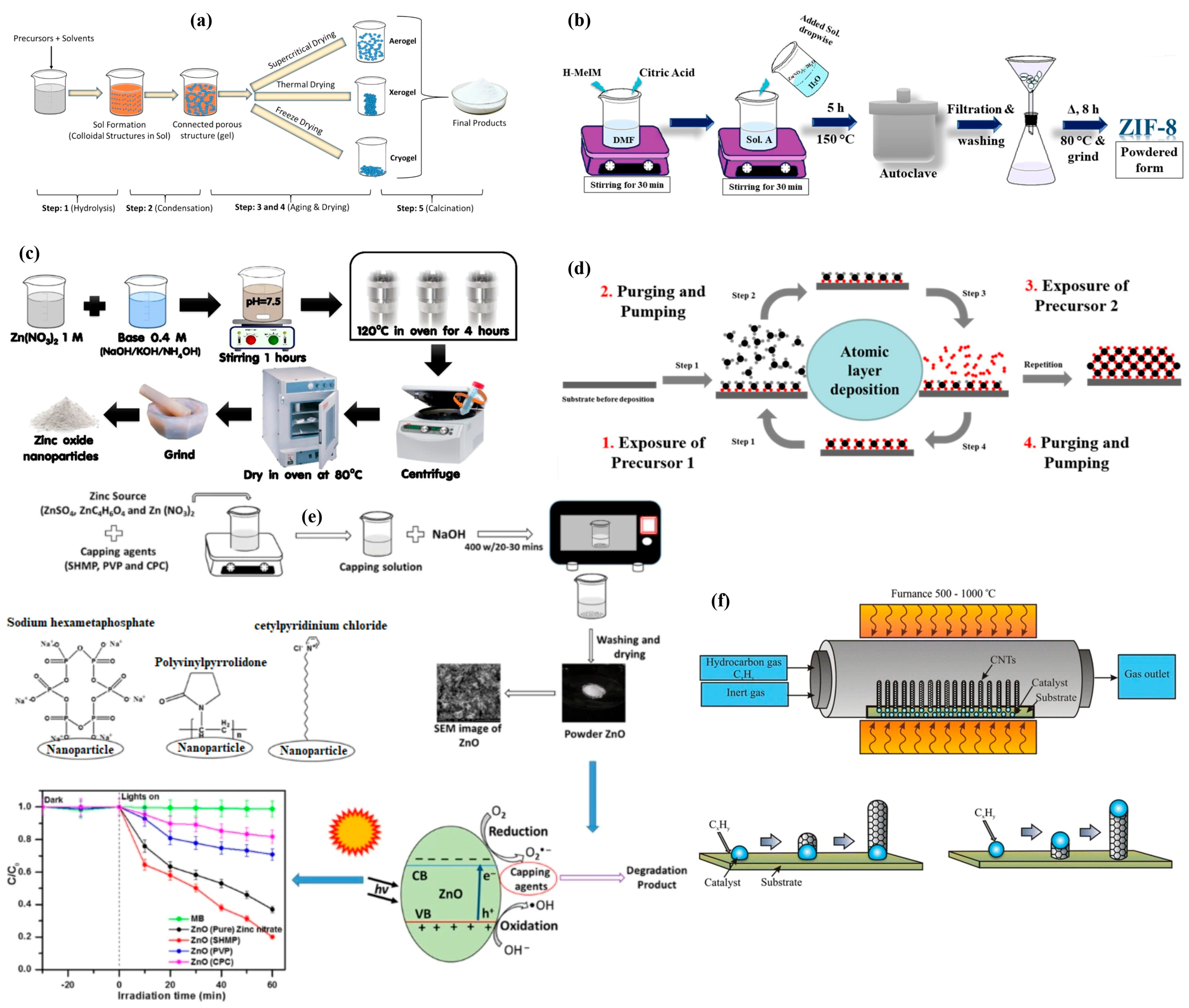
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Effect of calcination temperature on electrochemical behavior of pristine zeolitic imidazolate framework-8. Ionics 2022, 28, 5395–5404. [Google Scholar] [CrossRef]
- Wirunchit, S.; Koetniyom, W. ZnO Nanoparticles Synthesis and Characterization by Hydrothermal Process for Biological Applications. Phys. Status Solidi A 2022, 220, 2200364. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cha, B.J.; Saqlain, S.; Seo, H.O.; Kim, Y.D. Atomic Layer Deposition for Preparation of Highly Efficient Catalysts for Dry Reforming of Methane. Catalysts 2019, 9, 266. [Google Scholar] [CrossRef]
- Mageswari, K.; Prabukanthan, P.; Madhavan, J. Microwave-assisted synthesis of ZnO nanoparticles using different capping agents and their photocatalytic application. Environ. Sci. Pollut. Res. 2023, 30, 40174–40188. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2020, 31, 2006291. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal–Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Lal, S.; Singh, P.; Singhal, A.; Kumar, S.; Gahlot, A.P.S.; Gandhi, N.; Kumari, P. Advances in metal–organic frameworks for water remediation applications. RSC Adv. 2024, 14, 3413–3446. [Google Scholar] [CrossRef] [PubMed]
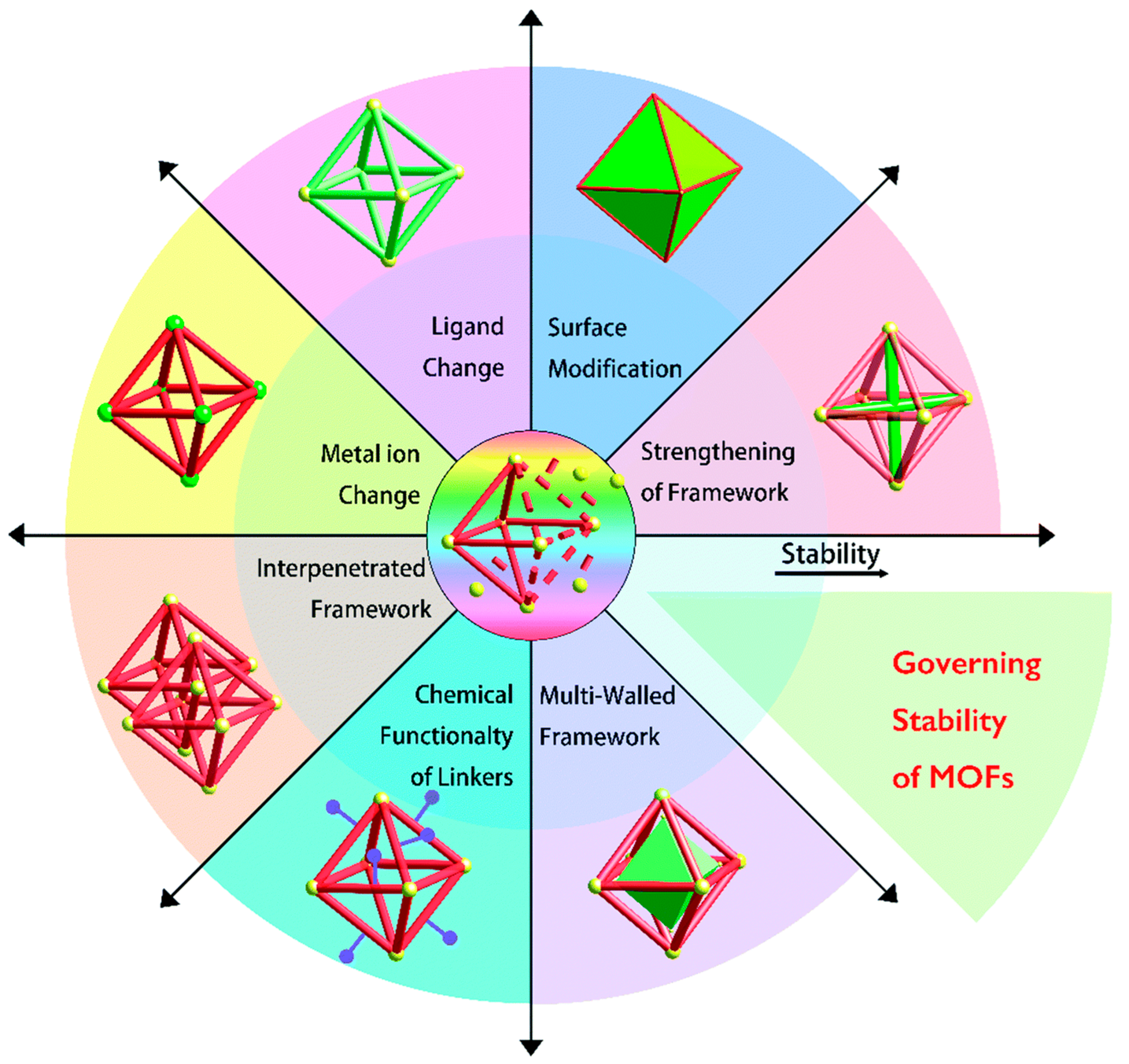
- Li, N.; Xu, J.; Feng, R.; Hu, T.-L.; Bu, X.-H. Governing metal–organic frameworks towards high stability. Chem. Commun. 2016, 52, 8501–8513. [Google Scholar] [CrossRef] [PubMed]
- James, J.B.; Lang, L.; Meng, L.; Lin, J.Y.S. Postsynthetic Modification of ZIF-8 Membranes via Membrane Surface Ligand Exchange for Light Hydrocarbon Gas Separation Enhancement. ACS Appl. Mater. Interfaces 2019, 12, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Yang, M.; Ma, Q.; Qi, Y.; Gao, H.; Wu, Z.; Wang, G. Introduction of an organic acid phase changing material into metal–organic frameworks and the study of its thermal properties. J. Mater. Chem. A 2016, 4, 7641. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Functionalization, protonation and ligand extension on MIL-53 (Al) MOFs to boost water adsorption and thermal energy storage for heat transformations. Chem. Eng. J. 2023, 472, 145137. