Growth of ZnO Nanoparticles Using Microwave Hydrothermal Method—Search for Defect-Free Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Synthesis
2.2. Nanoparticle Characterization
3. Results and Discussion
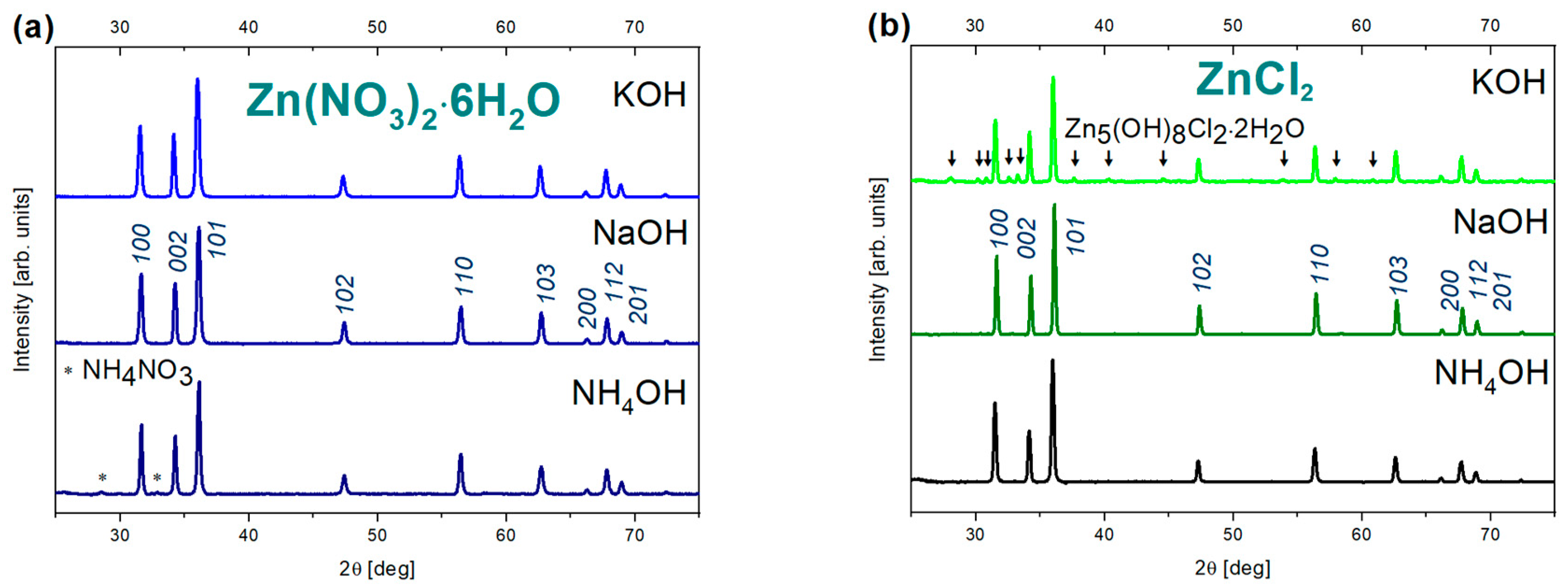
3.1. Crystal Structure
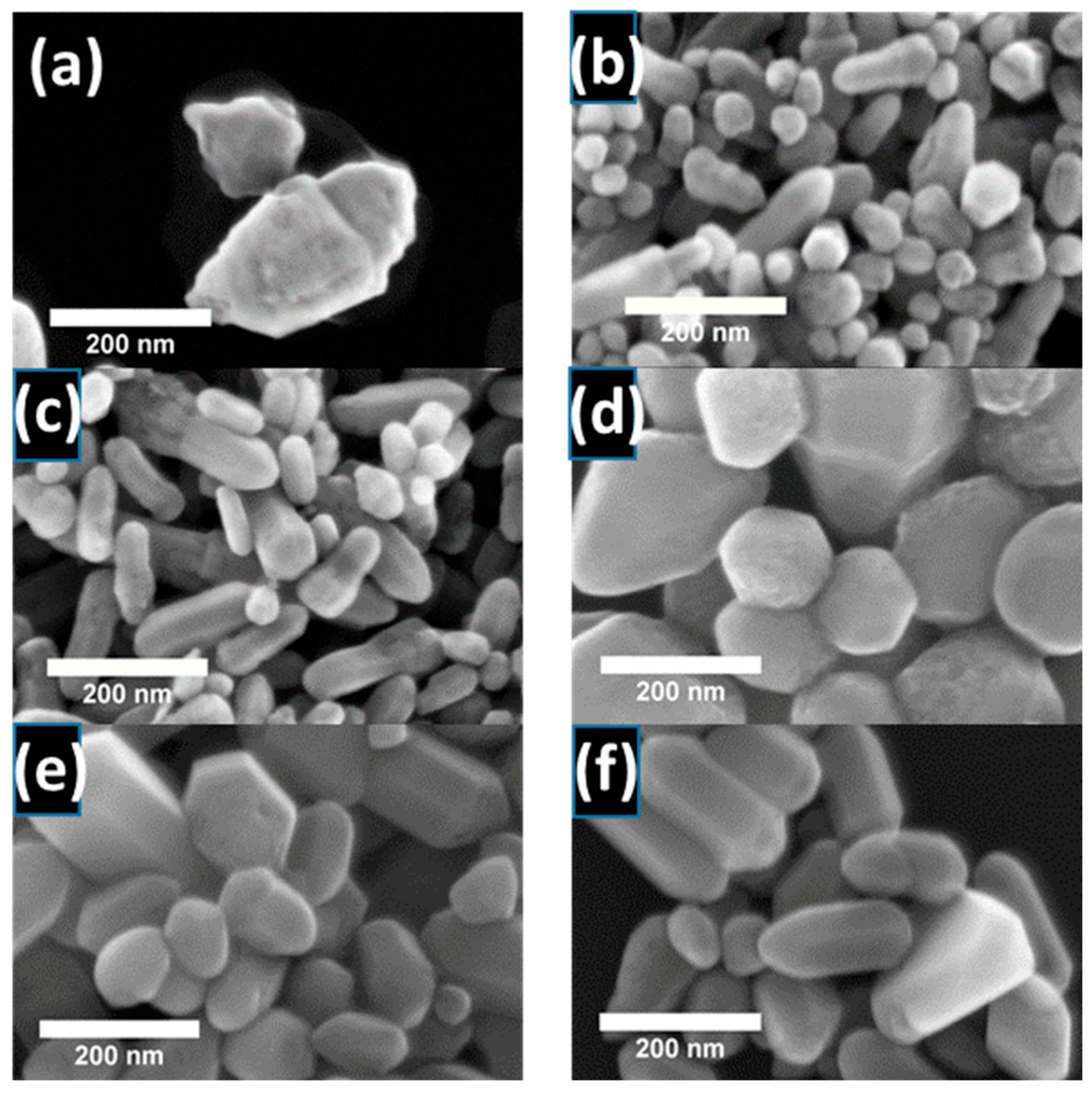
3.2. Morphology Characteristics—Scanning Electron Microscopy (SEM)
3.3. Grain Size Distributions
3.4. Chemical Composition and Thermogravimetry
3.4.1. EDX Measurements
3.4.2. TGA-DTA Measurements
- Sublimation with possible hydrolysis of [52].
3.5. Spectroscopic Properties
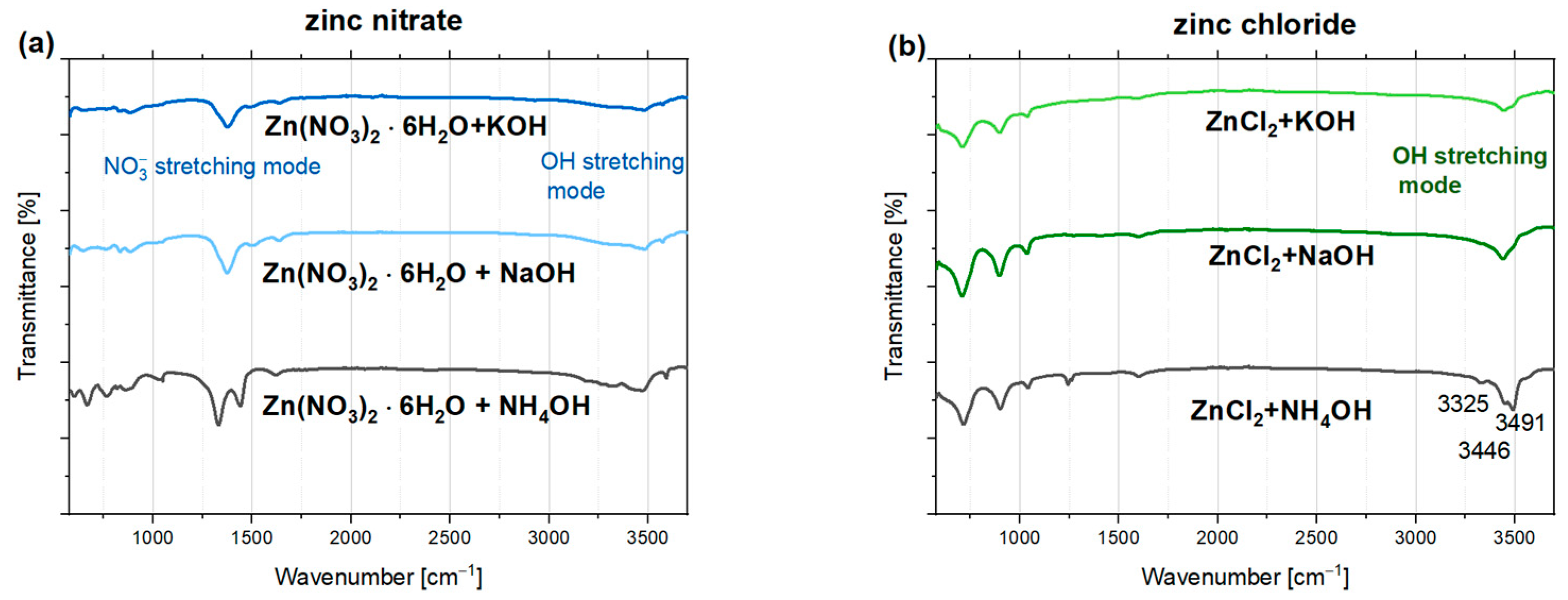
3.5.1. Fourier-Transform Infrared Spectroscopy (FTIR)
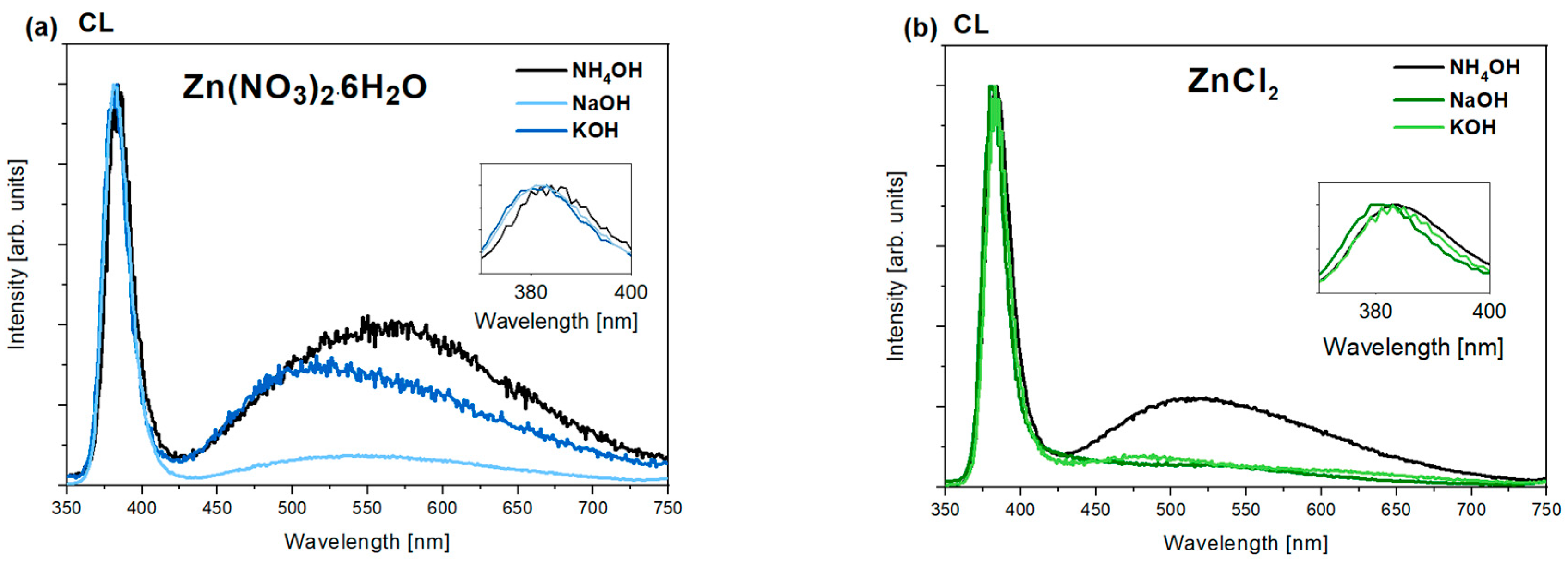
3.5.2. CL and PL Measurements
3.5.3. Dependence of ZnO Luminescence Spectra on Excitation Power Density—Measurements with an OPO Laser
3.5.4. Effect of Annealing on Optical Properties and Role of Chloride Ions in Defect Passivation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mousa, S.A.; Wissa, D.A.; Hassan, H.H.; Ebnalwaled, A.A.; Khairy, S.A. Enhanced Photocatalytic Activity of Green Synthesized Zinc Oxide Nanoparticles Using Low-Cost Plant Extracts. Sci. Rep. 2024, 14, 16713. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Hashem, A.H.; Elhussieny, N.I.; Saied, E. Green Biosynthesis of Zinc Oxide Nanoparticles Using Pluchea indica Leaf Extract: Antimicrobial and Photocatalytic Activities. Molecules 2023, 28, 4679. [Google Scholar] [CrossRef] [PubMed]
- Listewnik, P.; Hirsch, M.; Struk, P.; Weber, M.; Bechelany, M.; Jędrzejewska-Szczerska, M. Preparation and characterization of microsphere ZnO ALD coating dedicated for the fiber-optic refractive index sensor. Nanomaterials 2019, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Praveenkumar, S.; Manikandan, S.; Lingaraja, D.; Sugapriya, T. A review of doped and undoped ZnO nanoparticles for fabrication of gas sensor. Sens. Lett. 2018, 16, 889–900. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T. Impact of temperature and UV irradiation on dynamics of NO2 sensors based on ZnO nanostructures. Nanomaterials 2017, 7, 312. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO nanostructures for drug delivery and theranostic applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef] [PubMed]
