Boron Nitride-Supported Metal Catalysts for the Synthesis and Decomposition of Ammonia and Formic Acid
Abstract
:1. Introduction
2. Catalytic Application of Boron Nitride-Supported Metal Nanoparticles
2.1. Ammonia Synthesis
2.1.1. Effect of Barium Doping on Ru/BN Catalysts
2.1.2. Comparison of Boron Nitride-Supported Catalysts with Other Supports
| Ref. | Catalyst | Molar Ratio | wt% Ru | T (°C) | Catalytic Activity (ml·g−1·h−1) |
|---|---|---|---|---|---|
| [102] | Ba-Ru/BN | Ba/Ru: 1:1 | 4.5 | 400 | 2981 |
| [104] | Ba1-Ru4.3/HCVNH3 | Ba/Ru: 1:1 | 4.3 | 400 | 1865 |
| [77] | Ru-Ba/BN | Ba/Ru: 1:1 | 4 | 475 | 2061 |
| [109] | Ba-Ru/MgO/h-BN [8:2] | Ba/Ru: 1:1 | 5 | 450 | 473 |
| [105] | Ba-Ru9.1/C | - | 9.1 | 400 | 4959 |
| [105] | Ba-Cs-Ru9.1/C | - | 9.1 | 400 | 9835 |
| [105] | Ba-Cs-Ru23.1/C | - | 23.1 | 400 | 17,960 |
| [108] | Ba-(Ru/AC) | Ba/Ru: 1 | 4.3 | 400 | 929 |
| [92] | Ru/MgO | - | 3.4 | 315 | 532 |
| [107] | Cs-Ru/MCP | Cs/Ru: 2.5 | 10 | 370 | 358 |
| [107] | Cs-Ru/MCP | Cs/Ru: 2.5 | 10 | 410 | 1047 |
| [113] | K/Ru/MWNTs | K/Ru: 1:3 | - | 420 | 37 |
2.2. Ammonia Decomposition
2.3. Formic Acid Synthesis
2.4. Formic Acid Decomposition
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Badakhsh, A.; Cha, J.; Park, Y.; Lee, Y.J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; Park, C.W.; et al. Autothermal Recirculating Reactor (ARR) with Cu-BN Composite as a Stable Reactor Material for Sustainable Hydrogen Release from Ammonia. J. Power Sources 2021, 506, 230081. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Abbasian Hamedani, E.; Alenabi, S.A.; Talebi, S. Hydrogen as an Energy Source: A Review of Production Technologies and Challenges of Fuel Cell Vehicles. Energy Rep. 2024, 12, 3778–3794. [Google Scholar] [CrossRef]
- Ishaq, H.; Crawford, C. Review of ammonia production and utilization: Enabling clean energy transition and net-zero climate targets. Energy Convers. Manag. 2024, 300, 117869. [Google Scholar] [CrossRef]
- Boddien, A.; Loges, B.; Junge, H.; Gärtner, F.; Noyes, J.R.; Beller, M. Continuous Hydrogen Generation from Formic Acid: Highly Active and Stable Ruthenium Catalysts. Adv. Synth. Catal. 2009, 351, 2517–2520. [Google Scholar] [CrossRef]
- Nayak-Luke, R.M.; Bañares-Alcántara, R. Long-Term Energy Storage: What Is the Need and Is Ammonia a Solution? In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 1843–1848. [Google Scholar] [CrossRef]
- Enthaler, S.; Von Langermann, J.; Schmidt, T. Carbon Dioxide and Formic Acid - The Couple for Environmental-Friendly Hydrogen Storage? Energy Environ. Sci. 2010, 3, 1207–1217. [Google Scholar] [CrossRef]
- Loges, B.; Boddien, A.; Junge, H.; Beller, M. Controlled Generation of Hydrogen from Formic Acid Amine Adducts at Room Temperature and Application in H2/O2 Fuel Cells. Angew. Chem. Int. Ed. 2008, 47, 3962–3965. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, M.; Laurenczy, G. Formic Acid as a Hydrogen Source—Recent Developments and Future Trends. Energy Environ. Sci. R. Soc. Chem. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Dong, J.; Gao, L.; Fu, Q. Hexagonal Boron Nitride Meeting Metal: A New Opportunity and Territory in Heterogeneous Catalysis. J. Phys. Chem. Lett. 2021, 12, 9608–9619. [Google Scholar] [CrossRef] [PubMed]
- Postole, G.; Gervasini, A.; Guimon, C.; Auroux, A.; Bonnetot, B. Influence of the Preparation Method on the Surface Characteristics and Activity of Boron-Nitride-Supported Noble Metal Catalysts. J. Phys. Chem. B 2006, 110, 12572–12580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Sun, K.; Li, S.; Zhou, J.; Liu, S.; Wei, H.; Liu, B.; Xie, L.; Li, B.; et al. Applications and Theory Investigation of Two-Dimensional Boron Nitride Nanomaterials in Energy Catalysis and Storage. EnergyChem 2023, 5, 100108. [Google Scholar] [CrossRef]
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Nanomaterials for Hydrogen Storage Applications: A Review. J. Nanomater. 2008, 2008, 950967. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Li, L.; Lin, J.; Yang, X.; Yu, C.; Liu, Z.; Fang, Y.; Huang, Y.; Tang, C. CuCo Binary Metal Nanoparticles Supported on Boron Nitride Nanofibers as Highly Efficient Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane. J. Power Sources 2019, 431, 135–143. [Google Scholar] [CrossRef]
- Sutter, P.; Lahiri, J.; Albrecht, P.; Sutter, E. Chemical Vapor Deposition and Etching of High-Quality Monolayer Hexagonal Boron Nitride Films. ACS Nano 2011, 5, 7303–7309. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Lv, X.; Feng, J.; Zhang, S.; Bai, J.; Lu, R.; Liu, J. Cobalt Nickel Nanoparticles Encapsulated within Hexagonal Boron Nitride as Stable, Catalytic Dehydrogenation Nanoreactor. Int. J. Hydrogen Energy 2017, 42, 11312–11320. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; He, H.; Zhang, N.; Liu, Z.; Zhang, G. A Novel Two-Dimensional MgO-h-BN Nanomaterial Supported Pd Catalyst for CO Oxidation Reaction. Catal. Today 2019, 332, 214–221. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Yu, X.; Wu, X.; Xie, Z.; Wang, X.; Lu, Z.; Zhang, X.; Huang, Y.; Yang, X. Ultrafine Platinum Particles Anchored on Porous Boron Nitride Enabling Excellent Stability and Activity for Oxygen Reduction Reaction. Chem. Eng. J. 2020, 399, 125827. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron Nitride Nanotubes and Nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Moussa, G.; Salameh, C.; Bruma, A.; Malo, S.; Demirci, U.B.; Bernard, S.; Miele, P. Nanostructured Boron Nitride: From Molecular Design to Hydrogen Storage Application. Inorganics 2014, 2, 396–409. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Singh, A.K.; Ahmaruzzaman, M. Advances in Boron Nitride-Based Nanomaterials for Environmental Remediation and Water Splitting: A Review. RSC Adv. 2024, 14, 3447–3472. [Google Scholar] [CrossRef]