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Subhan, S.; Yu, X.; Liu, Z.; Chen, R.; Deng, J.; Ji, H.; Zhao, Z.; Zhao, Z. Construction of HKUST-1@Cu nanofibers with thermal conductive adsorption sites for synchronous enhancement of toluene adsorption and desorption efficiency. Sep. Purif. Technol. 2024, 339, 126624. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Experimental investigation for water adsorption characteristics on functionalized MIL-125 (Ti) MOFs: Enhanced water transfer and kinetics for heat transformation systems. Int. J. Heat Mass Transf. 2022, 186, 122473. [Google Scholar] [CrossRef]
- Huo, Y.; Xiu, S.; Meng, L.-Y.; Quan, B. Solvothermal synthesis and applications of micro/nano carbons: A review. Chem. Eng. J. 2023, 451, 138572. [Google Scholar] [CrossRef]
- Begum, S.; Hassan, Z.; Bra, S.; Wo, C.; Tsotsalas, M. Metal–Organic Framework-Templated Biomaterials: Recent Progress in Synthesis, Functionalization, and Applications. Acc. Chem. Res. 2019, 52, 1598–1610. [Google Scholar] [CrossRef]
- Severino, M.I.; Gkaniatsou, E.; Nouar, F.; Pinto, M.L.; Serre, C. MOFs industrialization: A complete assessment of production costs. Faraday Discuss. 2021, 231, 326–341. [Google Scholar] [CrossRef]
- Shahvari, S.Z.; Kalkhorani, V.A.; Wade, C.R.; Clark, J.D. Benefits of metal–organic frameworks sorbents for sorbent wheels used in air conditioning systems. Appl. Therm. Eng. 2022, 210, 118407. [Google Scholar] [CrossRef]
- Thomas-Hillman, I.; Laybourn, A.; Dodds, C.; Kingman, S.W. Realising the environmental benefits of metal–organic frameworks: Recent advances in microwave synthesis. J. Mater. Chem. A 2018, 6, 11564–11581. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Wang, J.J.; Cai, Z.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Enhanced Performance of Sulfonated Poly(ether ether Ketone) Hybrid Membranes by Introducing Sulfated MOF-808/Graphene Oxide Composites. ACS Appl. Energy Mater. 2021, 4, 9664–9672. [Google Scholar] [CrossRef]