- Khatir, N.M.; Sabbagh, F. Green Facile Synthesis of Silver-Doped Zinc Oxide Nanoparticles and Evaluation of Their Effect on Drug Release. Materials 2022, 15, 5536. [Google Scholar] [CrossRef]
- Kielbik, P.; Kaszewski, J.; Dominiak, B.; Damentko, M.; Serafińska, I.; Rosowska, J.; Gralak, M.A.; Krajewski, M.; Witkowski, B.S.; Gajewski, Z.; et al. Preliminary Studies on Biodegradable Zinc Oxide Nanoparticles Doped with Fe as a Potential Form of Iron Delivery to the Living Organism. Nanoscale Res. Lett. 2019, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Kiełbik, P.; Jończy, A.; Kaszewski, J.; Gralak, M.; Rosowska, J.; Sapierzyński, R.; Witkowski, B.; Wachnicki, L.; Lawniczak-Jablonska, K.; Kuzmiuk, P.; et al. Biodegradable Zinc Oxide Nanoparticles Doped with Iron as Carriers of Exogenous Iron in the Living Organism. Pharmaceuticals 2021, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, M.M.; Kaszewski, J.; Kielbik, P.; Olszewski, J.; Lipinski, W.; Slonska-Zielonka, A.; Rosowska, J.; Witkowski, B.S.; Gralak, M.A.; Gajewski, Z.; et al. New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics. Nanotechnol. Rev. 2020, 9, 274–302. [Google Scholar] [CrossRef]
- Wolska, E.; Kaszewski, J.; Kiełbik, P.; Grzyb, J.; Godlewski, M.M.; Godlewski, M. Rare earth activated ZnO nanoparticles as biomarkers. Opt. Mater. 2014, 36, 1655–1659. [Google Scholar] [CrossRef]
- Kielbik, P.; Kaszewski, J.; Rosowska, J.; Wolska, E.; Witkowski, B.S.; Gralak, M.A.; Gajewski, Z.; Godlewski, M.; Godlewski, M.M. Biodegradation of the ZnO: Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Narcy, A.; Durosoy, S.; Bordes, C.; Chevalier, Y. Dissolution kinetics of zinc oxide and its relationship with physicochemical characteristics. Powder Technol. 2021, 378, 746–759. [Google Scholar] [CrossRef]
