Nano Cobalt-Loaded Porous Carbon Derived from Waste Plastic for Efficient Persulfate Activation and Tetracycline Degradation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Characterizations
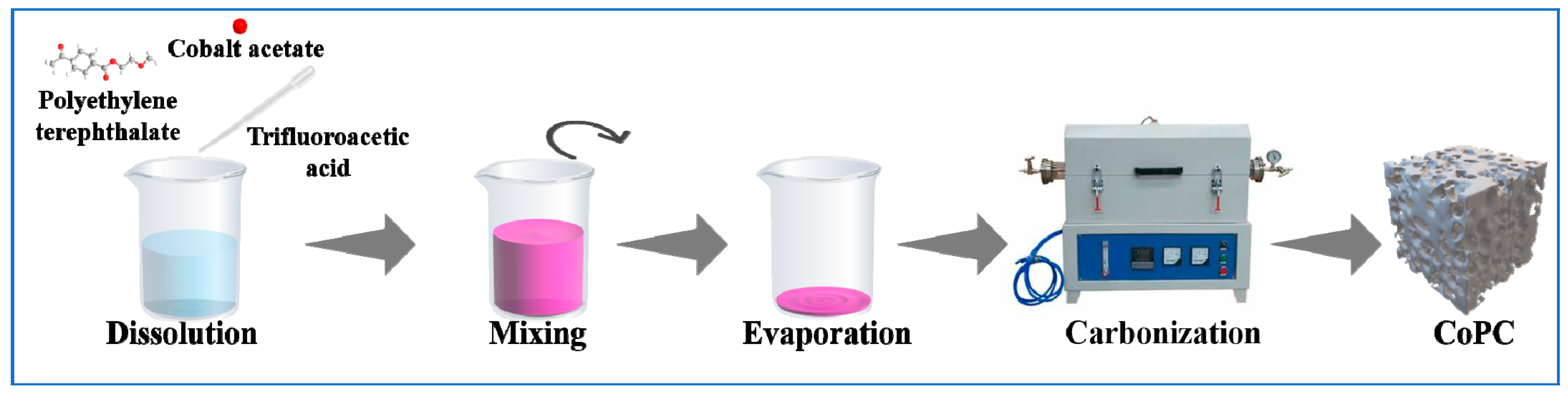
2.2. Preparation Method of CoPC Catalysts
2.3. Catalytic Degradation Test
3. Results and Discussion
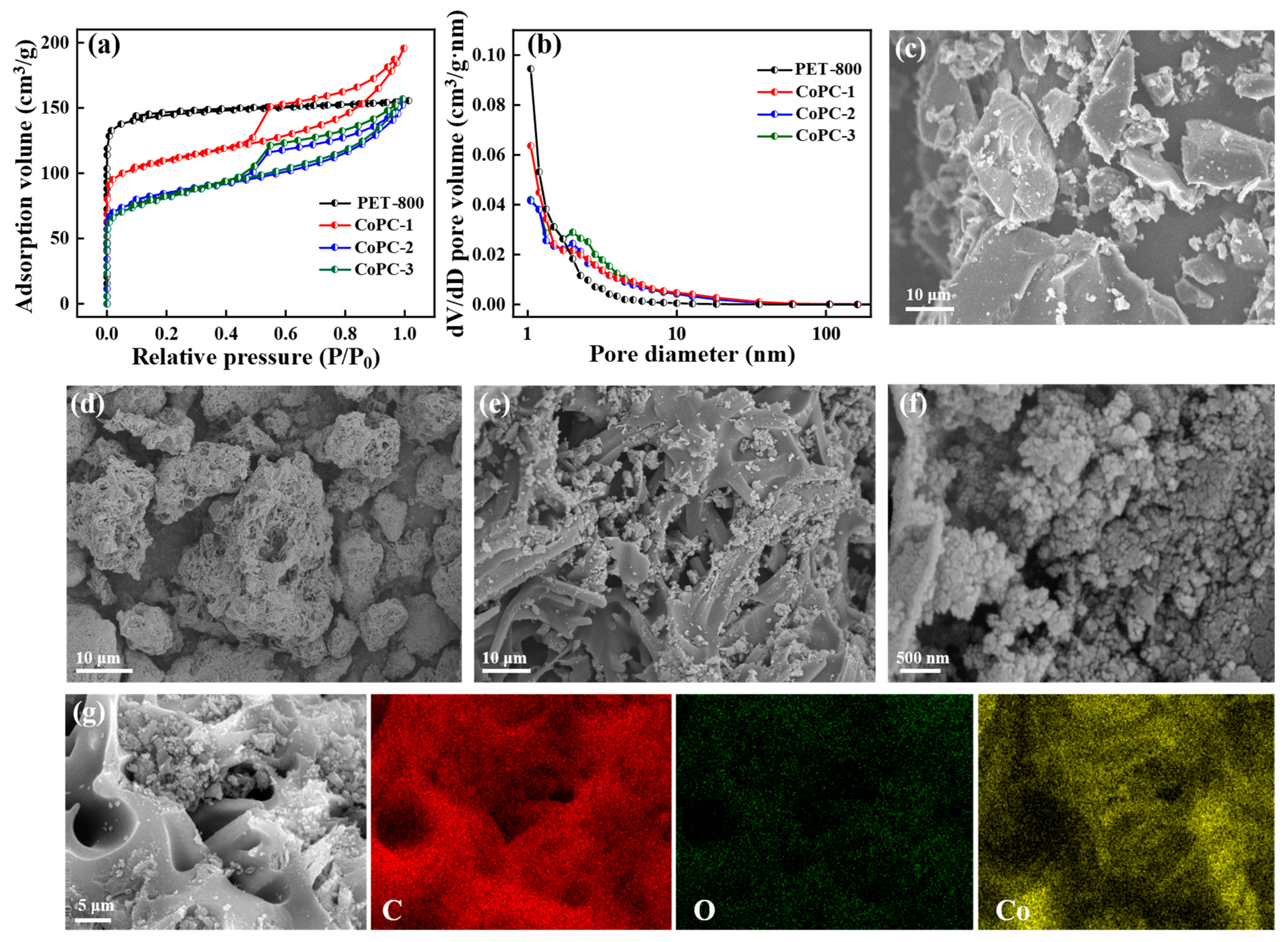
3.1. Characterization of the Nanocatalyst
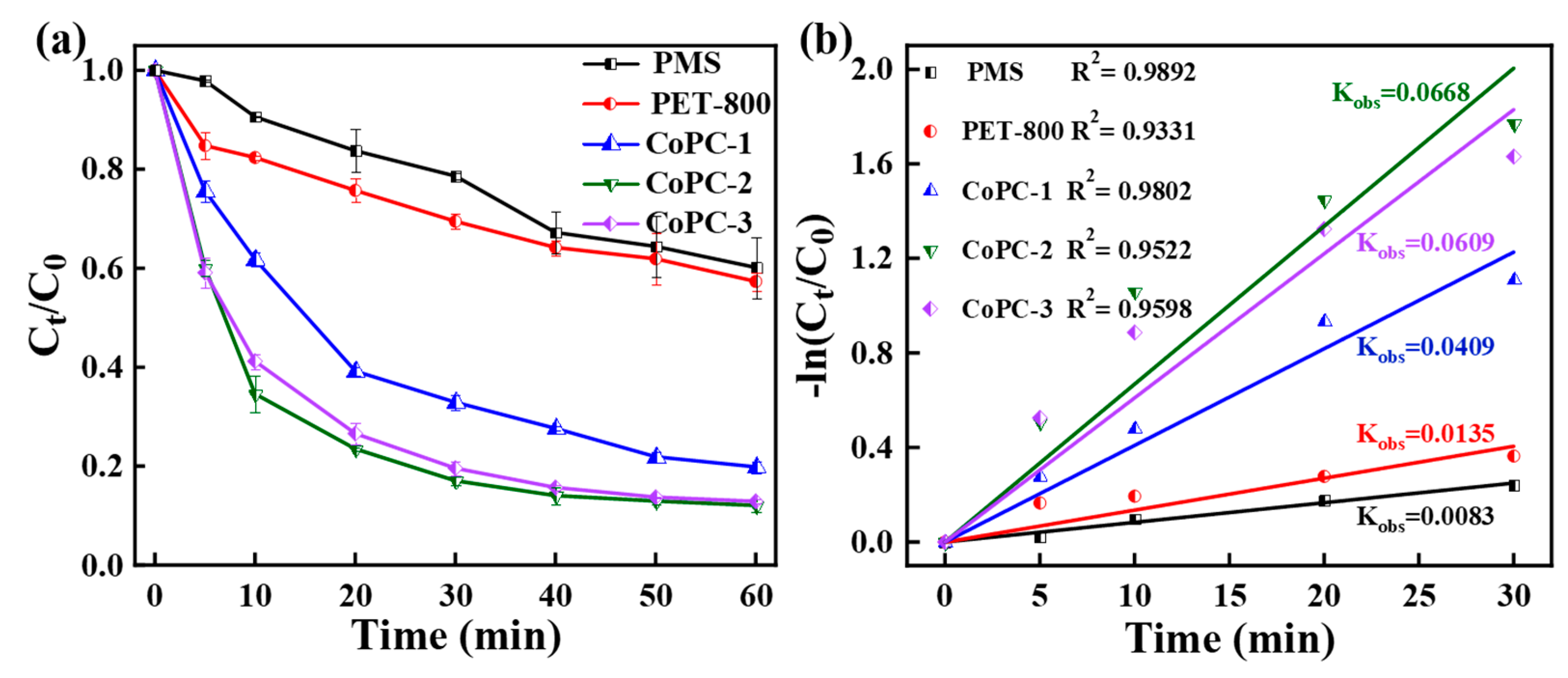
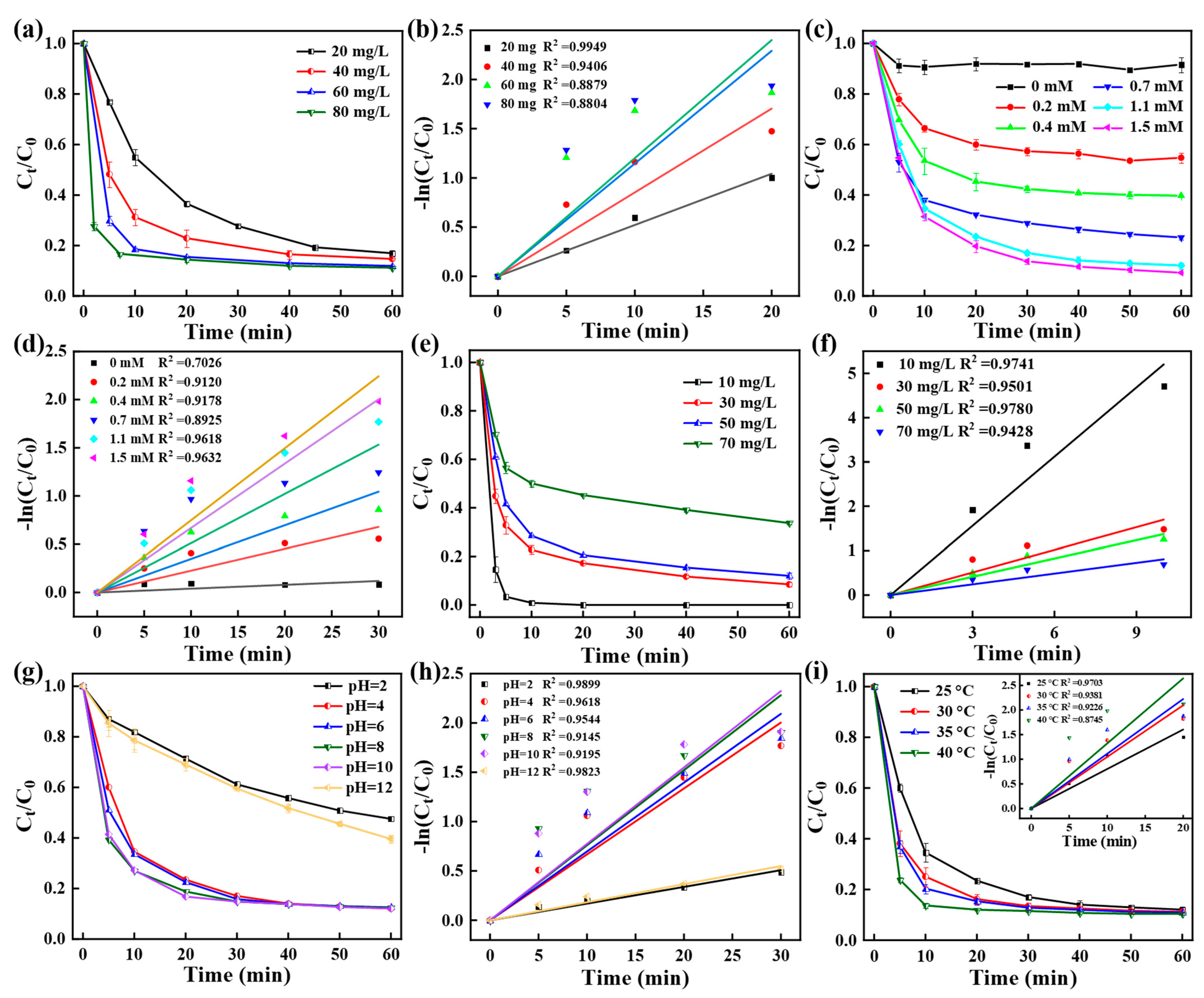
3.2. Catalytic Performance of CoPC Catalyst
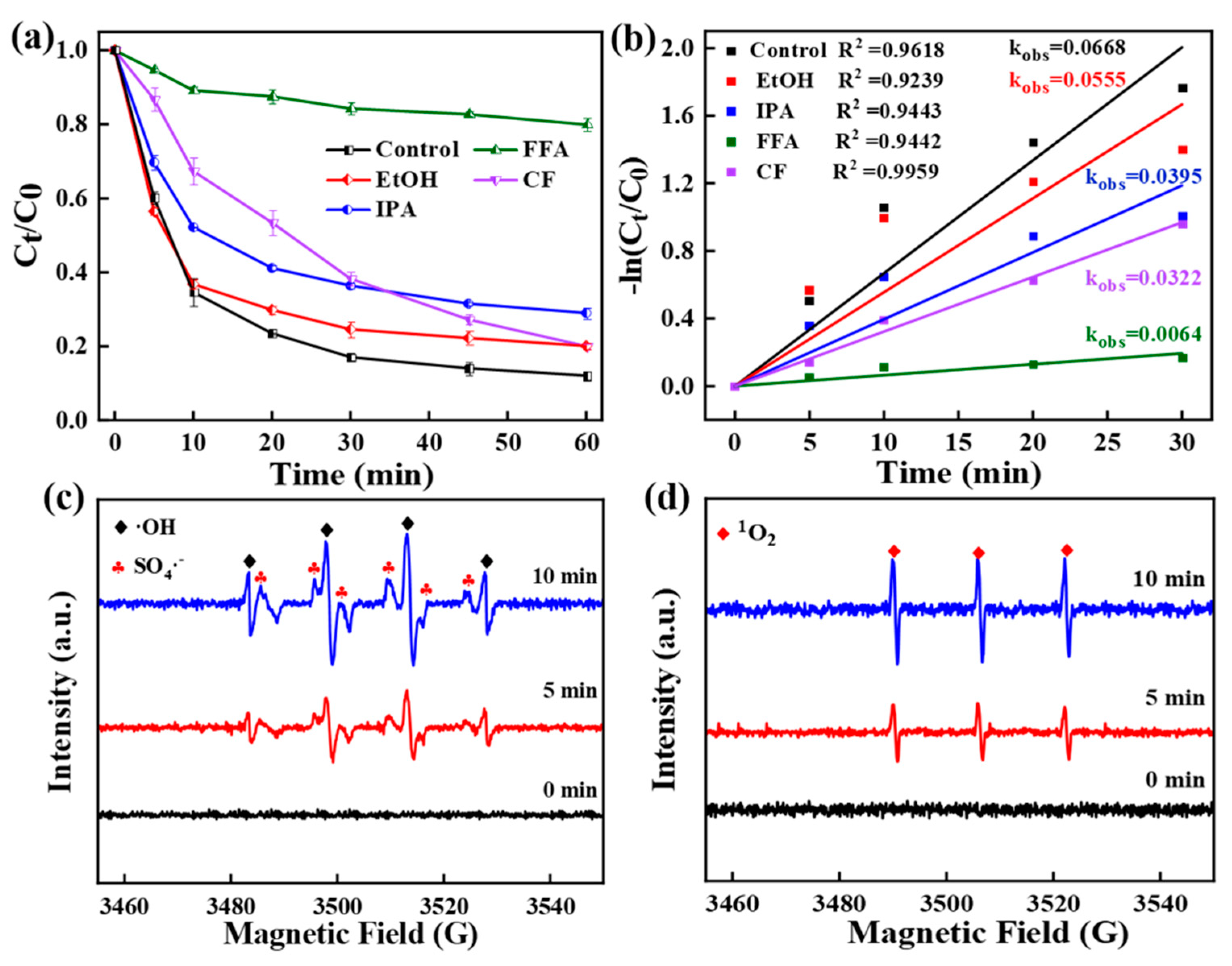
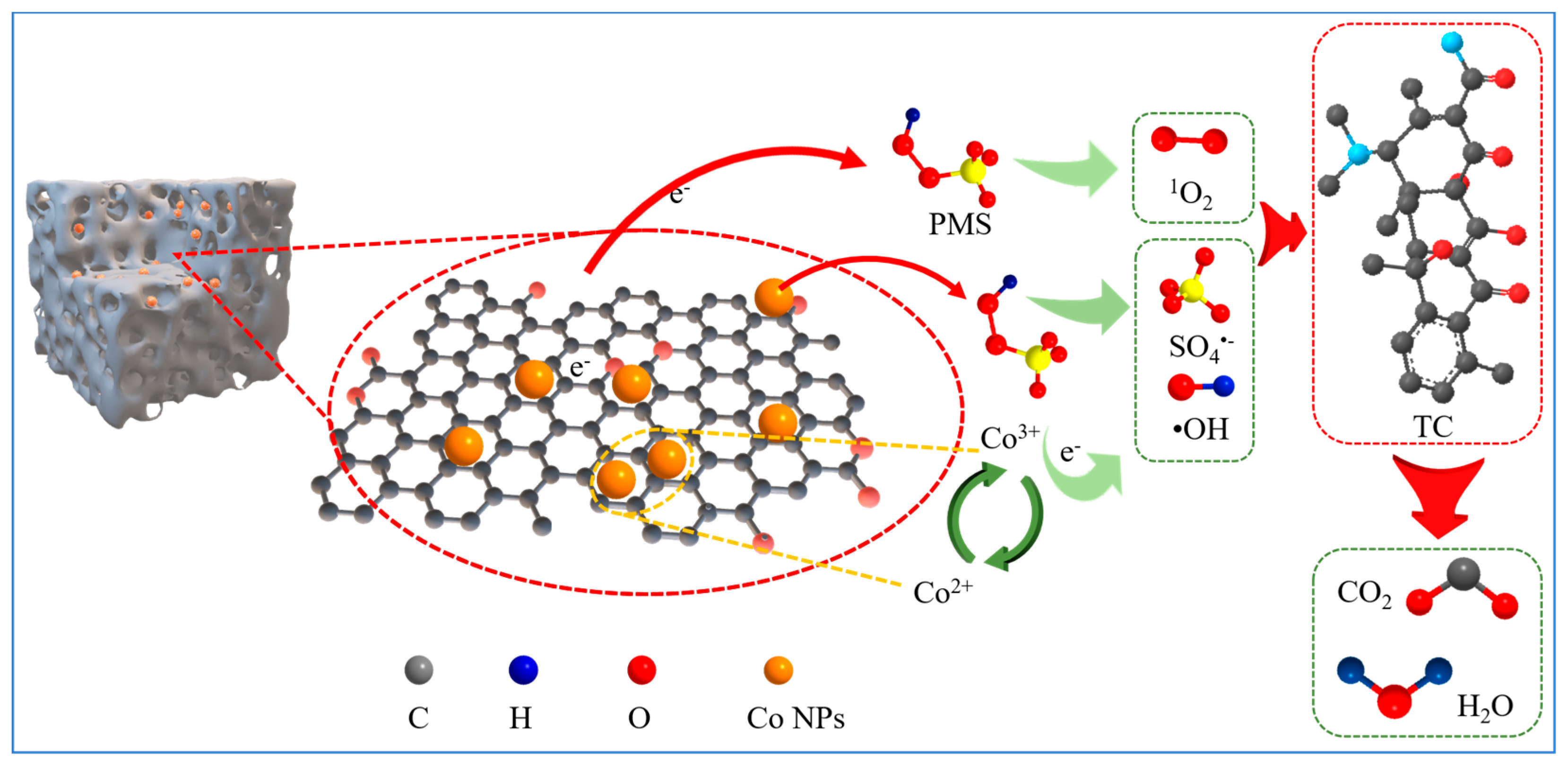
3.3. Discussion of Degradation Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Tong, Z.; Xie, Y.; Sun, H.; Gong, X.; Qin, P.; Liang, Y.; Yuan, X.; Zou, D.; Jiang, L. Efficient degradation of tetracycline by persulfate activation with Fe, Co and O co−doped g−C3N4: Performance, mechanism and toxicity. Chem. Eng. J. 2022, 434, 134732. [Google Scholar] [CrossRef]
- Zhang, H.; Smith, R.L.; Guo, H.; Qi, X. Cobalt cross-linked ordered mesoporous carbon as peroxymonosulfate activator for sulfamethoxazole degradation. Chem. Eng. J. 2023, 472, 145060. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Bao, S.; Tian, F.; Sheng, J.; Yang, W.; Yu, Y. Visible-light enhanced peroxymonosulfate activation on Co3O4/MnO2 for the degradation of tetracycline: Cooperation of radical and non-radical mechanisms. Sep. Purif. Technol. 2023, 316, 123779. [Google Scholar] [CrossRef]
- Cheng, M.; Huo, Y.; Diao, Z.; Song, G.; Chen, D.; Hang, L.; Kong, L. The role of the Co reduction and Zn evaporation of ZnCo-MOF carbonization in peroxymonosulfate activation for levofloxacin purification from wastewater. Sep. Purif. Technol. 2023, 327, 124976. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Jin, H.; Qian, L.; Wang, K.; Shen, Y.; Zhao, M.; Liu, R. Multivalent cobalt species supported on graphene aerogel for degradation of sulfamethoxazole via high-valent cobalt-oxo species. Chem. Eng. J. 2023, 463, 142367. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Li, M.; Zhu, Y.; Liang, J.; Yu, D.; Wang, Z.; Pei, J. Mechanistic insight into the degradation of sulfadiazine by electro-Fenton system: Role of different reactive species. J. Hazard. Mater. 2024, 469, 134063. [Google Scholar] [CrossRef]