- Álvarez, J.R.; Sánchez-González, E.; Pérez, E.; Schneider-Revueltas, E.; Martínez, A.; Tejeda-Cruz, A.; Islas-Jácome, A.; González-Zamora, E.; Ibarra, I.A. Structure stability of HKUST-1 towards water and ethanol and their effect on its CO2 capture properties. Dalton Trans. 2017, 46, 9192–9200. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Jiang, H.-L. Improving Water Stability of Metal–Organic Frameworks by a General Surface Hydrophobic Polymerization. CCS Chem. 2021, 3, 2740–2748. [Google Scholar] [CrossRef]
- Khouki, M.; Al Khatib, O. Performance of Desiccant-Based Cooling Systems in Hot-Humid Climates: A Review. Energy Eng. 2021, 118, 875–909. [Google Scholar] [CrossRef]
- Zhao, L.H.; Wang, R.Z.; Ge, T.S. Desiccant coated heat exchanger and its applications. Int. J. Refrig. 2021, 130, 217–232. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, T.S.; Wang, R.Z. Recent progress on desiccant materials for solid desiccant cooling systems. Energy 2014, 74, 280–294. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Activated carbon/metal-organic framework composite as a bio-based novel green adsorbent: Preparation and mathematical pollutant removal modeling. J. Mol. Liq. 2019, 277, 310–322. [Google Scholar] [CrossRef]
- Hu, L.; Ge, T.; Jiang, Y.; Wang, R. Performance study on composite desiccant material coated fin-tube heat exchangers. Int. J. Heat Mass Transf. 2015, 90, 109–120. [Google Scholar] [CrossRef]
- Cui, S.; Marandi, A.; Lebourleux, G.; Thimon, M.; Bourdon, M.; Chen, C.; Severino, M.I.; Steggles, V.; Nouar, F.; Serre, C. Heat properties of a hydrophilic carboxylate-based MOF for water adsorption applications. Appl. Therm. Eng. 2019, 161, 114135. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tome, J.P.C. Multifunctional metal–organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.J.; Huang, L.J.; Han, Z.Y.; Wang, Y.W.; Zhang, Z.J.; Wang, Y.; Chang, Q.R.; Wei, N.; Kipper, M.J.; Tang, J.G. A review of graphene-oxide/metal–organic framework composites materials: Characteristics, preparation and applications. J. Porous Mater. 2021, 28, 1837–1865. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of hybrid graphene-MOF materials. J. Colloid Interface Sci. 2018, 514, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.; Chen, J.; Li, X.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. Recent development and applications of electrical conductive MOFs. Nanoscale 2020, 13, 485–509. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Chang, S.J.; Berardi, U.; Kim, K.-H.; Kim, S. Potential utility of HKUST-1-graphite nanocomposite to endow alkane with high thermal properties and low electrical resistivity. J. Hazard. Mater. 2021, 402, 123695. [Google Scholar] [CrossRef]