- Komarneni, S.; Roy, R.; Li, Q.H. Microwave Hydrothermal Synthesis of Ceramic Powders. Mater. Res. Bull. 1992, 27, 1393–1405. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- Mohammadi, E.; Aliofkhazraei, M.; Hasanpoor, M.; Chipara, M. Hierarchical and Complex ZnO Nanostructures by Microwave-Assisted Synthesis: Morphologies, Growth Mechanism and Classification. Crit. Rev. Solid State Mater. Sci. 2018, 43, 475–541. [Google Scholar] [CrossRef]
- Witkowski, B.; Dluzewski, P.; Kaszewski, J.; Wachnicki, L.; Gieraltowska, S.; Kurowska, B.; Godlewski, M. Ultra-fast epitaxial growth of ZnO nano/microrods on a GaN substrate, using the microwave-assisted hydrothermal method. Mater. Chem. Phys. 2018, 205, 16–22. [Google Scholar] [CrossRef]
- Ortega, N.P.; Silva, C.C.; Ramirez, M.A.; Biasotto, G.; Foschini, C.R.; Simões, A.Z. Multifunctional Environmental Applications of ZnO Nanostructures Synthesized by the Microwave-Assisted Hydrothermal Technique. Appl. Surf. Sci. 2021, 542, 148723. [Google Scholar] [CrossRef]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef]
- Nandi, A.; Nag, P.; Saha, H.; Majumdar, S. Precursor dependent morphologies of microwave-assisted ZnO nanostructures and their VOC detection properties. Mater. Today Proc. 2018, 5, 9831–9838. [Google Scholar] [CrossRef]
- Caglar, Y.; Gorgun, K.; Aksoy, S. Effect of deposition parameters on the structural properties of ZnO nanopowders prepared by microwave-assisted hydrothermal synthesis. Spectrochim. Acta A 2015, 138, 617–622. [Google Scholar] [CrossRef]
- Rosowska, J.; Kaszewski, J.; Witkowski, B.; Wachnicki, Ł.; Godlewski, M. The effect of synthesis pressure on properties of Eu-doped ZnO nanopowders prepared by microwave hydrothermal method. Acta Phys. Pol. A 2016, 130, 1205–1208. [Google Scholar] [CrossRef]
- Demoisson, F.; Piolet, R.; Ariane, M.; Leybros, A.; Bernard, F. Influence of the pH on the ZnO nanoparticle growth in supercritical water: Experimental and simulation approaches. J. Supercrit. Fluids 2014, 95, 75–83. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Meyer, B.K.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Forster, D.; Bertram, F.; Christen, J.; Hoffmann, A.; Straßburg, M.; Dworzak, M.; et al. Bound exciton and donor-acceptor pair recombinations in ZnO. Phys. Status Solidi B 2004, 241, 231–260. [Google Scholar] [CrossRef]