- Erdem, H.; Erdem, M. Ciprofloxacin Degradation with Persulfate Activated with the Synergistic Effect of the Activated Carbon and Cobalt Dual Catalyst. Arab. J. Sci. Eng. 2022, 48, 8401–8415. [Google Scholar] [CrossRef]
- Qian, W.; Deng, Y.-L.; Liu, X.-L.; Liu, H.; Ye, M.-Y.; Li, Y.-Y.; Zhang, Y.-Z.; Diao, Z.-H.; Liang, J.-L. Degradation of ofloxacin by activation of persulfate with metal-N co-doped modified peanut shell biochar: The key role of cobalt doping. J. Water Process. Eng. 2025, 70, 106967. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Wang, Y.; Cai, Y.; Liu, Y.; Xu, Z.; Diao, Z. Persulfate oxidation of norfloxacin by cobalt doped water hyacinth biochar composite: The key role of cobalt and singlet oxygen. J. Water Process. Eng. 2024, 59, 104967. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Show, P.L.; Mahlknecht, J.; Wang, C. Degradation of tetracycline by nitrogen-doped biochar as a peroxydisulfate activator: Nitrogen doping pattern and non-radical mechanism. Sustain. Horiz. 2024, 10, 100091. [Google Scholar] [CrossRef]
- Long, S.; Hamilton, P.B.; Wang, C.; Li, C.; Xue, X.; Zhao, Z.; Wu, P.; Gu, E.; Uddin, M.M.; Li, B.; et al. Bioadsorption, bioaccumulation and biodegradation of antibiotics by algae and their association with algal physiological state and antibiotic physicochemical properties. J. Hazard. Mater. 2024, 468, 133787. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, S.; Qiu, X.; Zhao, S.; Wang, R.; Wang, Y. Electroactive Ultrafiltration Membrane for Simultaneous Removal of Antibiotic, Antibiotic Resistant Bacteria, and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2022, 56, 15120–15129. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, J.; Zhou, Q.; Qin, L.; Zhao, D.; Liu, H.; Yang, M.; Zhang, Y. Microwave assisted plastic waste derived O vacancies enriched cobalt oxide/porous carbon material for highly efficient carbamazepine degradation via peroxymonosulfate activation. Chem. Eng. J. 2024, 489, 151256. [Google Scholar] [CrossRef]