- Ghanbari, T.; Patah, M.F.A.; Wong, Y.H.; Abnisa, F.; Daud, W.M.A.W. Probing the capability of the MOF-74(Ni)@GrO composite for CO2 adsorption and CO2/N2 separation: A combination of experimental and molecular dynamic simulation studies. Fuel 2024, 372, 131837. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Prabhakaran, P.K.; Pré, P. Hydrogen adsorption and kinetics in MIL-101(Cr) and hybrid activated carbon-MIL-101(Cr) materials. Int. J. Hydrogen Energy 2017, 42, 8021–8031. [Google Scholar] [CrossRef]
- Qadir, N.U.; Said, S.A.M.; Mansour, R.B.; Mezghani, K.; Ul-Hamid, A. Synthesis, characterization, and water adsorption properties of a novel multi-walled carbon nanotube/MIL-100(Fe) composite. Dalton Trans. 2016, 45, 15621–15633. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, Z.; Li, H.; Liu, X. MIL-100(Fe) and its derivatives: From synthesis to application for wastewater decontamination. Environ. Sci. Pollut. Res. 2020, 27, 4703–4724. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Alalm, M.G. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 127152. [Google Scholar] [CrossRef]
- Kazemi, A.; Pordsari, M.A.; Tamtaji, M.; Afshari, M.H.; Keshavarz, S.; Zeinali, F.; Baesmat, H.; Zahiri, S.; Manteghi, F.; Ghaemi, A.; et al. Unveiling the power of defect engineering in MOF-808 to enhance efficient carbon dioxide adsorption and separation by harnessing the potential of DFT analysis. Chem. Eng. J. 2024, 494, 153049. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Wei, Y.; He, P.; Lv, B.; Ma, H.; Lei, Z. MIL-53(Fe)-graphene nanocomposites: Efficient visible-light photocatalysts for the selective oxidation of alcohols. Appl. Catal. B Environ. 2016, 198, 112–123. [Google Scholar] [CrossRef]
- Serventi, D.R. MIL-53 (Al) and Graphene Oxide Nanocomposites for Dye Adsorption. 2020. Available online: http://hdl.handle.net/11122/11877 (accessed on 14 November 2024).