- Lale, A.; Bernard, S.; Demirci, U.B. Boron Nitride for Hydrogen Storage. Chempluschem 2018, 83, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Orimo, S.; Matsushima, T.; Fujii, H.; Majer, G. Hydrogen in Mechanically Prepared Nanostructured H-BN: A Critical Comparison with That in Nanostructured Graphite. Appl. Phys. Lett. 2002, 80, 318–320. [Google Scholar] [CrossRef]
- Dolati, S.; Fereidoon, A.; Kashyzadeh, K.R. A Comparison Study between Boron Nitride Nanotubes and Carbon Nanotubes. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 470. [Google Scholar]
- Kovalskii, A.M.; Manakhov, A.M.; Afanasev, P.A.; Popov, Z.I.; Matveev, A.T.; Al-Qasim, A.S. Hydrogen Storage Ability of Hexagonal Boron Nitride. Front. Mater. 2024, 11, 1375977. [Google Scholar] [CrossRef]
- Kostoglou, N.; Tampaxis, C.; Charalambopoulou, G.; Constantinides, G.; Ryzhkov, V.; Doumanidis, C.; Matovic, B.; Mitterer, C.; Rebholz, C. Boron Nitride Nanotubes versus Carbon Nanotubes: A Thermal Stability and Oxidation Behavior Study. Nanomaterials 2020, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Hmcj, N.; Kr, K. A Comparative Study of Carbon Nanotubes and Boron Nitride Nanotubes as Nanomaterial for Hydrogen Storage. Int. J. Res. Publ. Rev. 2024, 5, 540–543. [Google Scholar]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Boron Nitride Nanotubes. In Materials Science and Engineering R: Reports; Elsevier: Amsterdam, The Netherlands, 2010; Volume 70, pp. 92–111. [Google Scholar] [CrossRef]
- Lee, C.H.; Bhandari, S.; Tiwari, B.; Yapici, N.; Zhang, D.; Yap, Y.K. Boron Nitride Nanotubes: Recent Advances in Their Synthesis, Functionalization, and Applications. Molecules 2016, 21, 922. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Ding, X.; Qi, S.; Golberg, D. Catalyzed Collapse and Enhanced Hydrogen Storage of BN Nanotubes. J. Am. Chem. Soc. 2002, 124, 14550–14551. [Google Scholar] [CrossRef] [PubMed]
- Jhi, S.H. Activated Boron Nitride Nanotubes: A Potential Material for Room-Temperature Hydrogen Storage. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 155424. [Google Scholar] [CrossRef]
- Jhi, S.-H.; Kwon, Y.-K. Hydrogen Adsorption on Boron Nitride Nanotubes: A Path to Room-Temperature Hydrogen Storage. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 69, 245401–245407. [Google Scholar] [CrossRef]
- Auwärter, W. Hexagonal Boron Nitride Monolayers on Metal Supports: Versatile Templates for Atoms, Molecules and Nanostructures. Surf. Sci. Rep. 2019, 74, 1–95. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, L.; Qiao, R.; Wu, M.; Wang, Z.; Zhang, S.; Liang, J.; Zhang, Z.; Zhang, Z.; et al. Epitaxial Growth of a 100-Square-Centimetre Single-Crystal Hexagonal Boron Nitride Monolayer on Copper. Nature 2019, 570, 91–95. [Google Scholar] [CrossRef]
- Meziani, M.J.; Sheriff, K.; Parajuli, P.; Priego, P.; Bhattacharya, S.; Rao, A.M.; Quimby, J.L.; Qiao, R.; Wang, P.; Hwu, S.J.; et al. Advances in Studies of Boron Nitride Nanosheets and Nanocomposites for Thermal Transport and Related Applications. ChemPhysChem 2022, 23, e202100645. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, L.; Jiang, W.; Wu, Y.; Guo, X.; Li, Z.; Yuan, H.; Luo, M. Recent Advances of Boron Nitride Nanosheets in Hydrogen Storage Application. J. Mater. Res. Technol. 2023, 26, 2028–2042. [Google Scholar] [CrossRef]
- Lvova, N.A.; Ananina, O.Y. Theoretical Study of the Adsorption Properties of Porous Boron Nitride Nanosheets. Comput. Mater. Sci. 2016, 115, 11–17. [Google Scholar] [CrossRef]
- Fan, D.; Feng, J.; Liu, J.; Gao, T.; Ye, Z.; Chen, M.; Lv, X. Hexagonal Boron Nitride Nanosheets Exfoliated by Sodium Hypochlorite Ball Mill and Their Potential Application in Catalysis. Ceram. Int. 2016, 42, 7155–7163. [Google Scholar] [CrossRef]
- Sun, W.; Meng, Y.; Fu, Q.; Wang, F.; Wang, G.; Gao, W.; Huang, X.; Lu, F. High-Yield Production of Boron Nitride Nanosheets and Its Uses as a Catalyst Support for Hydrogenation of Nitroaromatics. ACS Appl. Mater. Interfaces 2016, 8, 9881–9888. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, X.; Bando, Y.; Golberg, D. One-Step Template-Free Synthesis of Highly Porous Boron Nitride Microsponges for Hydrogen Storage. Adv. Energy Mater. 2014, 4, 1301525. [Google Scholar] [CrossRef]
- Naresh Muthu, R.; Rajashabala, S.; Kannan, R. Hexagonal Boron Nitride (h-BN) Nanoparticles Decorated Multi-Walled Carbon Nanotubes (MWCNT) for Hydrogen Storage. Renew. Energy 2016, 85, 387–394. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Xu, X.; Zhang, X.; Xue, Y.; Mi, J.; Mo, Z.; Fan, Y.; Hu, L.; Yang, X.; et al. Porous Boron Nitride with a High Surface Area: Hydrogen Storage and Water Treatment. Nanotechnology 2013, 24, 155603. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, X.; Zhi, C.; Bando, Y.; Golberg, D. Boron Nitride Porous Microbelts for Hydrogen Storage. ACS Nano 2013, 7, 1558–1565. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.; Sato, T.; Golberg, D.; Zhu, H.; Xu, C.; Wu, D. Synthesis of Boron Nitride Nanofibers and Measurement of Their Hydrogen Uptake Capacity. Appl. Phys. Lett. 2002, 81, 5225–5227. [Google Scholar] [CrossRef]
- Lin, J.; Xu, L.; Huang, Y.; Li, J.; Wang, W.; Feng, C.; Liu, Z.; Xu, X.; Zou, J.; Tang, C. Ultrafine Porous Boron Nitride Nanofibers Synthesized via a Freeze-Drying and Pyrolysis Process and Their Adsorption Properties. RSC Adv. 2016, 6, 1253–1259. [Google Scholar] [CrossRef]
- Jindal, R.; Sharma, V.; Shukla, A. Density Functional Theory Study of the Hydrogen Evolution Reaction in Haeckelite Boron Nitride Quantum Dots. Int. J. Hydrogen Energy 2022, 47, 41783–41794. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Sato, T. Catalytic Growth of Boron Nitride Nanotubes. Chem. Phys. Lett. 2002, 362, 185–189. [Google Scholar] [CrossRef]