- Fang, C.; Nie, L.; Chen, H.; Yang, Y. Magnetic recyclable Co-MOF derivate-modified steel slag for tetracycline removal by integrated adsorption and Fenton-like catalysis. J. Water Process. Eng. 2024, 63, 105434. [Google Scholar] [CrossRef]
- Li, J.; Peng, X.; Zeng, P.; Shen, L.; Li, M.; Guo, Y. Removal of sulfonamides by persulfate-based advanced oxidation: A mini review. Chemosphere 2025, 370, 143874. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, X.; Wang, H.; Yu, J.; Sun, Y.; Chen, L.; He, M.; Liu, J.; Shi, H. MOFs-derived MnOx@C nanosheets for peroxymonosulfate activation: Synergistic effect and mechanism. Chem. Eng. J. 2022, 433, 133806. [Google Scholar] [CrossRef]
- Li, Q.; Wei, G.; Duan, G.; Zhang, L.; Li, Z.; Yan, F. Valorization of ball-milled waste red mud into heterogeneous catalyst as effective peroxymonosulfate activator for tetracycline hydrochloride degradation. J. Environ. Manag. 2022, 324, 116301. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.D.; Wang, C. Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Zhang, Y.; Mahlknecht, J.; Wang, C. A review of metallurgical slags as catalysts in advanced oxidation processes for removal of refractory organic pollutants in wastewater. J. Environ. Manag. 2024, 352, 120051. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, Y.; Li, C.; Jin, R.; Mutabazi, E. Different peroxymonosulfate activation and utilization pathways of typical cobalt oxides, cobalt-carbon and carbonaceous composites derived from metal-organic frameworks for pollutant oxidation in wastewater. Chem. Eng. J. 2023, 475, 146234. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, W.; Lan, Y.; Li, Y. Reutilization of waste self-heating pad by loading cobalt: A magnetic and green peroxymonosulfate activator for naphthalene degradation. J. Hazard. Mater. 2022, 439, 129572. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Xiao, Z.-J.; Zhou, B.-Q.; Feng, X.-C.; Shi, H.-T.; Zhu, Y.-N.; Wang, C.-P.; Van der Bruggen, B.; Ren, N.