- Abdi, J.; Banisharif, F.; Khataee, A. Amine-functionalized Zr-MOF/CNTs nanocomposite as an efficient and reusable photocatalyst for removing organic contaminants. J. Mol. Liq. 2021, 334, 116129. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Wu, Y.; Li, Y.; Yan, Y.; Zhu, J. In-situ synthesis of CNT/UiO-66-NH2-based molecularly imprinted nanocomposite membranes for selective recognition and separation of sulfamethoxazole: A synergistic promotion system. Surf. Interfaces 2022, 31, 101986. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Bakhtiari, M.; Oveisi, M.; Mahmoodi, B.; Hayati, B. Green synthesis of eco-friendly magnetic metal-organic framework nanocomposites (AlFum-graphene oxide) and pollutants (dye and pharmaceuticals) removal capacity from water. Mater. Chem. Phys. 2023, 302, 127720. [Google Scholar] [CrossRef]
- Abdi, S.; Nasiri, M. Enhanced Hydrophilicity and Water Flux of Poly(ether sulfone) Membranes in the Presence of Aluminum Fumarate Metal–Organic Framework Nanoparticles: Preparation and Characterization. ACS Appl. Mater. Interfaces 2019, 11, 15060–15070. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Zhao, J.; Yang, J.; Zhou, Z.; Du, X.; Lu, X. Evaluation and development of GO/UiO-67@PtNPs nanohybrid-based electrochemical sensor for invisible arsenic (III) in water samples. Microchem. J. 2022, 181, 107765. [Google Scholar] [CrossRef]
- Chen, Z.; Wasson, M.C.; Drout, R.J.; Robison, L.; Idrees, K.B.; Knapp, J.G.; Son, F.A.; Zhang, X.; Hierse, W.; Kühn, C.; et al. The state of the field: From inception to commercialization of metal–organic frameworks. Faraday Discuss. 2021, 225, 9–69. [Google Scholar] [CrossRef]
- Kong, X.J.; Li, J.R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Yan, X.; Lv, Y. Recent advances in metal-organic frameworks: Synthesis, application and toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef]
- Jahan, I.; Rupam, T.H.; Palash, M.; Rocky, K.A.; Saha, B.B. Energy efficient green synthesized MOF-801 for adsorption cooling applications. J. Mol. Liq. 2022, 345, 117760. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thünemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical Synthesis of Metal−Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical synthesis of metal–organic frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Almohasin, J.A.; Balag, J.; Miral, V.G.; Moreno, R.V.; Tongco, L.J.; Lopez, E.C.R. Green Solvents for Liquid–Liquid Extraction: Recent Advances and Future Trends. Eng. Proc. 2023, 56, 174. [Google