- Bläsing, B.; Calvo-Merino, B.; Cross, E.S.; Jola, C.; Honisch, J.; Stevens, C.J. Understanding the Active Sites of Boron Nitride for CWPO: An Experimental and Computational Approach. Chem. Eng. J. 2012, 406, 300–308. [Google Scholar] [CrossRef]
- Biswas, A.; Kapse, S.; Thapa, R.; Dey, R.S. Oxygen Functionalization-Induced Charging Effect on Boron Active Sites for High-Yield Electrocatalytic NH3 Production. Nanomicro Lett. 2022, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, D.E.; Yang, Z.; Dai, S. Engineering Nanostructured Interfaces of Hexagonal Boron Nitride-Based Materials for Enhanced Catalysis. Acc. Chem. Res. 2023, 56, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sergio, C.S.; de Campos, M.; Pansini, F.N.N. Hydrogen Storage Capacity of the Niobium Atom Adsorbed on Carbon and Boron Nitride Planar Nanoflakes. Int. J. Hydrogen Energy 2022, 48, 8189–8197. [Google Scholar] [CrossRef]
- Guo, R.; Hu, C.; Li, Q.; Liu, L.L.; Tung, C.H.; Kong, L. BN Analogue of Butadiyne: A Platform for Dinitrogen Release and Reduction. J. Am. Chem. Soc. 2023, 145, 18767–18772. [Google Scholar] [CrossRef] [PubMed]
- Terrones, M.; Romo-Herrera, J.M.; Cruz-Silva, E.; López-Urías, F.; Muñoz-Sandoval, E.; Velázquez-Salazar, J.J.; Terrones, H.; Bando, Y.; Golberg, D. Pure and Doped Boron Nitride Nanotubes. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Chagoya, K.L.; Nash, D.J.; Jiang, T.; Le, D.; Alayoglu, S.; Idrees, K.B.; Zhang, X.; Farha, O.K.; Harper, J.K.; Rahman, T.S.; et al. Mechanically Enhanced Catalytic Reduction of Carbon Dioxide over Defect Hexagonal Boron Nitride. ACS Sustain. Chem. Eng. 2021, 9, 2447–2455. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Liu, X.; Gao, N.; Song, X.; Zhao, Z. AuPd Nanocatalysts Supported on Citric Acid-Modified Boron Nitride to Boost Hydrogen Generation from Formic Acid Dehydrogenation. ACS Appl. Nano Mater. 2022, 6, 3285–3292. [Google Scholar] [CrossRef]
- Baierle, R.J.; Piquini, P.; Schmidt, T.M.; Fazzio, A. Hydrogen Adsorption on Carbon-Doped Boron Nitride Nanotube. J. Phys. Chem. B 2006, 110, 21184–21188. [Google Scholar] [CrossRef]
- Wu, X.; Yang, J.L.; Zeng, X.C. Adsorption of Hydrogen Molecules on the Platinum-Doped Boron Nitride Nanotubes. J. Chem. Phys. 2006, 125, 2210933. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zhang, H.; Wu, Y.; Zhang, B.; Liu, D.; Qin, S.; Liu, Z.; Liu, L.; Ma, Y.; Chen, Y. Oxygen-Doped Boron Nitride Nanosheets with Excellent Performance in Hydrogen Storage. Nano Energy 2014, 6, 219–224. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, M.; Luo, Z.; Wakeel, M.; Asiri, A.M.; Wang, X. Template-Free Synthesis of Carbon-Doped Boron Nitride Nanosheets for Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B 2019, 241, 246–255. [Google Scholar] [CrossRef]
- Durgun, E.; Jang, Y.-R.; Ciraci, S. Hydrogen Storage Capacity of Ti-Doped Boron-Nitride and B Be -Substituted Carbon Nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 073413. [Google Scholar] [CrossRef]
- Mao, X.; Zhou, S.; Yan, C.; Zhu, Z.; Du, A. A Single Boron Atom Doped Boron Nitride Edge as a Metal-Free Catalyst for N2 Fixation. Phys. Chem. Chem. Phys. 2019, 21, 1110–1116. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Zhang, J. Applied Surface Science Fe Doped BN Monolayer: A Promising Low-Cost Single Atom Catalyst for Promoted CO Oxidation Activity. Appl. Surf. Sci. 2020, 525, 146567. [Google Scholar] [CrossRef]
- Shentu, Q.; Wu, Z.; Song, W.; Pan, S.; Zhou, Z.; Lv, W.; Song, C.; Yao, Y. Carbon Doped Boron Nitride Nanosheet as Efficient Metal-Free Catalyst for Peroxymonosulfate Activation: Important Role of B-N-C Moieties. Chem. Eng. J. 2022, 446 Pt 3, 137274. [Google Scholar] [CrossRef]
- Esra, M.D.; Mousavian, P.; Arjomandi, F. Adsorption of Formamide over Pristine and Al-Doped Boron Nitride Nanosheets: A Dispersion-Corrected DFT Study. J. Mol. Graph. Model. 2018, 82, 101–107. [Google Scholar] [CrossRef]
- Lim, S.H.; Luo, J.; Ji, W.; Lin, J. Synthesis of Boron Nitride Nanotubes and Its Hydrogen Uptake. Catal. Today 2007, 120, 346–350. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, J.; Chen, Z.; Gao, X.; Yan, T.; Wen, B.; Von Ragué Schleyer, P. Comparative Study of Hydrogen Adsorption on Carbon and BN Nanotubes. J. Phys. Chem. B 2006, 110, 13363–13369. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, X.; Kuo, J.L. Metal Free Hydrogenation Reaction on Carbon Doped Boron Nitride Fullerene: A DFT Study on the Kinetic Issue. Int. J. Hydrogen Energy 2012, 37, 14336–14342. [Google Scholar] [CrossRef]
- Gou, J.; Liu, C.; Lin, J.; Yu, C.; Fang, Y.; Liu, Z.; Guo, Z.; Tang, C.; Huang, Y. Densification and Pelletization of Porous Boron Nitride Fibers for Effective CO2 Adsorption. Ceram. Int. 2022, 48, 11636–11643. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Chen, Y.; Liu, D.; Huang, S.; Lei, W. One-Step Template-Free Synthesis of 3D Functionalized Flower-like Boron Nitride Nanosheets for NH3 and CO2 Adsorption. Nanoscale 2018, 10, 10979–10985. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Rhee, K.Y.; Park, S.J. Effect of Triblock Copolymer on Carbon-Based Boron Nitride Whiskers for Efficient CO2 Adsorption. Polymers 2019, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, X.; Chen, H.; Fang, W.; He, X.; Li, W.; Huang, Z.; Zhao, L. Porous Hexagonal Boron Nitride Nanosheets from G-C3N4 Templates with a High Specific Surface Area for CO2 Adsorption. Ceram. Int. 2020, 46, 27627–27633. [Google Scholar] [CrossRef]