- Kohan, A.; Ceder, G.; Morgan, D.; Van de Walle, C.G. First-principles study of native point defects in ZnO. Phys. Rev. B 2000, 61, 15019–15027. [Google Scholar] [CrossRef]
- Egelhaaf, H.J.; Oelkrug, D. Luminescence and non-radiative deactivation of excited states involving oxygen defect centers in polycrystalline ZnO. J. Cryst. Growth 1996, 161, 190–194. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, P.; Shi, C.; Xu, F.; Pan, H.; Lu, E. A FP-LMTO study on the native shallow donor in ZnO. J. Electron Spectrosc. Relat. Phenom. 2001, 114–116, 1123–1125. [Google Scholar]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1995, 68, 403–405. [Google Scholar] [CrossRef]
- Leiter, F.H.; Alves, H.R.; Hofmann, D.M.; Meyer, B.K. The oxygen vacancy as the origin of a green emission in undoped ZnO. Phys. Status Solidi B 2001, 226, R4–R5. [Google Scholar] [CrossRef]
- Xu, P.S.; Sun, M.; Shi, C.S. The electronic structure and spectral properties of ZnO and its defects. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 199, 286–290. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, K.; Zuo, J.; Oian, Y. Synthesis and luminescent property of single-crystal ZnO nanobelts by a simple low-temperature evaporation route. Appl. Phys. A 2004, 79, 1847–1851. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Choy, W.C.H.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Rao, T.K.G.; Chan, W.K.; Lui, H.F.; Surya, C. Photoluminescence and electron paramagnetic resonance of ZnO tetrapod structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Djurisić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Li, D.; Leung, Y.; Djurisić, A.B.; Liu, Z.; Xie, M.; Shi, L.; Xu, S.; Chan, W. Different Origins of Visible Luminescence in ZnO Nanostructures Fabricated by the Chemical and Evaporation Methods. Appl. Phys. Lett. 2004, 85, 1601–1603. [Google Scholar] [CrossRef]
- Fan, H.; Scholz, R.; Kolb, F.; Zacharias, M. Two-Dimensional Dendritic ZnO Nanowires from Oxidation of Zn Microcrystals. Appl. Phys. Lett. 2004, 85, 4142–4144. [Google Scholar] [CrossRef]