Scholar] [CrossRef]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure stability of metal-organic framework MIL-53 (Al) in aqueous solutions. Int. J. Hydrogen Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Alvares, E.; Tantoro, S.; Wijaya, C.J.; Cheng, K.C.; Soetaredjo, F.E.; Hsu, H.Y.; Angkawijaya, A.E.; Go, A.W.; Hsieh, C.W.; Santoso, S.P. Preparation of MIL100/MIL101-alginate composite beads for selective phosphate removal from aqueous solution. Int. J. Biol. Macromol. 2023, 231, 123322. [Google Scholar] [CrossRef] [PubMed]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Advances in Microwave Synthesis of Nanoporous Materials. Adv. Mater. 2021, 33, 2103477. [Google Scholar] [CrossRef] [PubMed]
- Boukayouht, K.; Bazzi, L.; El Hankari, S. Sustainable synthesis of metal-organic frameworks and their derived materials from organic and inorganic wastes. Coord. Chem. Rev. 2023, 478, 214986. [Google Scholar] [CrossRef]
- Pham, H.K.; Sim, Y.; Carboni, M.; Meyer, D.; Mathews, N. Generating metal-organic frameworks (MOFs) from photovoltaic modules for wastewater remediation. J. Environ. Chem. Eng. 2022, 10, 108346. [Google Scholar] [CrossRef]

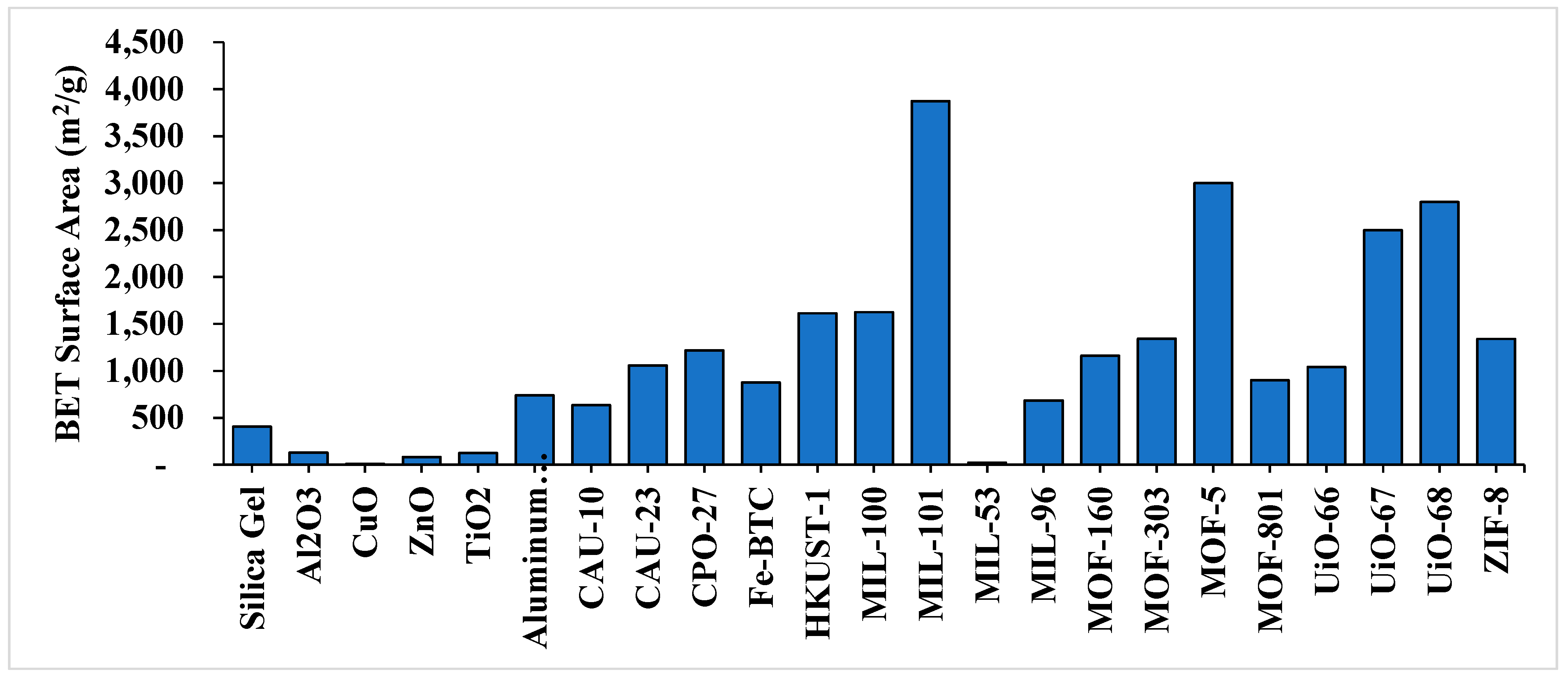
| S. No. | MOF | Thermal Conductivity in W/(mK) | Main Findings | Ref. |
|---|---|---|---|---|
| 1 | Aluminum Fumarate | 0.07 |
| [98] |
| 2 | Cu-BTC UiO-66 UiO-67 | 0.39 0.11 0.19 |
| [99] |
| 3 | HKUST-1 | 0.44–0.73 |
| [100] |
| 4 | Idealized MOF structures featuring various pore shapes, including cubic pores, triangular channels, and hexagonal channels | 0.03 |
| [101] |
| 5 | MIL-101/20% Few Layer Graphene (FLG) | 0.8322–0.8603 |
| [102] |
| 6 | MOF-5 | 0.1218–0.1477 |
| [103] |
| 7 | MOF-5 | 0.32 |
| [104] |
| 8 | MOF-5 | 0.33 |
| [105] |
| 9 | Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2 (Ni3(HITP)2) a 2D MOF | 0.21 |
| [106] |
| 10 | Tetracyanoquinodimethane (TCNQ) @HKUST-1 | 0.23–0.31 |
| [107] |
| 11 | ZIF-8 | 0.165–0190 |
| [108] |
| 12 | ZIF-8(H)ZIF-8 (Cl)ZIF-8 (Br)ZIF-8 (CH3) | 0.165 0.138 0.142 0.174 |
| [109] |
| 13 | ZIF-8 | 0324–0.328 |
| [110] |
| S. No. | Composite Name | Description | Applications | Ref. |
|---|---|---|---|---|
| 1 | ZIF-8/Polyimide (PI) Composite | ZIF-8 embedded in polyimide enhances thermal conductivity while maintaining the flexibility and robustness of the polymer. | HVAC systems for efficient heat transfer and moisture management, as well as in gas separation applications. | [127,128,129] |