- Shaybanizadeh, S.; Najafi Chermahini, A.; Luque, R. Boron Nitride Nanosheets Supported Highly Homogeneous Bimetallic AuPd Alloy Nanoparticles Catalyst for Hydrogen Production from Formic Acid. Nanotechnology 2022, 33, 275601. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, G.; Chen, X.; Yin, J.; Zhang, P.; Yao, Y.; Shen, J.; Wu, Y.; Huang, J. Perspectives on Environmental Applications of Hexagonal Boron Nitride Nanomaterials. Nano Today 2022, 44, 101486. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Cui, T. Carbon and Oxygen Coordinating Atoms Adjust Transition Metal Single-Atom Catalysts Based on Boron Nitride Monolayers for Highly Efficient CO2 Electroreduction. ACS Appl. Mater. Interfaces 2021, 13, 18934–18943. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zhang, R.; Li, H.; Wu, Y.; Sun, Z.; Yu, Y.; Sun, Z. Catalytic Formic Acid Dehydrogenation via Hexagonal-Boron Nitride Supported Palladium. Int. J. Hydrogen Energy 2024, 49, 602–612. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Yu, C.; Li, R.; Tang, C.; Huang, Y. Pd Nanoparticles Anchored on Amine-Functionalized Boron Nitride Co-Doped with Carbon and Oxygen as Catalysts for Formic Acid Dehydrogenation. ACS Appl. Nano Mater. 2024, 7, 16245–16255. [Google Scholar] [CrossRef]
- Xu, C.; Ouyang, L.; Zhang, J.; Zhou, B.; Li, Y.; Liu, H. Effect of Boron Nitride Support on Catalytic Activity of Ru-Ba/BN for Ammonia Synthesis. Cuihua Xuebao/Chin. J. Catal. 2010, 31, 677–682. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wei, H.; Wang, C.; Liu, T.; Wu, X.; Ashraf, S.; Mehdi, S.; Guan, S.; Fan, Y.; et al. Atomic-Bridge Structure in B-Co-P Dual-Active Sites on Boron Nitride Nanosheets for Catalytic Hydrogen Generation. Appl. Catal. B 2022, 314, 121495. [Google Scholar] [CrossRef]
- Guan, S.; Liu, Y.; Zhang, H.; Shen, R.; Wen, H.; Kang, N.; Zhou, J.; Liu, B.; Fan, Y.; Jiang, J.; et al. Recent Advances and Perspectives on Supported Catalysts for Heterogeneous Hydrogen Production from Ammonia Borane. Adv. Sci. 2023, 10, 2300726. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhu, X.; Cheng, B.; Yu, J.; Jiang, C. Few-Layered Graphene-like Boron Nitride: A Highly Efficient Adsorbent for Indoor Formaldehyde Removal. Environ. Sci. Technol. Lett. 2017, 4, 20–25. [Google Scholar] [CrossRef]
- Epelle, E.I.; Desongu, K.S.; Obande, W.; Adeleke, A.A.; Ikubanni, P.P.; Okolie, J.A.; Gunes, B. A Comprehensive Review of Hydrogen Production and Storage: A Focus on the Role of Nanomaterials. Int. J. Hydrogen Energy 2022, 47, 20398–20431. [Google Scholar] [CrossRef]
- Kalay, S.; Yilmaz, Z.; Sen, O.; Emanet, M.; Kazanc, E.; Çulha, M. Synthesis of Boron Nitride Nanotubes and Their Applications. Beilstein J. Nanotechnol. 2015, 6, 84–102. [Google Scholar] [CrossRef]
- Arroyo-Caire, J.; Diaz-Perez, M.A.; Lara-Angulo, M.A.; Serrano-Ruiz, J.C. A Conceptual Approach for the Design of New Catalysts for Ammonia Synthesis: A Metal—Support Interactions Review. Nanomaterials 2023, 13, 2914. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Matveev, A.T.; Permyakova, E.S.; Leybo, D.V.; Konopatsky, A.S.; Sorokin, P.B. Recent Progress in Fabrication and Application of BN Nanostructures and BN-Based Nanohybrids. Nanomaterials 2022, 12, 2810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W. Bin. B-Doped Pd Catalyst: Boosting Room-Temperature Hydrogen Production from Formic Acid-Formate Solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef] [PubMed]
- Erfani, N.; Baharudin, L.; Watson, M. Recent Advances and Intensifications in Haber-Bosch Ammonia Synthesis Process. Chem. Eng. Process. Process Intensif. 2024, 2024, 109962. [Google Scholar] [CrossRef]
- Li, W.Q.; Xu, M.; Chen, J.S.; Ye, T.N. Enabling Sustainable Ammonia Synthesis: From Nitrogen Activation Strategies to Emerging Materials. Adv. Mater. 2024, 36, 2408434. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Zeinalipour-Yazdi, C.D. Recent Advances in Ammonia Synthesis Modeling and Experiments on Metal Nitrides and Other Catalytic Surfaces. Crystals 2024, 14, 818. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Xu, C.; Kong, D.; Tsubouchi, N. Decomposition of Ammonia with Iron and Calcium Catalysts Supported on Coal Chars. Fuel 2004, 83, 685–692. [Google Scholar] [CrossRef]
- Homs, N.; Fierro, J.L.G.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I.; Ramírez de la Piscina, P. Study of the Activation Process and Catalytic Behaviour of a Supported Iron Ammonia Synthesis Catalyst. Appl. Surf. Sci. 1993, 72, 103–111. [Google Scholar] [CrossRef]
- Truszkiewicz, E.; Raróg-Pilecka, W.; Schmidt-Szałowski, K.; Jodzis, S.; Wilczkowska, E.; Łomot, D.; Kaszkur, Z.; Karpiński, Z.; Kowalczyk, Z. Barium-Promoted Ru/Carbon Catalyst for Ammonia Synthesis: State of the System When Operating. J. Catal. 2009, 265, 181–190. [Google Scholar] [CrossRef]
- Bielawa, H.; Hinrichsen, O.; Birkner, A.; Muhler, M. The Ammonia-Synthesis Catalyst of the next Generation: Barium-Promoted Oxide-Supported Ruthenium. Angew. Chem. Int. Ed. 2001, 40, 1061–1063. [Google Scholar] [CrossRef]
- Yu, X.; Lin, B.; Gong, B.; Lin, J.; Wang, R.; Wei, K. Effect of Nitric Acid Treatment on Carbon Nanotubes (CNTs)-Cordierite Monoliths Supported Ruthenium Catalysts for Ammonia Synthesis. Catal. Lett. 2008, 124, 168–173. [Google Scholar] [CrossRef]
- Ni, J.; Wang, R.; Kong, F.; Zhang, T.; Lin, J.; Lin, B.; Wei, K. Highly Efficient Ru/Ba/AC Catalyst Promoted by Magnesium for Ammonia Synthesis. Cuihua Xuebao/Chin. J. Catal. 2011, 32, 436–439. [Google Scholar] [CrossRef]
- Hill, A.K.; Torrente-Murciano, L. In-Situ H2 Production via Low Temperature Decomposition of Ammonia: Insights into the Role of Cesium as a Promoter. Int. J. Hydrogen Energy 2014, 39, 7646–7654. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Jodzis, S.; Âg, R.; Âski, J.Z.; Pielaszek, J. Effect of Potassium and Barium on the Stability of a Carbon-Supported Ruthenium Catalyst for the Synthesis of Ammonia. Appl. Catal. 1998, 173, 153–160. [Google Scholar] [CrossRef]