-Q. Anchored Co–oxo generated by cobalt single atoms outperformed aqueous species from the counterparts in peroxymonosulfate treatment. Appl. Catal. B Environ. 2023, 328, 122483. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Wang, S.; Wang, C. A review of cobalt-based heterogeneous catalysts for peroxomonosulfate activation and antibiotics degradation: Catalyst synthesis, characterizations, regulation, and mechanism. Sep. Purif. Technol. 2025, 356, 122483. [Google Scholar] [CrossRef]
- Yin, K.; Wu, R.; Shang, Y.; Chen, D.; Wu, Z.; Wang, X.; Gao, B.; Xu, X. Microenvironment modulation of cobalt single-atom catalysts for boosting both radical oxidation and electron-transfer process in Fenton-like system. Appl. Catal. B Environ. 2023, 329, 122558. [Google Scholar] [CrossRef]
- Wang, J.; Hasaer, B.; Yang, M.; Liu, R.; Hu, C.; Liu, H.; Qu, J. Anaerobically-digested sludge disintegration by transition metal ions-activated peroxymonosulfate (PMS): Comparison between Co2+, Cu2+, Fe2+ and Mn2+. Sci. Total Environ. 2020, 713, 136530. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Huang, P.; Lei, B.; Qiao, L.; Li, T.; Lin, K.-Y.A.; Wang, H. Mechanochemical synthesis of biochar encapsulated FeMn nanoparticles with strong metal–carbon interactions for efficient degradation of tetracycline via activating peroxymonosulfate. Chem. Eng. J. 2024, 479, 147525. [Google Scholar] [CrossRef]
- Gao, M.; Wang, L.; Yang, Y.; Sun, Y.; Zhao, X.; Wan, Y. Metal and Metal Oxide Supported on Ordered Mesoporous Carbon as Heterogeneous Catalysts. ACS Catal. 2023, 13, 4060–4090. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Liu, S.; Wang, D.; Zhou, X.; Zhang, M.; Li, L.; Li, X.; Xu, F.; Nie, J. Metal-carbon hybrid materials induced persulfate activation: Application, mechanism, and tunable reaction pathways. Water Res. 2023, 234, 119808. [Google Scholar] [CrossRef]
- Nan, H.; Huang, R.; Zhang, X.; Wang, C. How does ball-milling elevate biochar as a value-added peroxydisulfate activator for antibiotics removal? Ind. Crops Prod. 2024, 214, 118569. [Google Scholar] [CrossRef]