- Wen, F.; Li, W.; Moon, J.-H.; Kim, J.H. Hydrothermal synthesis of ZnO: Zn with green emission at low temperature with reduction process. Solid State Commun. 2005, 135, 34–37. [Google Scholar] [CrossRef]
- Gomi, M.; Oohira, N.; Ozaki, K.; Koyano, M. Photoluminescent and structural properties of precipitated ZnO fine particles. Jpn. J. Appl. Phys. 2003, 42, 481–485. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Cao, H.; Chang, R.P.H. Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2004, 95, 3141–3147. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.; Liu, C.; Teke, A.; Reshchikov, M.; Dogan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Chemical Manipulation of Oxygen Vacancy and Antibacterial Activity in ZnO. Mater. Sci. Eng. C 2017, 77, 1027–1034. [Google Scholar]
- Strachowski, T.; Baran, M.; Małek, M.; Kosturek, R.; Grzanka, E.; Mizeracki, J.; Romanowska, A.; Marynowicz, S. Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Different Chemical Reaction Stimulation Methods and Their Influence on Process Kinetics. Materials 2022, 15, 7661. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, F.; Sokolova, E. Simonkolleite, Zn5(OH)8Cl2(H2O), a Decorated Interrupted-Sheet Structure of the Form [MΦ2]4. Can. Mineralog. 2002, 40, 939–946. [Google Scholar] [CrossRef]
- Barreto, G.; Morales, G.; Quintanilla, M. Microwave Assisted Synthesis of ZnO Nanoparticles: Effect of Precursor Reagents, Temperature, Irradiation Time, and Additives on Nano-ZnO Morphology Development. J. Mater. 2013, 1, 478681. [Google Scholar] [CrossRef]
- Nistor, S.V.; Ghica, D.; Stefan, M.; Vlaicu, I.; Barascu, J.N.; Bartha, C. Magnetic Defects in Crystalline Zn(OH)2 and Nanocrystalline ZnO Resulting from Its Thermal Decomposition. J. Alloys Compd. 2013, 548, 222–227. [Google Scholar] [CrossRef]
- Reinders, N.; Cousy, S.; Svoboda, L.; Zelenka, J. Peculiarities of Thermal Decomposition of Synthetic Simonkolleite. In Proceedings of the 3rd Central and Eastern European Conference on Thermal Analysis and Calorimetry, Ljubljana, Slovenia, 25–28 August 2015. [Google Scholar]
- Nejati, K.; Akbari, A.R.; Davari, S.; Asadpour-Zeynali, K.; Rezvani, Z. Zn–Fe-layered double hydroxide intercalated with vanadate and molybdate anions for electrocatalytic water oxidation. New J. Chem. 2018, 42, 2889–2895. [Google Scholar] [CrossRef]