- Paine, R.T.; Narulat, C.K. Synthetic Routes to Boron Nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Raróg, W.; Kowalczyk, Z.; Sentek, J.; Składanowski, D.; Zieli´nski, J.Z. Effect of K, Cs and Ba on the Kinetics of NH 3 Synthesis over Carbon-Based Ruthenium Catalysts. Catal. Lett. 2000, 68, 163–168. [Google Scholar] [CrossRef]
- Tsyrul’nikov, P.G.; Iost, K.N.; Shitova, N.B.; Temerev, V.L. Methanation of the Carbon Supports of Ruthenium Ammonia Synthesis Catalysts: A Review. Catal. Ind. 2016, 8, 341–347. [Google Scholar] [CrossRef]
- Iost, K.N.; Temerev, V.L.; Smirnova, N.S.; Shlyapin, D.A.; Borisov, V.A.; Muromtsev, I.V.; Trenikhin, M.V.; Kireeva, T.V.; Shilova, A.V.; Tsyrul’nikov, P.G. Synthesis and Study of Ru–Ba–Cs/Sibunit Ternary Catalysts for Ammonia Synthesis. Russ. J. Appl. Chem. 2017, 90, 887–894. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H. Boron Nitride: A Novel Support for Ruthenium-Based Ammonia Synthesis Catalysts. J. Catal. 2001, 200, 3200. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Dahl, S.; Hansen, P.L.; Törnqvist, E.; Jensen, L.; Topsøe, H.; Prip, D.V.; Møenshaug, P.B.; Chorkendorff, I. Structure Sensitivity of Supported Ruthenium Catalysts for Ammonia Synthesis. J. Mol. Catal. A Chem. 2000, 163, 19–26. [Google Scholar] [CrossRef]
- Szmigiel, D.; Raróg-Pilecka, W.; Miśkiewicz, E.; MacIejewska, E.; Kaszkur, Z.; Sobczak, J.W.; Kowalczyk, Z. Ammonia Synthesis over the Ba-Promoted Ruthenium Catalysts Supported on Boron Nitride. Catal. Lett. 2005, 100, 79–87. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Krukowski, M.; Raróg-Pilecka, W.; Szmigiel, D.; Zielinski, J. Carbon-Based Ruthenium Catalyst for Ammonia Synthesis: Role of the Barium and Caesium Promoters and Carbon Support. Appl. Catal. A Gen. 2003, 248, 67–73. [Google Scholar] [CrossRef]
- Raróg, W.; Lenarcik, I.; Kowalczyk, Z.; Sentek, J.; Krukowski, M.; Zieliński, J. Carbon-Based Ruthenium Catalyst for NH3 Synthesis. Effect of Carbon Treatment. Pol. J. Chem. 2000, 74, 1473–1478. [Google Scholar]
- Chen, S.Y.; Wang, L.Y.; Chen, K.C.; Yeh, C.H.; Hsiao, W.C.; Chen, H.Y.; Nishi, M.; Keller, M.; Chang, C.L.; Liao, C.N.; et al. Ammonia Synthesis over Cesium-Promoted Mesoporous-Carbon-Supported Ruthenium Catalysts: Impact of Graphitization Degree of the Carbon Support. Appl. Catal. B 2024, 346, 123725. [Google Scholar] [CrossRef]
- Ni, J.; Shi, S.; Zhang, C.; Fang, B.; Wang, X.; Lin, J.; Liang, S.; Lin, B.; Jiang, L. Enhanced Catalytic Performance of the Carbon-Supported Ru Ammonia Synthesis Catalyst by an Introduction of Oxygen Functional Groups via Gas-Phase Oxidation. J. Catal. 2022, 409, 78–86. [Google Scholar] [CrossRef]
- Yang, X.; Tang, L.; Xia, C.; Xiong, X.; Mu, X.; Hu, B. Effect of MgO/h-BN Composite Support on Catalytic Activity of Ba-Ru/MgO/h-BN for Ammonia Synthesis. Cuihua Xuebao/Chin. J. Catal. 2012, 33, 447–453. [Google Scholar] [CrossRef]
- Hansen, T.W.; Wagner, J.B.; Hansen, P.L.; Dahl, S.; Topsøe, H.; Jacobsen, C.J.H. Atomic-Resolution in Situ Transmission Electron Microscopy of a Promoter of a Heterogeneous Catalyst. Science 2001, 294, 1508–1510. [Google Scholar] [CrossRef]
- Senthamaraikannan, T.G.; Yoon, C.W.; Lim, D.H. Morphology-Dependent Adsorption Energetics of Ru Nanoparticles on Hcp-Boron Nitride (001) Surface—A First-Principles Study. Nanoscale Adv. 2023, 5, 2422–2426. [Google Scholar] [CrossRef] [PubMed]
- Gavnholt, J.; Schiøtz, J. Structure and Reactivity of Ruthenium Nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 035404. [Google Scholar] [CrossRef]
- Chen, H.-B.; Lin, J.-D.; Cai, Y.; Wang, X.-Y.; Yi, J.; Wang, J.; Wei, G.; Lin, Y.-Z.; Liao, D.-W. Novel Multi-Walled Nanotubes-Supported and Alkali-Promoted Ru Catalysts for Ammonia Synthesis under Atmospheric Pressure. Appl. Surf. Sci. 2001, 180, 328–335. [Google Scholar] [CrossRef]
- Kang, S.; Cha, J.; Jo, Y.S.; Lee, Y.J.; Sohn, H.; Kim, Y.; Song, C.K.; Kim, Y.; Lim, D.H.; Park, J.; et al. Heteroepitaxial Growth of B5-Site-Rich Ru Nanoparticles Guided by Hexagonal Boron Nitride for Low-Temperature Ammonia Dehydrogenation. Adv. Mater. 2023, 35, 2203364. [Google Scholar] [CrossRef]
- Bell, T.E.; Torrente-Murciano, L. H2 Production via Ammonia Decomposition Using Non-Noble Metal Catalysts: A Review. Top. Catal. 2016, 59, 1438–1457. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for Hydrogen Storage; A Review of Catalytic Ammonia Decomposition and Hydrogen Separation and Purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Xi, S.; Wu, W.; Yao, W.; Han, R.; He, S.; Wang, W.; Zhang, T.; Yu, L. Hydrogen Production from Ammonia Decomposition: A Mini-Review of Metal Oxide-Based Catalysts. Molecules 2024, 29, 3817. [Google Scholar] [CrossRef]
- García-García, F.R.; Álvarez-Rodríguez, J.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. The Use of Carbon Nanotubes with and without Nitrogen Doping as Support for Ruthenium Catalysts in the Ammonia Decomposition Reaction. Carbon 2010, 48, 267–276. [Google Scholar] [CrossRef]
- García-García, F.R.; Ma, Y.H.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. High Purity Hydrogen Production by Low Temperature Catalytic Ammonia Decomposition in a Multifunctional Membrane Reactor. Catal. Commun. 2008, 9, 482–486. [Google Scholar] [CrossRef]
- García-García, F.R.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Goguet, A.; Shekhtman, S.O.; Hardacre, C. TAP Studies of Ammonia Decomposition over Ru and Ir Catalysts. Phys. Chem. Chem. Phys. 2011, 13, 12892–12899. [Google Scholar] [CrossRef] [PubMed]
- Ganley, J.C.; Thomas, F.S.; Seebauer, E.G.; Masel, R.I. A Priori Catalytic Activity Correlations: The Difficult Case of Hydrogen Production from Ammonia. Catal. Lett. 2004, 96, 117–122. [Google Scholar] [CrossRef]