- Yao, C.; Qin, Y.; Li, Y.; An, Q.; Xiao, Z.; Wang, C.; Zhai, S. Activation of peroxymonosulfate by cobalt-embedded carbon aerogels: Preparation and singlet oxygen-dominated catalytic degradation insight. Sep. Purif. Technol. 2023, 307, 122728. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Wang, M.; Kang, J.; Zhang, J.; Liu, S.; Tang, Y.; Li, S. Peroxymonosulfate activation by cobalt particles embedded into biochar for levofloxacin degradation: Efficiency, stability, and mechanism. Sep. Purif. Technol. 2022, 294, 121082. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, X.; Lichtfouse, E.; Jiang, H.; Wang, C. Conversion of waste plastics into value-added carbon materials. Environ. Chem. Lett. 2023, 21, 3127–3158. [Google Scholar] [CrossRef]
- Dai, L.; Karakas, O.; Cheng, Y.; Cobb, K.; Chen, P.; Ruan, R. A review on carbon materials production from plastic wastes. Chem. Eng. J. 2023, 453, 139725. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Xi, W.; Zhou, H.; Yang, P.; Yao, J.; Jiang, X.; Wu, D. Upcycling plastic waste to carbon materials for electrochemical energy storage and conversion. Chem. Eng. J. 2023, 461, 141962. [Google Scholar] [CrossRef]
- Mong, G.R.; Tan, H.; Sheng, D.D.C.V.; Kek, H.Y.; Nyakuma, B.B.; Woon, K.S.; Othman, M.H.D.; Kang, H.S.; Goh, P.S.; Wong, K.Y. A review on plastic waste valorisation to advanced materials: Solutions and technologies to curb plastic waste pollution. J. Clean. Prod. 2024, 434, 140180. [Google Scholar] [CrossRef]
- Zhu, H.; He, D.; Duan, H.; Yin, H.; Chen, Y.; Chao, X.; Zhang, X.; Gong, H. Study on coupled combustion behaviors and kinetics of plastic pyrolysis by-product for oil. Energy 2023, 262, 125452. [Google Scholar] [CrossRef]
- Su, J.; Li, T.; Xie, W.; Wang, C.; Yin, L.; Yan, T.; Wang, K. Emerging Technologies for Waste Plastic Treatment. ACS Sustain. Chem. Eng. 2023, 11, 8176–8192. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.H. Advancements and future directions in waste plastics recycling: From mechanical methods to innovative chemical processes. Chem. Eng. J. 2024, 493, 152727. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Jiang, S.; Hou, H. Carbonization: A feasible route for reutilization of plastic wastes. Sci. Total Environ. 2020, 710, 136250. [Google Scholar] [CrossRef]