| 2 | MIL-101(Cr)/Polysulfone (PSU) Composite | MIL-101(Cr) combined with PSU improves mechanical strength and thermal stability. The high surface area of MIL-101(Cr) contributes to superior adsorption capabilities. | Air purification, nano-filtration, and gas separation. | [130,131,132] |
| 3 | UiO-66/Polyethylene Oxide (PEO) Composite | UiO-66 incorporated into PEO enhances thermal conductivity and mechanical properties, along with increasing moisture resistance. | Lithium-ion batteries, proton exchange membranes, drug delivery, and water filtration systems. | [133,134,135] |
| 4 | MOF-5/Polymethyl Methacrylate (PMMA) Composite | MOF-5 embedded in PMMA improves the thermal and mechanical properties of the polymer, enhancing its use in structural applications. | Lightweight thermal insulation panels and coatings. | [136,137] |
| 5 | HKUST-1/PDMS Composite | HKUST-1 integrated with PDMS increases thermal conductivity while retaining the flexibility and elasticity of PDMS. | Heat exchangers, wearable electronics, and gas sensors. | [138,139] |
| 6 | MOF-801/Polyurethane (PU) Composite | MOF-801 embedded in PU improves thermal stability and adsorption properties, making it ideal for applications requiring flexibility and durability. | HVAC systems, energy-efficient coatings, and adsorptive cooling systems. | [140,141] |
| S. No. | MOF Used | Heat Exchanger Type | Main Findings | Ref. |
|---|---|---|---|---|
| 1 | Sodium polyacrylate | Single-row finned-tube |
| [145] |
| 2 | MIL-100 (Fe) | Rectangular finned heat sinks |
| [146] |
| 3 | Aluminum Fumarate MIL-100 (Fe) MIL-100 (Fe)/G MOF-303/G MOF-801 MOF-801/G | Wire-finned heat exchanger |
| [147] |
| 4 | Aluminum Fumarate | Wire-finned heat exchanger |
| [13] |
| 5 | Aluminum Fumarate | Packed heat exchanger |
| [148] |
| 6 | MIL-100 (Fe) | Packed heat exchanger |
| [51] |
| 7 | CAU-23 CAU-10 Co2Cl2 (BTDD) | Plate-type heat exchanger |
| [149] |
| 8 | MIL-101 (Cr) | Cross-flow heat exchanger |
| [150] |
| 9 | CPO27 (NI) MIL100 (Fe) MIL-101 (Cr) Aluminum Fumarate silica gel | Cross-flow heat exchanger |
| [151] |
| 10 | MOF–801 Aluminum Fumarate MIL-100 (Fe) | - |
| [152] |
| S. No. | MOF Used | Main Findings | Ref. |
|---|---|---|---|
| 1 | ZIF-8@PVDF45 HKUST-1@PVDF45 |
| [129] |
| 2 | MIL-110 |
| [155] |
| 3 | CuZn-MOF-74 (PBMA) |
| [156] |
| 4 | GO/ZIF-8 MOF |
| [157] |
| 5 | PDMS@MOF@Cu |
| [158] |
| S. No. | MOFs Used | Main Findings | Ref. |
|---|---|---|---|
| 1 | Aluminum Fumarate |
| [98] |
| 2 | CAU-23 CAU-10 Co2Cl2 (BTDD) |
| [149] |
| 3 | MIL-100 (Fe) |
| [146] |