- Khan, M.H.; Liu, H.K.; Sun, X.; Yamauchi, Y.; Bando, Y.; Golberg, D.; Huang, Z. Few-Atomic-Layered Hexagonal Boron Nitride: CVD Growth, Characterization, and Applications. Materials 2017, 20, 611–628. [Google Scholar] [CrossRef]
- Hossein Ali, Y.R.; Shin, D. Green Hydrogen Production Technologies from Ammonia Cracking. Energies 2022, 15, 8246. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Ng, C.F.; Au, C.T. Nano Ru/CNTs: A Highly Active and Stable Catalyst for the Generation of COx-Free Hydrogen in Ammonia Decomposition. Appl. Catal. B 2004, 48, 237–241. [Google Scholar] [CrossRef]
- Huang, X.; Lei, K.; Mi, Y.; Fang, W.; Li, X. Recent Progress on Hydrogen Production from Ammonia Decomposition: Technical Roadmap and Catalytic Mechanism. Molecules 2023, 28, 5245. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Gu, L.L.; Zhong, A.H.; Yao, Y.; Ji, W.J.; Ding, W.P.; Au, C.T. Layered Double Hydroxide Derived Mg2Al-LDO Supported and K-Modified Ru Catalyst for Hydrogen Production via Ammonia Decomposition. Catal. Lett. 2018, 148, 894–903. [Google Scholar] [CrossRef]
- Yu, P.; Guo, J.; Liu, L.; Wang, P.; Chang, F.; Wang, H.; Ju, X.; Chen, P. Effects of Alkaline Earth Metal Amides on Ru in Catalytic Ammonia Decomposition. J. Phys. Chem. C 2016, 120, 2822–2828. [Google Scholar] [CrossRef]
- Sima, D.; Wu, H.; Tian, K.; Xie, S.; Foo, J.J.; Li, S.; Wang, D.; Ye, Y.; Zheng, Z.; Liu, Y.Q. Enhanced Low Temperature Catalytic Activity of Ni/Al–Ce0.8Zr0.2O2 for Hydrogen Production from Ammonia Decomposition. Int. J. Hydrogen Energy 2020, 45, 9342–9352. [Google Scholar] [CrossRef]
- Yin, S.F.; Zhang, Q.H.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Au, C.T. Investigation on the Catalysis of COx-Free Hydrogen Generation from Ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Zhou, X.P.; Au, C.T. Carbon Nanotubes-Supported Ru Catalyst for the Generation of CO x-Free Hydrogen from Ammonia. Catal. Today 2004, 93, 27–38. [Google Scholar] [CrossRef]
- Zhang, H.; Alhamed, Y.A.; Kojima, Y.; Al-Zahrani, A.A.; Miyaoka, H.; Petrov, L.A. Structure and Catalytic Properties of Ni/MWCNTs and Ni/AC Catalysts for Hydrogen Production via Ammonia Decomposition. Int. J. Hydrogen Energy 2014, 39, 277–287. [Google Scholar] [CrossRef]
- Yao, L.; Shi, T.; Li, Y.; Zhao, J.; Ji, W.; Au, C.T. Core-Shell Structured Nickel and Ruthenium Nanoparticles: Very Active and Stable Catalysts for the Generation of COx-Free Hydrogen via Ammonia Decomposition. Catal. Today 2011, 164, 112–118. [Google Scholar] [CrossRef]
- Chellappa, A.S.; Fischer, C.M.; Thomson, W.J. Ammonia Decomposition Kinetics over Ni-Pt/Al2O3 for PEM Fuel Cell Applications. Appl. Catal. A Gen. 2002, 227, 231–240. [Google Scholar] [CrossRef]
- García-García, F.R.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Role of B5-Type Sites in Ru Catalysts Used for the NH3 Decomposition Reaction. Top. Catal. 2009, 52, 758–764. [Google Scholar] [CrossRef]
- Cha, J.; Lee, T.; Lee, Y.J.; Jeong, H.; Jo, Y.S.; Kim, Y.; Nam, S.W.; Han, J.; Lee, K.B.; Yoon, C.W.; et al. Highly Monodisperse Sub-Nanometer and Nanometer Ru Particles Confined in Alkali-Exchanged Zeolite Y for Ammonia Decomposition. Appl. Catal. B 2021, 283, 119627. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Wang, S.J.; Ng, C.F.; Au, C.T. Magnesia-Carbon Nanotubes (MgO-CNTs) Nanocomposite: Novel Support of Ru Catalyst for the Generation of CO x-Free Hydrogen from Ammonia. Catal. Lett. 2004, 96, 113–116. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, K.; Huang, H.; Cao, C.F.; Luo, Y.; Chen, C.Q.; Lin, L.; Au, C.; Jiang, L. Spatial Confinement of Electron-Rich Ni Nanoparticles for Efficient Ammonia Decomposition to Hydrogen Production. ACS Catal. 2021, 11, 10345–10350. [Google Scholar] [CrossRef]
- Hansgen, D.A.; Vlachos, D.G.; Chen, J.G. Using First Principles to Predict Bimetallic Catalysts for the Ammonia Decomposition Reaction. Nat. Chem. 2010, 2, 484–489. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Jin, X.; Ge, Q.; Li, W. Characterizations and Activities of the Nano-Sized Ni/Al2O3 and Ni/La-Al2O3 Catalysts for NH3 Decomposition. Appl. Catal. A Gen. 2005, 290, 87–96. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Sivadinarayana, C.; Goodman, D.W. Catalytic Ammonia Decomposition: COx-Free Hydrogen Production for Fuel Cell Applications. Catal. Lett. 2001, 72, 197–201. [Google Scholar] [CrossRef]
- Leela Mohana Reddy, A.; Tanur, A.E.; Walker, G.C. Synthesis and Hydrogen Storage Properties of Different Types of Boron Nitride Nanostructures. Int. J. Hydrogen Energy 2010, 35, 4138–4143. [Google Scholar] [CrossRef]
- Lo, J.S.C.; Chen, X.; Chen, S.; Miao, Y.; Daoud, W.A.; Tso, C.Y.; Firdous, I.; Deka, B.J.; Lin, C.S.K. Fabrication of Biodegradable PLA-PHBV Medical Textiles via Electrospinning for Healthcare Apparel and Personal Protective Equipment. Sustain. Chem. Pharm. 2024, 39, 101536. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.D. Pesticides in Wastewater Treatment Plant Effluents in the Yeongsan River Basin, Korea: Occurrence and Environmental Risk Assessment. Sci. Total Environ. 2024, 946, 174388. [Google Scholar] [CrossRef] [PubMed]
- Achour, M.; Álvarez-Hernández, D.; Ruiz-López, E.; Megías-Sayago, C.; Ammari, F.; Ivanova, S.; Centeno, M.Á. Formic Acid as Renewable Reagent and Product in Biomass Upgrading. Tetrahedron Green Chem 2023, 2, 100020. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and Prospects in the Chemical Recycling of Carbon Dioxide to Fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Ogo, S.; Kabe, R.; Hayashi, H.; Harada, R.; Fukuzumi, S. Mechanistic Investigation of CO2 Hydrogenation by Ru(Ii) and Ir(Iii) Aqua Complexes under Acidic Conditions: Two Catalytic Systems Differing in the Nature of the Rate Determining Step. Dalton Trans. 2006, 39, 4657–4663. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Qi, W.; Huber, G.W. Production of Furfural and Carboxylic Acids from Waste Aqueous Hemicellulose Solutions from the Pulp and Paper and Cellulosic Ethanol Industries. Energy Environ. Sci. 2011, 4, 2193–2205. [Google Scholar] [CrossRef]