- Padha, B.; Verma, S.; Prerna; Ahmed, A.; Patole, S.P.; Arya, S. Plastic turned into MXene–based pyro-piezoelectric hybrid nanogenerator-driven self-powered wearable symmetric supercapacitor. Appl. Energy 2024, 356, 122402. [Google Scholar] [CrossRef]
- Ren, S.; Xu, X.; Zhu, Z.-S.; Yang, Y.; Tian, W.; Hu, K.; Zhong, S.; Yi, J.; Duan, X.; Wang, S. Catalytic transformation of microplastics to functional carbon for catalytic peroxymonosulfate activation: Conversion mechanism and defect of scavenging. Appl. Catal. B Environ. 2024, 342, 123410. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Wang, L.; Liu, G.; Boczkaj, G. Valorization of waste plastics to a novel metal-organic framework derived cobalt/carbon nanocatalyst as peroxymonosulfate activator for antibiotics degradation. J. Clean. Prod. 2025, 486, 144539. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, Y.; Shen, K.; Xue, Z.; Wu, Y.; Yang, Z.; Bao, Y. Synthesis of high-value porous carbon from waste plastics and structure regulation promote solar interface water evaporation. Sep. Purif. Technol. 2025, 355, 129622. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, L.; Dong, W.; Jiang, M. Solving two environmental problems simultaneously: Microporous carbon derived from mixed plastic waste for CO2 capture. Chemosphere 2023, 345, 140546. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, Y.; Yang, Y.; Tong, X.; Liu, W. Plastic/rubber waste-templated carbide slag pellets for regenerable CO2 capture at elevated temperature. Appl. Energy 2019, 242, 919–930. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Ren, Z.; Wang, Z.; Mao, F.; Wu, H.; Zhou, R.; Bu, Y. Catalytic degradation of antibiotic by Co nanoparticles encapsulated in nitrogen-doped nanocarbon derived from Co-MOF for promoted peroxymonosulfate activation. Chem. Eng. J. 2022, 429, 132269. [Google Scholar] [CrossRef]
- Luo, J.; Bo, S.; Qin, Y.; An, Q.; Xiao, Z.; Zhai, S. Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation. Chem. Eng. J. 2020, 395, 125063. [Google Scholar] [CrossRef]