- Lavrov, E.V. Infrared Absorption Spectroscopy of Hydrogen-Related Defects in ZnO. Phys. B 2003, 340, 195. [Google Scholar] [CrossRef]
- Goebbert, D.J.; Garand, E.; Wende, T.; Bergmann, R.; Meijer, G.; Asmis, K.R.; Neumark, D.M. Infrared Spectroscopy of the Microhydrated Nitrate Ions NO(3)−(H2O)(1−6). J. Phys. Chem. A. 2009, 113, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, O.K.; Secco, E.A. Studies on Metal Hydroxy Compounds. II. Infrared Spectra of Zinc Derivatives ε-Zn(OH)2, β-ZnOHCl, ZnOHF, Zn5(OH)8Cl2, and Zn5(OH)8Cl2·H2O. Can. J. Chem. 1967, 45, 585–588. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujioka, A.; Futoyu, A.; Kandori, K.; Ishikawa, T. Synthesis and Characterization of Layered Zinc Hydroxychlorides. J. Solid State Chem. 2007, 180, 2061–2066. [Google Scholar] [CrossRef]
- Herklotz, F.; Hupfer, A.; Johansen, K.M.; Svensson, B.G.; Koch, S.G.; Lavrov, E.V. Infrared Absorption on a Complex Comprising Three Equivalent Hydrogen Atoms in ZnO. Phys. Rev. B 2015, 92, 155203. [Google Scholar] [CrossRef]
- Wardle, M.G.; Goss, J.P.; Briddon, P.R. Theory of Fe, Co, Ni, Cu, and Their Complexes with Hydrogen in ZnO. Phys. Rev. B 2005, 72, 155108. [Google Scholar] [CrossRef]
- Mohan, S.; Vellakkat, M.; Aravind, A. Hydrothermal Synthesis and Characterization of Zinc Oxide Nanoparticles of Various Shapes under Different Reaction Conditions. Nano Express 2020, 1, 030028. [Google Scholar] [CrossRef]
- Linhua, X.; Xiangyin, L.; Jun, Y. Effect of K-Doping on Structural and Optical Properties of ZnO Thin Films. Superlattices Microstruct. 2008, 44, 276–281. [Google Scholar]
- Eixenberger, J.E.; Anders, C.B.; Wada, K.; Reddy, K.M.; Brown, R.J.; Moreno-Ramirez, J.G.; Weltner, A.E.; Karthik, C.; Tenne, D.A.; Fologea, D.; et al. Defect Engineering of ZnO Nanoparticles for Bio-imaging Applications. ACS Appl. Mater. Interfaces 2019, 11, 24933–24944. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, K.E.; Galeckas, A.; Zubiaga, A.; Tuomisto, F.; Farlow, G.; Svensson, B.; Yu, A.; Kuznetsov, A. Zinc Vacancy and Oxygen Interstitial in ZnO Revealed by Sequential Annealing and Electron Irradiation. Phys. Rev. B 2012, 86, 121203. [Google Scholar] [CrossRef]