- Andérez-Fernández, M.; Ferrero, S.; Queiroz, J.P.S.; Pérez, E.; Álvarez, C.M.; Martín, Á.; Bermejo, M.D. Formic Acid Production by Simultaneous Hydrothermal CO2 Reduction and Conversion of Glucose and Its Derivatives. J. Taiwan. Inst. Chem. Eng. 2022, 139, 104504. [Google Scholar] [CrossRef]
- Jin, F.; Enomoto, H. Rapid and Highly Selective Conversion of Biomass into Value-Added Products in Hydrothermal Conditions: Chemistry of Acid/Base-Catalysed and Oxidation Reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
- Hietala, J.; Vuori, A.; Johnsson, P.; Pollari, I.; Reutemann, W.; Kieczka, H. Formic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Cao, Y.; Geng, Z.; Chen, W.; Cai, F.; Wang, G.; Wang, Z.; Zeng, J. Introduction of Carbon-Boron Atomic Groups as an Efficient Strategy to Boost Formic Acid Production toward CO2 Electrochemical Reduction. Chem. Commun. 2018, 54, 3367–3370. [Google Scholar] [CrossRef]
- Mani, D.; Mathivanan, D.; Chang, H.; Sakthivel, K.; Elangovan, E.; Sivakumar, T.; Arivanandhan, M.; Jayavel, R. A Facile Synthesis of Novel ϵ-Fe2O3grafted 2D h-BN Nanostructures for Enhanced Visible Active Photocatalytic Applications. New J. Chem. 2020, 44, 12289–12298. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, M.; Dong, G.; Wei, T.; Feng, J.; Ren, Y.; Luan, T. Photocatalytic Nitrate Reduction by a Non-Metal Catalyst h-BN: Performance and Mechanism. Chem. Eng. J. 2022, 429, 132216. [Google Scholar] [CrossRef]
- Injongkol, Y.; Intayot, R.; Yodsin, N.; Montoya, A.; Jungsuttiwong, S. Mechanistic Insight into Catalytic Carbon Dioxide Hydrogenation to Formic Acid over Pt-Doped Boron Nitride Nanosheets. Mol. Catal. 2021, 510, 111675. [Google Scholar] [CrossRef]
- Sinthika, S.; Kumar, E.M.; Surya, V.J.; Kawazoe, Y.; Park, N.; Iyakutti, K.; Thapa, R. Activation of CO and CO2 on Homonuclear Boron Bonds of Fullerene-like BN Cages: First Principles Study. Sci. Rep. 2015, 5, 17460. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhuo, Z.; Lv, H.; Wu, X. Enhanced Activation of CO2 on H-BN Nanosheets via Forming a Donor-Acceptor Heterostructure with 2D M2X Electrenes. J. Phys. Chem. C 2021, 125, 18762–18769. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Dutta, S. Review and Outlook of Hydrogen Production through Catalytic Processes. Energy and Fuels. Am. Chem. Soc. 2024, 38, 2601–2629. [Google Scholar] [CrossRef]
- Li, H.; Song, D.; Wang, X.; Li, X.; Lei, G. Recent Progress on Heterogeneous Catalytic Formic Acid Decomposition for Hydrogen Production. Fuel 2025, 383, 133824. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Dongil, A.B.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Tandem Catalysts for the Selective Hydrogenation of Butadiene with Hydrogen Generated from the Decomposition of Formic Acid. Chem. Commun. 2021, 57, 6479–6482. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q. Gold-Containing Metal Nanoparticles for Catalytic Hydrogen Generation from Liquid Chemical Hydrides. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 1594–1599. [Google Scholar] [CrossRef]
- Lang, C.; Jia, Y.; Yao, X. Recent Advances in Liquid-Phase Chemical Hydrogen Storage. Energy Storage Mater. 2020, 26, 290–312. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of Metal-Support Interactions in Heterogeneous Catalysts to Enhance Activity and Selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Yruela-Garrido, M.; Martín-Rodríguez, N.; Castillejos, E.; Campos-Castellanos, E.; Conesa, J.M.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Formic Acid Dehydration Using Mechanochemically Prepared TiO2-Graphite Composites. ChemCatChem 2024, 16, e202400897. [Google Scholar] [CrossRef]
- Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic Acid as a Hydrogen Storage Material-Development of Homogeneous Catalysts for Selective Hydrogen Release. Chemical Society Reviews. R. Soc. Chem. 2016, 21, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Connell, J.W. Advances in 2D Boron Nitride Nanostructures: Nanosheets, Nanoribbons, Nanomeshes, and Hybrids with Graphene. Nanoscale 2012, 4, 6908–6939. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Williams, T.V.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Defect Functionalization of Hexagonal Boron Nitride Nanosheets. J. Phys. Chem. C 2010, 114, 17434–17439. [Google Scholar] [CrossRef]
- Sainsbury, T.; Satti, A.; May, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.K.; Coleman, J. Oxygen Radical Functionalization of Boron Nitride Nanosheets. J. Am. Chem. Soc. 2012, 134, 18758–18771. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Nurazar, R. Metal-Free Decomposition of Formic Acid on Pristine and Carbon-Doped Boron Nitride Fullerene: A DFT Study. J. Clust. Sci. 2015, 26, 595–608. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Zhang, Z.; Yang, G.; Jin, M.; Chen, Q.; Yin, Y. Construction of Au-Pd Alloy Shells for Enhanced Catalytic Performance toward Alkyne Semihydrogenation Reactions. Mater. Horiz. 2017, 4, 584–590. [Google Scholar] [CrossRef]
- Faroldi, B.M.; Conesa, J.M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Efficient Nickel and Copper-Based Catalysts Supported on Modified Graphite Materials for the Hydrogen Production from Formic Acid Decomposition. Appl. Catal. A Gen. 2022, 629, 118419. [Google Scholar] [CrossRef]