- Lam, D.V.; Nguyen, U.N.T.; Roh, E.; Choi, W.; Kim, J.H.; Kim, H.; Lee, S.M. Graphitic Carbon with MnO/Mn7C3 Prepared by Laser-Scribing of MOF for Versatile Supercapacitor Electrodes. Small 2021, 17, 2100670. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.G.; Ehlen, N.; Senkovskiy, B.V.; Hasdeo, E.H.; Fedorov, A.; Dombrowski, D.; Busse, C.; Michely, T.; di Santo, G.; Petaccia, L.; et al. Resonance Raman Spectrum of Doped Epitaxial Graphene at the Lifshitz Transition. Nano Lett. 2018, 18, 6045–6056. [Google Scholar] [CrossRef] [PubMed]
- Mortezaeikia, V.; Tavakoli, O.; Khodaparasti, M.S. A review on kinetic study approach for pyrolysis of plastic wastes using thermogravimetric analysis. J. Anal. Appl. Pyrolysis 2021, 160, 105340. [Google Scholar] [CrossRef]
- Zhu, Y.; Miao, J.; Zhang, Y.; Li, C.; Wang, Y.; Cheng, Y.; Long, M.; Wang, J.; Wu, C. Carbon nanotubes production from real-world waste plastics and the pyrolysis behaviour. Waste Manag. 2023, 166, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Martín, D.; Varela, A.; González-Calbet, J.M.; Matesanz, E.; Parras, M. Revisiting the Decomposition Process of Tetrahydrate Co(II) Acetate: A Sample’s Journey through Temperature. Appl. Sci. 2022, 12, 6786. [Google Scholar] [CrossRef]
- Dai, L.; Karakas, O.; Lata, S.; Cobb, K.; Lei, H.; He, C.; Cheng, Y.; Chen, P.; Ruan, R. Holistic utilization of waste plastics through a tandem process. J. Environ. Chem. Eng. 2023, 11, 110547. [Google Scholar] [CrossRef]
- Chen, M.-M.; Niu, H.-Y.; Niu, C.-G.; Guo, H.; Liang, S.; Yang, Y.-Y. Metal-organic framework-derived CuCo/carbon as an efficient magnetic heterogeneous catalyst for persulfate activation and ciprofloxacin degradation. J. Hazard. Mater. 2022, 424, 127196. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, M.; Song, Z.; Guan, Y.; Wang, L.; Zhang, Q. Coral-like carbon-based composite derived from layered structure Co-MOF-71 with outstanding impedance matching and tunable microwave absorption performance. J. Mater. Sci. Technol. 2022, 108, 10–17. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, X.; Li, Y.; Li, R.; Wu, C.; Wang, L.; Wang, C.; Yang, T.; Ge, M.; He, Z. Cobalt-loaded carbon nanofibers as magnetic catalyst for tetracycline degradation through peroxydisulfate activation: Non-radical dominated mechanism. J. Water Process. Eng. 2024, 57, 104600. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Gao, Y.; Lin, P.; Liu, J.; Zhang, J.; Huang, T. The catalyst derived from the sulfurized Co-doped metal–organic framework (MOF) for peroxymonosulfate (PMS) activation and its application to pollutant removal. Sep. Purif. Technol. 2022, 285, 120362. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, N.; Wang, D.; Wang, L.; Song, Y.; Chen, Z.; Ma, J.; Zhang, T. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways. Appl. Catal. B Environ. 2020, 263, 118350. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, Q.; Ren, S.; Zhang, H.; Tian, W.; Sun, H.; Wang, S. Effects of cobalt salts on biomass conversion to functional carbon-based catalysts for peroxymonosulfate activation. Chem. Eng. J. 2023, 469, 143856. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, X.; Zhang, Y.; Wang, J.; Wang, C. Nano Cobalt-Loaded Porous Carbon Derived from Waste Plastic for Efficient Persulfate Activation and Tetracycline Degradation. Nanomaterials 2025, 15, 371. https://doi.org/10.3390/nano15050371
Luo Y, Zhang X, Zhang Y, Wang J, Wang C. Nano Cobalt-Loaded Porous Carbon Derived from Waste Plastic for Efficient Persulfate Activation and Tetracycline Degradation. Nanomaterials. 2025; 15(5):371. https://doi.org/10.3390/nano15050371
Chicago/Turabian StyleLuo, Yueyue, Xiuxiu Zhang, Yu Zhang, Jianchao Wang, and Chongqing Wang. 2025. "Nano Cobalt-Loaded Porous Carbon Derived from Waste Plastic for Efficient Persulfate Activation and Tetracycline Degradation" Nanomaterials 15, no. 5: 371. https://doi.org/10.3390/nano15050371
APA StyleLuo, Y., Zhang, X., Zhang, Y., Wang, J., & Wang, C. (2025). Nano Cobalt-Loaded Porous Carbon Derived from Waste Plastic for Efficient Persulfate Activation and Tetracycline Degradation. Nanomaterials, 15(5), 371. https://doi.org/10.3390/nano15050371