- Patra, M.; Manzoor, K.; Manoth, M.; Vadera, S.; Kumar, N. Studies of Luminescence Properties of ZnO and ZnO: Zn Nanorods Prepared by Solution Growth Technique. J. Lumin. 2008, 128, 267–272. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms Behind Green Photoluminescence in ZnO Phosphor Powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Cui, J.B.; Soo, Y.C.; Chen, T.P.; Gibson, U.J. Low-Temperature Growth and Characterization of Cl-Doped ZnO Nanowire Arrays. J. Phys. Chem. C 2008, 112, 4475. [Google Scholar] [CrossRef]
- Lehr, D.; Wagner, M.R.; Flock, J.; Reparaz, J.S.; Torres, C.M.S.; Klaiber, A.; Dekorsy, T.; Polarz, S. A Single-Source Precursor Route to Anisotropic Halogen-Doped Zinc Oxide Particles as a Promising Candidate for New Transparent Conducting Oxide Materials. Beilstein J. Nanotechnol. 2015, 6, 2161–2172. [Google Scholar] [CrossRef]
- Liu, B.; Gu, M.; Liu, X.; Huang, S.; Ni, C. Defect Formation in Chlorine-Doped Zinc Oxide. Solid State Commun. 2013, 171, 30–33. [Google Scholar] [CrossRef]
- Azarov, A.; Galeckas, A.; Venkatachalapathy, V.; Mei, Z.; Du, X.; Monakhov, E.; Kuznetsov, A. Acceptor Complex Signatures in Oxygen-Rich ZnO Thin Films Implanted with Chlorine Ions. J. Appl. Phys. 2020, 128, 125701. [Google Scholar] [CrossRef]


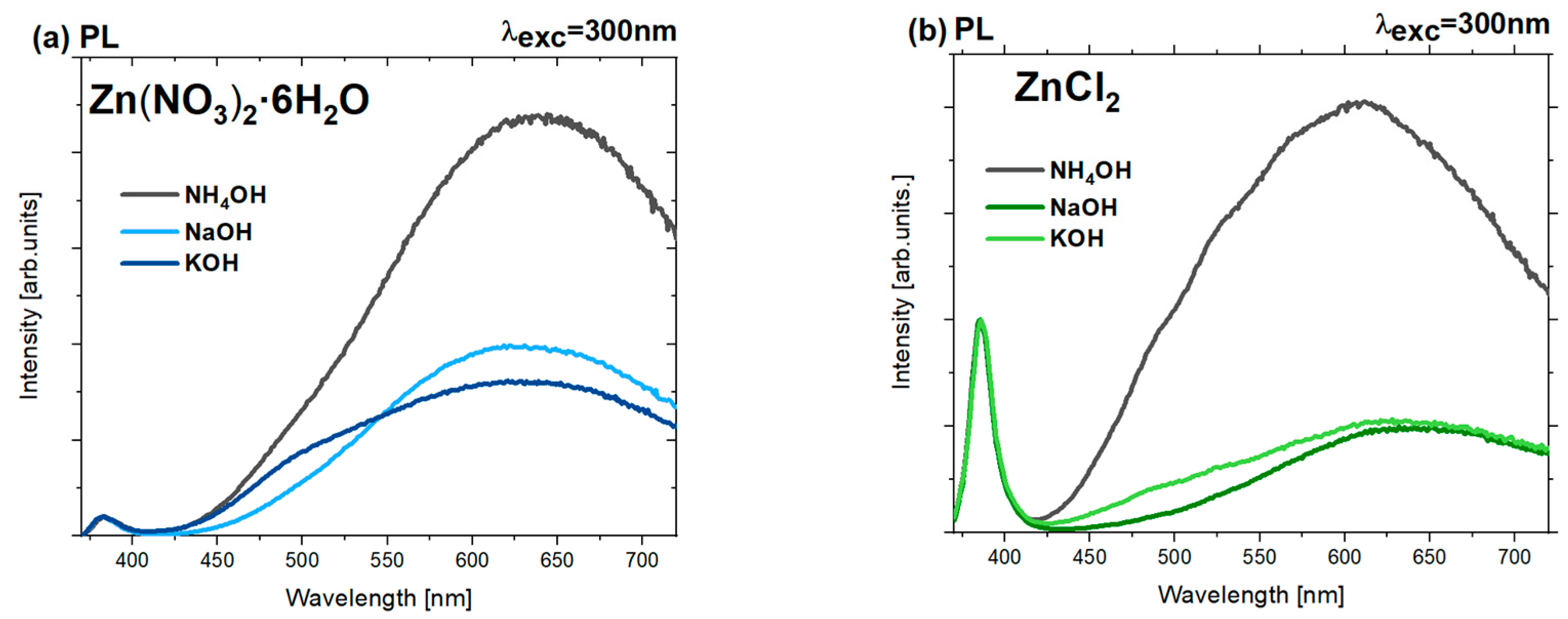
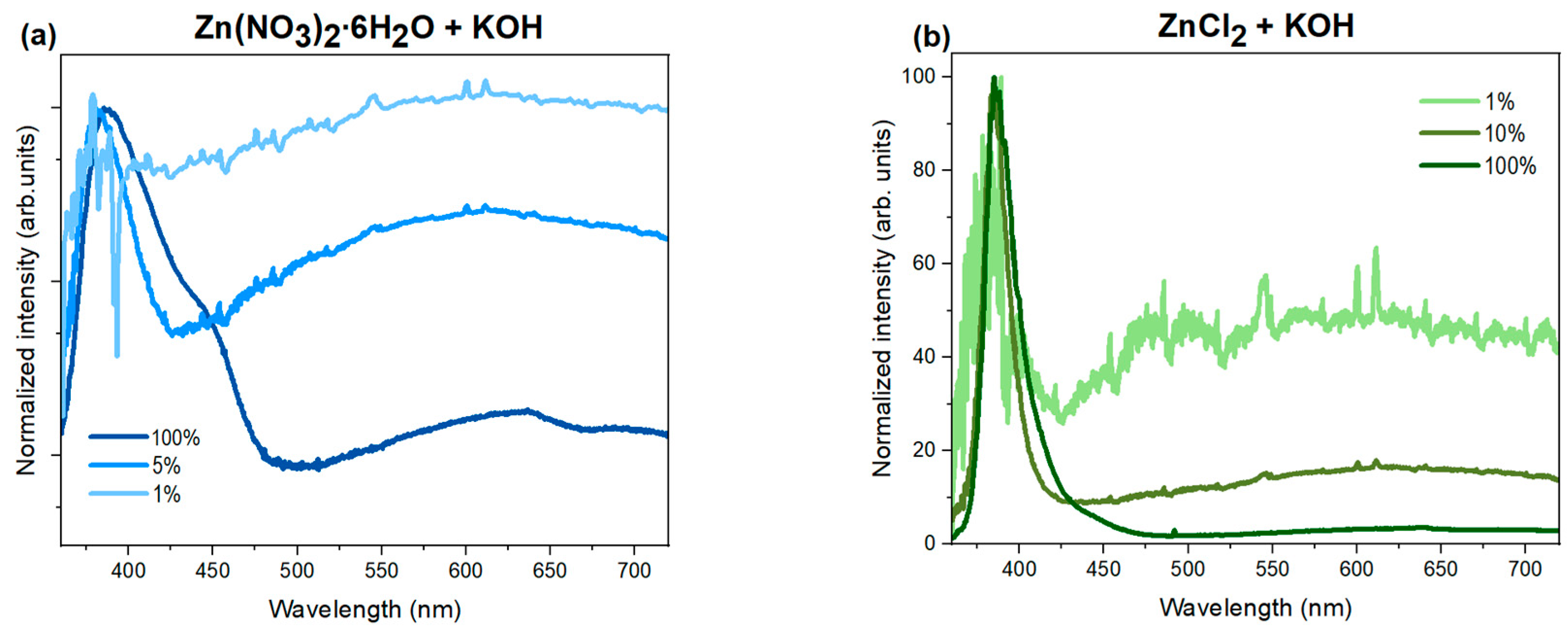
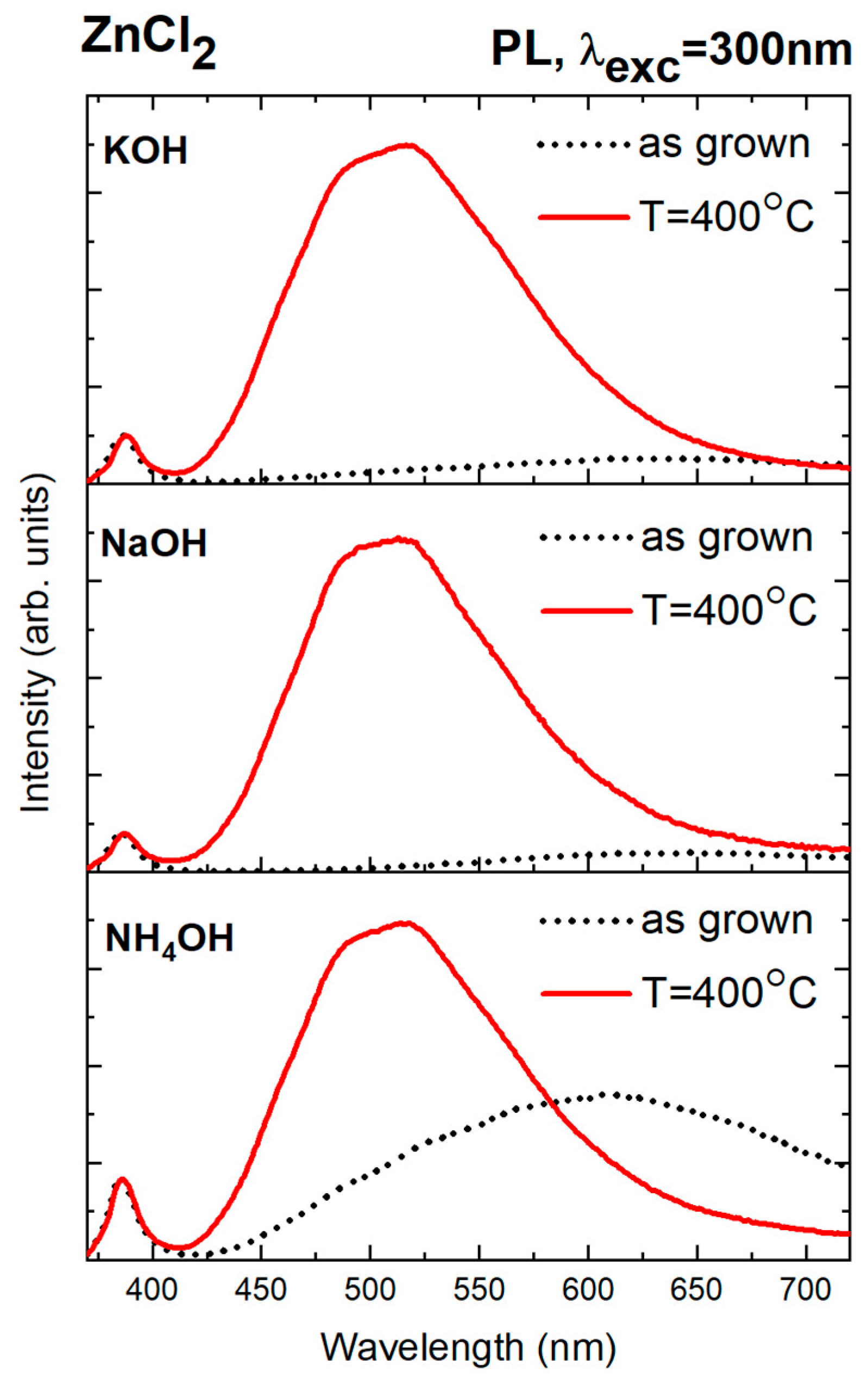
| Precursor | Precipitation Agent | MCS (002) [nm] | Average SEM Size [nm] | Average TEM Size [nm] | Length-to-Width Ratio L/D from SEM |
|---|---|---|---|---|---|
| Zn(NO3)2·6H2O | NH4OH | 107 | 306 ± 11 | 266 ± 25 | 1.75 |
| Zn(NO3)2·6H2O | NaOH | 88 | 130 ± 5 | 144 ± 6 | 2.4 |
| Zn(NO3)2·6H2O | KOH | 90 | 148 ± 6 | 144 ± 7 | 2.86 |
| ZnCl2 | NH4OH | 107 | 258 ± 8 | 247 ± 7 | 1.23 |
| ZnCl2 | NaOH | 219 | 169 ± 4 | 177 ± 5 | 1.88 |
| ZnCl2 | KOH | 143 | 236 ± 8 | 187 ± 7 | 1.79 |
| Precursor | P. agent | Zn | O | N | Cl |
|---|---|---|---|---|---|