| S. No. | MOFs Used | Main Findings | Ref. |
|---|---|---|---|
| 1 | MIL-160 (Al) |
| [165] |
| 2 | MIL-100 (Fe) |
| [166] |
| 3 | Aluminum Fumarate |
| [167] |
| 4 | Aluminum Fumarate |
| [168] |
| S. No. | MOFs Used | Main Findings | Ref. |
|---|---|---|---|
| 1 | Aluminum Fumarate MIL-100 (Fe) MIL-100 (Fe)/G MOF-303/G MOF-801 MOF-801/G |
| [147] |
| 2 | Aluminum Fumarate |
| [13] |
| 3 | Aluminum Fumarate |
| [169] |
| 4 | MIL-101 (Cr) |
| [170] |
| S. No. | MOF Used | Function Group Added | Main Findings | Ref. |
|---|---|---|---|---|
| 1 | UiO-66 (Zr) MIL-125 (Ti) | amino (-NH2) |
| [77] |
| 2 | MIL-101-NH2 | (Cr) |
| [192] |
| 3 | MIL-53 | (Al) |
| [193] |
| 4 | HKUST-1 | (Cu) |
| [194] |
| 5 | MIL-125 (Ti) | hydroxyl (-OH), amino (-NH2), nitro (-NO2), bromo (-Br), and pyridine (-C5H5N) |
| [195] |
| S. No. | Multifunctional MOFs | Enhancement in | Applications | Ref. | |||
|---|---|---|---|---|---|---|---|
| Thermal Properties | Mechanical Stability | Electrical Conductivity | Adsorption Characteristics | ||||
| 1 | HKUST-1 with CNT | ✔ | ✔ | - | - |
| [215] |
| 2 | MOF-5 with GO | - | ✔ | ✔ | ✔ |
| [214] |
| 3 | MOF-74(Ni) with GO | - | ✔ | - | ✔ |
| [216] |
| 4 | MIL-101 (Cr) with AC | - | ✔ | - | ✔ |
| [217] |
| 5 | MIL-100 (Fe) with CNT | ✔ | ✔ | - | ✔ |
| [218,219] |
| 6 | MOF-808 with CNT | - | ✔ | - | ✔ |
| [220,221] |
| 7 | MIL-53 with Graphene Oxide | ✔ | ✔ | - | ✔ |
| [222,223] |
| 8 | UiO-66 with CNT | - | ✔ | - | ✔ |
| [224,225] |
| 9 | Aluminum Fumarate | ✔ | ✔ | - | ✔ |
| [226,227] |
| 10 | UiO-67 with Graphene Oxide | - | ✔ | - | ✔ |
| [228] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, T.B.; Imran, M.; Tandis, E. Applications of MOF-Based Nanocomposites in Heat Exchangers: Innovations, Challenges, and Future Directions. Nanomaterials 2025, 15, 205. https://doi.org/10.3390/nano15030205
Nadeem TB, Imran M, Tandis E. Applications of MOF-Based Nanocomposites in Heat Exchangers: Innovations, Challenges, and Future Directions. Nanomaterials. 2025; 15(3):205. https://doi.org/10.3390/nano15030205
Chicago/Turabian StyleNadeem, Talha Bin, Muhammad Imran, and Emad Tandis. 2025. "Applications of MOF-Based Nanocomposites in Heat Exchangers: Innovations, Challenges, and Future Directions" Nanomaterials 15, no. 3: 205. https://doi.org/10.3390/nano15030205
APA StyleNadeem, T. B., Imran, M., & Tandis, E. (2025). Applications of MOF-Based Nanocomposites in Heat Exchangers: Innovations, Challenges, and Future Directions. Nanomaterials, 15(3), 205. https://doi.org/10.3390/nano15030205