- Mori, K.; Naka, K.; Masuda, S.; Miyawaki, K.; Yamashita, H. Palladium Copper Chromium Ternary Nanoparticles Constructed In Situ within a Basic Resin: Enhanced Activity in the Dehydrogenation of Formic Acid. ChemCatChem 2017, 9, 3456–3462. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Say, Z.; Kivrak, H.; Kaya, M.; Gulcan, M.; Ozensoy, E.; Zahmakiran, M. MnOx-Promoted PdAg Alloy Nanoparticles for the Additive-Free Dehydrogenation of Formic Acid at Room Temperature. ACS Catal. 2015, 5, 6099–6110. [Google Scholar] [CrossRef]
- Wang, Z.L.; Ping, Y.; Yan, J.M.; Wang, H.L.; Jiang, Q. Hydrogen Generation from Formic Acid Decomposition at Room Temperature Using a NiAuPd Alloy Nanocatalyst. Int. J. Hydrog. Energy 2014, 39, 4850–4856. [Google Scholar] [CrossRef]
- Faroldi, B.; Paviotti, M.A.; Camino-Manjarrés, M.; González-Carrazán, S.; López-Olmos, C.; Rodríguez-Ramos, I. Hydrogen Production by Formic Acid Decomposition over ca Promoted Ni/SiO2 Catalysts: Effect of the Calcium Content. Nanomaterials 2019, 9, 1516. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Li, Q.; Lin, J.; Yu, C.; Gao, X.; Fang, Y.; Liu, Z.; Guo, Z.; Tang, C.; Huang, Y. Bimetallic AuPd Nanoparticles Loaded on Amine-Functionalized Porous Boron Nitride Nanofibers for Catalytic Dehydrogenation of Formic Acid. ACS Appl. Nano Mater. 2021, 4, 1849–1857. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Hu, E.; Xu, L.; Gan, S.; Zhai, J. Catalytic Performance of CuPd/BNNS Nanocatalysts Prepared by Microwave-Assisted Synthesis for the Reduction of Cr(VI). Nano 2020, 15, 2050142. [Google Scholar] [CrossRef]
- Wang, H.; Chi, Y.; Gao, D.; Wang, Z.; Wang, C.; Wang, L.; Wang, M.; Cheng, D.; Zhang, J.; Wu, C.; et al. Enhancing Formic Acid Dehydrogenation for Hydrogen Production with the Metal/Organic Interface. Appl. Catal. B 2019, 255, 117776. [Google Scholar] [CrossRef]
- Liu, P.; Gu, X.; Zhang, H.; Cheng, J.; Song, J.; Su, H. Visible-Light-Driven Catalytic Activity Enhancement of Pd in AuPd Nanoparticles for Hydrogen Evolution from Formic Acid at Room Temperature. Appl. Catal. B 2017, 204, 497–504. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Role of Water in Formic Acid Decomposition. AIChE J. 1998, 44, 405–415. [Google Scholar] [CrossRef]
- Gao, D.; Wang, Z.; Wang, C.; Wang, L.; Chi, Y.; Wang, M.; Zhang, J.; Wu, C.; Gu, Y.; Wang, H.; et al. CrPd Nanoparticles on NH2-Functionalized Metal-Organic Framework as a Synergistic Catalyst for Efficient Hydrogen Evolution from Formic Acid. Chem. Eng. J. 2019, 361, 953–959. [Google Scholar] [CrossRef]
- Laskowski, R.; Blaha, P.; Schwarz, K. Bonding of Hexagonal BN to Transition Metal Surfaces: An Ab Initio Density-Functional Theory Study. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 78, 045409. [Google Scholar] [CrossRef]
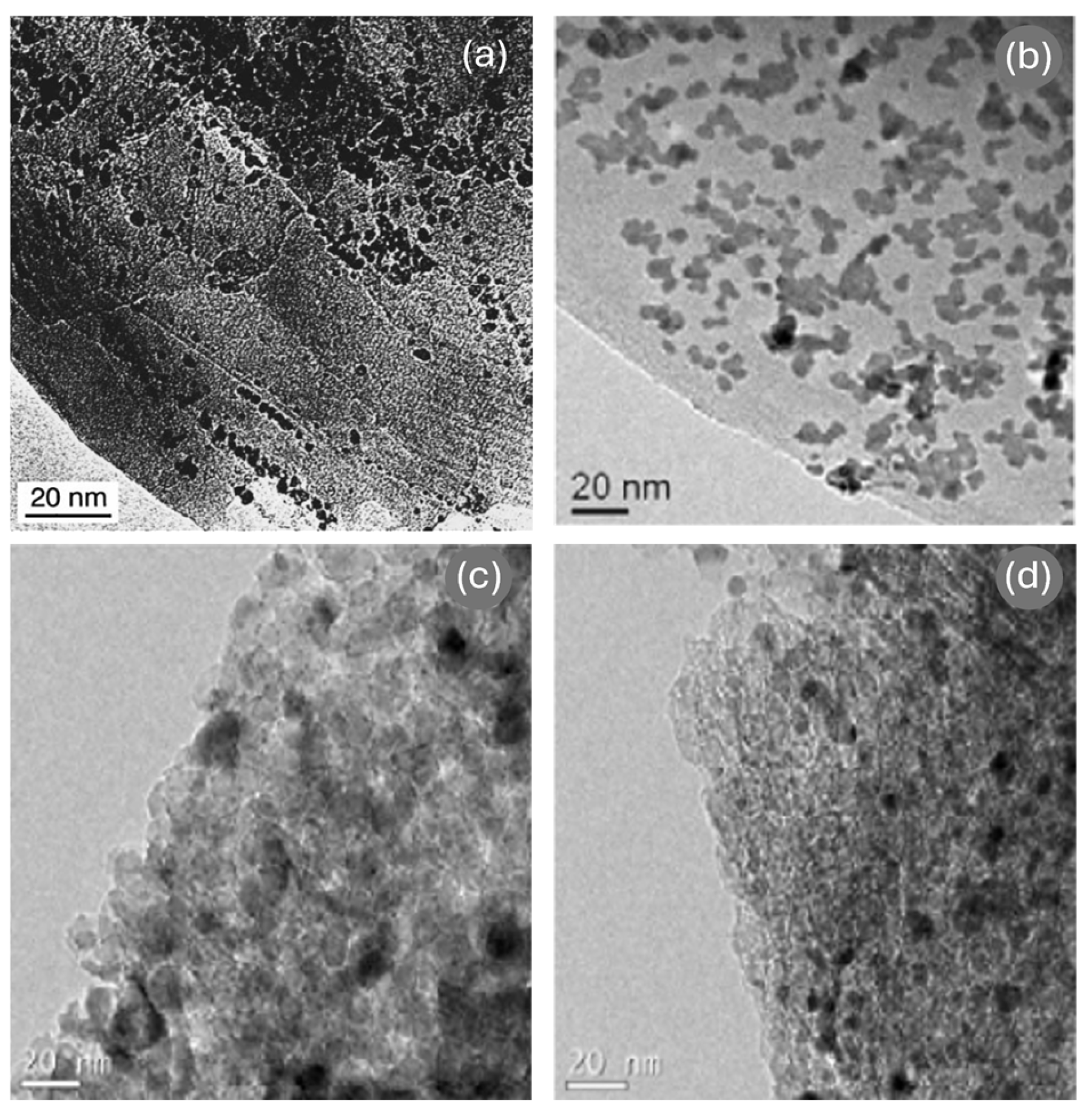
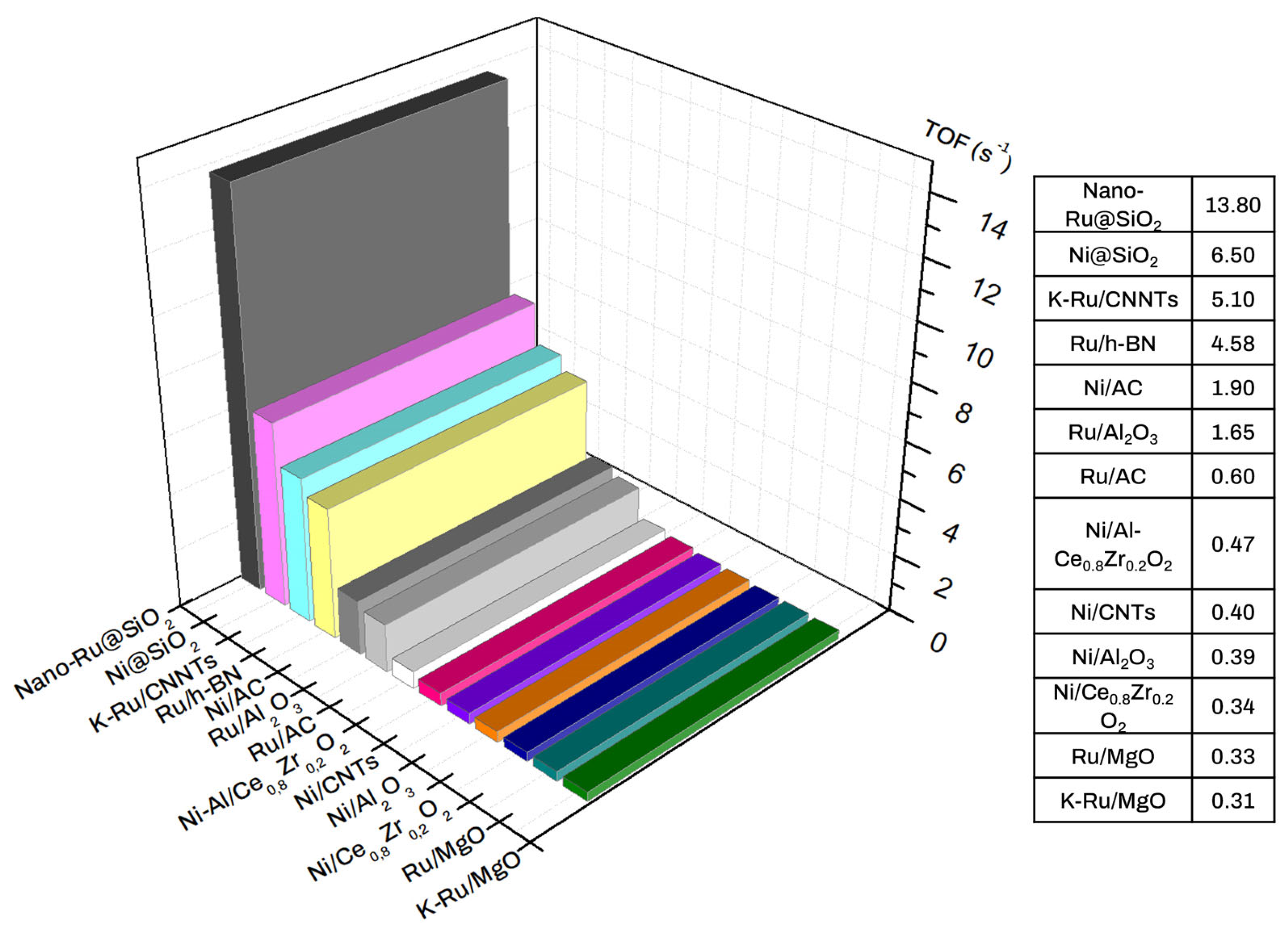
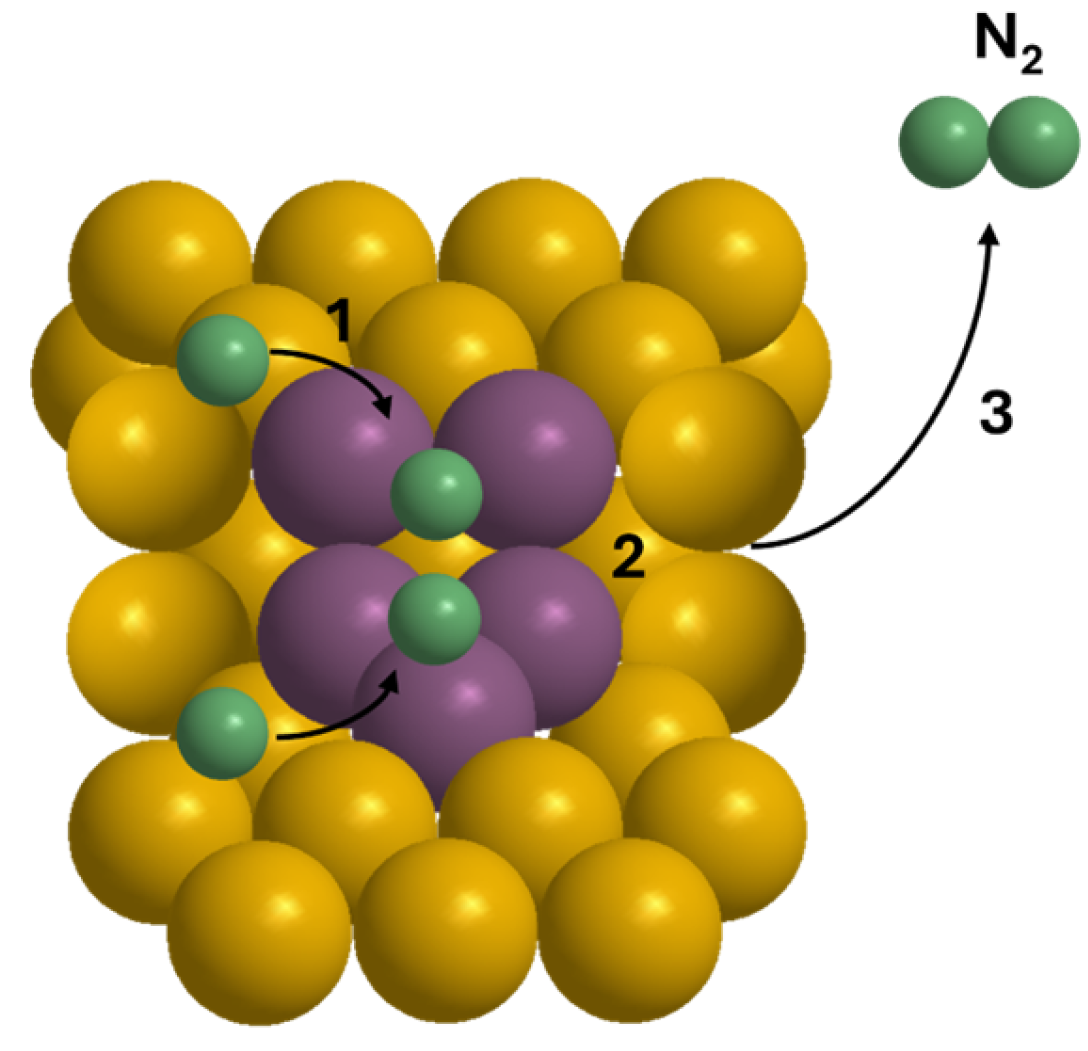
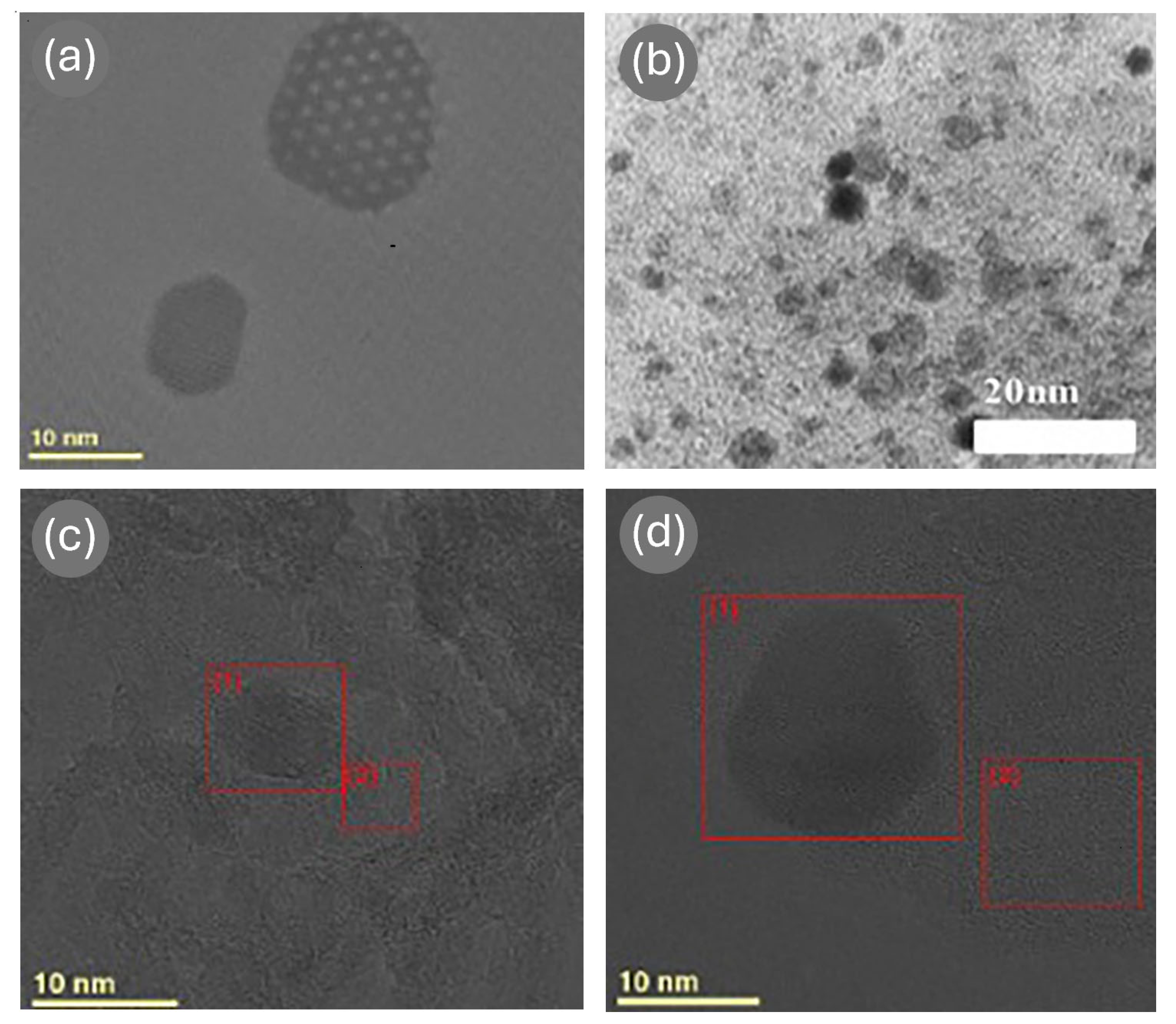
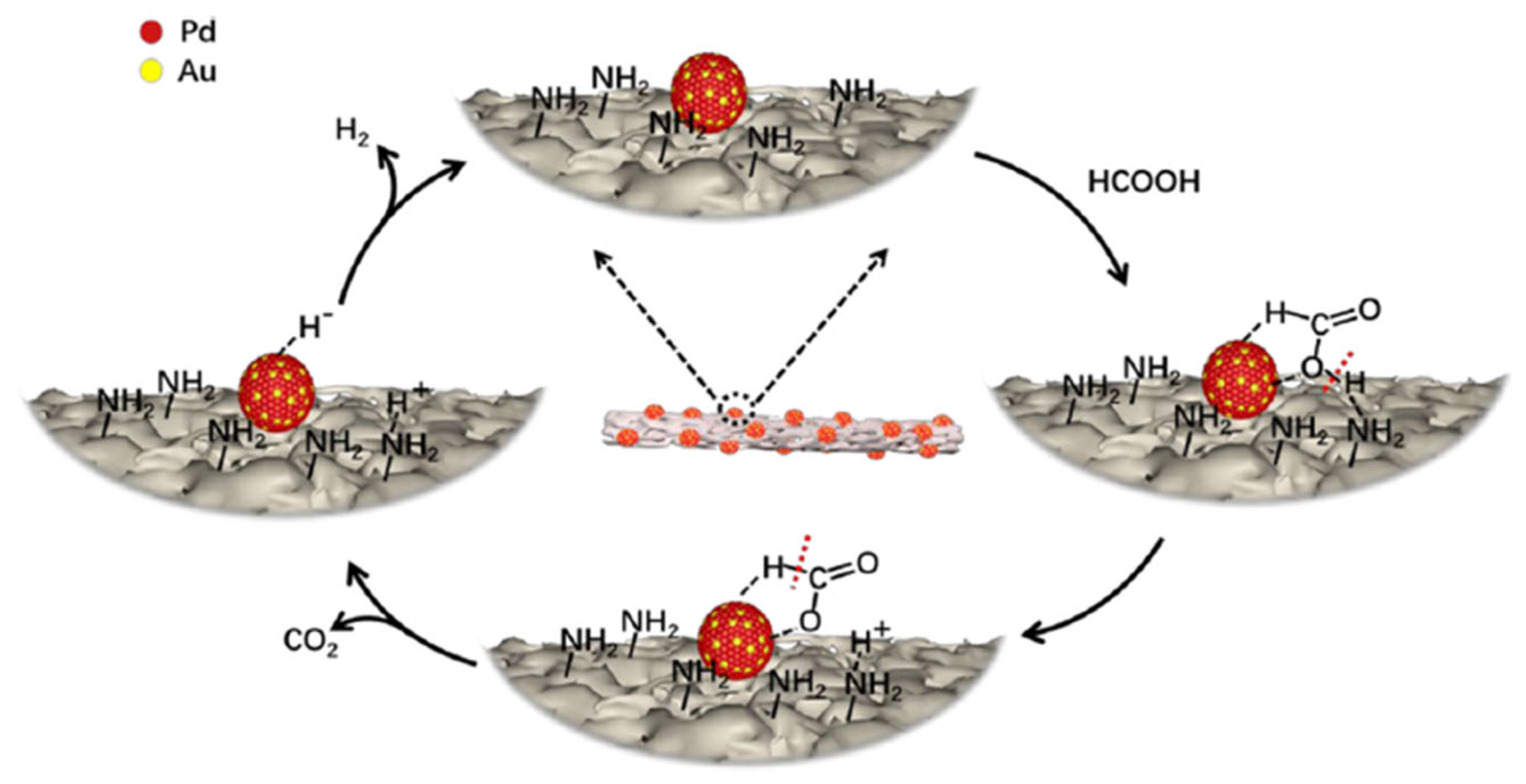
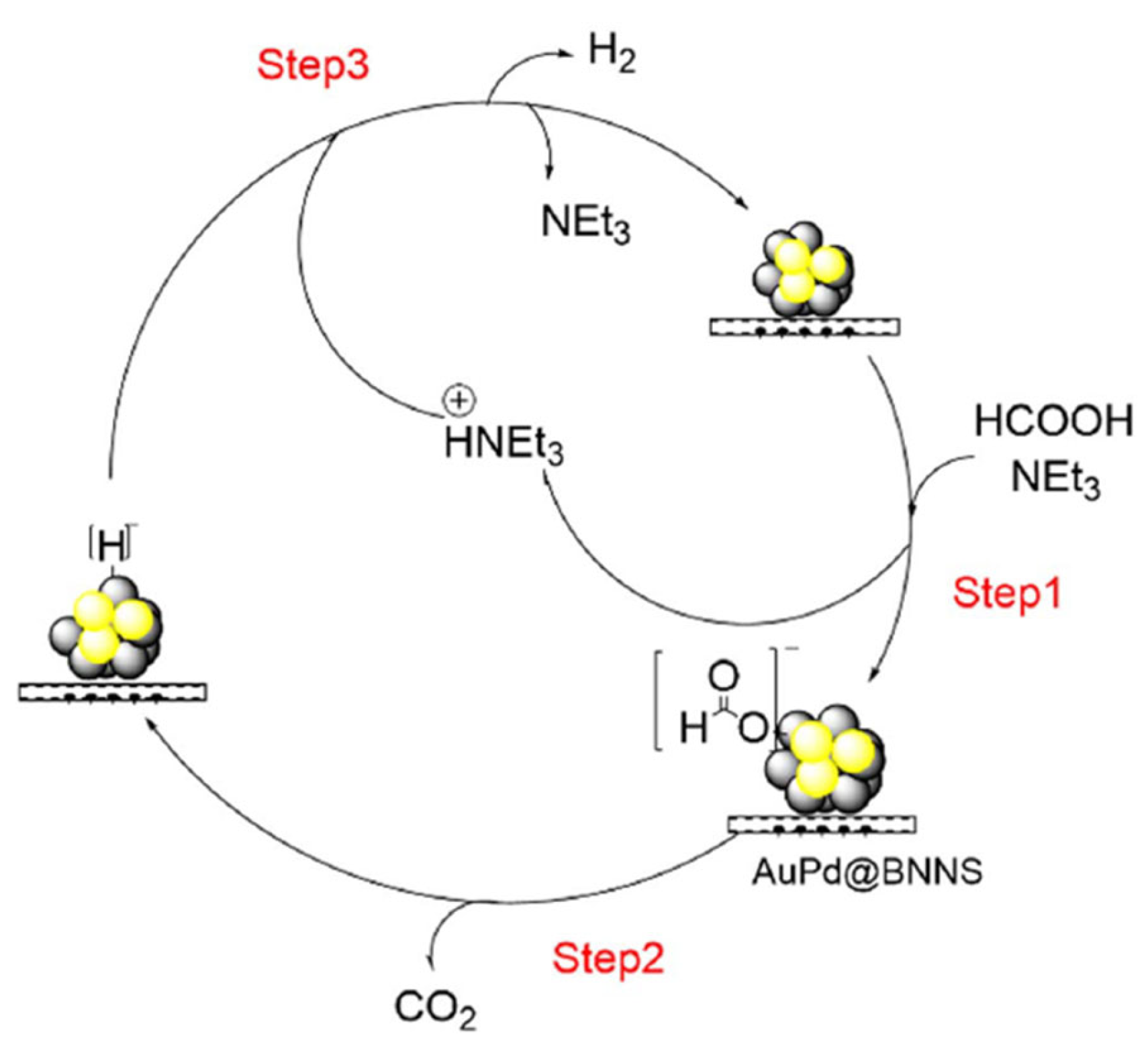

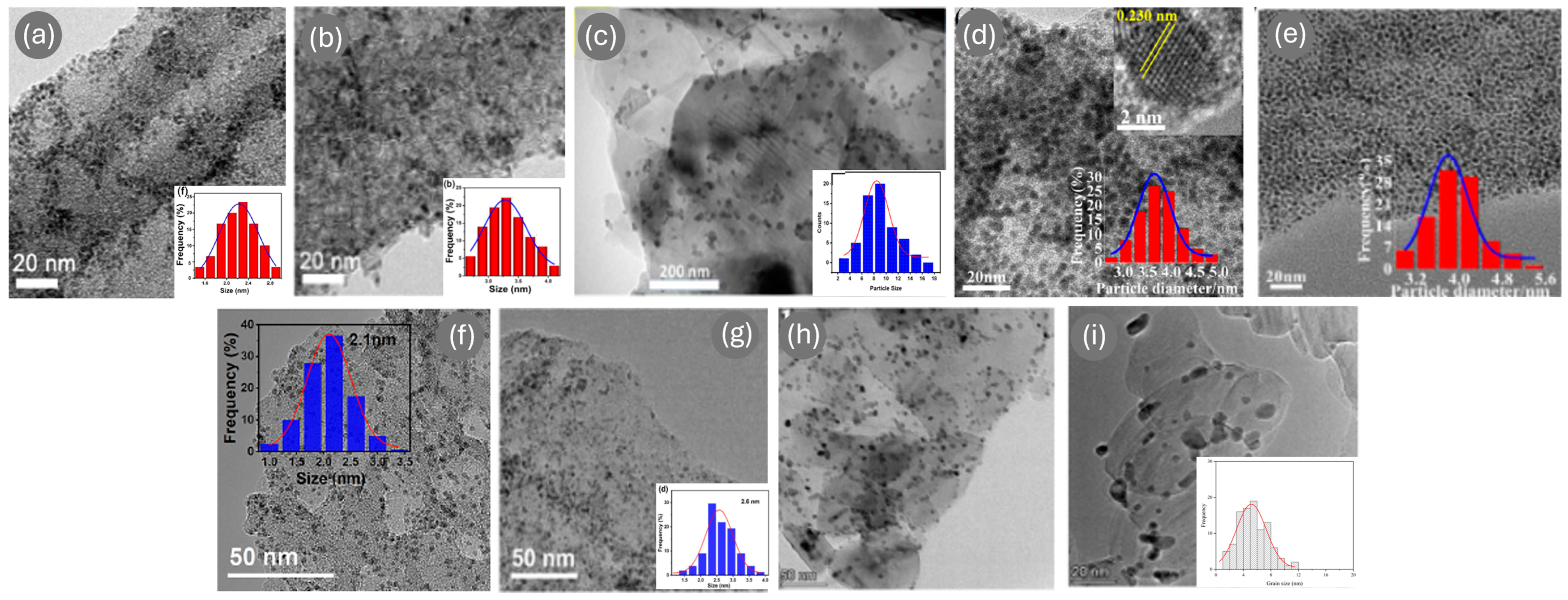
| Ref. | Catalyst | GHSV (ml/gcat·h) | wt% | T (°C) | H2 Production Rate (mmol/gcat·s) |
|---|---|---|---|---|---|
| [114] | Ru/h-BN | - | 1 | 450 | 0.32 |
| [132] | Nano-Ru@SiO2 | 30,000 | - | 450 | 0.38 |
| [130] | Ru/AC | 30,000 | 4.80 | 450 | 0.02 |
| [129] | Ru/Al2O3 | 150,000 | 5.00 | 450 | 0.13 |
| [130] | K-Ru/CNNTs | 30,000 | 4.80 | 450 | 0.54 |
| [136] | K-Ru/MgO-CNTs | 60,000 | 4.85 | 450 | 0.94 |
| [127] | Ru/MgO | 60,000 | 4.70 | 400 | 0.07 |
| [126] | K-Ru/MgO | 30,000 | 4.60 | 400 | 0.06 |
| Ref. | Catalyst | GHSV (ml/gcat·h) | wt% | T (°C) | H2 Rate (mmol/gcat·s) |
|---|---|---|---|---|---|
| [137] | Ni/CeO₂-BN | 30,000 | 9.5 | 600 | 0.49 |
| [132] | Ni@SiO2 | 30,000 | - | 500 | 0.20 |
| [131] | Ni/AC | - | 5 | 500 | - |
| [139] | Ni/Al2O3 | 30,000 | - | 450 | 0.08 |
| [128] | Ni/Al2O3 | - | 8.9 | 500 | 1.02 |
| [128] | Ni/Al-Ce0.8Zr0.2O2 | - | 8 | 500 | 2.20 |
| [128] | Ni/Ce0.8Zr0.2O2 | - | 10.7 | 1.80 | |
| [129] | Ni/CNTs | 30,000 | - | 500 | 0.05 |
| [140] | Ni/SiO2 | - | 10 | 600 | 0.19 |
| [140] | Ni/SiO2/ Al2O3 | - | 65 | 600 | 0.41 |
| Ref. | Catalyst | Ea (kJ/mol) | T (°C) | TOF (h−1) | NP Size (nm) |
|---|---|---|---|---|---|
| [175] | Au1Pd3/BNNFs-A | 20.1 | 25 | 1181 | 2.2 |
| [72] | Au0.03Pd0.05@BNNSs | - | 50 | 6848 | 8.4 |
| [55] | Au0.3Pd0.7/CA-BN-NH2 | 29.7 | 20 | 7046 | 3.6 |
| [76] | Pd/BNC,O-1-A | 23.6 | 65 | 1561 | 2.1 |
| [176] | Cu8Pd2/BNNSs | 26.07 | 25 | - | 3.4 |
| [76] | 1-Pd/h-BN | - | 160 | 913 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yruela-Garrido, M.; Campos-Castellanos, E.; Morales, M.V.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Boron Nitride-Supported Metal Catalysts for the Synthesis and Decomposition of Ammonia and Formic Acid. Nanomaterials 2025, 15, 212. https://doi.org/10.3390/nano15030212
Yruela-Garrido M, Campos-Castellanos E, Morales MV, Rodríguez-Ramos I, Guerrero-Ruiz A. Boron Nitride-Supported Metal Catalysts for the Synthesis and Decomposition of Ammonia and Formic Acid. Nanomaterials. 2025; 15(3):212. https://doi.org/10.3390/nano15030212
Chicago/Turabian StyleYruela-Garrido, Marta, Eduardo Campos-Castellanos, María V. Morales, Inmaculada Rodríguez-Ramos, and Antonio Guerrero-Ruiz. 2025. "Boron Nitride-Supported Metal Catalysts for the Synthesis and Decomposition of Ammonia and Formic Acid" Nanomaterials 15, no. 3: 212. https://doi.org/10.3390/nano15030212
APA StyleYruela-Garrido, M., Campos-Castellanos, E., Morales, M. V., Rodríguez-Ramos, I., & Guerrero-Ruiz, A. (2025). Boron Nitride-Supported Metal Catalysts for the Synthesis and Decomposition of Ammonia and Formic Acid. Nanomaterials, 15(3), 212. https://doi.org/10.3390/nano15030212