| Zn(NO3)2·6H2O | NH4OH | 40.5 ± 0.5 | 54.6 ± 0.4 | 4.8 ± 0.4 | 0 |
| NaOH | 41.5 ± 0.5 | 55.3 ± 0.4 | 3.2 ± 0.4 | 0 | |
| KOH | 44.4 ± 0.5 | 52.6 ± 0.4 | 2.8 ± 0.4 | 0 | |
| ZnCl2 | NH4OH | 44.2 ± 0.5 | 49.2 ± 0.4 | 2.8 ± 0.5 | 2.9 ± 0.1 |
| NaOH | 40.1 ± 0.5 | 49.2 ± 0.4 | 0 | 10.7 ± 0.1 | |
| KOH | 50.4 ± 0.6 | 46.4 ± 0.4 | 0 | 3.2 ± <0.1 |
| Reagents Used in Synthesis | ~INBE/IDLE | |
|---|---|---|
| Zn(NO3)2·6H2O | NH4OH | 0.005 |
| Zn(NO3)2·6H2O | NaOH | 0.0104 |
| Zn(NO3)2·6H2O | KOH | 0.0128 |
| ZnCl2 | NH4OH | 0.54 |
| ZnCl2 | NaOH | 1.59 |
| ZnCl2 | KOH | 1.32 |
| Precursor | P. agent | Zn (%) | O (%) | N (%) | Cl (%) |
|---|---|---|---|---|---|
| ZnCl2 | NH4OH | 59.8 ± 0.6 | 40.1 ± 0.4 | 0 | 0 |
| NaOH | 62.3 ± 0.6 | 34.7 ± 0.4 | 0 | 0 | |
| KOH | 59.7 ± 0.6 | 37.2 ± 0.4 | 0 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosowska, J.; Kaszewski, J.; Krajewski, M.; Małolepszy, A.; Witkowski, B.S.; Wachnicki, Ł.; Bulyk, L.-I.; Sybilski, P.; Godlewski, M.; Godlewski, M.M. Growth of ZnO Nanoparticles Using Microwave Hydrothermal Method—Search for Defect-Free Particles. Nanomaterials 2025, 15, 230. https://doi.org/10.3390/nano15030230
Rosowska J, Kaszewski J, Krajewski M, Małolepszy A, Witkowski BS, Wachnicki Ł, Bulyk L-I, Sybilski P, Godlewski M, Godlewski MM. Growth of ZnO Nanoparticles Using Microwave Hydrothermal Method—Search for Defect-Free Particles. Nanomaterials. 2025; 15(3):230. https://doi.org/10.3390/nano15030230
Chicago/Turabian StyleRosowska, Julita, Jarosław Kaszewski, Marcin Krajewski, Artur Małolepszy, Bartłomiej S. Witkowski, Łukasz Wachnicki, Lev-Ivan Bulyk, Piotr Sybilski, Marek Godlewski, and Michał M. Godlewski. 2025. "Growth of ZnO Nanoparticles Using Microwave Hydrothermal Method—Search for Defect-Free Particles" Nanomaterials 15, no. 3: 230. https://doi.org/10.3390/nano15030230
APA StyleRosowska, J., Kaszewski, J., Krajewski, M., Małolepszy, A., Witkowski, B. S., Wachnicki, Ł., Bulyk, L.-I., Sybilski, P., Godlewski, M., & Godlewski, M. M. (2025). Growth of ZnO Nanoparticles Using Microwave Hydrothermal Method—Search for Defect-Free Particles. Nanomaterials, 15(3), 230. https://doi.org/10.3390/nano15030230







